Abstract
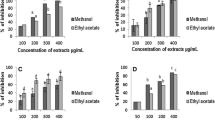
Viper bites cause high morbidity and mortality especially in tropical and subtropical regions, affecting a large number of the rural population in these areas. Even though anti-venoms are available, in most cases they fail to tackle viper venom-induced local manifestations that persist even after anti-venom administration. Several studies have been reported the use of plant products and approved drugs along side anti-venom therapy for efficient management of local tissue damage. In this regard, the present study focuses on the protective efficacy of Cassia auriculata L. (Leguminosae) against Echis carinatus venom (ECV) induced toxicity. C. auriculata is a traditional medicinal plant, much valued in alternative medicine for its wide usage in ayurveda, naturopathy, and herbal therapy. Further, it has been used widely by traditional healers for treatment of snake and scorpion bites in the Western Ghats of Karnataka, India. In the present study, C. auriculata leaf methanol extract (CAME) significantly inhibited enzymatic activities of ECV proteases (96 ± 1 %; P = 0.001), PLA2 (45 ± 5 %; P = 0.01) and hyaluronidases (100 %; P = 0.0003) in vitro and hemorrhage, edema and myotoxicity in vivo. Further, CAME effectively reduced the lethal potency of ECV and increased the survival time of mice by ~6 times (17 vs 3 h). These inhibitory potentials of CAME towards hydrolytic enzymes, mortal and morbid symptoms of ECV toxins clearly substantiates the use by traditional healers of C. auriculata as a folk medicinal remedy for snakebite.









Similar content being viewed by others
Abbreviations
- CAME:
-
Cassia auriculata leaf methanol extract
- CK:
-
Creatine kinase
- ECM:
-
Extracellular matrix
- ECV:
-
Echis carinatus venom
- LDH:
-
Lactate dehydrogenase
- PLA2 :
-
Phospholipase A2
- PPP:
-
Platelet-poor plasma
References
Gutierrez JM, Warrell DA, Williams DJ, Jensen S, Brown N, Calvete JJ, Harrison RA (2013) The need for full integration of snakebite envenoming within a global strategy to combat the neglected tropical diseases: the way forward. PLoS Negl Trop Dis 7:e2162
Simpson ID, Norris RL (2007) Snakes of medical importance in India: is the concept of the “Big 4″ still relevant and useful? Wilderness Environ Med 18:2–9
Urs NA, Yariswamy M, Joshi V, Nataraju A, Gowda TV, Vishwanath BS (2013) Implications of phytochemicals in snakebite management: present status and future prospective. Toxin Rev 33:60–83
Gomes A, Das R, Sarkhel S, Mishra R, Mukherjee S, Bhattacharya S, Gomes A (2010) Herbs and herbal constituents active against snake bite. Indian J Exp Biol 48:865–878
Pandey S, Toppo E, Chauhan P (2011) Comparative study of antitoxin activity of Calotropis gigentea Linn and Cassia fistula Linn against Naja naja (cobra) venom. Intl J Green Pharm 5:292–295
Dey A, De JN (2012) Traditional use of plants against snakebite in Indian subcontinent: a review of the recent literature. Afr J Tradit Complement Altern Med 9:153–174
Joy V, Peter M, YesuRaj J (2012) Ramesh. Medicinal values of avaram (Cassia auriculata Linn.): a review. Int J Curr Pharm Res 4:1–3
Ratnam KV, Raju RV (2008) Folk remedies for insect bites from Gundla Brahmeswaram wildlife sanctuary, Andhra Pradesh. Indian J Tradit Knowl 7:436–437
Kainsa S, Kumar P, Rani P (2012) Pharmacological potentials of Cassia auriculata and Cassia fistula plants: a review. Pak J Biol Sci 15:408–417
Surveswaran S, Cai Y-Z, Corke H, Sun M (2007) Systematic evaluation of natural phenolic antioxidants from 133 Indian medicinal plants. Food Chem 102:938–953
Anandan A, Eswaran R, Doss A, Sangeetha G, Anand S (2011) Chemical compounds investigation of Cassia auriculata leaves—a potential folklore medicinal plant. Bull Environ Pharmacol Life Sci 1:20–23
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Veerachari U, Bopaiah D (2012) Phytochemical investigation of the ethanol, methanol and ethyl acetate leaf extracts of six cassia species. Int J Pharma Bio Sci 3:260–270
Meier J, Theakston RD (1986) Approximate LD50 determinations of snake venoms using eight to ten experimental animals. Toxicon 24:395–401
Murata Y, Satake M, Suzuki T (1963) Studies on snake venom XII. Distribution of proteinase activities among Japanese and Formosan snake venoms. J Biochem 53:431–437
Mohamed R, Dharmappa KK, Tarannum S, Jameel NM, Kannum SA, Ashrafulla HS, Rai L, Souza CJ, Shekhar MA, Vishwanath BS (2010) Chemical modification of ascorbic acid and evaluation of its lipophilic derivatives as inhibitors of secretory phospholipase A(2) with anti-inflammatory activity. Mol Cell Biochem 345:69–76
Reissig JL, Storminger JL, Leloir LF (1955) A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem 217:959–966
Gowda CD, Shivaprasad HV, Kumar RV, Rajesh R, Saikumari YK, Frey BM, Frey FJ, Sharath BK, Vishwanath BS (2011) Characterization of major zinc containing myonecrotic and procoagulant metalloprotease ‘malabarin’ from non lethal Trimeresurus malabaricus snake venom with thrombin like activity: its neutralization by chelating agents. Curr Top Med Chem 11:2578–2588
Vishwanath BS, Kini RM, Gowda TV (1987) Characterization of three edema-inducing phospholipase A2 enzymes from habu (Trimeresurus flavoviridis) venom and their interaction with the alkaloid aristolochic acid. Toxicon 25:501–515
Yariswamy M, Shivaprasad HV, Joshi V, Nanjaraj Urs AN, Nataraju A, Vishwanath BS (2013) Topical application of serine proteases from Wrightia tinctoria R. Br. (Apocyanaceae) latex augments healing of experimentally induced excision wound in mice. J Ethnopharmacol 149:377–383
Girish KS, Jagadeesha DK, Rajeev KB, Kemparaju K (2002) Snake venom hyaluronidase: an evidence for isoforms and extracellular matrix degradation. Mol Cell Biochem 240:105–110
Ouyang C, Teng CM (1976) Fibrinogenolytic enzymes of Trimeresurus mucrosquamatus venom. Biochim Biophys Acta 420:298–308
Rajesh R, Shivaprasad HV, Gowda CD, Nataraju A, Dhananjaya BL, Vishwanath BS (2007) Comparative study on plant latex proteases and their involvement in hemostasis: a special emphasis on clot inducing and dissolving properties. Planta Med 73:1061–1067
Condrea E, Yang CC, Rosenberg P (1983) Anticoagulant activity and plasma phosphatidylserine hydrolysis by snake venom phospholipases A2. Thromb Haemost 49:151
Cantin CM, Moreno MA, Gogorcena Y (2009) Evaluation of the antioxidant capacity, phenolic compounds, and vitamin C content of different peach and nectarine [Prunus persica (L.) Batsch] breeding progenies. J Agric Food Chem 57:4586–4592
Wulf L, Nagel C (1976) Analysis of phenolic acids and flavonoids by high-pressure liquid chromatography. J Chromatogr A 116:271–279
Mukherjee AK (2012) Green medicine as a harmonizing tool to antivenom therapy for the clinical management of snakebite: the road ahead. Indian J Med Res 136:10
Ushakumari J, Ramana V, Reddy K (2012) Ethnomedicinal plants used for wounds and snake-bites by tribals of kinnerasani region, AP. India J Pharmacogn 3:79
Escalante T, Rucavado A, Fox JW, Gutierrez JM (2011) Key events in microvascular damage induced by snake venom hemorrhagic metalloproteinases. J Prot 74:1781–1794
Kornalik F, Blomback B (1975) Prothrombin activation induced by Ecarin—a prothrombin converting enzyme from Echis carinatus venom. Thromb Res 6:57–63
Yamada D, Sekiya F, Morita T (1996) Isolation and characterization of carinactivase, a novel prothrombin activator in Echis carinatus venom with a unique catalytic mechanism. J Biol Chem 271:5200–5207
Hiremath V, Yariswamy M, Nanjaraj Urs AN, Joshi V, Suvilesh KN, Ramakrishnan C, Nataraju A, Vishwanath BS (2013) Differential action of Indian BIG FOUR snake venom toxins on blood coagulation. Toxin Rev 33:23–32
Gutierrez JM, Rucavado A (2000) Snake venom metalloproteinases: their role in the pathogenesis of local tissue damage. Biochimie 82:841–850
Baldo C, Jamora C, Yamanouye N, Zorn TM, Moura-da-Silva AM (2010) Mechanisms of vascular damage by hemorrhagic snake venom metalloproteinases: tissue distribution and in situ hydrolysis. PLoS Negl Trop Dis 4:e727
Maruyama M, Sugiki M, Yoshida E, Shimaya K, Mihara H (1992) Broad substrate specificity of snake venom fibrinolytic enzymes: possible role in haemorrhage. Toxicon 30:1387–1397
Lomonte B, Gutierrez JM (2011) Phospholipases A2 from viperidae snake venoms: how do they induce skeletal muscle damage? Acta Chim Slov 58:647–659
Arruda EZ, Silva NM, Moraes RA, Melo PA (2002) Effect of suramin on myotoxicity of some crotalid snake venoms. Braz J Med Biol Res 35:723–726
Nok AJ, Balogun E, Lori JA, Abubakar MS (2002) Inhibition of Naja nigricolis venom acidic phospholipase A2 catalysed hydrolysis of ghost red blood cells by columbin. J Enzyme Inhib Med Chem 17:55–59
Acknowledgments
This work was supported by the Department of Biotechnology (DBT), New Delhi (Order No. BT/43/NE/TBP/2010; Dated 14-03-2011), SAP and UGC-BSR New Delhi.
Conflict of interest
The authors have no conflicts of interests to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nanjaraj Urs, A.N., Yariswamy, M., Joshi, V. et al. Local and systemic toxicity of Echis carinatus venom: neutralization by Cassia auriculata L. leaf methanol extract. J Nat Med 69, 111–122 (2015). https://doi.org/10.1007/s11418-014-0875-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-014-0875-3