Abstract
Background
AB-CHMINACA is a cannabimimetic indazole derivative. In 2013, it was reported in different countries as a substance of abuse.
Purpose
This study evaluated the subacute toxic effects of AB-CHMINACA on the liver and kidneys and measured its blood level in adult male mice.
Methods
The histological and biochemical subacute toxic effects on the liver and kidneys were assessed after four weeks of daily intraperitoneal injections of one of the following doses: 0.3 mg/kg, 3 mg/kg, or 10 mg/kg as the highest dose in adult male albino mice. In addition, the blood concentration level of AB-CHMINACA was determined by GC–MS-MS.
Results
The histological effects showed congestion, hemorrhage, degeneration, and cellular infiltration of the liver and kidney tissues. Considering the control groups as a reference, biochemical results indicated a significant increase in the serum AST only in the highest dose group, while the ALT and creatinine levels did not significantly change. The mean values of AB-CHMINACA blood levels were 3.05 ± 1.16, 15.08 ± 4.30, and 54.43 ± 8.70 ng/mL for the three treated groups, respectively, one hour after the last dose of intraperitoneal injection. The calibration curves were linear in the 2.5–500 ng/mL concentration range. The intra-assay precision and accuracy of the method were less than 7.0% (RSD) and ± 9.2% (Bias).
Conclusion
This research supports the available case reports on AB-CHMINACA toxicity that it has low lethality; still, the chronic administration causes evident liver and kidney histotoxic effects even at low doses with unnoticeable clinical effects in mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
AB-CHMINACA (N-[(2S)-1-amino-3-methyl-1-oxobutan-2-yl]-1-(cyclohexyl-methyl) indazole-3-carboxamide) is a third-generation synthetic cannabinoid (SC) which is an indazole carboxamide derivative containing L-Valinamide at the 3-carboxamide position [1]. In 2009, AB-CHMINACA was first synthesized as a potential medicinal drug, but in 2013, it was reported in different countries as a substance of abuse [2].
Cannabinoids exert their effects primarily through activating two cannabinoid receptors, cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2) [3]. AB-CHMINACA has a high affinity to bind to those receptors and is estimated to be 11 to 58 times more potent than tetrahydrocannabinol (THC) in mice [4]. Numerous observational studies have reported symptoms of acute AB-CHMINACA toxicity, with neuropsychiatric symptoms being the most frequently reported. These included symptoms ranging from mild depression and disorientation to severe agitation, convulsions, and acute psychosis. These were followed by gastrointestinal symptoms and a wide range of cardiorespiratory presentations [5,6,7], and to a lesser extent, acute renal injury, fulminant liver failure, and stroke were observed [8,9,10].
Accurate estimation of the toxic and lethal doses of AB-CHMINACA for humans is challenging due to the nature of the commercial manufacturing method. The substance is usually dissolved in a vehicle and sprayed over herbs, making it unevenly distributed, which results in highly variable consumption levels of the illicit substance [11]. Most reported acute toxicity cases ended with complete recovery; death was uncommon [12]. Deaths from toxicity by SC compounds were either due to direct vital organ toxicity or severe CNS depression. In most cases, alcohol or other drugs of abuse were consumed concurrently, which may have augmented the toxic effects, or reduced the toxic dose of SCs [13].
Conventional THC detection kits cannot identify synthetic cannabinoids due to differences in their chemical structure, so sensitive and precise chromatographic techniques are favored, such as gas-chromatography mass spectrometry (GC–MS), high-performance liquid chromatography (HPLC) and liquid chromatography time of flight mass spectrometry (LCTOF-MS) [14, 15]. SCs levels can be detected in various biological samples, such as blood, urine, hair, and some tissues [16].
This study aimed to evaluate the subacute toxic effects of AB-CHMINACA on the liver and kidneys in adult male albino mice. A secondary aim was the detection of the AB-CHMINACA levels in the blood samples of treated mice in relation to different given doses.As the lethal dose of AB-CHMINACA was unknown, a preliminary experiment was done to detect the median lethal dose (LD50). LD50 was used as a guide for properly selecting doses used in the main experiment. In this study, we try to shed some light on the possible sublethal toxic effects of long-term drug administration that may impact chronic drug users and to understand better the acute toxic effects of SCs reported by various emergency units.
Material and method
Animals
Adult drug-naïve male Swiss albino mice weighing 30 ± 5 g were used; 12 mice for the LD50 experiment and 50 mice for the subacute toxicity experiment. The animals, a maximum of five mice per cage, were acclimatized to the lab conditions one week before experimentation in a controlled room temperature (22 ± 2 °C) and 12-h dark–light cycles with free access to water and pellet feed.
Chemicals
A solid form of AB-CHMINACA was purchased from Cayman Chemicals, USA. The substance was dissolved in absolute ethanol followed by dilution in normal saline (ethanol: saline 1:9) immediately before injection. Human diagnostic kits for ALT and AST were from Roche Diagnostics USA.
Instrument
Cobas C311 autoanalyzer (Roche Diagnostics, USA) was used for serum ALT, AST and creatinine measurement. Agilent GC–MS/MS model 7890B GC equipped with 7000C MS, Autosampler (Agilent 7693), and capillary column (HP-5MS 5% Phenyl Methyl Silox: 30 m x 250 µm, 0.25 µm) were used for detection and quantification of AB-CHMINACA in blood. A Leica DM500 microscope with an ICC50 camera system was used for histological examination.
Method
The LD50 experiment
The LD50 was determined according to Lorke [17], which allows approximate LD50 detection using a limited number of animals in a two-step experiment. In the first step, nine mice were equally divided into three groups, each receiving intraperitoneal (IP) injection of AB-CHMINACA in a dose of 10, 100, or 1000 mg/kg. The animals were monitored continuously for eight hours, then every four hours for the remaining of the 1st 24 h, and daily for 14 days. Based on the number of deceased and living animals in the first step, further four doses (50, 100, 200, and 400 mg/kg) were chosen for the second step using a table proposed by Lorke. Each dose was given to a single mouse except for the 100 mg/kg dose which was already assessed in the first step. The animals were monitored using the same schedule as the first step, and then the LD50 was computed based on the dead-to-living animals ratio. LD50 was determined by taking the geometric mean of the two subsequent doses that showed 0% and 100% death (the highest nonlethal and the lowest lethal doses).
The subacute experiment
A total of fifty mice were allocated into five equal groups. Group A did not receive any treatment (negative control). Group B received the vehicle only (ethanol: saline) (positive control), while groups C, D, and E received daily IP AB-CHMINACA injections at doses of 0.3, 3, and 10 mg/kg/day, respectively, for four weeks. Clinical effects such as excitation and depression were recorded daily, and weekly body weights were taken. The animals were sacrificed under light anesthesia one hour after the last dose. Samples from jugular venous blood were collected for biochemical and toxicological analysis.
Biochemical analysis
Collected blood samples were centrifuged, and serum levels of AST, ALT, and creatinine were measured to assess liver and kidney functions.
Histopathological evaluation
Animals were dissected, and the liver and kidney were harvested and fixed in 10% neutral buffered formalin and transferred to 70% ethanol after two days. Tissues were processed, kept in paraffin blocks, and sectioned to a thickness of 4 µm. Hematoxylin and eosin (H&E) were used to stain the tissues before inspection under the light microscope.
Toxicological analysis
Whole blood samples were preserved with sodium fluoride (2 mg/mL) and potassium oxalate (2 mg/cc) as an anticoagulant and stored at -20 °C. Sample preparation started with adding 50 µL of Granisetron as internal standard (IS) (5.0 µg/mL) to a 200 µL blood sample. Acetonitrile (0.5 mL) was applied, vortexed for a minute, and centrifuged for 5 min. The supernatant was transferred to a 3-mL polypropylene tube with Phosphate buffer (2.5 mL, pH6) and then mixed for 1 min.
Extraction of samples was done by a C18 Solid-phase extraction (SPE) cartridge (CHROMABOND, Macherey–Nagel) [18]. The cartridge was conditioned by successively adding 3 mL of each; methanol, distilled water, and phosphate buffer (pH 6); the sample was left to flow under gravity. Washing was done by adding 3 mL of water followed by 3 mL of 5% acetonitrile. The cartridge was dried under a pressure of 20 psi for 20 min. 2 mL of freshly prepared dichloromethane: isopropanol (4:1) was used to separate AB-CHMINACA from the SPE cartridge. The solvent was evaporated by nitrogen steam at room temperature. Finally, samples were reconstituted by adding 50 µL of ethyl acetate and transferred to a GC vial for analysis. 4 µL was injected into the GC inlet.
GC–MS-MS analysis was performed by helium as a carrier gas at a flow rate of 1 mL/min. The oven column temperature started at 200º C, held for 2 min, and then increased at a ramp of 20º C/min to 320º C, and then held for 6 min. The total run time was 12 min. Tandem mass spectrometry (MS/MS) was set to positive chemical ionization (PCI) mode. The ion source temperature was 300 ̊C, and the transfer line temperature was 280 ̊C. The used ionization gas was methane. The collision gas was argon. The multiple reaction monitoring (MRM) mode was applied to detect and quantify the compounds. MRM transitions and collision energies (CE) for AB-CHMINACA and IS are shown in Fig. 1.
The specificity, sensitivity, limit of detection (LOD), limit of quantification (LOQ), Linearity, precision, and accuracy were determined according to FDA (2018) guidelines [19]. Specificity of the method was evaluated by analysis of 7 blank blood collected from the negative control group. LOD and LOQ were calculated at signal-to-noise ratios (S/N) ≥ 3 and ≥ 10, respectively. Linearity of the method was determined by evaluation of the correlation coefficient (r2) for calibration curve. The calibration curve was prepared over the 2.5, 5, 25, 50, 100, and 500 ng/mL concentration range. The calibration curve was established by plotting the peak area ratios (analyte/IS) against the analyte concentrations. The intra-assay accuracy (% bias) and precision (% RSD) of the method was performed by analyzing six replicating of three quality control samples at concentrations of 7.5, 75, and 450 ng/mL. The values of acceptance for bias and RSD were ± 15% and 15%, respectively of the target compound.
Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences “SPSS”software version 24. Normally distributed data were expressed as mean ± SD. Significance was tested by One-way ANOVA and post-Hoc tests at a P-value < 0.05. Data with skewed distribution were presented as median, minimum, and maximum. Significance was tested by Kruskal–Wallis H and Mann–Whitney U tests at a P-value < 0.05.
Results
Calculation of LD50
The numbers of dead animals in the first and second steps of the experiment are shown in Table 1. The calculated IP LD50 for AB-CHMINACA was 282.84 mg/kg.
Subacute toxicity
Mice from groups A, B, and C did not show any remarkable signs, while groups D and E demonstrated a short period of excitement followed by depression. The period of depression was brief in group D, about 15 min, and started to decrease gradually until complete disappearance on day ten. However, mice in group E showed a more extended period of depression, reaching about two hours in the experiment's early days, which started to slowly become briefer until reaching only 14 min on day 28. Certain animals in group E showed catalepsy, tachypnea or bradypnea, labored respiration, and generalized hair erection. There were no deaths except one mouse from group D, which died on the first day (mostly due to postural asphyxia secondary to CNS depression). Weekly weight measurements showed no statistically significant difference in animal weight across all groups, Table 2.
Biochemical analysis showed no significant differences in serum ALT across the five groups. However, serum AST was significantly higher in group E compared to the negative and positive control groups (p-value 0.034 and 0.004, respectively), with no significant differences between other study groups. As regards serum creatinine, there were no significant differences between the study groups, Table 3.
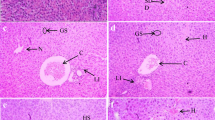
The histopathological evaluation of both control groups showed a typical architecture of the hepatic lobules. The hepatic vessels and intrahepatic bile ducts were on average diameters, and the hepatocytes had a regular polyhedral shape with acidophilic cytoplasm and basophilic vesicular nuclei. On the other hand, liver sections from group C revealed mild dilatation and congestion of the central vein, portal vein, and blood sinusoids, and the hepatocytes showed hydropic degeneration. Sections from group D showed further dilatation and congestion of the hepatic vasculature with an accidental intraluminal thrombus, inflammatory cellular infiltration with foci of aggregation and hydropic degeneration, and apoptosis of the hepatocytes. In group E, the effects were considerably worse, where the liver architecture was disturbed with more significant vascular dilatation, congestion, and interstitial hemorrhage. Significant cellular infiltration and many inflammatory foci were present, and the liver cells showed different stages of degeneration and apoptosis, particularly around the portal vein, Fig. 2.
Photomicrographs of liver tissue by H&E. a and b section from the negative control and positive control group showing normal hepatocytes, central vein (CV), and portal vein (P). c section from group C (0.3 mg/kg) showing congested and dilated portal vein (P) and sinusoids (S). d section from group D (3 mg/kg) showing severely congested and dilated portal vein (P) and sinusoids (S), large foci of inflammatory cell infiltration (IN), and hepatocytes showing degeneration and apoptosis (H). e section from group E (10 mg/kg) showing severely congested and dilated portal vein (P), foci of inflammatory cell infiltration (IN), and hepatocytes showing degeneration and apoptosis (H). There is an area of interstitial hemorrhage (hg). (Magnification × 200)
Examination of the kidney tissues in groups A and B showed preserved normal structure. The renal corpuscles and their capillary tufts, the proximal convoluted tubules, the distal convoluted tubules, the loop of Henle, and the collecting tubules were normal. Sections from group C showed dilated renal tubules with hydropic degeneration of the tubular epithelium in some tubules and flattening in others. The renal glomeruli were slightly affected, and the stroma was infiltrated by inflammatory cells. Group D showed expanded renal tubular lumens with flattened and degenerated epithelium, widened glomerular space, dilated and congested renal vasculature, and interstitial hemorrhage. Similar but more severe changes were also detected in group E, Fig. 3.
Photomicrographs of kidney tissue by H&E. a and b section from the negative control and positive control group showing normal proximal tubules (P.T.), distal tubules (D.T.) and renal glomeruli (G). c: section from group C (0.3 mg/kg) showing dilated proximal tubules (P.T.) and distal tubules (D.T.) with flattening of the lining epithelium. The renal glomeruli (G) are slightly affected. There is an area of inflammatory cell infiltration (IN). d section from group D (3 mg/kg) showing dilated proximal tubules (P.T.) and distal tubules (D.T.) with flattening of the lining epithelium. The renal glomeruli (G) show widened space. There are areas of hemorrhage (hg) and congestion (C.O.). e section from group E (10 mg/kg) showing dilated proximal tubules (P.T.) and distal tubules (D.T.) with flattening of the lining epithelium. The renal glomeruli (G) show widened space. There are areas of hemorrhage (hg) and congestion (C.O.). (Magnification × 200)
GC–MS-MS analysis showed no interfering peaks in blank samples at the retention times for AB-CHMINACA and IS, Fig. 4. The LOD and LOQ for AB-CHMINACA were 1.25 and 2.5 ng/mL, respectively. The calibration curve of AB-CHMINACA was linear over the range of 2.5–500 ng/mL (r2 > 0.99). Table 4 illustrates the values of accuracy and precision for AB-CHMINACA. As shown in the table, the values of RSD and bias were within the acceptable limit recommended by FDA (2018) guidelines.
MRM chromatogram for the analysis of AB-CHMINACA in blood samples of the studied groups. a Spiked blood sample at 50 ng/mL of AB-CHMINACA. b Blank blood sample. c Blood sample from group C. d Blood sample from group D. e Blood sample from group E. The mean values of AB-CHMINACA levels in groups C, D, and E were 3.05 ± 1.16, 15.08 ± 4.30, and 54.43 ± 8.70 ng/mL, respectively
The mean values of AB-CHMINACA level after one hour from the last IP injection in groups C, D, and E were 3.05 ± 1.16, 15.08 ± 4.30, and 54.43 ± 8.70 ng/mL, respectively.
Discussion
SCs pose a public health hazard due to the lack of knowledge of their toxicity and unidentified negative health impacts and are often ingested in combination with other substances [20].
This study found that AB-CHMINACA has low lethality according to the toxicity classification of substances for IP administration by Berezovskaya [21], as the calculated LD50 was 282.84 mg/kg. Although there are no LD50 studies for AB-CHMINACA in the literature, our findings are comparable to those of studies on THC and other cannabinoids [22,23,24], even though the LD50 of a substance can vary depending on several variables, including the animal used, the route of administration, and the vehicle used [22, 25].
Although the lethal dose is high, the clinical effects of SCs appear at low doses. AB-CHMINACA (dose range 0.03 to 3 mg/kg, IP injection) showed complete substitution of THC and produced the tetrad response; motor depression, catalepsy, decreased pain sensation, and hypothermia in mice [4, 26, 27] which was similar to our results. In our experiment, the duration of action was notably short, which can be explained by the rapid metabolism of AB-CHMINACA [28], and the period of depression had much decreased with repeated injections, suggesting the development of tolerance as described with the synthetic cannabinoid AB-FUBINACA [29, 30]. Drug tolerance in our experiment could also be due to the concurrent use of ethanol which may have led to decreased sensitivity to SC [31], a common combination used by addicts.
The present study showed no effect of AB-CHMINCA on mice's body weight, reflecting no impact on the appetite. Interestingly, previous reports by De Vry et al. [32], Williams and Kirkham [33], Freedland et al. [34], Foltin et al. [35], and Beal et al. [36] showed that THC and its derivatives increase appetite in both humans and animals while cannabinoid antagonists are appetite suppressants. The different results could be attributed to the varying routes of administration, as reported by Manwell et al. [37], who found that vapored THC was associated with increased food intake in rats, while IP administration of THC did not affect food consumption.
The liver is the primary site of most exogenous and endogenous compounds metabolism; hence, it is a potential target for xenobiotic toxicity [38]. Cases of liver toxicity following a history of SCs consumption have been reported, which were diagnosed by elevated ALT and AST, bilirubin level, alkaline phosphatase, and INR. Variable degrees of liver toxicity were reported; however, most of those patients eventually improved, which suggests that the liver damage was at least partially reversible [39, 40].
The available literature did not have any histopathological evaluation of the chronic toxicity of AB-CHMINACA. However, the histopathological results of the present study are close to those reported in acute toxicity studies of other SCs, as mentioned by Abass et al. [23] and Bakdash et al. [24], who explored the LD50 of voodoo extract and THJ-2201 in rodents. Similarly, Abdelmoneim et al. [41] mentioned comparable results after chronic oral administration of Strox in Rats. The biochemical assay in the present study showed an increase in serum AST in the higher-dose group and no change in serum ALT which can be explained by injecting low doses of AB-CHMINACA and the liver's capacity to compensate for damage to an extent to preserve its function. Furthermore, AST is elevated not only by liver damage but by other organ affection, such as the heart and muscles, which is expected to be present [41,42,43].
Most substances are urinary excreted, so it is crucial to evaluate the toxic effects of any drug on the renal tissue [44]. Bhanushali et al. [45], Buser et al. [46], and Gudsoorkar and Perez [47], who supported our results, reported symptoms of acute kidney injury and renal failure in many cases after consumption of different types of SCs with elevated serum urea, creatinine and BUN, while urine analysis was positive for proteins and casts. Examination of the renal biopsy showed dilated renal tubules with epithelial degeneration and crystals inside the lumen and inflammatory cell infiltration in the surrounding stroma. Also, Abbas et al. [23] and Bakdash et al. [24], who explored the LD50 of THJ-2201 and voodoo extract, showed similar histopathological changes in the kidney.
Tissue damage caused by AB-CHMINACA could be attributed to the oxidative stress caused by the direct effect on mitochondrial respiratory enzymes with the decrease in the rate of O2 consumption and increase in the levels of hydrogen peroxide [48]. In addition to the direct toxic effect, nephrotoxicity in some cases could be due to rhabdomyolysis from convulsion and agitation [49].
This study proposed a GC–MS-MS detection method that could detect AB-CHMINACA in whole blood samples of the treated animals, one hour after the last dose of repeated IP injection. Some researchers found it possible to identify the parent drug in postmortem human blood samples [28, 50], while others could not [51]. The different results could be attributed to many factors, including the dose of exposure (which is difficult to be calculated in abusers due to the manufacturing process), the frequency of exposure, and the time between exposure and sampling, which is lacking in most studies. It is known that AB-CHMINACA is rapidly metabolized in the body [28], although there is no information about how long the parent drug would take to disappear from blood samples.
Conclusion and recommendations
AB-CHMINACA is a synthetic cannabinoid substance of low lethality with LD50 of 282.84 mg/kg via the IP route. The central nervous system and respiratory systems were the most obviously clinically affected. There were evident histotoxic effects on the liver and kidney even at low dosages without clinically significant consequences, and these effects increased with exposure time and dose.
The proposed GC–MS-MS method for detecting AB-CHMINACA in blood samples was successfully applied to mice blood samples in the present study. However, further research is recommended to find the parent compound blood levels at different time intervals and correlate them to the levels of its major metabolites to establish the detection window for AB-CHMINACA as it is rapidly metabolized.
References
Longworth M, Banister SD, Mack JBC, Glass M, Connor M, Kassiou M (2016) The 2-alkyl-2H-indazole regioisomers of synthetic cannabinoids AB-CHMINACA, AB-FUBINACA, AB-PINACA, and 5F-AB-PINACA are possible manufacturing impurities with cannabimimetic activities. Forensic Toxicol 34:286–303. https://doi.org/10.1007/s11419-016-0316-y
Uchiyama N, Shimokawa Y, Kawamura M, Kikura-Hanajiri R, Hakamatsuka T (2014) Chemical analysis of a benzofuran derivative, 2-(2-ethylaminopropyl) benzofuran (2-EAPB), eight synthetic cannabinoids, five cathinone derivatives, and five other designer drugs newly detected in illegal products. Forensic Toxicol 32:266–281. https://doi.org/10.1007/s11419-014-0238-5
Hudson S, Ramsey J (2011) The emergence and analysis of synthetic cannabinoids. Drug Test Anal 3:466–478. https://doi.org/10.1002/dta.268
Wiley JL, Marusich JA, Lefever TW, Antonazzo KR, Wallgren MT, Cortes RA, Patel PR, Grabenauer M, Moore KN, Thomas BF (2015) AB-CHMINACA, AB-PINACA, and FUBIMINA: affinity and potency of novel synthetic cannabinoids in producing Δ9-Tetrahydrocannabinol-like effects in mice. J Pharmacol Exp Ther 354:328–339. https://doi.org/10.1124/jpet.115.225326
Solimini R, Busardo FP, Rotolo MC, Ricci S, Mastrobattista L, Mortali C, Graziano S, Pellegrini M, Di Luca NM, Palmi I (2017) Hepatotoxicity associated to synthetic cannabinoids use. Eur Rev Med Pharmacol Sci 21:1–6
Hamilton RJ, Keyfes V, Banka SS (2017) Synthetic cannabinoid abuse resulting in S.T. segment elevation myocardial infarction requiring percutaneous coronary intervention. J Emerg Med 52:496–498. https://doi.org/10.1016/j.jemermed.2016.09.023
Hassen GW, Fernandez D, Dunn N, Bulbena-Cabre A, Chirurgi R, Li L, Dittmar M, Aldous KM, Su M (2017) Analysis of k2 products sold as incense. Am J Emerg Med 36:1307–1309. https://doi.org/10.1016/j.ajem.2017.11.030
Thornton SL, Wood C, Friesen MW, Gerona RR (2013) Synthetic cannabinoid use associated with acute kidney injury. Clin Toxicol 51:189–190. https://doi.org/10.3109/15563650.2013.770870
Moeller S, Lucke C, Struffert T, Schwarze B, Gerner ST, Schwab S, Köhrmann M, Machold K, Philipsen A, Müller HH (2017) Ischemic stroke associated with the use of a synthetic cannabinoid (spice). Asian J Psychiatr 25:127–130. https://doi.org/10.1016/j.ajp.2016.10.019
Silva JP, Carmo H, Carvalho F (2017) In vitro nephrotoxicity of synthetic cannabinoids. Toxicol Lett 280:S137. https://doi.org/10.1016/j.toxlet.2017.07.384
Moosmann B, Angerer V, Auwärter V (2015) Inhomogeneities in herbal mixtures: a serious risk for consumers. Forensic Toxicol 33:54–60. https://doi.org/10.1007/s11419-014-0247-4
Darke S, Duflou J, Farrell M, Peacock A, Lappin J (2020) Characteristics and circumstances of synthetic cannabinoid-related death. Clin toxicol 58:368–374. https://doi.org/10.1080/15563650.2019.1647344
Angerer V, Jacobi S, Franz F, Auwarter V, Pietsch J (2017) Three fatalities associated with the synthetic cannabinoids 5F-ADB, 5F-PB-22, and AB-CHMINACA. Forensic Sci Int 281:9–15. https://doi.org/10.1016/j.forsciint.2017.10.042
Namera A, Kawamura M, Nakamoto A, Saito T, Nagao M (2015) Comprehensive review of the detection methods for synthetic cannabinoids and cathinones. Forensic Toxicol 33:175–194. https://doi.org/10.1007/s11419-015-0270-0
Logan BK, Reinhold LE, Xu A, Diamond FX (2012) Identification of synthetic cannabinoids in herbal incense blends in the united states. J Forensic Sci 57:1168–1180. https://doi.org/10.1111/j.1556-4029.2012.02207.x
Tsujikawa K, Yamamuro T, Kuwayama K, Kanamori T, Iwata YT, Inoue H (2014) Thermal degradation of a new synthetic cannabinoid QUPIC during analysis by gas chromatography–mass spectrometry. Forensic Toxicol 32:201–207. https://doi.org/10.1007/s11419-013-0221-6
Lorke D (1983) A new approach to practical acute toxicity testing. Arch Toxicol 54:275–287. https://doi.org/10.1007/BF01234480
Chimalakonda KC, Moran CL, Kennedy PD, Endres GW, Uzieblo A, Dobrowolski PJ, Fifer EK, Lapoint J, Nelson LS, Hoffman RS, James LP, Radominska-Pandya A, Moran JH (2011) Solid-phase extraction and quantitative measurement of omega and omega-1 metabolites of JWH-018 and JWH-073 in human urine. Anal Chem 83(16):6381–6388. https://doi.org/10.1021/ac201377m
Food and drug administration (2018) Bioanalytical Method Validation Guidance for Industry. US. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Veterinary Medicine (CVM), Biopharmaceutics, 1–41
Zapata F, Matey JM, Montalvo G, García-Ruiz C (2021) Chemical classification of new psychoactive substances (NPS). Microchem J 163:105877. https://doi.org/10.1016/j.microc.2020.105877
Berezovskaya IV (2003) Classification of substances with respect to acute toxicity for parenteral administration. Pharm Chem J 37:139–141. https://doi.org/10.1023/A:1024586630954
Rosenkrantz H, Heyman IA, Braude MC (1974) Inhalation, parenteral and oral LD50 values of Δ9-tetrahydrocannabinol in Fischer rats. Toxicol Appl Pharmacol 28:18–27. https://doi.org/10.1016/0041-008X(74)90126-4
Abass M, Hassan M, Abd Elhaleem M, Abd Elaziz H, Abd-Allah R (2017) Acute toxicity of a novel class of hallucinogen “voodoo” (clinical and experimental study). Ain-Shams J Forensic Med Clin Toxicol 28:62–73. https://doi.org/10.21608/ajfm.2017.18280
Bakdash A, Al-Mathloum AMK, Abdelgadir ElAmin EH, Abu Taha NMT, Kumar S, Nasr FA (2018) Single-dose acute toxicity of THJ-2201 designer Cannabis drug: LD50 and hematological and histological changes in mice. Egypt J Forensic Sci 8:1–6. https://doi.org/10.1186/s41935-018-0079-1
Thompson GR, Rosenkrantz H, Schaeppi UH, Braude MC (1973) Comparison of acute oral toxicity of cannabinoids in rats, dogs and monkeys. Toxicol Appl Pharmacol 25:363–372. https://doi.org/10.1016/0041-008X(73)90310-4
Lefever TW, Marusich JA, Thomas BF, Barrus DG, Peiper NC, Kevin RC, Wiley JL (2017) Vaping synthetic cannabinoids: a novel preclinical model of E-Cigarette use in mice. Subst Abuse Res Treat 11:1178221817701739. https://doi.org/10.1177/1178221817701739
Wiley JL, Lefever TW, Glass M, Thomas BF (2019) Do you feel it now? Route of administration and Δ9-tetrahydrocannabinol-like discriminative stimulus effects of synthetic cannabinoids in mice. Neurotoxicol 73:161–167. https://doi.org/10.1016/j.neuro.2019.04.002
Maeda H, Kikura-Hanajiri R, Kawamura M, Nagashima E, Yoshida K-I (2018) AB-CHMINACA-induced sudden death from non-cardiogenic pulmonary edema. Clin Toxicol 56:143–145. https://doi.org/10.1080/15563650.2017.1340648
Ford BM, Tai S, Fantegrossi WE, Prather PL (2017) Synthetic pot: not your grandfather’s marijuana. Trends Pharmacol Sci 38:257–276. https://doi.org/10.1016/j.tips.2016.12.003
Trexler KR, Vanegas SO, Poklis JL, Kinsey SG (2020) The short-acting synthetic cannabinoid AB-FUBINACA induces physical dependence in mice. Drug Alcohol Depend 214:108179. https://doi.org/10.1016/j.drugalcdep.2020.108179
Pava MJ, Blake EM, Green ST, Mizroch BJ, Mulholland PJ, Woodward JJ (2012) Tolerance to cannabinoid-induced behaviors in mice treated chronically with ethanol. Psychopharmacol 219:137–147. https://doi.org/10.1007/s00213-011-2387-0
De Vry J, Schreiber R, Eckel G, Jentzsch KR (2004) Behavioral mechanisms underlying inhibition of food-maintained responding by the cannabinoid receptor antagonist/inverse agonist SR141716A. Eur J Pharmacol 483:55–63. https://doi.org/10.1016/j.ejphar.2003.10.012
Williams CM, Kirkham TC (2002) Observational analysis of feeding induced by Δ9-THC and anandamide. Physiol Behav 76:241–250. https://doi.org/10.1016/S0031-9384(02)00725-4
Freedland CS, Poston JS, Porrino LJ (2000) Effects of SR141716A, a central cannabinoid receptor antagonist, on food-maintained responding. Pharmacol Biochem Behav 67:265–270. https://doi.org/10.1016/S0091-3057(00)00359-2
Foltin RW, Brady JV, Fischman MW (1986) Behavioral analysis of marijuana effects on food intake in humans. Pharmacol Biochem Behav 25:577–582. https://doi.org/10.1016/0091-3057(86)90144-9
Beal JE, Olson R, Lefkowitz L, Laubenstein L, Bellman P, Yangco B, Morales JO, Murphy R, Powderly W, Plasse TF (1997) Long-term efficacy and safety of dronabinol for acquired immunodeficiency syndrome-associated anorexia. J Pain Symptom Manage 14:7–14. https://doi.org/10.1016/S0885-3924(97)00038-9
Manwell LA, Ford B, Matthews BA, Heipel H, Mallet PE (2014) A vapourized Δ9-tetrahydrocannabinol (Δ9-THC) delivery system part II: comparison of behavioural effects of pulmonary versus parenteral cannabinoid exposure in rodents. J Pharmacol Toxicol Methods 70:112–119. https://doi.org/10.1016/j.vascn.2014.06.004
Almazroo OA, Miah MK, Venkataramanan R (2017) Drug metabolism in the liver. Clin Liver Dis 21:1–20. https://doi.org/10.1016/j.cld.2016.08.001
Sheikh IA, Lukšič M, Ferstenberg R, Culpepper-Morgan JA (2014) Spice/K2 synthetic marijuana-induced toxic hepatitis treated with N-acetylcysteine. Am J Case Rep 15:584–588. https://doi.org/10.12659/AJCR.891399
Armenian P, Darracq M, Gevorkyan J, Clark S, Kaye B, Brandehoff NP (2018) Intoxication from the novel synthetic cannabinoids AB-PINACA and ADB-PINACA: a case series and review of the literature. Neuropharmacol 134:82–91. https://doi.org/10.1016/j.neuropharm.2017.10.017
Abdelmoneim WM, Bakr MH, Ghandour NM, Mohammed MK, Fawzy M, Ramadan AG, Abdellah NZ (2023) Cytotoxicity associated with acute and chronic administration of synthetic cannabinoids “Strox” in the brain, liver, heart, and testes of male albino rats: histological and immunohistochemical study. Egypt J Forensic Sci 13:1–21. https://doi.org/10.1186/s41935-023-00331-8
Mousa R, Gebri S, Masoud K, Radwan R, Mohamad S (2021) Acute toxic effects of AB-CHMINACA on lung, heart and liver: an experimental pilot study. Ain-Shams J Forensic Med Clin Toxicol 37:128–135. https://doi.org/10.21608/AJFM.2021.182715
Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB (2000) Diagnosis and monitoring of hepatic injury. I. Performance characteristics of laboratory tests. Clin Chem 46:2027–2049. https://doi.org/10.1093/clinchem/46.12.2050
George B, You D, Joy MS, Aleksunes LM (2017) Xenobiotic transporters and kidney injury. Adv Drug Deliv Rev 116:73–91. https://doi.org/10.1016/j.addr.2017.01.005
Bhanushali GK, Jain G, Fatima H, Leisch LJ, Thornley-Brown D (2013) AKI associated with synthetic cannabinoids: a case series. Clin J Am Soc Nephrol 8:523–526. https://doi.org/10.2215/CJN.05690612
Buser GL, Gerona RR, Horowitz BZ, Vian KP, Troxell ML, Hendrickson RG, Houghton DC, Rozansky D, Su SW, Leman RF (2014) Acute kidney injury associated with smoking synthetic cannabinoid. Clin Toxicol 52:664–673. https://doi.org/10.3109/15563650.2014.932365
Gudsoorkar VS, Perez JA Jr (2015) A new differential diagnosis: synthetic cannabinoids-associated acute renal failure. Methodist Debakey Cardiovasc J 11:189–191. https://doi.org/10.14797/mdcj-11-3-189
Athanasiou A, Clarke AB, Turner AE, Kumaran NM, Vakilpour S, Smith PA, Bagiokou D, Bradshaw TD, Westwell AD, Fang L (2007) Cannabinoid receptor agonists are mitochondrial inhibitors: a unified hypothesis of how cannabinoids modulate mitochondrial function and induce cell death. Biochem Biophys Res Commun 364:131–137. https://doi.org/10.1016/j.bbrc.2007.09.107
Tait RJ, Caldicott D, Mountain D, Hill SL, Lenton S (2016) A systematic review of adverse events arising from the use of synthetic cannabinoids and their associated treatment. Clin toxicol 54:1–13. https://doi.org/10.3109/15563650.2015.1110590
Peterson BL, Couper FJ (2015) Concentrations of AB-CHMINACA and AB-PINACA and driving behavior in suspected impaired driving cases. J Anal Toxicol 39:642–647. https://doi.org/10.1093/jat/bkv09
Hasegawa K, Wurita A, Minakata K, Gonmori K, Nozawa H, Yamagishi I, Watanabe K, Suzuki O (2015) Postmortem distribution of AB-CHMINACA, 5-fluoro-AMB, and diphenidine in body fluids and solid tissues in a fatal poisoning case: usefulness of adipose tissue for detection of the drugs in unchanged forms. Forensic Toxicol 33:45–53. https://doi.org/10.1007/s11419-014-0245-6
Acknowledgements
The authors would like to thank Abeer Hussein Mohammed El-Galas for her assistance with animal observation.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This project received no external funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study's conception and design. REAM carried out the animal experiment. SMG performed the histological tissue preparation and interpretation. KMMM performed GC–MS-MS analysis and interpretation. RAR wrote the first draft of the manuscript. The Project Administrator and principal supervisor was SAM. All authors commented on previous versions of the manuscript and read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare. All co-authors have seen and agree with the contents of the manuscript. We certify that the submission is original work and is not under review at any other publication.
Ethical approval
Approval was obtained from the ethics committee of Sohag Faculty of Medicine (OHRP #: IRB00013006).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohammad, S.A., Mousa, R.E.A., Gebril, S.M. et al. Toxic effects of AB-CHMINACA on liver and kidney and detection of its blood level in adult male mice. Forensic Toxicol 42, 7–17 (2024). https://doi.org/10.1007/s11419-023-00670-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11419-023-00670-0