Abstract
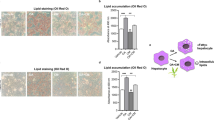
We previously synthesized two retinoid X receptor (RXR) agonists, 4′-hydroxy-3′-propyl-[1,1′-biphenyl]-3-propanoic acid ethyl ester (4′OHE) and 6-hydroxy-3′-propyl-[1,1′-biphenyl]-3-propanoic acid ethyl ester (6OHE), based on the structure of magnaldehyde B, a natural product obtained from Magnolia obovata. 4′OHE and 6OHE exhibited different selectivities for peroxisome proliferator-activated receptor (PPAR)/RXR heterodimers. To examine the regulatory effects of these compounds in adipogenesis, 3T3–L1 mouse preadipocytes were treated with a differentiation cocktail with or without test compounds to induce differentiation, and subsequently treated with test compounds in insulin-containing medium every alternate day. Lipid droplets were stained with Oil Red O to examine lipid accumulation. In addition, adipogenesis-related gene expression was measured using RT–qPCR and immunoblotting. The results showed that a PPARγ agonist, 4′OHE, which exerts agonistic effects on PPARγ and RXRα, enhanced adipogenesis similar to rosiglitazone. However, unlike GW501516, a PPARδ agonist, 6OHE and its hydrolysis product (6OHA), which exert agonistic effects on PPARδ and RXRα, suppressed adipogenesis. In a manner similar to 6OHE and 6OHA, bexarotene, an RXR agonist, suppressed adipocyte differentiation, and its anti-adipogenic effect was reversed by an RXR antagonist. Furthermore, 6OHA and bexarotene inhibited the increase in Pparγ2 and Cebpa mRNA levels 2 days after the induction of differentiation. We demonstrated the adipogenic effect of 4′OHE and anti-adipogenic effects of 6OHE and 6OHA in 3T3–L1 cells. Previously, RXR agonists have been reported to positively regulate the differentiation of mesenchymal stem cells into adipocytes, but our current data showed that they inhibited the differentiation of preadipocytes, at least 3T3–L1 cells, into adipocytes.
Graphical abstract








Similar content being viewed by others

Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
GDB 2015 Obesity Collaborators (2017) Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 377:13–27. https://doi.org/10.1056/NEJMoa1614362
NCD Risk Factor Collaboration (NCD-RisC) (2017) Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 390:2627–2642. https://doi.org/10.1016/S0140-6736(17)32129-3
Brown RE, Sharma AM, Ardern CI, Mirdamadi P, Mirdamadi P, Kuk JL (2016) Secular differences in the association between caloric intake, macronutrient intake, and physical activity with obesity. Obes Res Clin Pract 10:243–255. https://doi.org/10.1016/j.orcp.2015.08.007
Ludwig DS, Ebbeling CB (2018) The carbohydrate-insulin model of obesity: beyond “calories in, calories out.” JAMA Intern Med 178:1098–1103. https://doi.org/10.1001/jamainternmed.2018.2933
Ludwig DS, Hu FB, Tappy L, Brand-Miller J (2018) Dietary carbohydrates: role of quality and quantity in chronic disease. BMJ 361:2340. https://doi.org/10.1136/bmj.k2340
Locke AE et al (2015) Genetic studies of body mass index yield new insights for obesity biology. Nature 518:197–206. https://doi.org/10.1038/nature14177
Egusquiza RJ, Blumberg B (2020) Environmental obesogens and their impact on susceptibility to obesity: new mechanisms and chemicals. Endocrinology 161:bqaa24. https://doi.org/10.1210/endocr/bqaa024
Heindel JJ, Blumberg B (2019) Environmental obesogens: mechanisms and controversies. Annu Rev Pharmacol Toxicol 59:89–106. https://doi.org/10.1146/annurev-pharmtox-010818-021304
Heindel JJ (2019) History of the obesogen field: looking back to look forward. Front Endocrinol 10:14. https://doi.org/10.3389/fendo.2019.00014
Kirchner S, Kieu T, Chow C, Casey S, Blumberg B (2010) Prenatal exposure to the environmental obesogen tributyltin predisposes multipotent stem cells to become adipocytes. Mol Endocrinol 24:526–539. https://doi.org/10.1210/me.2009-0261
Milton FA, Lacerda MG, Sinoti SBP, Mesquita PG, Prakasan D, Coelho MS, de Lima CL, Martini AG, Pazzine GT, Borin MF, Amato AA, Neves FAR (2017) Dibutyltin compounds effects on PPARγ/RXRα activity, adipogenesis, and inflammation in mammalians cells. Front Pharmacol 8:507. https://doi.org/10.3389/fphar.2017.00507
Shoucri BM, Martinez ES, Abreo TJ, Hung VT, Moosova Z, Shioda T, Blumberg B (2017) Retinoid X receptor activation alters the chromatin landscape to commit mesenchymal stem cells to the adipose lineage. Endocrinology 158:3109–3125. https://doi.org/10.1210/en.2017-00348
Sagara C, Takahashi K, Kagechika H, Takahashi N (2013) Molecular mechanism of 9-cis-retinoic acid inhibition of adipogenesis in 3T3-L1 cells. Biochem Biophys Res Commun 433:102–107. https://doi.org/10.1016/j.bbrc.2013.02.057
Kotani H, Tanabe H, Mizukami H, Makishima M, Inoue M (2010) Identification of a naturally occurring rexinoid, honokiol, that activates the retinoid X receptor. J Nat Prod 73:1332–1336. https://doi.org/10.1021/np100120c
Nakashima K, Murakami T, Tanabe H, Inoue M (2014) Identification of a naturally occurring retinoid X receptor agonist from Brazilian green propolis. Biochim Biophys Acta 1840:3034–3041. https://doi.org/10.1016/j.bbagen.2014.06.011
Inoue M, Tanabe H, Nakashima K, Ishida Y, Kotani H (2014) Rexinoids isolated from Sophora tonkinensis with a gene expression profile distinct from the synthetic rexinoid bexarotene. J Nat Prod 77:1670–1677. https://doi.org/10.1021/np5002016
Nakashima K, Yamaguchi E, Noritake C, Mitsugi Y, Goto M, Hirai T, Abe N, Sakai E, Oyama M, Itoh A, Inoue M (2020) Discovery and SAR of natural-product-inspired RXR agonists with heterodimer selectivity to PPARδ-RXR. ACS Chem Biol 15:1526–1534. https://doi.org/10.1021/acschembio.0c00146
Taki M, Kajiwara K, Yamaguchi E, Sato Y, Yamaguchi S (2021) Fused thiophene-S, S-dioxide-based super-photostable fluorescent marker for lipid droplets. ACS Materials Lett 3:42–49. https://doi.org/10.1021/acsmaterialslett.0c00451
Rosen ED, MacDougald OA (2006) Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 7:885–896. https://doi.org/10.1038/nrm2066
Tontonoz P, Spiegelman BM (2008) Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem 77:289–312. https://doi.org/10.1146/annurev.biochem.77.061307.091829
Tang QQ, Lane MD (2012) Adipogenesis: from stem cell to adipocyte. Annu Rev Biochem 81:715–736. https://doi.org/10.1146/annurev-biochem-052110-115718
Choi SS, Cha BY, Lee YS, Yonezawa T, Teruya T, Nagai K, Woo J (2009) Magnolol enhances adipocyte differentiation and glucose uptake in 3T3-L1 cells. Life Sci 84:908–914. https://doi.org/10.1016/j.lfs.2009.04.001
Lebovitz HE (2019) Thiazolidinediones: the forgotten diabetes medications. Curr Diab Rep 19:151. https://doi.org/10.1007/s11892-019-1270-y
Gijsen HSV (2019) Adipocyte biology: it is time to upgrade to a new model. J Cell Physiol 234:2399–2425. https://doi.org/10.1002/jcp.27266
Shoucri BM, Hung VT, Chamorro-Garcia R, Shioda T, Blumberg B (2018) Retinoid X receptor activation during adipogenesis of female mesenchymal stem cells programs a dysfunctional adipocyte. Endocrinology 159:2863–2883. https://doi.org/10.1210/en.2018-00056
Zhang JW, Klemm DJ, Vinson C, Lane MD (2004) Role of CREB in transcriptional regulation of CCAAT/enhancer-binding protein β gene during adipogenesis. J Biol Chem 279:4471–4478. https://doi.org/10.1074/jbc.M311327200
Zhang JW, Tang QQ, Vinson C, Lane MD (2004) Dominant-negative C/EBP disrupts mitotic clonal expansion and differentiation of 3T3-L1 preadipocytes. Proc Natl Acad Sci U S A 101:43–47. https://doi.org/10.1073/pnas.0307229101
Tang QQ, Otto TC, Lane MD (2003) CCAAT/enhancer-binding protein beta is required for mitotic clonal expansion during adipogenesis. Proc Natl Acad Sci U S A 100:850–855. https://doi.org/10.1073/pnas.0337434100
Tang QQ, Gronborg M, Huang H, Kim JW, Otto TC, Pandey A, Lane MD (2005) Sequential phosphorylation of CCAAT enhancer-binding protein beta by MAPK and glycogen synthase kinase 3beta is required for adipogenesis. Proc Natl Acad Sci U S A 102:9766–9771. https://doi.org/10.1073/pnas.0503891102
de Almeida NR, Conda-Sheridan M (2019) A review of the molecular design and biological activities of RXR agonists. Med Res Rev 39:1372–1397. https://doi.org/10.1002/med.21578
Worldwide Bexarotene Study Group, Duvic M, Martin AG, Kim Y, Olsen E, Wood GS, Crowley CA, Yocum RC (2001) Phase 2 and 3 clinical trial of oral bexarotene (Targretin capsules) for the treatment of refractory or persistent early-stage cutaneous T-cell lymphoma. Arch Dermatol 137:581–593
Bexarotene Worldwide Study Group, Duvic M, Hymes K, Heald P, Breneman D, Martin AG, Myskowski P, Crowley C, Yocum RC (2001) Bexarotene is effective and safe for treatment of refractory advanced-stage cutaneous T-cell lymphoma: multinational phase II–III trial results. J Clin Oncol 19:2456–2471. https://doi.org/10.1200/JCO.2001.19.9.2456
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Funding
This study was supported by a research grant from the Institute of Pharmaceutical Life Sciences at Aichi Gakuin University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nakashima, Ki., Okamura, M., Matsumoto, I. et al. Regulation of adipogenesis through retinoid X receptor and/or peroxisome proliferator-activated receptor by designed lignans based on natural products in 3T3–L1 cells. J Nat Med 77, 315–326 (2023). https://doi.org/10.1007/s11418-022-01674-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-022-01674-7