Abstract
Purpose
Pejão Mining Complex locates in Castelo de Paiva municipality and, until its closure in 1994, was one of the most important coal mines in the Douro Coalfield. This work aims to study the presence, quantify, and evaluate the dissemination of mercury (Hg), a potentially toxic element (PTE) of major public health concern by the World Health Organization (WHO), from a waste pile affected by coal fires.
Materials and methods
Samples from areas affected and unaffected by the combustion and from surrounding soil were collected from Fojo waste pile region. First, the Hg pseudo-total concentration was estimated for all collected samples by soil microwave–assisted digestion with aqua regia (USEPA 3051A). Then, a sequential extraction procedure (SEP), the USEPA 3200, was applied for Hg fractionation and speciation aiming to evaluate Hg mobility and bioavailability to surrounding ecosystems.
Results and discussion
The results obtained showed a Hg enrichment in soil samples when compared to Portuguese and international reference values for soils. Relatively to the Hg availability and mobility, although it predominates in the semi-mobile fraction, the waste pile materials exposed to combustion showed a concerning increase of Hg levels in the mobile fraction that contains the more labile Hg species, being a major source of environmental contamination by Hg.
Conclusions
This study allowed to conclude that combustion of mining residues increased Hg mobility, toxicity, and bioavailability, increasing the contamination potential of the coal waste pile. The methodology applied in this work can be replicated in other abandoned mines to monitor, control, and/or mitigate the Hg environmental impact in the surrounding soils and waters.
Similar content being viewed by others
1 Introduction
The mining activity is considered one of the major anthropogenic sources of environmental contamination. The exploitation of mineral resources drastically changes the layout of the soil, altering its properties, the ecosystem and produces huge amounts of waste that are deposited in the surrounding areas of active or deactivated mines in the form of heaps and tailings (Demková et al. 2017; Farjana et al. 2019; Worlanyo and Jiangfeng 2021). The lack of proper monitoring and treatment of the heaps and tailings and their weathering make those residues a source of heavy metals to the environment that can be easily mobilized by acid mine drainage (AMD) (Farjana et al. 2019; Worlanyo and Jiangfeng 2021; Alvarenga et al. 2021) and, therefore, contaminate the surrounding soil and aquifers.
Heavy metals naturally occur in earth’s crust and, although some of those metals are essential for some plants and animals to maintain their biological processes, they are considered potentially toxic elements (PTEs) to organisms at high concentration levels. On the other hand, other non-essential metals and metalloids, such as As, Sb, Hg, and Pb, are highly toxic even at low concentrations (Musilova et al. 2016; Sarwar et al. 2017; Worlanyo and Jiangfeng 2021).
Mercury (Hg) is highly toxic, can persist in the environment for long periods (bioaccumulation) (Li et al. 2021; Jin et al. 2023), being listed as one of the major public health concerns by the World Health Organization (WHO) (WHO 2020; Li et al. 2021). It naturally occurs in metal-rich geologic deposits and coal and its emission to the environment from anthropogenic sources mainly comes from fossil-fuel combustion and mining activity, such as extraction of gold and silver through amalgamation processes (Li et al. 2021).
The Fojo waste pile is one of the many residue deposits that resulted from mining activities in the Pejão Mining Complex, located in the Castelo de Paiva Municipality, being distributed along approximately 9 km from Germunde to the Fojo Paraduça area. The Pejão Mining Complex, closed since 1994, was one of the most relevant extractive industries in Northern Portugal. These mines exploited anthracite A (ISO 5667-3:2018) from the Douro Carboniferous Basin (DCB) latter used for power generation. DCB hosts the most relevant continental carboniferous deposits in Portugal, aged Upper Pennsylvanian (Lower Stephanian C) (Lemos de Sousa and Wagner 1983; Correia et al. 2018). This coal-bearing sequence was deposited in a limnic basin controlled by variations in tectonic activity. The sedimentary sequence includes a basal breccia, followed by fossiliferous shales, siltstones, and sandstones, along with interlayered conglomerates and coal seams (Lemos de Sousa and Wagner 1983; Medeiros et al. 1980). DCB length is approximately 53 km, following a NW–SE alignment, extended from São Pedro de Fins until Janarde, with a thickness that can range from 30 to 250 m (Lemos de Sousa and Wagner 1983; Pinto De Jesus 2003, 2019).
Coal fires in Fojo waste pile occurred between the years of 2017 and 2019, after ignition caused by forest fires, and obliged a technical intervention in 2019 by a Portuguese company responsible for environmental monitoring and rehabilitation of degraded mining areas (EDM—Empresa de Desenvolvimento Mineiro). The coal fires ceased after the wastes remobilization and cooling with water mixed with a cooling agent (see Fig. 1).
Previous studies conducted on self-burning coal waste piles from the DCB highlighted environmental concerns (Ribeiro et al. 2010, 2011, 2013, 2015, 2022; Stracher et al. 2015; Mansilha et al. 2021; Marques et al. 2021; Teodoro et al. 2021; Ribeiro and Flores 2021; Santos et al. 2023) related to high concentration levels of PTEs (As, Pb, Cd, Mn, etc.) in mining residues, some of them significantly higher than reference values defined by the Portuguese Environmental Agency (APA) (APA 2019). However, no information about Hg levels (Li et al. 2021; He et al. 2023) in mine waste piles from this region was reported so far. Thus, this work aims to assess the contamination and dissemination of Hg from untreated mining residues of Pejão Mining Complex, providing information about the risks to surrounding soils, aquifers, and potentially to human health. The approach consisted of first submitting soil sub-samples to microwave-assisted digestion with aqua regia to obtain the “pseudo-total content” of Hg (USEPA 3051A procedure) (US EPA 2007). However, although the PTEs “total concentration” provides a fair indication of soil contamination in risk assessment studies, the information obtained by total concentration analysis is insufficient for precise risk assessment since PTEs toxicity, bioavailability, and environmental mobility are strongly dependents on its chemical form (Quintanilla-Villanueva et al. 2020; Mourinha et al. 2022; Abdul Rashid et al. 2023). Thus, sequential extraction procedures (SEPs) (Du Laing 2010; Reis et al. 2016; Costa Ferreira et al. 2019; Du et al. 2020; Srithongkul et al. 2020; Mourinha et al. 2022; Wang et al. 2022) can be applied for metal fractioning and speciation according to species mobility by successively applying extraction solutions with increasing reactivity to the same soil sample in a way that the obtained fractions contain species ranging from higher to lower mobility.
In this work, to study Hg mobility and bioavailability, a SEP provided by the U. S. Environmental Protection Agency (EPA), the USEPA 3200 procedure (EPA 2014), was applied to fractionate Hg in mine soil samples into (i) mobile, (ii) semi-mobile, and (iii) non-mobile, where the mobile fraction contains the most concerning labile mercury species.
2 Materials and methods
2.1 Chemicals and material
The chemicals used in this work were: sodium borohydride (99%, Sigma-Aldrich, Merck), sodium hydroxide (≥ 98%, analytical grade, Sigma-Aldrich, Merck), ethanol (EtOH, ≥ 99.8%, HPLC grade, Fisher Chemical, Thermo Fisher Scientific), hydrochloric acid (37% w/v, laboratory grade, Fisher Chemical, Thermo Fisher Scientific), and nitric acid (69% w/v, ACS grade, Fisher Chemical, Thermo Fisher Scientific). All reagents and solvents were used without further purification.
All aqueous solutions used in this work were prepared with water purified with a Milli-Q purification system (resistivity ≥ 18 MΩ.cm).
2.2 Soil sampling and samples pre-treatment
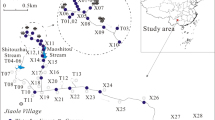
The mining residues (overburden and rejected materials) from the Fojo mine are essentially composed by fragments of carbonaceous shales rich in carbon and lithic arenites. A total of 25 soil surface samples (0–20 cm) were taken from the waste pile and its surrounding region. The spatial distribution of collected samples is shown in Fig. 2 and englobes:
- 10 samples from the waste pile affected by combustion (burnt waste, BW). These sample residues include fine- to coarse-grained reddish-colored material rich in iron oxides and ash (see Fig. 1);
- 5 samples from pile unaffected by the coal fires (unburnt waste, UW). The samples were composed by coarse-grained dark lithic fragments;
- 5 soil samples from the downstream region (downhill soil, DS) to evaluate Hg leaching from the waste pile;
- 5 soil samples from the woods upstream to the waste pile (uphill soil, US) to serve as local background samples.
Each superficial sample was collected using a stainless-steel shovel and consisted of approximately 1.5 kg of soil, stored in a polyethylene bag. The geographic coordinates of the sampling locations were identified using a global positioning system (GPS). Soil samples were dried at room temperature to minimize volatiles loss and sieved at 2 mm to remove gravel and organic residues.
The collected samples were dried for several days at room temperature to minimize losses of volatile Hg. Then, samples were sieved at 2 mm and homogenized. Subsampling was performed using the coning and quartering method (Horwitz 1990; Campos-M and Campos-C 2017). Samples were stored in a refrigerator (4 °C) until use.
2.3 Mercury analysis in soil
Cold vapor atomic absorption spectroscopy (CV-AAS) was used for determination of Hg in soil extracts. The measurements were performed using a Thermo Scientific iCE 3000 Series double-beam Atomic Absorption Spectrometer combined with the VP100 accessory, a continuous flow vapor generation (hydride) system. For the cold vapor generation, solutions of 10% (w/v) hydrochloric acid and 1% (w/v) sodium borohydride, stabilized in 0.5% (w/v) sodium hydroxide, were used. The reductant solution was freshly prepared prior to the measurements. The operational conditions of the CV-AAS system are depicted in Table 1.
For Hg quantification, a calibration curve ranging from 2.00 to 20.0 µg L−1 was built. The standard solutions were prepared by dilution of a stock solution (C = 1004 ± 7 µg L−1, AAS standard solution, SpecPure, Hg 1000 g/mL, Hg in 5% HNO3, Alfa Aesar), using an intermediate solution of 2.00 mg L−1, and making up the volume with 10% w/v HCl. The typical graphic profile and a representative calibration curve obtained for Hg analysis by CV-AAS are shown in Fig. S1 and Fig. S2, respectively, along with information on the sensitivity of the method, linearity, limit of detection (LOD), limit of quantification (LOQ) (see Table S1, SI), and precision of the analytical result.
2.4 Pseudo-total concentration of Hg in soil
The Hg pseudo-total concentration in samples was determined by soil microwave–assisted digestion with aqua regia (HCl/HNO3, molar ratio 3:1) according to experimental conditions detailed in USEPA 3051 method (US EPA 2007). Briefly, about 0.3000 g of homogenized soil sub-samples was weighted to digestion vessels followed by addition of HCl (V = 6.0 mL) and HNO3 (V = 2.0 mL). Then, the samples were submitted to microwave dissolution at 175 °C for 10 min using a microwave system (Analytikjena TOPwave). After cooling of the samples, the final solutions were filtered through a 0.45-μm nylon membrane syringe filter, transferred into 25 mL (or 50 mL) volumetric flasks, and the volume made up with ultrapure water prior to analysis by CV-AAS.
For validation of the digestion procedure for mercury analysis, a certified reference material (CRM), RTC CRM005: Trace Metals—Sewage Amended Soil (Sigma-Aldrich, Merck), was submitted to digestion (5 replications). The obtained Hg recovery range varied from 86 to 118% with a mean value of 97% ± 11% (see Table S3, SI). Thus, the procedure applied for digestion was considered efficient for Hg analysis in soils.
2.5 Mercury fractioning (SEP USEPA 3200)
In this work, the USEPA 3200 (EPA 2014) procedure was applied for the sequential extraction of Hg species in three fractions: mobile Hg, semi-mobile Hg, and non-mobile Hg. Briefly, the mobile fraction contains organic and inorganic Hg2+ species mainly bioavailable for soil-to-plant exchange. The semi-mobile faction mainly contains elemental Hg and possibly amalgams, while the non-mobile fraction contains sulfides and calomels. The extraction solutions and experimental conditions employed are summarized in Table 2.
2.6 Geoaccumulation index
The geoaccumulation index (Igeo) is commonly used to classify soil pollution (Ackah 2019; Zhao et al. 2021; John et al. 2022). The Igeo is calculated by the following equation:
where Ci represents the measured concentration of the contaminant (in mg kg−1), B represents its background average concentration, and 1.5 is the correction factor for soil heterogeneity. Based on the calculated value, samples are then classified according to Igeo classification chart (see Table S2, SI).
3 Results and discussion
The determination of Hg concentration levels in DCB mining waste piles is of great interest for assessing its impact in the health of surrounding soils, aquifers, and ecosystems. In particular, this work aims to provide an accurate insight on the environmental effects induced by the coal fires on this mine region and investigate any correlation with Hg mobility. Therefore, the first stage of this study focused on the characterization of “pseudo-total” concentrations of Hg in soil samples. Then, a sequential extraction procedure (SEP) was applied to gather information about Hg mobility and bioavailability in the waste pile residues (affected and non-affected by combustion) and in the surrounding soils.
3.1 Mercury pseudo-total concentration in mine soil
The Hg content for the 25 soil samples collected from the Fojo waste pile region was estimated and the results are represented in Fig. 3A (see Table S4, SI). As can be seen, the Hg pseudo-total values (i.e. the aqua regia soluble content) were all above the background values proposed by the FOREGS project for European top soils (of 0.061 mg kg−1) (Salminen 2013), averaging 1.0 ± 0.1 mg kg−1 and ranging from 0.1 ± 0.1 to 1.8 ± 0.1 mg kg−1.
A Hg pseudo-total concentrations obtained for the Fojo coal waste pile region samples: burnt waste (BW), unburnt waste (UW), uphill soil (US), and downhill soil (DS). The red line in the graphic represents the APA’s reference value for Hg. B Spatial distribution of Hg modeled by IDW using the ArcGIS Pro software
All samples, excluding DS1, returned Hg pseudo-total values above the APA reference value (of 0.25 mg kg−1) (APA 2019), considering coarse soils and agricultural use, suggesting a natural enrichment of Hg in the soil of the region. Even samples located uphill the waste pile, without influence from the mining residues, presented Hg values above the national background average concentration of Hg in topsoil for Portugal (of 0.048 mg kg–1), reported in the FOREGS Geochemical Atlas (Salminen 2013). This natural enrichment can be explained by the occurrence of abnormal enrichment of Hg associated with cinnabar present in coals from Douro Carboniferous Basin, as previously described (Moura et al. 2018; Costa et al. 2022), also identified by SEM–EDS in the coal residues of the Fojo waste pile.
Furthermore, the mean values of the samples from regions affected (BW1-10, 1.2 mg kg−1) and unaffected (UW1-5, 1.2 mg kg−1) by combustion are about 120% higher than the mean value of the reference region (US1-5, 0.6 mg kg−1), revealing a concerning anthropogenic enrichment of Hg in these regions with high potential environmental risks for surrounding ecosystems. Moreover, the mean pseudo-total values for the downstream soil samples (DS1-5, 1.0 mg kg−1) are about 25% higher than the mean value of local background samples (US1-5), possibly indicating the spread of the contamination (due to erosion and/or leaching by runoff effects) from the waste pile to the nearby region. Samples DS4 and DS2 show higher concentration levels probably due to terrain irregularities in the water flow patterns. The statistical comparison of the average mercury concentration values can be found in Table S5 (SI).
For better visualization of the Hg spatial distribution over the mine region, an inverse distance weighted (IDW) map for the Fojo region is shown in Fig. 3B, where the blue areas possess lower levels of Hg and the red areas very high degree of Hg contamination.
The mean concentration of Hg in the local background, based on the samples collected uphill from the waste pile, was used for the geoaccumulation index (Igeo) calculation. Based on the Igeo classification chart (Table S2, SI) and considering average Igeo values (see Table S6, SI), the samples of the downstream region (SJ) were classified as “unpolluted” and the waste piles, both affected and unaffected by combustion (BW1-10 and UW1-5), were classified as “unpolluted to moderately polluted,” confirming the waste piles regions as main source for potential contamination of surrounding areas.
Considering that most pseudo-total concentration values for Hg exceeded the APA´s national reference values proposed for agriculture soils, further studies applying a sequential extraction procedure (SEP) were conducted to investigate the mobility of Hg in the mine soil.
3.2 Mercury fractioning and speciation in mine soil
Mercury can be retained in soils by adsorption onto organic matter and mineral surfaces (Fe, Mn, Al oxides, and silicates) affecting its environmental mobility in soil (O’Connor et al. 2019). Furthermore, its toxicity depends on its speciation (see Table 2). The species that belong to the “semi-mobile” category are less toxic than extractable species from the “mobile” fraction. For example, Hg2+ complexes, elemental Hg and mercury chloride are not readily available for plants and for organisms uptake (Jing et al. 2007; Zhu et al. 2018). However, these inorganic species of soluble Hg can serve as substrate for Hg methylation (Li et al. 2022). Alkyl species, such as methylmercury, are more mobile than inorganic Hg species, being more toxic and easily bioaccumulated. By opposition, the “non-mobile” species, such as Hg sulfides, are chemically stable for long periods and less toxic.
In this work, the SEP USEPA 3200 (EPA 2014) was selected and applied to mining waste pile materials to provide insight on the mobility and availability of Hg, particularly in the areas of major environment concern (BW and UW). The estimated concentration for Hg in the mobile, semi-mobile, and non-mobile phases is graphically represented in Fig. 4 (see Table S4, SI).
A relevant aspect in mercury fractioning studies is the fair correlation between the pseudo-total concentration of mercury and the sum of the Hg fractions (mobile + semi-mobile + non-mobile). In this work, despite the fact that each sample was thoroughly homogenized prior to analysis, sometimes a discrepancy between the total Hg and the mercury extracted the by SEP method was observed. Potential reasons for such discrepancies include (Sánchez et al. 2005; Liu et al. 2006; Issaro et al. 2009; Reis et al. 2010) (i) incomplete extraction, since not all forms of mercury in the soil may be fully extracted in the SEP process; (ii) losses during the extraction and analysis since some mercury species are volatile at room temperature or higher; and (iii) sample heterogeneity, if the sample used for extraction does not represent the overall soil composition. Analytical errors such as calibration errors, matrix effects, or interference from other elements are also a possible source of this error. However, in this work, the stringent analytical quality control measures in place make this possibility unlikely.
After application of the 3-stage Hg SEP, the results revealed that the dominant fraction is the “semi-mobile” one, throughout the Fojo region, with an average mercury concentration value of 0.50 mg kg−1, ranging from 0.05 to 1.04 mg kg−1. The Hg levels found in the “semi-mobile” fraction are greater than the APA’s reference value (of 0.25 mg kg−1), except for the samples from local background (B1-5). Even though this fraction mainly contains species of mercury that are not readily mobilized, they can be easily converted into more labile species (Reis et al. 2010, 2016) and constitute a threat to the environment.
Interestingly, the Hg fractioning profiles in samples affected by coal fires (BW1-10) contrasted with samples unaffected by combustion (UW1-5). For the waste pile samples that had experienced the effects of coal fires, the second most dominant fraction (16% of the sum of the fractions; see Fig. S3, SI) was the “mobile” one, with a Hg average concentration of 0.15 ± 0.03 mg kg−1, ranging from 0.10 ± 0.02 to 0.22 ± 0.02 mg kg−1. For the unburnt samples, the second most dominant fraction (17% of the sum of the fractions; see Fig. S3, SI) was the “non-mobile,” with a Hg average concentration of 0.11 ± 0.03 mg kg−1 (ranging from 0.08 ± 0.01 to 0.17 ± 0.01 mg kg−1), while the “mobile” phase was the less representative (8% of the total sum; average concentration of 0.05 ± 0.02 mg kg−1). Despite the natural waste heterogeneity, the results showed significant statistical differences (see Table S5, SI) in Hg content in the mobile fractions within samples that had been affected or not by coal fires, strongly suggesting that heat from coal fires is responsible for the increase of Hg labile species from 8% (unburnt waste materials, UW) to 16% (burnt waste materials, BW). So, in terms of hazardous perception, higher Hg leaching rates are expected in waste pile areas that have experienced combustion, resulting in a greater dispersion potential to the underlying aquifers and surrounding soils, which means with higher toxicity and available Hg.
These results are in close agreement with the literature. Mashyanov et al. (2017) showed that the diverse Hg species (organic matrix-bound Hg, adsorbed elemental Hg, silicate and pyrite-bound Hg, HgS, etc.) existing in coal have different thermal stabilities and releasing behaviors when subjected to heating. Luo et al. (2013) reported the release of pyrite-bound Hg in coal at a temperature range of 350–400 °C, after destruction of the FeS2 lattice.
Overall, several processes can contribute to increase the concentration of mobile Hg species in mine soil samples affected by coal fires, namely: (i) the combustion process generates partial consumption of carbon present in the wastes tending to concentrate the inorganic fractions; (ii) the combustion of fossil organic matter can liberate Hg species originally bound to the organic matrix, as well as adsorbed species; and (iii) liberation of Hg pyrite-bound species from the lattice.
As stated before, Hg species in mobile fraction are highly toxic and bioavailable. Thus, the results obtained suggest that strict monitoring may be advisable for the Fojo mine region, particularly in the waste pile affected by combustion (BW) to control and minimize the Hg environmental impact in surrounding soils and aquifers.
4 Conclusions
The study conducted in the Fojo waste pile, included in the Pejão Mining Complex, revealed a soil enrichment in Hg when compared to European background soils, with all samples exceeding the Portuguese Hg reference values threshold proposed as limits for contaminated soils for agriculture.
To fully assess Hg mobility and bioavailability in mine soil, a sequential extraction procedure (SEP), the USEPA 3200, was successfully applied to all soil samples from the waste pile and surrounding areas. The results obtained allowed to conclude that the Hg dominant fraction in the mine soil was the semi-mobile. Nonetheless, an increase of Hg levels in the mobile fraction, containing the more labile Hg species, was observed for the soil samples affected by coal fires, suggesting that combustion of mining residues increases Hg mobility, toxicity, and bioavailability, increasing the contamination potential of the coal waste pile. Thus, the present study should be replicated in abandoned mines with a similar problem in order to monitor and control the Hg environmental impact in the surrounding soils and waters. Moreover, to mitigate the potential toxicity of Hg to humans and ecosystems, several physical, chemical, and biological soil remediation approaches (Eckley et al. 2020; Wang et al. 2020) can be implemented, including thermal desorption, electrokinetic removal, use of emerging materials and nanomaterials (such as CNTs, graphene, nanocomposites, MOFs, COFs, LDHs, clay minerals, manganese oxides), and innovative technologies based on biochar immobilization or involving organisms (phytoremediation, algae-based mercury removal, microbial reduction, constructed wetlands, among others).
References
Abdul Rashid SR, Wan Yaacob WZ, Umor MR (2023) Assessments of heavy metals accumulation, bioavailability, mobility, and toxicity in serpentine soils. Sustainability 15:1218. https://doi.org/10.3390/su15021218
Ackah M (2019) Soil elemental concentrations, geoaccumulation index, non-carcinogenic and carcinogenic risks in functional areas of an informal e-waste recycling area in Accra, Ghana. Chemosphere 235:908–917. https://doi.org/10.1016/j.chemosphere.2019.07.014
Alvarenga P, Guerreiro N, Simões I et al (2021) Assessment of the environmental impact of acid mine drainage on surface water, stream sediments, and macrophytes using a battery of chemical and ecotoxicological indicators. Water 13:1436. https://doi.org/10.3390/W13101436
APA (2019) Valores De Referência Para O Solo. Solos Contam - Guia Técnico 2019:1–73
Campos-M M, Campos-C R (2017) Applications of quartering method in soils and foods. Int J Eng Res Appl 7:35–39. https://doi.org/10.9790/9622-0701023539
Correia P, Šimůnek Z, Sá AA, Flores D (2018) A new Late Pennsylvanian floral assemblage from the Douro Basin, Portugal. Geol J 53:2507–2531. https://doi.org/10.1002/gj.3086
Costa M, Moura H, de Jesus A et al (2022) Effects of magmatic fluids in coals of São Pedro da Cova Coalfield, Douro Carboniferous Basin, Portugal: insights from inorganic geochemistry. Minerals 12. https://doi.org/10.3390/min12020275
Costa Ferreira SL, dos Anjos JP, Assis Felix CS et al (2019) Speciation analysis of antimony in environmental samples employing atomic fluorescence spectrometry-review. TrAC Trends Anal Chem 110:335–343. https://doi.org/10.1016/j.trac.2018.11.017
Demková L, Jezný T, Bobulská L (2017) Assessment of soil heavy metal pollution in a former mining area-before and after the end of mining activities. Soil Water Res 12:229–236. https://doi.org/10.17221/107/2016-SWR
Du X, Gao L, Xun Y, Feng L (2020) Comparison of different sequential extraction procedures to identify and estimate bioavailability of arsenic fractions in soil. J Soils Sediments 20:3656–3668. https://doi.org/10.1007/s11368-020-02694-0
Eckley CS, Gilmour CC, Janssen S et al (2020) The assessment and remediation of mercury contaminated sites: a review of current approaches. Sci Total Environ 707:136031. https://doi.org/10.1016/j.scitotenv.2019.136031
EPA (2014) Method 3200 - Mercury species fractionation by microwave assisted extraction, selective solvent extraction and/or solid phase extraction, U. S. Envronmental Protection Agency, p. 34. Implement Sci 39:1–24
Farjana SH, Huda N, Parvez Mahmud MA, Saidur R (2019) A review on the impact of mining and mineral processing industries through life cycle assessment. J Clean Prod 231:1200–1217. https://doi.org/10.1016/j.jclepro.2019.05.264
He KQ, Zhang XR, Li YP et al (2023) Identification of mercury species in coal combustion by-products from power plants using thermal desorption-atomic fluorescence spectrometry on-line coupling system. Chemosphere 312:137206. https://doi.org/10.1016/j.chemosphere.2022.137206
Horwitz W (1990) No Title. Pure Appl Chem 62:1193–1208. https://doi.org/10.1351/pac199062061193
Issaro N, Abi-Ghanem C, Bermond A (2009) Fractionation studies of mercury in soils and sediments: a review of the chemical reagents used for mercury extraction. Anal Chim Acta 631:1–12. https://doi.org/10.1016/j.aca.2008.10.020
Jin X, Yan J, Ali MU et al (2023) Mercury biogeochemical cycle in Yanwuping Hg Mine and source apportionment by Hg isotopes. Toxics 11. https://doi.org/10.3390/toxics11050456
Jing YD, He ZL, Yang XE (2007) Effects of pH, organic acids, and competitive cations on mercury desorption in soils. Chemosphere 69:1662–1669. https://doi.org/10.1016/j.chemosphere.2007.05.033
John PM, Murali V, Chakraborty K et al (2022) Spatial and seasonal trends of trace metals in the surficial sediments from off Kochi - geochemistry and environmental implications. Mar Pollut Bull 182. https://doi.org/10.1016/j.marpolbul.2022.114029
Du Laing G (2010) Analysis and fractionation of trace elements in soils. In: Hooda PS (ed) Trace elements in soils. Wiley-Blackwell, Chichester, UK, pp 53–80
Lemos de Sousa MJ, Wagner RH (1983) General description of the Terrestrial Carboniferous Basins in Portugal and history of investigations. Lemos Sousa, MJ, Oliveira, JT (Eds), Carbonif Port 29 Memórias dos Serviços Geológicos Port 117–126
Li Z, Chen B, Li Y, Le XC (2021) Reduction of mercury emissions from anthropogenic sources including coal combustion. J Environ Sci (china) 100:363–368. https://doi.org/10.1016/j.jes.2020.11.002
Li Y, Li D, Song B, Li Y (2022) The potential of mercury methylation and demethylation by 15 species of marine microalgae. Water Res 215:1–8. https://doi.org/10.1016/j.watres.2022.118266
Liu G, Cabrera J, Allen M, Cai Y (2006) Mercury characterization in a soil sample collected nearby the DOE Oak Ridge Reservation utilizing sequential extraction and thermal desorption method. Sci Total Environ 369:384–392. https://doi.org/10.1016/j.scitotenv.2006.07.011
Luo G, Ma J, Han J et al (2013) Hg occurrence in coal and its removal before coal utilization. Fuel 104:70–76. https://doi.org/10.1016/j.fuel.2010.04.004
Mansilha C, Melo A, Flores D et al (2021) Irrigation with coal mining effluents : sustainability and water. Water 13:1–22
Marques JE, Martins V, Santos P et al (2021) Changes induced by self-burning in technosols from a coal mine waste pile: a hydropedological approach. Geosci 11. https://doi.org/10.3390/geosciences11050195
Mashyanov NR, Pogarev SE, Panova EG et al (2017) Determination of mercury thermospecies in coal. Fuel 203:973–980. https://doi.org/10.1016/j.fuel.2017.03.085
Medeiros A, Pereira E, Moreira A (1980) Notícia Explicativa da Folha 9-D Penafiel da Carta Geológica de Portugal à Escala 1:50000. Lisboa
Moura H, Ribeiro J, Flores D et al (2018) Occurrence of mercury and enrichment source in coals from the Douro Carboniferous Basin in São Pedro da Cova area (NW Portugal). 70th Annual ICCP Meeting, International Comitee for Coal and Organic Petrology Proceedings, Brisbane, Australia
Mourinha C, Palma P, Alexandre C et al (2022) Potentially toxic elements’ contamination of soils affected by mining activities in the Portuguese sector of the Iberian Pyrite Belt and optional remediation actions: a review. Environ - MDPI 9. https://doi.org/10.3390/environments9010011
Musilova J, Arvay J, Vollmannova A et al (2016) Environmental contamination by heavy metals in region with previous mining activity. Bull Environ Contam Toxicol 97:569–575. https://doi.org/10.1007/s00128-016-1907-3
O’Connor D, Hou D, Ok YS et al (2019) Mercury speciation, transformation, and transportation in soils, atmospheric flux, and implications for risk management: a critical review. Environ Int 126:747–761. https://doi.org/10.1016/j.envint.2019.03.019
Pinto De Jesus A (2003) Evolu̧c̃ao sedimentar e tect́onica da Bacia Carbońifera do Douro (Estefaniano C inferior, NW de Portugal). Cad Do Lab Xeol Laxe 28:107–125
Pinto De Jesus A (2019) Carboniferous Intermontane Basins of Portugal. In: Oliveira J, Quesada C (eds) The Geology of Iberia: a geodynamic approach, Vol. 2. pp 402–408
Quintanilla-Villanueva GE, Villanueva-Rodríguez M, Guzmán-Mar JL et al (2020) Mobility and speciation of mercury in soils from a mining zone in Villa Hidalgo, SLP, Mexico: a preliminary risk assessment. Appl Geochem 122. https://doi.org/10.1016/j.apgeochem.2020.104746
Reis AT, Rodrigues SM, Davidson CM et al (2010) Extractability and mobility of mercury from agricultural soils surrounding industrial and mining contaminated areas. Chemosphere 81:1369–1377. https://doi.org/10.1016/j.chemosphere.2010.09.030
Reis AT, Davidson CM, Vale C, Pereira E (2016) Overview and challenges of mercury fractionation and speciation in soils. TrAC - Trends Anal Chem 82:109–117. https://doi.org/10.1016/j.trac.2016.05.008
Ribeiro J, Flores D (2021) Occurrence, leaching, and mobility of major and trace elements in a coal mining waste dump: the case of Douro Coalfield, Portugal. Energy Geosci 2:121–128. https://doi.org/10.1016/j.engeos.2020.09.005
Ribeiro J, da Silva EF, Flores D (2010) Burning of coal waste piles from Douro Coalfield (Portugal): petrological, geochemical and mineralogical characterization. Int J Coal Geol 81:359–372. https://doi.org/10.1016/j.coal.2009.10.005
Ribeiro J, Ferreira da Silva E, de Jesus AP, Flores D (2011) Petrographic and geochemical characterization of coal waste piles from Douro Coalfield (NW Portugal). Int J Coal Geol 87:226–236. https://doi.org/10.1016/j.coal.2011.06.014
Ribeiro J, Moura R, Flores D et al (2013) The Douro Coalfield Fires of Portugal. In: Coal and peat fires: a global perspective. Elsevier 313–337
Ribeiro J, Suárez-Ruiz I, Flores D (2022) Coal related fires in Portugal: new occurrences and new insights on the characterization of thermally affected and non-affected coal waste piles. Int J Coal Geol 252:103941. https://doi.org/10.1016/j.coal.2022.103941
Ribeiro J, Sant’Ovaia H, Gomes C et al (2015) Chapter 18. Mineralogy and magnetic parameters of materials resulting from the mining and consumption of coal from the Douro Coalfield, Northwest Portugal. In: Coal and peat fires: a global perspective
Salminen R (2013) Foregs Geochemical Atlas. http://weppi.gtk.fi/publ/foregsatlas/. Accessed 23 Aug 2022
Sánchez DM, Quejido AJ, Fernández M et al (2005) Mercury and trace element fractionation in Almaden soils by application of different sequential extraction procedures. Anal Bioanal Chem 381:1507–1513. https://doi.org/10.1007/s00216-005-3058-y
Santos P, Espinha Marques J, Ribeiro J et al (2023) Geochemistry of soils from the surrounding area of a coal mine waste pile affected by self-burning (Northern Portugal). Minerals 13:1–27. https://doi.org/10.3390/min13010028
Sarwar N, Imran M, Shaheen MR et al (2017) Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 171:710–721. https://doi.org/10.1016/j.chemosphere.2016.12.116
Srithongkul C, Krongchai C, Santasup C, Kittiwachana S (2020) An investigation of the effect of operational conditions on a sequential extraction procedure for arsenic in soil in Thailand. Chemosphere 242:125230. https://doi.org/10.1016/j.chemosphere.2019.125230
Stracher GB, Prakash A, Sokol EV (2015) Mineralogy and magnetic parameters of materials resulting from the mining and consumption of coal from the Douro Coalfield, Northwest Portugal Coal and peat fires: a global perspective Elsevier Boston 493-508
Teodoro A, Santos P, Marques JE et al (2021) An integrated multi-approach to environmental monitoring of a self-burning coal waste pile: the São Pedro da Cova Mine (Porto, Portugal) study case. Environ - MDPI 8. https://doi.org/10.3390/environments8060048
US EPA (2007) Method 3051A (SW-846): Microwave Assisted Acid Digestion of Sediments, Sludges, and Oils. Revision 1. Washington, DC
Wang L, Hou D, Cao Y et al (2020) Remediation of mercury contaminated soil, water, and air: a review of emerging materials and innovative technologies. Environ Int 134:105281. https://doi.org/10.1016/j.envint.2019.105281
Wang J, Wang X, Li G et al (2022) Speciation analysis method of heavy metals in organic fertilizers: a review. Sustain 14. https://doi.org/10.3390/su142416789
WHO - World Health Organization (2020) Ten chemicals of major health concern. https://www.who.int/news-room/photo-story/photo-story-detail/10-chemicals-of-public-health-concern?msclkid=067ddc83a5e911ec892510bb660d4b1a
Worlanyo AS, Jiangfeng L (2021) Evaluating the environmental and economic impact of mining for post-mined land restoration and land-use: a review. J Environ Manage 279. https://doi.org/10.1016/j.jenvman.2020.111623
Zhao J, Wu E, Zhang B et al (2021) Pollution characteristics and ecological risks associated with heavy metals in the Fuyang river system in North China. Environ Pollut 281. https://doi.org/10.1016/j.envpol.2021.116994
Zhu S, Zhang Z, Žagar D (2018) Mercury transport and fate models in aquatic systems: a review and synthesis. Sci Total Environ 639:538–549. https://doi.org/10.1016/j.scitotenv.2018.04.397
Funding
Open access funding provided by FCT|FCCN (b-on). This research was supported by SHS Project, financed by NORTE-45–2020-75—SISTEMA DE APOIO À INVESTIGAÇÃO CIENTÍFICA E TECNOLÓGICA—“PROJETOS ESTRUTURADOS DE I&D”—HORIZONTE EUROPA, Ref. NORTE-01–0145-FEDER-000056 and framed by ICT – GI3 (UIDB/04683/2020 and UIDP/04683/2020), CIQUP (UIDB/00081/2020) and IMS (LA/P/0056/2020) activities. José A. Ribeiro (ref. SFRH/BPD/105395/2014) acknowledges FCT under the QREN e POPH e Advanced Training, subsidized by European Union and national MEC funds. Marcus Monteiro acknowledges SHS project and FCT for the grant and financial support. The authors acknowledge Paulo Ferreira, Aracelys Narayan, and Joana Ribeiro for the support given in the sampling campaign.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Marcus Monteiro: writing—original draft, material preparation, data collection and analysis. Patrícia Santos: writing—review and editing, data analysis, conceptualization. Jorge E. Marques: sample collection, material preparation. Deolinda Flores: writing—review and editing, funding acquisition, conceptualization, supervision. Carlos M. Pereira: funding acquisition, conceptualization, supervision. José A. Ribeiro: writing—review and editing, methodology, data analysis, conceptualization, supervision. Manuel Azenha: writing—review and editing, methodology, data analysis, conceptualization, supervision.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Sarah Pariente
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Monteiro, M., Santos, P., Marques, J.E. et al. Assessment of mobile mercury concentration in soils of an abandoned coalfield waste pile in Douro region: the Fojo waste pile (Portugal) study case. J Soils Sediments (2024). https://doi.org/10.1007/s11368-024-03786-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11368-024-03786-x