Abstract
Purpose
This work reports the synthesis and pharmacological and analytical data for a new series of recently identified azaindole-adamantyl-derived synthetic cannabinoids (SCs).
Methods
Each SC was synthesised using an efficient and divergent synthesis, and assessed by electron ionisation mass spectrometry (EIMS). The cannabimimetic activity of each compound was conducted using a fluorometric imaging plate reader (FLIPR) assay.
Results
The described EIMS method and retention time by gas chromatography were able to effectively differentiate each of the analogues regardless of the bicyclic core. For the first time in these SC structures, the bicyclic ring system was shown to have an impact on the cannabimimetic activities in the fluorometric assay of membrane potential. Analogues ranged from moderately potent at both CB1 and CB2 (e.g., AP4AIC EC50 = 160 nM and EC50 = 64 nM, respectively) to not active at either cannabinoid receptor (AP4AICA, AP5AICA, and APIC).
Conclusions
Further investigation into receptor selectivity surrounding these bicyclic cores could prove useful for future therapeutic applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
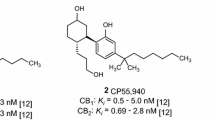
The field of synthetic cannabinoids (SCs) has been constantly changing since their emergence in the early 2000s. These compounds are reported to elicit effects similar to ∆9-THC (1), the psychoactive component of cannabis, by activating the two known cannabinoid receptors: cannabinoid type-1 (CB1) and cannabinoid type-2 receptors (CB2). JWH-018 (2) was one of the first SCs to be identified as a drug of abuse. The capacity for diversification of the aminoalkylindole-derived SC scaffold has resulted in clandestine chemists making modifications to the N-pentyl and 3-acyl substituents in an attempt to avoid detection by law enforcement agencies. As a result, SCs have become one of the most prominent classes of novel psychoactive substances (NPS) [1]. Because of the limited understanding of their pharmacological effects, these compounds have caused a number of unpredictable adverse events, posing a major risk to public health [2,3,4,5,6,7,8,9]. The rate at which new chemical entities are appearing, along with the plethora of potential substitution patterns, has made it virtually impossible for law enforcement agencies to stay ahead of newly appearing SCs (Fig. 1).
Our group has reported the pharmacological and analytical data for a variety of identified indole- and indazole-derived SCs containing N-substituents, including pentyl, 5-fluoropentyl, butyl, methylcyclohexyl, and p-fluorobenzyl, and various 3-acyl-substituted skeletons containing adamantyl, l-valinate, l-tert-leucinate, and cumyl functionalities (examples include 4 to 6 in Fig. 1) [10,11,12,13]. Whereas ∆9-THC shows only partial agonist activity at CB1, these SCs have proven to be highly potent and efficacious agonists at both CB1 and CB2. There have also been reports of SC CB1 activation resulting in promiscuous G-protein coupling and off-target receptor activation, potentially contributing to their toxicological profiles [14, 15].
In 2013, a new modification to the SC scaffold was observed, incorporating an ester linker, seen in analogues such as PB-22 (QUPIC), 5F-PB-22, and BB-22 (QUCHIC), identified in Japan and Russia [16, 17]. This modification was subsequently identified in another series of other compounds including FUB-PB-22, NM-2201, and 5F-NM-2201 [18]. PB-22 was detected in illicit samples received at Queensland Health Forensic and Scientific Services (QHFSS) in mid-2013, along with 5F-PB-22, and became a prominent compound in the Queensland SC market throughout 2013 and 2014.
Additional SC modifications incorporating an azaindole scaffold have begun to appear in recent years. CUMYL-5F-P7AICA, the 7-azaindole isomer of CUMYL-5F-PINACA, has been reported in forensic samples from 2015 to 2017 in Switzerland, Germany, and Australia, and was detected in illicit samples received at QHFSS in late 2015 [19,20,21]. Other 7-azaindole SCs have been reported in the NPS market, including NNL-1, NNL-3, 5F-NPB-22-7N, 5F-AKB-48-7N, CUMYL-4CN-B7AICA, and AB-5F-P7AICA [20, 22,23,24]. While the majority of azaindole SCs reported have been the 7-azaindole, the potential for other isomers to enter the NPS market has been demonstrated with the reporting of 5F-PCN, the 5-azaindole isomer of 5F-MN-18 [25]. The presence of azaindole/indazole structural isomers in the NPS market can present analytical challenges for forensic identification, as electron ionisation mass spectrometry (EIMS) data are likely to be similar and may not provide clear discrimination between each isomer. In early 2015, QHFSS obtained an illicit sample of plant material portraying an EIMS consistent with a 1-N-pentyl azaindole/indazole and an adamantyl carboxylate substituent at the 3-position. As nothing with these chemical characteristics has previously been reported, EIMS was unable to distinguish which indazole/azaindole ring system was present in the sample. Detection of this compound was subsequently reported in South Korea in 2016 and Russia in 2017 [26, 27].
A series of reference samples with an indazole or azaindole core were synthesised to assess whether the EIMS fragmentation patterns could differentiate between the indazole/azaindole isomers. In conjunction, derivatives incorporating the amide functionality (7–11), along with the indole bicyclic ring system (4 and 12) (see structures in Fig. 2), were also synthesised and assessed in vitro at the CB1 and CB2 receptors to determine functional trends within this new class of SCs. Each analogue was designated an abbreviation following a protocol similar to lead compounds 4 and 7 (e.g., Adamantan-1-yl 1-Pentyl-1H-4-AzaIndole-3-Carboxylate was given the abbreviation AP4AIC).
Materials and methods
General chemical synthesis details
All reactions were performed under an atmosphere of nitrogen or argon unless otherwise specified. Anhydrous methanol (MeOH), acetonitrile, and dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO, USA) were used as received. Anhydrous tetrahydrofuran (THF) and toluene were obtained from a PureSolv MD 7 solvent purification system (Innovative Technology, Inc., Oldham, UK). Other commercially available chemicals (Sigma-Aldrich) were used as received. Analytical thin-layer chromatography was performed using Merck aluminum-backed silica gel 60 F254 (0.2 mm) plates (Merck, Darmstadt, Germany), which were visualised using shortwave (254 nm) UV fluorescence. Flash chromatography was performed using Merck Kieselgel 60 (230–400 mesh) silica gel, with the eluent mixture reported as the volume/volume ratio (%). Melting point ranges (m.p.) were measured in open capillaries using a Stanford Research Systems (SRS, Sunnyvale, CA, USA) MPA160 melting point apparatus with a ramp rate of 0.5–2.0 °C/min. Nuclear magnetic resonance (NMR) spectra were recorded at 300 K using either a Bruker AVANCE DRX 300 (300 MHz), AVANCE DRX400 (400.1 MHz), or AVANCE III 500 Ascend (500.1 MHz) spectrometer (Bruker, Billerica, MA, USA). The data are reported as chemical shift (δ ppm) relative to the residual protonated solvent resonance, relative integral, multiplicity (s = singlet, br s = broad singlet, d = doublet, t = triplet, quat. = quartet, quin. = quintet, m = multiplet), coupling constants (J Hz), and assignment. Assignment of signals was assisted by correlation spectroscopy (COSY), distortionless enhancement by polarisation transfer (DEPT), heteronuclear single quantum coherence (HSQC), and heteronuclear multiple-bond correlation (HMBC) experiments where necessary. Low-resolution mass spectra (LRMS) were recorded using electrospray ionisation (ESI) on a Finnigan LCQ ion trap mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). High-resolution mass spectra (HRMS) were run on a Bruker 7T Apex-Qe Fourier transform (FT) ion cyclotron resonance mass spectrometer equipped with an Apollo II ESI/APCI/MALDI dual source (Bruker) by the Mass Spectrometry Facility of the School of Chemistry at the University of Sydney. Infrared (IR) absorption spectra were recorded on a Bruker ALPHA FT-IR spectrometer (Bruker) as solid or thin film from ethanol, and the data are reported as vibrational frequencies (cm−1).
Preparation of N-pentyl-3-trifluoroacetylindole (19)
Sodium hydride (60% dispersion in mineral oil, 137 mg, 3.42 mmol) was added portion-wise to an ice-cold solution of indole (200 mg, 1.71 mmol) in N,N-dimethylformamide (DMF, 6 mL) and stirred for 10 min. 1-Bromopentane (225 µL, 1.80 mmol) was added, and the mixture was warmed to ambient temperature and stirred for 1 h. The mixture was re-cooled to 0 °C, and trifluoroacetic anhydride (600 µL, 4.28 mmol) was added dropwise. The reaction mixture was warmed to ambient temperature and stirred for 1 h. The reaction mixture was poured onto ice (75 mL) and extracted with CH2Cl2 (3 × 75 mL). The combined organic extracts were washed with H2O (100 mL) and brine (100 mL), dried (MgSO4), and concentrated under reduced pressure, giving 19, following purification by flash chromatography [hexane/ethyl acetate (EtOAc), 94:6 v/v], as a yellow oil (450 mg, 94%).
1H NMR (300 MHz, CDCl3): δ 8.43 (1H, d, J = 8.7 Hz), 7.93 (1H, s), 7.42–7.36 (3H, m), 4.21 (2H, t, J = 6.9 Hz), 1.95 (2H, quin., J = 7.2 Hz), 1.43–1.32 (4H, m), 0.92 (3H, t, J = 6.6 Hz); 13C NMR (75 MHz, CDCl3): δ 174.8 (q, CO, J = 34.5 Hz), 137.5 (d, CH, J = 4.5 Hz), 136.8 (quat.), 127.3 (quat.), 124.6 (CH), 124.0 (CH), 122.8 (CH), 117.3 (q, CF3, J = 289.5 Hz), 110.5 (CH), 109.5 (quat.), 47.8 (CH2), 29.5 (CH2), 29.0 (CH2), 22.3 (CH2), 14.0 (CH3); 19F NMR (282 MHz, CDCl3): δ −72.2 (3F, s); LRMS (+ ESI): m/z 306 ([M + Na]+, 100%); IR (diamond cell, thin film): 3124 (w), 2959 (m), 2933 (m), 2863 (w), 1662 (s), 1527 (s), 1397 (m), 1286 (m), 1181 (s), 1132 (s), 878 (m), 751 (m).
Preparation of 1-N-pentyl-3-indazole methyl carboxylate (22)
Potassium tert-butoxide (350 mg, 3.12 mmol) was added to an ice-cold solution of methyl-3-indazole carboxylate (500 mg, 2.84 mmol) in THF (15 mL), warmed to ambient temperature, and stirred for 1 h. 1-Bromopentane (415 µL, 2.98 mmol) was added and stirred at ambient temperature for 48 h and then heated to reflux for 24 h. The reaction mixture was cooled to ambient temperature, poured into H2O (100 mL), and extracted with diethyl ether (Et2O, 3 × 100 mL). The combined organic extracts were dried (MgSO4) and concentrated under reduced pressure, giving 22, following purification by flash chromatography (hexane/EtOAc, 90:10 v/v), as a colourless oil (585 mg, 84%).
1H NMR (300 MHz, CDCl3): δ 8.24 (1H, d, J = 8.0 Hz), 7.50–7.44 (2H, m), 7.35–7.27 (1H, m), 4.47 (2H, t, J = 7.4 Hz), 4.04 (3H, s), 1.97 (2H, quin., J = 7.0 Hz), 1.32 (4H, m), 0.87 (3H, t, J = 6.6 Hz); 13C NMR (75 MHz, CDCl3): δ 163.3 (CO), 140.6 (quat.), 134.6 (quat.), 126.8 (CH), 123.9 (quat.), 123.1 (CH), 122.3 (CH), 109.7 (CH), 52.1 (CH2), 50.1 (CH3), 29.7 (CH2), 29.0 (CH2), 22.4 (CH2), 14.0 (CH3); LRMS (+ ESI): m/z 269 ([M + Na]+, 60%), 515 ([2M + Na]+, 100%); IR (diamond cell, thin film): 2954 (m), 2932 (m), 2860 (w), 1709 (s), 1477 (s), 1215 (s), 1159 (s), 1117 (s), 751 (s).
General procedure A: 1-N-alkylation of azaindoles
The appropriate azaindole (1.00 g, 8.46 mmol) in DMF (15 mL) was slowly added to an ice-cold solution of sodium hydride (60% dispersion in mineral oil, 675 mg, 16.9 mmol) in DMF (5 mL) and stirred for 10 min. 1-Bromopentane (1.15 mL, 9.31 mmol) was added, and the mixture was warmed to ambient temperature and stirred for 1 h. The reaction mixture was slowly added to H2O (75 mL) and extracted with EtOAc (3 × 75 mL). The combined organic extracts were dried (MgSO4) and concentrated under reduced pressure.
Preparation of 1-N-pentyl-4-azaindole (28)
Treating 4-azaindole (1.00 g, 8.46 mmol) according to general procedure A gave 28, following purification by flash chromatography (hexane/EtOAc, 70:30 v/v), as an orange oil (1.28 g, 80%).
1H NMR (300 MHz, CDCl3): δ 8.57 (1H, d, J = 5.4 Hz), 8.18 (1H, d, J = 8.4 Hz), 7.67 (1H, d, J = 3.3 Hz), 7.46 (1H, dd, J = 8.4, 5.7 Hz), 7.00 (1H, d, J = 3 Hz), 4.26 (2H, t, J = 7.2 Hz), 1.86 (2H, quin., J = 7.5 Hz), 1.29 (4H, m), 0.88 (3H, t, J = 7.0 Hz); 13C NMR (75 MHz, CDCl3): δ 138.3 (quat.), 136.8 (CH), 135.3 (CH), 132.1 (quat.), 123.9 (CH), 116.1 (CH), 98.3 (CH), 47.5 (CH2), 30.1 (CH2), 28.9 (CH2), 22.2 (CH2), 13.9 (CH3); LRMS (+ ESI): 189 ([M + H]+, 100%); IR (diamond cell, thin film): 2956 (m), 2929 (m), 2860 (w), 1604 (m), 1417 (s), 1322 (m), 1290 (m), 771 (s), 723 (m).
Preparation of 1-N-pentyl-5-azaindole (29)
Treating 5-azaindole (250 mg, 2.12 mmol) according to general procedure A gave 29, following purification by flash chromatography (EtOAc), as a red oil (285 mg, 71%).
1H NMR (300 MHz, CDCl3): δ 8.89 (1H, s), 8.29 (1H, d, J = 5.7 Hz), 7.22 (1H, d, J = 5.7 Hz), 7.10 (1H, d, J = 2.4 Hz), 6.57 (1H, d, J = 1.2 Hz), 4.12 (2H, t, J = 6.9 Hz), 1.82 (2H, quint., J = 7.2 Hz), 1.30 (4H, m), 0..89 (3H, t, J = 7.1 Hz); 13C NMR (75 MHz, CDCl3): δ 144.1 (CH), 140.6 (CH), 139.6 (quat.), 128.8 (CH), 125.5 (quat.), 104.9 (CH), 100.9 (CH), 46.4 (CH2), 30.1 (CH2), 29.1 (CH2), 22.4 (CH2), 14.3 (CH3); LRMS (+ ESI): m/z 189 ([M + H]+, 100%); IR (diamond cell, thin film): 2956 (m), 2929 (m), 2859 (w), 1602 (m), 1476 (m), 1453 (m), 1319 (m), 1295 (m), 890 (m), 801 (m), 724 (s).
Preparation of 1-N-pentyl-6-azaindole (30)
Treating 6-azaindole (1.00 g, 8.46 mmol) according to general procedure A gave 30, following purification by flash chromatography (EtOAc), as a red oil (1.35 g, 85%).
1H NMR (300 MHz, CDCl3): δ 8.77 (1H, s), 8.22 (1H, d, J = 5.4 Hz), 7.50 (1H, d, J = 5.4 Hz), 7.49 (1H, m), 6.47 (1H, m), 4.19 (2H, t, J = 7.2 Hz), 1.87 (2H, quint., J = 7.2 Hz), 1.33 (4H, m), 0.89 (3H, t, J = 6.8 Hz); 13C NMR (75 MHz, CDCl3): δ 138.5 (CH), 133.3 (quat.), 133.2 (quat.), 133.0 (CH), 131.5 (CH), 115.4 (CH), 100.5 (CH), 46.9 (CH2), 30.3 (CH2), 29.2 (CH2), 22.4 (CH2), 14.0 (CH3); LRMS (+ ESI): m/z 189 ([M + H]+, 100%); IR (diamond cell, thin film): 3091 (w), 2956 (s), 2930 (s), 2870 (m), 1682 (m), 1500 (s), 1470 (s), 1320 (s), 1030 (m), 814 (s), 733 (s).
Preparation of 1-N-pentyl-7-azaindole (31)
Treating 7-azaindole (250 mg, 2.12 mmol) according to general procedure A gave 31, following purification by flash chromatography (hexane/EtOAc, 65:35 v/v), as a red oil (217 mg, 68%).
1H NMR (300 MHz, CDCl3): δ 8.32 (1H, d, J = 3.3 Hz), 7.89 (1H, d, J = 7.8 Hz), 7.22 (1H, m), 7.04 (1H, t, J = 4.8 Hz), 6.44 (1H, m), 4.29 (2H, t, J = 7.2 Hz), 1.87 (2H, quint., J = 6.9 Hz), 1.26 (4H, m), 0.88 (3H, t, J = 6.6 Hz); 13C NMR (75 MHz, CDCl3): δ 147.6 (quat.), 142.8 (CH), 128.8 (CH), 128.1 (CH), 120.7 (quat.), 115.6 (CH), 99.3 (CH), 44.7 (CH2), 30.3 (CH2), 29.2 (CH2), 22.5 (CH2), 14.1 (CH3); LRMS (+ ESI): m/z 189 ([M + H]+, 100%); IR (diamond cell, thin film): 3051 (w), 2956 (s), 2926 (s), 2858 (m), 1509 (s), 1425 (s), 1272 (s), 772 (s), 715 (s).
General procedure B: 3-trifluoroacylation of azaindoles
A suspension of aluminium chloride (4.42 g, 33.2 mmol) and the appropriate 1-N-pentyl azaindole (1.25 g, 6.64 mmol) in DMF (25 mL) was stirred at ambient temperature for 1 h. The reaction was cooled to 0 °C and trifluoroacetic anhydride (1.41 mL, 9.96 mmol) was added dropwise, warmed to ambient temperature, and stirred for 5 h. The reaction mixture was slowly added to H2O (75 mL) and extracted with CH2Cl2 (3 × 75 mL). The combined organic extracts were dried (MgSO4) and concentrated under reduced pressure.
Preparation of 1-N-pentyl-3-trifluoroacyl-4-azaindole (32)
Treating 28 (1.55 g, 7.17 mmol) according to general procedure B gave 32, following purification by flash chromatography (hexane/EtOAc, 70:30 v/v), as an off-white solid (1.48 g, 78%).
m.p. 101.5–103.0 °C; 1H NMR (300 MHz, CDCl3): δ 8.75 (1H, d, J = 4.5 Hz), 8.10 (1H, s), 7.76 (1H, d, J = 8.4 Hz), 7.29 (1H, t, J = 6.6 Hz), 4.23 (2H, t, J = 7.2 Hz), 1.92 (2H, quin., J = 6.9 Hz), 1.41–1.32 (4H, m), 0.90 (3H, t, J = 6.9 Hz); 13C NMR (75 MHz, CDCl3): δ 173.8 (q, CO, J = 35.3 Hz), 147.1 (CH), 144.8 (quat.), 139.0 (q, CH, J = 4.5 Hz), 130.0 (quat.), 118.8 (CH), 118.1 (CH), 116.9 (q, CF3, J = 288.8 Hz), 109.4 (quat.), 48.1 (CH2), 29.5 (CH2), 28.9 (CH2), 22.2 (CH2), 13.9 (CH3); 19F NMR (282 MHz, CDCl3): δ −72.69 (3F, s); LRMS (+ ESI): m/z 591 ([2 M + Na]+, 100%); IR (diamond cell, thin film): 3027 (w), 2956 (m), 2932 (m), 2871 (w), 1674 (s), 1525 (s), 1382 (s), 1282 (s), 1176 (s), 1135 (s), 880 (s), 785 (s), 725 (s).
Preparation of 1-N-pentyl-3-trifluoroacyl-5-azaindole (33)
Treating 29 (1.25 g, 6.64 mmol) according to general procedure B gave 33, following purification by flash chromatography (hexane/EtOAc, 45:55 v/v), as a red oil (1.18 g, 63%).
1H NMR (300 MHz, CDCl3): δ 9.63 (1H, s), 8.55 (1H, d, J = 4.5 Hz), 7.94 (1H, m), 7.34 (1H, d, J = 5.7 Hz), 4.21 (2H, t, J = 7.2 Hz), 1.92 (2H, quin., J = 7.2 Hz), 1.42–1.31 (4H, m), 0.92 (3H, t, J = 7.1 Hz); 13C NMR (75 MHz, CDCl3): δ 174.8 (q, CO, J = 36.3 Hz), 145.8 (CH), 143.9 (CH), 141.0 (quat.), 137.9 (q, CH, J = 4.6 Hz), 123.4 (quat.), 116.9 (q, quat., J = 290.8 Hz), 109.8 (quat.), 105.6 (CH), 47.7 (CH2), 29.6 (CH2), 28.9 (CH2), 22.3 (CH2), 13.9 (CH3); 19F NMR (282 MHz, CDCl3): δ −72.7 (3F, s); LRMS (+ ESI): m/z 307 ([M + Na]+, 100%); IR (diamond cell, thin film): 2959 (m), 2933 (m), 2864 (w), 1675 (s), 1527 (s), 1394 (m), 1295 (m), 1184 (s), 1141 (s), 1112 (s), 881 (s).
Preparation of 1-N-pentyl-3-trifluoroacyl-6-azaindole (34)
Treating 30 (1.25 g, 6.64 mmol) according to general procedure B gave 34, following purification by flash chromatography (hexane/EtOAc, 55:45 v/v), as a red oil (217 mg, 95%).
m.p. 80.5–82.5 °C; 1H NMR (300 MHz, CDCl3): δ 8.89 (1H, s), 8.53 (1H, d, J = 5.1 Hz), 8.25 (1H, d, J = 5.4 Hz), 8.01 (1H, s), 4.31 (2H, t, J = 7.2 Hz), 1.97 (2H, quin., J = 6.9 Hz), 1.42-1.30 (4H, m), 0.92 (3H, t, J = 6.9 Hz); 13C NMR (75 MHz, CDCl3): δ 174.8 (q, CO, J = 35.3 Hz), 143.1 (CH), 139.3 (q, CH, J = 4.5 Hz), 133.7 (CH), 133.7 (quat.), 132.6 (quat.), 116.8 (q, CF3, J = 288.8 Hz), 116.7 (CH), 109.1 (quat.), 48.3 (CH2), 29.7 (CH2), 28.9 (CH2), 22.2 (CH2), 13.8 (CH3); 19F NMR (282 MHz, CDCl3): δ −72.9 (3F, s); LRMS (+ ESI): m/z 307 ([M + Na]+, 100%); IR (diamond cell, thin film): 3118 (w), 2960 (m), 2938 (m), 2867 (m), 1681 (s), 1606 (s), 1525 (s), 1294 (s), 1188 (s), 1147 (s), 995 (m), 829 (m).
Preparation of 1-N-pentyl-3-trifluoroacyl-7-azaindole (35)
Treating 31 (200 mg, 1.06 mmol) according to general procedure B gave 35, following purification by flash chromatography (hexane/EtOAc, 80:20 v/v), as a red oil (235 mg, 78%).
1H NMR (300 MHz, CDCl3): δ 8.63 (1H, d, J = 7.8 Hz), 8.46 (1H, d, J = 1.5 Hz), 8.07 (1H, s), 7.32 (1H, t, J = 4.5 Hz), 4.37 (2H, t, J = 7.2 Hz), 1.95 (2H, quint., J = 6.6 Hz), 1.37 (4H, m), 0.91 (3H, t, J = 6.3 Hz); 13C NMR (75 MHz, CDCl3): δ 148.3 (quat,), 145.7 (CH), 137.3 (q, CH, J = 4.4 Hz), 131.1 (CH), 119.8 (CH), 119.6 (quat.), 117.0 (q, quat., J = 290.7 Hz), 108.1 (quat.), 46.1 (CH2), 29.8 (CH2), 29.0 (CH2), 22.3 (CH2), 14.0 (CH3), unresolved CO due to F splitting; 19F NMR (282 MHz, CDCl3): δ −72.5 (3F, s); LRMS (+ ESI): m/z 307 ([M + Na]+, 100%); IR (diamond cell, thin film): 3119 (w), 2958 (w), 2933 (w), 2863 (w), 1671 (s), 1527 (s), 1385 (s), 1128 (s), 1104 (s), 882 (s).
General procedure C: hydrolysis of methyl esters, trifluoroacetyl and benzyl sulfonyl groups
Aqueous NaOH (4 M, 3.87 mL, 15.5 mmol) was added dropwise to a stirred solution of the appropriately substituted methyl ester, trifluoroacetyl compound, or benzyl sulfonyl-protected indole (2.58 mmol) in MeOH (20 mL), and allowed to stir at ambient temperature (indazole derivatives) or reflux (indole/azaindole derivatives) for 18 h. The reaction mixture was added to H2O (75 mL) and washed with Et2O (75 mL). The aqueous layer was acidified to pH ~2 using 1 M aq. HCl and extracted with Et2O (3 × 75 mL). The combined organic extracts were dried (MgSO4) and concentrated under reduced pressure.
Preparation of N-pentyl-3-indole carboxylic acid (20)
Treating 19 (450 mg, 1.59 mmol) according to general procedure C gave 20 as a white solid (323 mg, 88%).
m.p. 106.5–108.0 °C; 1H NMR (300 MHz, MeOD-d4): δ 8.09 (1H, dd, J = 6.5, 1.9 Hz), 7.93 (1H, s), 7.42–7.28 (3H, m), 4.17 (2H, t, J = 7.2 Hz), 1.90 (2H, quin., J = 7.0 Hz), 1.40–1.32 (4H, m), 0.90 (3H, t, J = 6.6 Hz), COOH signal not observed; 13C NMR (75 MHz, MeOD-d4): δ 168.8 (CO2H), 138.1 (quat.), 136.3 (CH), 128.3 (quat.), 123.6 (CH), 122.6 (CH), 122.4 (CH), 111.3 (CH), 107.7 (quat.), 47.7 (CH2), 30.7 (CH2), 30.0 (CH2), 23.3 (CH2), 14.2 (CH3); LRMS (– ESI): m/z 230 ([M–H]−, 100%); IR (diamond cell, thin film): 3106 (w), 2925 (m), 2856 (w), 2525 (bs), 1649 (s), 1526 (s), 1461 (m), 1273 (m), 1204 (s), 1117 (m), 940 (m), 731 (s).
Preparation of 1-N-pentyl-3-indazole carboxylic acid (23)
Treating 22 (600 mg, 2.58 mmol) according to general procedure C gave 23 as a white solid (510 mg, 85%).
m.p. 76.5–78.0 °C; 1H NMR (300 MHz, MeOD-d4): δ 8.26 (1H, d, J = 8.1 Hz), 7.52–7.44 (2H, m), 7.35 (1H, t, J = 7.8 Hz), 4.48 (2H, t, J = 7.2 Hz), 1.99 (2H, quin., J = 7.2 Hz), 1.34 (4H, m), 0.89 (3H, t, J = 6.0 Hz), COOH signal not observed; 13C NMR (75 MHz, MeOD-d4): δ 165.4 (CO2H), 142.0 (quat.), 135.9 (quat.), 127.9 (CH), 124.5 (quat.), 124.0 (CH), 122.9 (CH), 111.0 (CH), 49.7 (CH2), 30.4 (CH2), 29.8 (CH2), 23.1 (CH2), 14.1 (CH3); LRMS (– ESI): m/z 231 ([M–H]−, 100%); IR (diamond cell, thin film): 3053 (bs), 2956 (m), 2931 (m), 2860 (w), 1687 (s), 1503 (s), 1218 (s), 1176 (s), 1121 (s), 752 (s).
Preparation of 1-N-pentyl-4-azaindole-3-carboxylic acid (36)
Treating 32 (1.48 g, 5.21 mmol) according to general procedure C gave 36 as an off-white solid (1.18 g, 97%).
m.p. 112.0–113.5 °C; 1H NMR (300 MHz, MeOD-d4): δ 8.48 (1H, d, J = 4.5 Hz), 8.06 (1H, s), 7.78 (1H, d, J = 8.3 Hz), 7.26 (1H, dd, J = 8.6, 4.8 Hz), 4.18 (2H, t, J = 7.1 Hz), 1.87 (2H, quin., J = 7.2 Hz), 1.38–1.24 (4H, m), 0.87 (3H, t, J = 7.1 Hz), COOH signal not observed; 13C NMR (75 MHz, MeOD-d4): δ 164.4 (CO2H), 144.4 (quat.), 143.7 (CH), 136.1 (CH), 129.2 (quat.), 118.9 (CH), 117.9 (CH), 106.6 (quat.), 47.8 (CH2), 29.8 (CH2), 28.9 (CH2), 22.3 (CH2), 13.9 (CH3); LRMS (+ ESI): m/z 255 ([M + Na]+, 100%); IR (diamond cell, thin film): 3358 (bs), 3120 (w), 2948 (m), 2928 (m), 2864 (m), 1666 (m), 1538 (m), 1373 (m), 1143 (m), 1032 (m), 962 (m), 782 (s), 723 (s), 491 (s).
Preparation of 1-N-pentyl-5-azaindole-3-carboxylic acid (37)
33 (600 mg, 2.11 mmol) was treated according to general procedure C. Once the reaction was complete, the reaction mixture was concentrated under reduced pressure and immediately subjected to flash chromatography (hexane/EtOAc/acetic acid, 91:8:1 v/v), giving 37 as an off-white solid (425 mg, 87%).
m.p. 185.0–188.0 °C; 1H NMR (300 MHz, MeOD-d4): δ 9.36 (1H, s), 8.36 (1H, d, J = 6.9 Hz), 8.18 (1H, s), 7.84 (1H, d, J = 6.3 Hz), 4.34 (2H, t, J = 7.2 Hz), 1.89 (2H, quint., J = 7.4 Hz), 1.39-1.28 (4H, m), 0.89 (3H, t, J = 7.1 Hz), COOH signal not observed; 13C NMR (75 MHz, MeOD-d4): δ 168.0 (CO2H), 143.3 (quat.), 141.2 (CH), 139.0 (CH), 136.8 (CH), 125.0 (quat.), 113.1 (quat.), 108.5 (CH), 48.1 (CH2), 30.8 (CH2), 29.9 (CH2), 23.3 (CH2), 14.2 (CH3); LRMS (+ ESI): m/z 233 ([M + H]+, 100%); IR (diamond cell, thin film): 3105 (w), 2956 (m), 2859 (w), 2391 (bs), 1677 (w), 1538 (m), 1475 (m), 1186 (s), 1007 (s), 805 (s).
Preparation of 1-N-pentyl-6-azaindole-3-carboxylic acid (38)
34 (900 mg, 3.17 mmol) was treated according to general procedure C. Once the reaction was complete, the reaction mixture was concentrated under reduced pressure and immediately subjected to flash chromatography (CH2Cl2/MeOH, 94:6 v/v), giving 38 as an off-white solid (385 mg, 52%).
m.p. 159.0–160.5 °C; 1H NMR (300 MHz, MeOD-d4): δ 8.90 (1H, s), 8.26 (1H, d, J = 5.6 Hz), 8.22 (1H, s), 8.13 (1H, d, J = 5.7 Hz), 4.37 (2H, t, J = 7.1 Hz), 1.92 (2H, quin., J = 7.1 Hz), 1.41–1.26 (4H, m), 0.89 (3H, t, J = 7.1 Hz), COOH signal not observed; 13C NMR (75 MHz, MeOD-d4): δ 167.9 (CO2H), 140.6 (CH), 139.3 (CH), 135.1 (quat.), 134.5 (CH), 133.5 (CH), 117.5 (quat.), 109.4 (quat.), 48.3 (CH2), 31.0 (CH2), 29.9 (CH2), 23.2 (CH2), 14.2 (CH3); LRMS (+ ESI): m/z 233 ([M + H]+, 100%); IR (diamond cell, thin film): 2930 (w), 2861 (w), 1694 (s), 1479 (s), 1183 (m), 1131 (m), 1029 (s), 822 (m).
Preparation of 1-N-pentyl-7-azaindole-3-carboxylic acid (39)
Treating 35 (200 mg, 0.70 mmol) according to general procedure C gave 39 as an off-white solid (135 mg, 83%).
m.p. 143.0–147.5 °C; 1H NMR (300 MHz, MeOD-d4): δ 8.43 (1H, d, J = 7.2 Hz), 8.31 (1H, m), 8.23 (1H, m), 7.25 (1H, s), 4.33 (2H, t, J = 6.0 Hz), 1.88 (2H, quin., J = 6.0 Hz), 1.41–1.15 (4H, m), 0.88 (3H, m), COOH signal not observed; 13C NMR (75 MHz, MeOD-d4): δ 167.9 (CO2H), 148.8 (quat.), 144.5 (CH), 136.5 (CH), 131.3 (CH), 121.0 (quat.), 118.9 (CH), 106.8 (quat.), 46.2 (CH2), 30.9 (CH2), 29.9 (CH2), 23.3 (CH2), 14.3 (CH3); LRMS (–ESI): m/z 231 ([M–H)−, 100%); IR (diamond cell, thin film): 2930 (w), 2856 (w), 1685 (s), 1533 (s), 1257 (s), 1138 (s), 798 (s), 755 (s).
General procedure D: amidation of 1-N-pentyl-3-indole/indazole/azaindole carboxylic acids with adamantylamine hydrochloride via 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide) hydrochloride coupling
N,N-Diisopropylethylamine (340 µL, 1.95 mmol) was added dropwise to a stirred solution of the appropriate 1-N-pentyl-3-indole/indazole/azaindole carboxylic acid (0.39 mmol), adamantylamine hydrochloride (0.41 mmol), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC·HCl, 150 mg, 0.78 mmol), and 1-hydroxybenzotriazole (HOBt, 119 mg, 0.78 mmol) in DMSO (5 mL), and was stirred at ambient temperature for 18 h. The reaction mixture was added to sat. aq. NaHCO3 (75 mL) and extracted with EtOAc (3 × 75 mL). The combined organic extracts were dried (MgSO4) and concentrated under reduced pressure.
Preparation of N-(adamantan-1-yl)-1-pentyl-1H-indole-3-carboxamide (APICA, 4)
Treating 20 (576 mg, 2.50 mmol) according to general procedure D gave 4, following recrystallisation from isopropanol/H2O, as a white solid (787 mg, 86%).
m.p. 140.0–141.0 °C; 1H NMR (500 MHz, CDCl3): δ 7.87 (1H, d, J = 7.5 Hz), 7.65 (1H, s), 7.36 (1H, d, J = 8.0 Hz), 7.28–7.22 (2H, m), 5.71 (1H, br s, NH), 4.11 (2H, t, J = 7.2 Hz), 2.19 (6H, br s), 2.14 (3H, br s), 1.84 (2H, quin., J = 7.3 Hz), 1.76 (6H, m) 1.39–1.24 (4H, m), 0.88 (3H, t, J = 7.0 Hz); 13C NMR (125 MHz, CDCl3): δ 164.0 (CO), 136.7 (quat.), 131.5 (CH), 125.3 (quat.), 122.3 (CH), 121.2 (CH), 120.0 (CH), 112.3 (quat.), 110.4 (CH), 52.2 (quat.), 46.9 (CH2), 42.4 (CH2), 36.6 (CH2), 29.8 (CH), 29.7 (CH2), 29.1 (CH2), 22.4 (CH2), 14.0 (CH3).
Preparation of N-(adamantan-1-yl)-1-pentyl-1H-indazole-3-carboxamide (APINACA, 7)
Treating 23 (100 mg, 0.43 mmol) according to general procedure D gave 7, following purification by flash chromatography (hexane/EtOAc, 95:5 v/v), as a white solid (128 mg, 81%).
m.p. 66.1–68.8 °C; 1H NMR (300 MHz, CDCl3): δ 8.38 (1H, d, J = 8.1 Hz), 7.38–7.34 (2H, m), 7.27–7.22 (1H, m), 6.80 (1H, s), 4.34 (2H, t, 6.9 Hz), 2.23–2.18 (6H, m), 2.17–2.10 (3H, m), 1.93 (2H, quin., 6.9 Hz), 1.79–1.69 (6H, m), 1.40–1.30 (4H, m), 0.89 (3H, t, J = 6.3 Hz); 13C NMR (75 MHz, CDCl3): δ 162.2 (CO), 141.0 (quat.), 138.2 (quat.), 126.6 (CH), 123.3 (CH), 123.0 (quat.), 122.4 (CH), 109.2 (CH), 52.0 (CH2), 49.5 (quat.), 42.1 (CH2), 36.6 (CH2), 29.7 (CH), 29.6 (CH2), 29.1 (CH2), 22.4 (CH2), 12.0 (CH3); LRMS (+ ESI): m/z 388 ([M + Na]+, 100%); HRMS (+ ESI): m/z calculated [M + Na]+ 388.2359, found 388.2360; IR (diamond cell, thin film): 3303 (w), 2908 (s), 2846 (m), 1647 (s), 1529 (s), 1491 (m), 1451 (m), 1357 (m), 1341 (m), 1289 (m), 1218 (m), 1139 (m), 1005 (m), 859 (m), 772 (m), 748 (s).
Preparation of N-(adamantan-1-yl)-1-pentyl-1H-pyrrolo[3,2-b]pyridine-3-carboxamide (AP4AICA, 8)
Treating 36 (200 mg, 0.86 mmol) according to general procedure D gave 8, following purification by flash chromatography (hexane/EtOAc, 70:30 v/v), as a white solid (210 mg, 67%).
m.p. 212.0–214.0 °C; 1H NMR (300 MHz, CDCl3): δ 8.69 (1H, br s), 8.47 (1H, s), 7.97 (1H, s), 7.65 (1H, d, J = 8.1 Hz), 7.16 (1H, t, J = 3.9 Hz), 4.12 (2H, t, J = 6.3 Hz), 2.13 (6H, m), 2.04 (3H, m), 1.83–1.69 (8H, m), 1.35–1.21 (4H, m), 0.88 (3H, t, J = 6.9 Hz); 13C NMR (75 MHz, CDCl3): δ 163.4 (CO), 143.5 (CH), 134.8 (CH), 129.8 (quat.), 127.1 (quat.), 117.6 (CH), 116.9 (CH), 112.0 (quat.), 51.8 (CH2), 47.2 (quat.), 42.2 (CH2), 36.8 (CH2), 29.9 (CH2), 29.8 (CH2), 29.0 (CH2), 22.4 (CH2), 14.0 (CH3); LRMS (+ ESI): m/z 388 ([M + Na]+, 100%); HRMS (+ ESI): m/z calculated [M + Na]+ 388.2359, found 388.2362; IR (diamond cell, thin film): 3257 (w), 3065 (w), 2902 (s), 2849 (m), 1635 (s), 1562 (s), 1432 (m), 1276 (m), 1255 (m), 782 (s).
Preparation of N-(adamantan-1-yl)-1-pentyl-1H-pyrrolo[3,2-c]pyridine-3-carboxamide (AP5AICA, 9)
Treating 37 (70 mg, 0.30 mmol) according to general procedure D gave 9, following purification by flash chromatography (EtOAc), as a white solid (68 mg, 62%).
m.p. 155.5–156.5 °C; 1H NMR (300 MHz, CDCl3): δ 9.24 (1H, s), 8.38 (1H, d, J = 5.7 Hz), 7.63 (1H, s), 7.26 (1H, d, J = 6.0 Hz), 5.70 (1H, br s), 4.10 (2H, t, J = 7.2 Hz), 2.18 (6H, m), 2.14 (3H, m), 1.84 (2H, quin., J = 7.2 Hz), 1.75 (6H, m), 1.38–1.27 (4H, m), 0.88 (3H, t, J = 7.1 Hz); 13C NMR (75 MHz, CDCl3): δ 163.4 (CO), 143.4 (CH), 141.6 (CH), 140.5 (quat.), 131.7 (CH), 122.4 (quat.), 113.0 (quat.), 105.4 (CH), 52.2 (CH2), 46.8 (quat.), 42.2 (CH2), 36.6 (CH), 29.9 (CH2), 29.7 (CH3), 29.0 (CH2), 22.3 (CH2), 14.0 (CH3); LRMS (+ ESI): m/z 366 ([M + H]+, 100%); HRMS (+ ESI): m/z calculated [M + Na]+ 388.2359, found 388.2362; IR (diamond cell, thin film): 3343 (bs), 2905 (m), 2851 (m), 1619 (s), 1537 (s), 1516 (s), 1357 (m), 1196 (m), 1141 (m), 799 (m).
Preparation of N-(adamantan-1-yl)-1-pentyl-1H-pyrrolo[2,3-c]pyridine-3-carboxamide (AP6AICA, 10)
Treating 38 (70 mg, 0.30 mmol) according to general procedure D gave 10, following purification by flash chromatography (hexane/EtOAc, 80:20 v/v), as a white solid (88 mg, 80%).
m.p. 154.0–155.5 °C; 1H NMR (500 MHz, CDCl3): δ 8.79 (1H, d, J = 0.9 Hz), 8.35 (1H, d, J = 5.6 Hz), 7.75-7.73 (2H, m), 5.67 (1H, br s), 4.19 (2H, t, J = 7.2 Hz), 2.17 (9H, m), 1.88 (2H, quin., J = 7.3 Hz), 1.77–1.70 (6H, m), 1.36–1.27 (4H, m), 0.87 (3H, t, J = 7.3 Hz); 13C NMR (125 MHz, CDCl3): δ 163.6 (CO), 140.5 (CH), 134.1 (CH), 133.8 (CH), 133.7 (quat.), 130.2 (quat.), 114.5 (CH), 112.2 (quat.), 52.4 (CH2), 47.4 (quat.), 42.3 (CH2), 36.6 (CH2), 30.1 (CH2), 29.7 (CH), 29.1 (CH2), 22.4 (CH2), 14.0 (CH3); LRMS (+ ESI): m/z 366 ([M + H]+, 100%); HRMS (+ ESI): m/z calculated [M + Na]+ 388.2359, found 388.2362; IR (diamond cell, thin film): 3333 (bs), 2904 (s), 2851 (m), 1618 (s), 1537 (s), 1514 (s), 2180 (m), 1245 (m), 821 (m).
Preparation of N-(adamantan-1-yl)-1-pentyl-1H-pyrrolo[2,3-b]pyridine-3-carboxamide (AP7AICA, 11)
Treating 39 (75 mg, 0.32 mmol) according to general procedure D gave 11, following purification by flash chromatography (hexane/EtOAc, 80:20 v/v), as a white solid (88 mg, 75%).
m.p. 149.0–149.5 °C; 1H NMR (300 MHz, CDCl3): δ 9.24 (1H, m), 8.26 (1H, d, J = 8.1 Hz), 7.69 (1H, s), 7.17 (1H, t, J = 4.5 Hz), 5.55 (1H, br s), 4.29 (2H, t, J = 6.6 Hz), 2.17 (9H, m), 1.88 (2H, quin., J = 6.8 Hz), 1.74 (6H, m), 1.33 (4H, m), 0.88 (3H, t, J = 6.7 Hz); 13C NMR (75 MHz, CDCl3): δ 164.0 (CO), 147.8 (quat.), 143.7 (CH), 130.2 (CH), 128.8 (CH), 118.8 (quat.), 117.3 (CH), 110.6 (quat.), 52.3 (CH2), 45.1 (quat.), 42.3 (CH2), 36.6 (CH), 30.1 (CH2), 29.7 (CH), 29.1 (CH2), 22.4 (CH2), 14.1 (CH3); LRMS (+ ESI): m/z 388 ([M + Na]+, 100%); HRMS (+ ESI): m/z calculated [M + Na]+ 388.2359, found 388.2362; IR (diamond cell, thin film): 3322 (bs), 3097 (s), 2905 (s), 2850 (m), 1622 (s), 1538 (s), 1451 (m), 1187 (m).
General procedure E: esterification of 1-N-pentyl-3-indole/indazole/azaindole carboxylic acids with 1-bromoadamantane
A suspension of the appropriate 1-N-pentyl-indole, indazole, or azaindole-3-carboxylic acid (0.43 mmol), 1-bromoadamantane (101 mg, 0.47 mmol), and silver carbonate (178 mg, 0.65 mmol) in N,N-dimethylacetamide (4 mL) was stirred at reflux for 24 h. The reaction was cooled to ambient temperature and concentrated under reduced pressure. The residue was added to H2O (50 mL) and extracted with EtOAc (3 × 50 mL), and the combined organic extracts were dried (MgSO4) and concentrated under reduced pressure.
Preparation of adamantan-1-yl 1-pentyl-1H-indole-3-carboxylate (APIC, 12)
Treating 20 (100 mg, 0.43 mmol) according to general procedure E gave 12, following purification by flash chromatography (hexane/EtOAc, 93:7 v/v), as an off-white solid (68 mg, 41%).
m.p. 102.0–103.0 °C; 1H NMR (300 MHz, CDCl3): δ 8.21–8.11 (1H, m), 7.75 (1H, s), 7.38–7.19 (3H, m), 4.12 (2H, t, J = 7.1 Hz), 2.40–2.31 (6H, m), 2.28–2.19 (3H, m), 1.86 (2H, quin., J = 7.3 Hz), 1.80–1.65 (6H, m), 1.44–1.25 (4H, m), 0.89 (3H, t, J = 6.5 Hz); 13C NMR (75 MHz, CDCl3): 164.7 (CO), 136.7 (quat.), 134.2 (CH), 126.9 (quat.), 122.5 (CH), 122.0 (CH), 121.6 (CH), 110.0 (CH), 108.9 (quat.), 80.1 (quat.), 47.1 (CH2), 42.1 (CH2), 36.5 (CH), 31.1 (CH2), 29.8 (CH2), 29.1 (CH2), 22.4 (CH2), 14.0 (CH3); LRMS: m/z 388 ([M + Na]+, 100%); HRMS (+ ESI): m/z calculated [M + Na]+ 388.2247, found 388.2245; IR (diamond cell, thin film): 3112 (w), 2904 (s), 2847 (m), 1680 (s), 1530 (s), 1265 (s), 1102 (s), 1058 (s), 734 (s).
Preparation of adamantan-1-yl 1-pentyl-1H-indazole-3-carboxylate (APINAC, 13)
Treating 20 (500 mg, 1.54 mmol) according to general procedure E gave 13, following purification by flash chromatography (hexane/EtOAc, 92:8 v/v), as an off-white solid (215 mg, 38%).
m.p. 109.0–110.0 °C; 1H NMR (300 MHz, CDCl3): δ 8.15 (1H, d, J = 8.1 Hz), 7.46–7.37 (2H, m), 7.26 (1H, t, J = 6.9 Hz), 4.44 (2H, t, J = 7.5 Hz), 2.39–2.37 (6H, m), 2.26–2.23 (3H, m), 1.96 (2H, quin., J = 7.2 Hz), 1.80–1.69 (6H, m), 1.39–1.28 (4H, m), 0.88 (3H, t, J = 6.9 Hz); 13C NMR (300 MHz, CDCl3): δ 161.8 (CO), 140.7 (quat.), 136.3 (quat.), 126.5 (CH), 123.5 (quat.), 122.7(0) (CH), 122.6(9) (CH), 109.7 (CH), 81.9 (quat.), 49.9 (CH2), 41.8 (CH2), 36.4 (CH), 31.1 (CH2), 29.6 (CH2), 29.1 (CH2), 22.4 (CH2), 14.0 (CH3); LRMS (+ ESI): m/z 389 ([M + Na]+, 100%), 755 ([2 M + Na]+, 95%); HRMS (+ ESI): m/z calculated [M + Na]+ 389.2199, found 389.2196; IR (diamond cell, thin film): 2905 (s), 2849 (m), 1690 (m), 1644 (s), 1361 (m), 1305 (s), 1169 (m), 1124 (s), 866 (m), 756 (s), 544 (w).
Preparation of adamantan-1-yl 1-pentyl-1H-pyrrolo[3,2-b]pyridine-3-carboxylate (AP4AIC, 14)
Treating 36 (100 mg, 0.43 mmol) according to general procedure E gave 14, following purification by flash chromatography (hexane/EtOAc, 70:30 v/v), as a white solid (55 mg, 35%).
m.p. 83.5–85.0 °C; 1H NMR (300 MHz, CDCl3): δ 8.64 (1H, d, J = 4.5 Hz), 7.86 (1H, s), 7.62 (1H, d, J = 8.4 Hz), 7.15 (1H, dd, J = 7.8, 4.8 Hz), 4.11 (2H, t, J = 6.9 Hz), 2.34–2.31 (6H, m), 2.22–2.19 (3H, m), 1.85 (2H, quin., J = 7.2 Hz), 1.74–1.67 (6H, m), 1.38–1.25 (4H, m), 0.89 (3H, t, J = 6.3 Hz); 13C NMR (75 MHz, CDCl3): δ 162.6 (CO), 145.5 (CH), 144.7 (quat.), 136.1 (CH), 129.7 (quat.), 117.2 (CH), 117.1 (CH), 109.1 (quat.), 80.2 (quat.), 47.2 (CH2), 41.8 (CH2), 36.5 (CH2), 31.1 (CH), 29.8 (CH2), 29.0 (CH2), 22.3 (CH2), 13.9 (CH3); LRMS (+ ESI): m/z 389 ([M + Na]+, 100%); HRMS (+ ESI): m/z calculated [M + Na]+ 389.2199, found 389.2200; IR (diamond cell, thin film): 3107 (w), 2911 (m), 2856 (w), 1661 (s), 1640 (s), 1517 (s), 1329 (s), 1182 (m), 885 (m), 799 (m), 549 (m).
Preparation of adamantan-1-yl 1-pentyl-1H-pyrrolo[3,2-c]pyridine-3-carboxylate (AP5AIC, 15)
Treating 37 (100 mg, 0.43 mmol) according to general procedure E gave 15, following purification by flash chromatography (hexane/EtOAc, 60:40 v/v), as an off-white solid (79 mg, 50%).
m.p. 101.5–102.5 °C; 1H NMR (300 MHz, CDCl3): δ 9.56 (1H, d, J = 0.9 Hz), 8.52 (1H, d, J = 5.8 Hz), 7.72 (1H, s), 7.33 (1H, dd, J = 5.9, 0.8 Hz), 4.19 (2H, t, J = 7.1 Hz), 2.31–2.28 (6H, m), 2.11–2.06 (3H, m), 1.90 (2H, quin., J = 7.3 Hz), 1.72–1.61 (6H, m), 1.39–1.28 (4H, m), 0.90 (3H, t, J = 7.0 Hz); 13C NMR (75 MHz, CDCl3): δ 162.5 (CO) 145.3 (CH), 143.3 (CH), 141.6 (quat.), 137.8 (CH), 123.0 (quat.), 116.2 (quat.), 105.7 (CH), 74.2 (quat.), 47.5 (CH2), 40.1 (CH2), 36.4 (CH2), 30.1 (CH), 29.5 (CH2), 29.0 (CH2), 22.3 (CH2), 14.0 (CH3); LRMS (+ ESI): m/z 367 ([M + H]+, 100%); HRMS (+ ESI): m/z calculated [M + Na]+ 389.2199, found 389.2199; IR (diamond cell, thin film): 3109 (w), 2909 (m), 2852 (w), 1683 (s), 1533 (m), 1229 (m), 1132 (s), 1055 (s), 757 (m), 421 (m).
Preparation of adamantan-1-yl 1-pentyl-1H-pyrrolo[2,3-c]pyridine-3-carboxylate (AP6AIC, 16)
Treating 38 (100 mg, 0.43 mmol) according to general procedure E gave 16, following purification by flash chromatography (hexane/EtOAc, 50:50 v/v), as a white solid (68 mg, 43%).
m.p. 108.5–110.0 °C; 1H NMR (300 MHz, CDCl3): δ 8.78 (1H, s), 8.37 (1H, d, J = 5.1 Hz), 7.97 (1H, d, J = 5.4 Hz), 7.83 (1H, s), 4.21 (2H, t, J = 7.2 Hz), 2.35–2.30 (6H, m), 2.26–2.21 (3H, m), 1.90 (2H, quin., J = 6.9 Hz), 1.78–1.67 (6H, m), 1.37–1.30 (4H, m), 0.89 (3H, t, J = 6.3 Hz); 13C NMR (75 MHz, CDCl3): δ 163.8 (CO), 140.9 (CH), 136.8 (CH), 133.8 (quat.), 133.4 (CH), 131.9 (CH), 116.2 (quat.), 109.0 (quat.), 80.8 (quat.), 47.5 (CH2), 42.0 (CH2), 36.5 (CH2), 31.1 (CH), 30.0 (CH2), 29.0 (CH2), 22.3 (CH2), 14.0 (CH3); LRMS (+ ESI): m/z 367 ([M + H]+, 100%); HRMS (+ ESI): m/z calculated [M + Na]+ 389.2199, found 389.2197; IR (diamond cell, thin film): 3109 (w), 2909 (s), 2848 (m), 1682 (s), 1603 (m), 1526 (s), 1443 (m), 1249 (s), 1194 (m), 1131 (s), 1055 (s), 820 (s), 626 (m), 424 (m).
Preparation of adamantan-1-yl 1-pentyl-1H-pyrrolo[2,3-b]pyridine-3-carboxylate (AP7AIC, 17)
Treating 39 (100 mg, 0.43 mmol) according to general procedure E gave 17, following purification by flash chromatography (hexane/EtOAc 70:30 v/v), as a yellow solid (60 mg, 38%).
m.p. 99.5–101.0 °C; 1H NMR (300 MHz, CDCl3): δ 8.38-8.34 (2H, m), 7.87 (1H, s), 7.17 (1H, dd, J = 8.1, 4.8 Hz), 4.29 (2H, t, J = 7.3 Hz), 2.34–2.29 (6H, m), 2.26–2.20 (3H, m), 1.89 (2H, quin., J = 7.2 Hz), 1.78–1.68 (6H, m), 1.39–1.28 (4H, m), 0.88 (3H, t, J = 7.0 Hz); 13C NMR (75 MHz, CDCl3): δ 164.0 (CO), 147.9 (quat.), 143.8 (CH), 133.9 (CH), 130.1 (CH), 119.3 (quat.), 117.8 (CH), 107.4 (quat.), 80.5 (quat.), 45.2 (CH2), 42.0 (CH2), 36.5 (CH2), 31.1 (CH), 30.0 (CH2), 29.0 (CH2), 22.4 (CH2), 14.0 (CH3); LRMS (+ ESI): m/z 389 ([M + Na]+, 100%); HRMS (+ ESI): m/z calculated [M + Na]+ 389.2199, found 389.2198; IR (diamond cell, thin film): 3105 (w), 2915 (m), 2852 (w), 1686 (s), 1528 (s), 1403 (m), 1256 (s), 1135 (s), 1117 (s), 1058 (s), 773 (m), 758 (s).
Gas chromatography–mass spectrometry
All final compounds were subjected to gas chromatography–electron ionisation–mass spectrometry (GC–EI-MS) using an Agilent 7890 gas chromatograph fitted with a 5975C inert mass selective detector (Agilent Technologies, Santa Clara, CA, USA). The column was an HP-5 capillary column (30 m × 0.25 mm, i.d., film thickness 0.25 µm), with helium as the carrier gas at a constant flow of 1.8 mL/min and a split ratio of 10:1 (Agilent Technologies). The oven temperature commenced at 65 °C with a hold time of 1 min, followed by a 40 °C/min ramp rate to 300 °C with a final hold time of 13 min. Mass spectra were collected over m/z 35–550 with an ionisation energy of 70 eV. Compounds were extracted in EtOAc at an approximate concentration of 1 mg/mL. The injection volume was 1 µL.
In vitro pharmacological assessment
Mouse AtT-20 neuroblastoma cells stably transfected with human CB1 or human CB2 have been described previously and were cultured in Dulbecco’s modified Eagle medium containing 10% fetal bovine serum (FBS), 100 U penicillin/streptomycin, and 80 μg/mL hygromycin [10]. Cells were passaged at 80% confluence as required. Cells for assays were grown in 75 cm2 flasks and used at 90% confluence. The day before, the assay cells were detached from the flask with trypsin/ethylenediaminetetraacetic acid (Sigma-Aldrich) and resuspended in 10 mL of Leibovitz’s L-15 media supplemented with 1% FBS, 100 U penicillin/streptomycin, and 15 mM glucose for the below membrane potential and Ca5 calcium assays. The cells were plated in a volume of 90 μL in black-walled, clear-bottomed 96-well microplates (Corning Inc., Corning, NY, USA). Cells were incubated overnight at 37 °C in ambient CO2.
Membrane potential was measured using a fluorometric imaging plate reader (FLIPR) membrane potential assay kit (blue) from Molecular Devices (San Jose, CA, USA), as described previously [28]. The dye was reconstituted with assay buffer of the following composition (mM): NaCl 145, HEPES 22, Na2HPO4 0.338, NaHCO3 4.17, KH2PO4 0.441, MgSO4 0.407, MgCl2 0.493, CaCl2 1.26, glucose 5.56 and bovine serum albumin (BSA) (pH 7.4 and osmolarity 315 ± 5). Prior to the assay, 90 μL/well of the dye solution was added, giving an initial assay volume of 180 μL/well.
Drugs were dissolved at 30 mM in DMSO and aliquoted for storage at −30 °C. On the day of experiment, an aliquot was unfrozen, and a portion diluted to 10 mM in DMSO. Serial dilutions were performed in HEPES-buffered saline (HBS) containing 0.1% BSA. The first 1:10 dilution was made into HBS plus BSA and 1% DMSO. Drugs were added to the cells at 10 times the final concentration, to give a final concentration of DMSO of 0.1% in all wells.
Plates were incubated at 37 °C at ambient CO2 for 60 min. Fluorescence was measured using a FlexStation 3 (Molecular Devices) microplate reader, with cells excited at a wavelength of 530 nm and emission measured at 565 nm. Baseline readings were taken every 2 s for at least 2 min, at which time either drug or vehicle was added in a volume of 20 μL. The background fluorescence of cells without dye or dye without cells was negligible. Changes in fluorescence were expressed as a percentage of predrug fluorescence after subtraction of the changes produced by vehicle addition.
Drugs were initially tested over a concentration range of 1 nM to 30 µM. Full concentration response curves were constructed for drugs which produced an effect greater than 50% of that of CP 55,940 (1 µM; Cayman Chemical, Ann Arbor, MI, USA) when tested at 10 µM. If drugs produced a response less than 50% of CP 55,940 (1 µM) when tested at 10 µM, experiments were repeated at 10 µM only.
Data were analysed with PRISM (GraphPad Software Inc., San Diego, CA, USA), using four-parameter nonlinear regression to fit concentration-response curves. In all plates, a maximum effective concentration of CP 55,940 (1 µM) was added to allow for a ready comparison between experiments.
Results and discussion
Preparation of APICA (4) is outlined in Fig. 3, and followed the same methodology as that previously reported [13]. Indole (18) was subjected to an N-pentyl alkylation with 1-bromopentane, followed by the addition of trifluoroacetic anhydride, to give 19 in good yield. Hydrolysis of the trifluoroacetate functionality under basic conditions gave carboxylic acid 20, before HOBt/EDC-mediated amide coupling with 1-aminoadamantane hydrochloride gave 4. Synthesis of the adamantyl ester derivative APIC (12) has not been reported previously, and required optimisation to obtain desirable amounts of material (full detail of attempts can be found in supplementary material). Given the bulky nature and relatively poor nucleophilicity of 1-hydroxyadamantane, traditional esterification conditions proved to be an ineffective way to access 12. Instead, halide abstraction of 1-bromoadamantane using silver(I) carbonate to form the tertiary carbocation in the presence of carboxylic acid 20 gave the desired product 12 in moderate yield.
Preparation of 4 and 12. Reagents and conditions: (a)(i) NaH, 1-bromopentane, N,N,-dimethylformamide (DMF), 0–25 °C, 1 h; (ii) (CF3CO)2O, 25 oC, 1 h, 94%; (b) 4 M aq. NaOH, methanol (MeOH), reflux, 18 h, 88%; (c) 1-aminoadamantane hydrochloride, 1-hydroxybenzotriazole (HOBt), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC-HCl), N,N-diisopropylethylamine (DIPEA), dimethyl sulfoxide (DMSO), 25 °C, 18 h, 86%; (d) 1-bromoadamantane, Ag2CO3, N,N-dimethylacetamide (DMA), reflux, 24 h, 41%
Synthesis of APINACA (7) also followed a methodology described previously (Fig. 4) [10]. N-Alkylation of methyl-3-indazole carboxylate (21) using potassium tert-butoxide gave predominantly the 1-N-alkyl regioisomer (22). Following hydrolysis of the methyl ester to give carboxylic acid 23, HOBt/EDC·HCl-mediated amide coupling with 1-aminoadamantane hydrochloride gave 7 in good yield. Synthesis of APINAC (13) utilised the optimised conditions employed in the preparation of 12, using 23 and 1-bromoadamantane to obtain the desired product in a sufficient quantity.
The azaindole-derived SCs were accessed through a synthetic approach similar to that outlined for 4 and 12. The appropriate azaindoles (24 to 27) were 1-N-pentylated with 1-bromopentane to give 28–31. Attempts to introduce the trifluoromethyl ketone in a one-pot reaction with the N-alkylation proved unsuccessful. This was attributed to the presence of the additional nitrogen in the bicyclic ring system, decreasing the nucleophilicity of the azaindole’s 3-position. Friedel-Crafts acylation conditions using aluminium chloride and trifluoroacetic anhydride proved successful, giving desired compounds 32–35 in good to excellent yield. Hydrolysis of the trifluoroacetate moieties to give carboxylic acids 36–39 was followed by the aforementioned amide coupling conditions to give 8–11, or esterification conditions to give 14–17 (Fig. 5).
Preparation of 8–11 and 14–17. Reagents and conditions: (a) 1-bromopentane, NaH, DMF, 0–25 °C, 2 h, 68–85%; (b) AlCl3, (CF3CO)2O, DMF, 25 °C, 2 h, 63–95%; (c) 4 M aq. NaOH, MeOH, reflux, 18 h, 52–97%; (d) 1-adamantylamine HCl, EDC·HCl, HOBt, DIPEA, DMSO, 25 °C, 18 h, 62–80%; (e) 1-bromoadamantane, Ag2CO3, DMA, reflux, 24 h, 35–50%
Representative total ion chromatograms (TICs) and EI mass spectra for carboxamides APINACA (7) and AP7AICA (11) are shown in Fig. 6, and carboxylates APINAC (13) and AP7AIC (17) in Fig. 7 (all other chromatograms and mass spectra can be found in the supplementary data). The GC method used was able to reliably differentiate each compound within the carboxamide series based exclusively on retention times, and although each derivative showed similar EI fragmentation patterns, the intensities of each fragment ion could be used as an additional source of identification. As demonstrated in Fig. 6, each of the carboxamides showed the parent ion (m/z 365) and a base peak at m/z 215 corresponding to fragmentation of the C–N amide bond. The carboxamide series also showed a tendency to cleave along the N-pentyl chain (e.g., APINACA m/z 294 and 336; and AP7AICA m/z 308).
Similar to the carboxamide series, each azaindole scaffold in the carboxylate series was able to be differentiated by retention time using the GC method described. However, APINAC (13) and AP7AIC (17) showed nearly identical retention times (see Fig. 7). The EIMS fragmentation ratios for the carboxylate analogues showed greater variability than their carboxamide counterparts, providing a more effective way to discriminate each structure. With all of the azaindole-derived carboxylates, the parent ion fragment was one of the major peaks recorded, whereas it was only a minor peak observed for the 13. The other major fragmentation peaks observed within this series include m/z 135/231 corresponding to cleavage of the adamantyl ring (the base peak for 13), m/z 215 corresponding to cleavage of the C–O ester bond (the base peak for 14), and m/z 145, presumably corresponding to the protonated state of the N-alkyl and C–O ester bond cleaved ion.
The in vitro activity of compounds 4 and 7–17 at CB1 and CB2 were compared to known agonist CP 55,940 (3) using a FLIPR assay (summary of results displayed in Table 1, n = 5 or more independent experiments) following the same methodology as that previously reported [5,6,7,8]. Previously identified SCs APICA (4) and APINACA (7) showed moderate potency at both hCB1 (EC50 = 109 and 142 nM, respectively) and hCB2 (EC50 = 56 and 141 nM, respectively), with 4 showing a similar preference for activating CB2, as previously reported [29]. Unlike previous series of SCs based on the indole scaffold, a number of compounds in the current series showed no activity at one or both cannabinoid receptors, indicating the importance of the bicyclic ring system to cannabimimetic activity. There was also no correlation between the activity of the amide series and the ester series. An example of this is known SC APICA (4), which had EC50 values of 109 and 56 nM at CB1 and CB2, respectively. Its ester counterpart APIC (12) showed no activities at the tested dosages at either cannabinoid receptor. The opposite was seen with the 4-azaindole derivatives. Whereas AP4AICA (8) showed no efficacy at either CB1 or CB2, AP4AIC (14) showed moderate activity at both CB1 and CB2 (EC50 = 160 and 64 nM, respectively). Based on these data, it is difficult to gauge any trend in azaindole and ester tolerability on cannabimimetic activity. Interestingly, a general trend for CB2 selectivity was seen with this series of compounds, indicating that a large aliphatic substituent at the 3-position of these bicyclic ring systems has enhanced activity at CB2. The most notable of these was AP7AICA (11), which showed potent activity at CB2 (EC50 = 20 nM), with no activity at CB1 in this FLIPR assay.
Conclusions
This work explored the synthesis, forensic analysis, and cannabimimetic tolerance of a series of adamantyl-containing SCs, incorporating a range of bicyclic cores and ester/amide linkages. For the first time, the importance of the bicyclic ring system to cannabinoid activity within the aminoalkylindole core structure has been illustrated. As there is no consistent structure-activity relationship within each scaffold, it is difficult to predict whether these bicyclic cores will readily appear in future drugs of abuse. However, the receptor selectivity for various analogues within these series may be of interest in the development of therapeutics.
References
United Nations Office on Drugs and Crime (2017). World Drug Report 2017, booklet 4. http://www.unodc.org/wdr2017/field/Booklet_4_ATSNP.pdf. Accessed 11 Oct 2018
Livny A, Cohen K, Tik N, Tsarfaty G, Rosca P, Weinstein A (2018) The effects of synthetic cannabinoids (SCs) on brain structure and function. Eur Neuropsychopharmacol 28:1047–1057
Müller H, Sperling W, Köhrmann M, Huttner HB, Kornhuber J, Maler JM (2010) The synthetic cannabinoid Spice as a trigger for an acute exacerbation of cannabis induced recurrent psychotic episodes. Schizophr Res 118:309–310
Schneir AB, Baumbacher T (2012) Convulsions associated with the use of a synthetic cannabinoid product. J Med Toxicol 8:62–64
Mir A, Obafemi A, Young A, Kane C (2011) Myocardial infarction associated with use of the synthetic cannabinoid K2. Pediatrics 128:e1622–e1627
Deng H, Verrico CD, Kosten TR, Nielsen DA (2018) Psychosis and synthetic cannabinoids. Psychiatry Res 268:400–412
De Luca MA, Fattore L (2018) Therapeutic use of synthetic cannabinoids: still an open issue? Clin Ther 40:1457–1466
Davidson C, Opacka-Juffry J, Arevalo-Martin A, Garcia-Ovejero D, Molina-Holgado E, Molina-Holgado F (2017) Spicing up pharmacology: a review of synthetic cannabinoids from structure to adverse events. Adv Pharmacol 80:135–168
Hess C, Schoeder CT, Pillaiyar T, Madea B, Müller CE (2016) Pharmacological evaluation of synthetic cannabinoids identified as constituents of spice. Forensic Toxicol 34:329–343
Banister SD, Longworth M, Kevin R, Sachdev S, Santiago M, Stuart J, Mack JBC, Glass M, McGregor IS, Connor M, Kassiou M (2016) Pharmacology of valinate and tert-leucinate synthetic cannabinoids 5F-AMBICA, 5F-AMB, 5F-ADB, AMB-FUBINACA, MDMB-FUBINACA, MDMB-CHMICA, and their analogues. ACS Chem Neurosci 7:1241–1254
Banister SD, Moir M, Stuart J, Kevin RC, Wood KE, Longworth M, Wilkinson SM, Beinat C, Buchanan AS, Glass M, Connor M, McGregor IS, Kassiou M (2015) Pharmacology of indole and indazole synthetic cannabinoid designer drugs AB-FUBINACA, ADB-FUBINACA, AB-PINACA, ADB-PINACA, 5F-AB-PINACA, 5F-ADB-PINACA, ADBICA, and 5F-ADBICA. ACS Chem Neurosci 6:1546–1559
Banister SD, Stuart J, Kevin RC, Edington A, Longworth M, Wilkinson SM, Beinat C, Buchanan AS, Hibbs DE, Glass M, Connor M, McGregor IS, Kassiou M (2015) Effects of bioisosteric fluorine in synthetic cannabinoid designer drugs JWH-018, AM-2201, UR-144, XLR-11, PB-22, 5F-PB-22, APICA, and STS-135. ACS Chem Neurosci 6:1445–1458
Banister SD, Wilkinson SM, Longworth M, Stuart J, Apetz N, English K, Brooker L, Goebel C, Hibbs DE, Glass M, Connor M, McGregor IS, Kassiou M (2013) The synthesis and pharmacological evaluation of adamantane-derived indoles: cannabimimetic drugs of abuse. ACS Chem Neurosci 4:1081–1092
Lauckner JE, Hille B, Mackie K (2005) The cannabinoid agonist WIN55,212-2 increases intracellular calcium via CB1 receptor coupling to Gq/11 G proteins. Proc Natl Acad Sci USA 102:19144–19149
Pertwee RG (2010) Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists. Curr Med Chem 17:1360–1381
Shevyrin V, Melkozerov V, Nevero A, Eltsov O, Shafran Y (2013) Analytical characterization of some synthetic cannabinoids, derivatives of indole-3-carboxylic acid. Forensic Sci Int 232:1–10
Uchiyama N, Matsuda S, Kawamura M, Kikura-Hanajiri R, Goda Y (2013) Two new-type cannabimimetic quinolinyl carboxylates, QUPIC and QUCHIC, two new cannabimimetic carboxamide derivatives, ADB-FUBINACA and ADBICA, and five synthetic cannabinoids detected with a thiophene derivative α-PVT and an opioid receptor agonist AH-7921 identified in illegal products. Forensic Toxicol 31:223–240
Shevyrin V, Melkozerov V, Nevero A, Eltsov O, Baranovsky A, Shafran Y (2014) Synthetic cannabinoids as designer drugs: new representatives of indol-3-carboxylates series and indazole-3-carboxylates as novel group of cannabinoids. Identification and analytical data. Forensic Sci Int 244:263–275
Banister SD, Kevin RC, Macdonald C, Glass M, Boyd R, Connor M, McGregor IS, Havel CM, Bright SJ, Ventura M, Gil C, Barrat MJ, Gerona RR (2019) Synthesis and pharmacology of new psychoactive substance 5F-CUMYL-P7AICA, a scaffold-hopping analogue of synthetic cannabinoid receptor agonists 5F-CUMYL-PICA and 5F-CUMYL-PINACA. Drug Test Anal 11:279–291
Bovens M, Bissig C, Staeheli SN, Poetzsch M, Pfeiffer B, Kraemer T (2017) Structural characterization of the new synthetic cannabinoids CUMYL-PINACA, 5F-CUMYL-PINACA, CUMYL-4CN-BINACA, 5F-CUMYL-P7AICA and CUMYL-4CN-B7AICA. Forensic Sci Int 281:98–105
Ernst L, Brandhorst K, Papke U, Altrogge A, Zodel S, Langer N, Beuerle T (2017) Identification and quantification of synthetic cannabinoids in ‘spice like’ herbal mixtures: update on the German situation in early 2017. Forensic Sci Int 277:51–58
Liu C, Jia W, Hua Z, Qian Z (2017) Identification and analytical characterization of six synthetic cannabinoids NNL-3, 5F-NPB-22-7N, 5F-AKB-47-7N, 5F-EDMB-PINACA, EMB-FUBINACA and EG-018. Drug Test Anal 9:1251–1261
Qian Z, Jia W, Li T, Hua Z, Liu C (2017) Identification and analytical characterization of four synthetic cannabinoids ADB-BICA, NNL-1, NNL-2 and PPA(N)-2201. Drug Test Anal 9:51–60
Schulze A, Boyd SE, Blakey K (2018) Characterization of two novel synthetic cannabinoid type compounds CUMYL CYBCZCA and 5-Fluoro AB-P7AICA. In: ANZFSS 24th international symposium on the forensic sciences, 9–13 Sep 2018, Perth, Australia
European project response to challenges in forensic analyses (2015) 5F-PCN. http://www.policija.si/apps/nfl_response_web/seznam.php. Accessed 18 Oct 2018
Lee JH, Park HN, Leem T, Jeon J, Cho S, Lee J, Baek SY (2017) Identification of new synthetic cannabinoid analogue APINAC (adamantan-1-yl 1-pentyl-1H-indazole-3-carboxylate) with other synthetic cannabinoid MDMB(N)-Bz-F in illegal products. Forensic Toxicol 35:45–55
Savchuk S, Appolonova S, Pechnikov A, Rizvanova L, Shestakova K, Tagliaro F (2017) In vivo metabolism of the new synthetic cannabinoid APINAC in rats by GC-MS and LC-QTOF-MS. Forensic Toxicol 35:359–368
Knapman A, Santiago M, Du YP, Bennallack PR, Christie MJ, Connor M (2013) A continuous, fluorescence-based assay of μ-opioid receptor activation in AtT-20 cells. J Biomol Screen 18:269–276
Longworth M, Connor M, Banister SD, Kassiou M (2017) Synthesis and pharmacological profiling of the metabolites of synthetic cannabinoid drugs APICA, STS-135, ADB-PINACA, and 5F-ADB-PINACA. ACS Chem Neurosci 8:1673–1680
Acknowledgements
This work was supported in part by NHMRC Project Grant 1107088.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no financial or other relations that could lead to a conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Longworth, M., Reekie, T.A., Blakey, K. et al. New-generation azaindole-adamantyl-derived synthetic cannabinoids. Forensic Toxicol 37, 350–365 (2019). https://doi.org/10.1007/s11419-019-00466-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11419-019-00466-1