Abstract
Purpose
This study explores the in situ variability of sediment thermal conductivity (K) in a pond, integrating field-deployed fibre optic sensing with laboratory analyses of sediment properties to enhance our understanding and management of aquatic systems.
Materials and methods
A 20-m cable setup, consisting of a fibre optic cable (FOC) and a heating tape, was buried at two depths within a channel-shaped section of a pond. Induced temperatures along the FOC were recorded during several heating and cooling periods using distributed temperature sensing (DTS). Thermal conductivity (K) was estimated at five locations along the FOC during the heating periods using the heat conduction theory for an infinite line source. Sediment core samples collected from these locations were analyzed to determine dry bulk density (DBD), organic matter content (OM), and particle size distribution (PSD), exploring their effects on K variability.
Results
Analysis of core samples identified three distinct layers, each with varying PSD, OM, and DBD. The study revealed substantial spatial differences in the thermal conductivity of sediments, even over very short distances along the FOC, attributed to variations in sediment properties. Through a combination of field and laboratory results, we developed quadratic regression models (R2 > 0.9) to characterize the influence of DBD and OM on K. These models enabled detailed vertical and horizontal characterization of K within specific sediment contexts.
Conclusion
The study demonstrates the effectiveness of active DTS in detecting in-situ variations in K, emphasizing the impact of OM and DBD on temperature propagation. This study highlights the necessity of considering sediment property variability in modelling heat transfer for accurate water resource management and environmental assessments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The determination of physical properties of sediments and soils holds significant value for assessments of environmental and hydrological water resources (Verstraeten and Poesen 2001), as well as subsurface characterization and modelling (Goto et al. 2017). Thermal conductivity, for instance, plays a crucial role in elucidating interactions like seepage and upwelling between groundwater and surface water (Vogt et al. 2010; Briggs et al. 2016; Sebok and Müller 2019; Del Val et al. 2021), and in the calculation of soil moisture (Sayde et al. 2010; Ciocca et al. 2012). This property is responsible for the temperature field and heat flow in sediment and soils as caused by heat conduction (Haigh 2012). Additionally, thermal conductivity is a parameter required for geothermal analysis (e.g. borehole heat exchangers) to assess soil heat transfer and optimize energy extraction or storage (Haigh 2012; Barry-Macaulay et al. 2013). Applications extend to fields like sea-bed oil pipelines’ flow performance prediction (Haigh 2012) and assessments of buried electrical power lines’ current capacity (De Lieto Vollaro et al. 2014).
Understanding spatial variability in sediment thermal conductivity (K) helps identify pivotal areas of groundwater-surface water flux exchange (Sebok et al. 2013; Sebok and Müller 2019). Accurate estimation of these fluxes is vital for water quality management, as such exchanges serve as pathways for pollutant transport (Bakx et al. 2019; Sebok and Müller 2019). Additionally, strong-upwelling regions function as crucial thermal refuges for surface water ecosystems, providing stable temperature conditions and counteracting the adverse effects of climate change (Briggs et al. 2016; Sebok and Müller 2019). Previous studies, such as those by Karan et al. (2014) and Tirado-Conde et al. (2019), underscore the significance of considering differentiated thermal properties of sediments in understanding groundwater-surface water interactions. These studies provide a compelling argument for the necessity of a refined approach in assessing thermal properties of sediments, taking into account their spatial and vertical variability, to better understand and model the dynamics of groundwater-surface water interactions. Similarly, Sebok et al. (2015) and Duque et al. (2018) present crucial evidence that small-scale spatial variability in sediment properties can have significant implications for groundwater-surface water interactions. They also emphasize the need for detailed sediment characterization, particularly in dynamic environments such as coastal lagoons and riverbeds, where sediment composition and hydraulic and thermal properties can vary drastically over short distances. These findings highlight the importance of considering meter-scale sediment heterogeneity in modelling and managing water resources.
Thermal-based methodologies, including numerical modelling, time series analysis, and steady-state analytical solutions, are commonly employed for groundwater-surface water flux exchange modelling. Regrettably, these models often rely on standard literature-based thermal conductivity values, despite various studies revealing the substantial impact of inaccurate thermal conductivity on flux exchange estimations. For instance, Duque et al. (2016) investigated the influence of thermal conductivity on groundwater-surface water flux estimations in low-flux settings. They found that employing literature-based thermal conductivity values led to significant flux overestimations (up to 89%) compared to field data. They further highlighted the natural vertical and horizontal variability of thermal conductivity, leading to a ± 25.4% fluctuation in mean flux. Similarly, Sebok and Müller (2019) observed that vertical fluxes based on measured thermal conductivities ranged from 64 to 75% of those using literature-based estimates of sand thermal conductivity. Notably, these studies emphasized the necessity of accurate thermal conductivity for precise flux exchange estimations under varying groundwater flow velocities.
Sediment or soil thermal conductivity can be measured in the laboratory using single or multiple needle probe methods (Barry-Macaulay et al. 2013; Goto et al. 2017; He et al. 2018). These methods are based on the infinite line source solution of radial heat flow, initially proposed by Carslaw and Jaeger (1959). Recent advances have seen the introduction of fibre optic sensing technology, mainly utilized in laboratory studies, to achieve real-time spatial determination of soil and sediment thermal conductivity (Ciocca et al. 2012; Sakaki et al. 2019; Del Val et al. 2021; Simon et al. 2021). Nonetheless, in situ measurement of sediment thermal conductivity has received limited attention, with existing research primarily focusing on vertical measurements employing point-based sensors or continuous fibre optic sensing (Kim et al. 2007; Sebok and Müller 2019; Del Val et al. 2021). Fibre optic sensors such as distributed temperature sensing (DTS) are based on optical time-domain reflectometry (OTDR). DTS employs a laser to transmit light pulses through kilometres of fibre optic cable (FOC), and by analyzing the returned scattered light, temperature variations along the FOC can be determined with a spatial resolution of up to 1 m (Selker et al. 2006; Hartog 2017). To apply the same principle of a line heat source using DTS technology, the FOC is equipped with either an integrated or external electrical resistor connected to an electrical source. This resistor elevates the FOC’s temperature dependent on the surrounding media’s thermal properties, facilitating estimates of the local thermal conductivity (Ciocca et al. 2012; Simon et al. 2021).
Numerous studies have explored the impact of some sediment/soil properties on thermal conductivity. For instance, Smits et al. (2010) measured the thermal conductivity of four different types of sand under various saturation conditions and compared results with existing empirical models. They found that thermal conductivity increases as saturation increases when water saturation is above its critical value. Sakaki et al. (2019) employed the active DTS method to investigate the relationship of thermal conductivity and dry bulk density (DBD) of granulated bentonite at different compaction levels. They demonstrated that active DTS can spatially determine thermal conductivity and infer dry density. The relationship between sediment/soil bulk density and thermal conductivity is directly proportional (Goto et al. 2017; Sakaki et al. 2019); as sediment becomes denser, the proportion of solid particles increases, leading to higher thermal conductivity. This is because solids are generally better conductors of heat compared to water and air (Smits et al. 2010; Barry-Macaulay et al. 2013). Barry-Macaulay et al. (2013) studied the effects of soil and rock properties and conditions (e.g. moisture, density, and mineralogy) on thermal conductivity, reporting positive correlations between thermal conductivity and density as well as water saturation. They also found that thermal conductivity was lower for fine grains soils due to lower quartz contents (< 60%).
Dry bulk density stands as another crucial property of sediments, playing a pivotal role in determining sediment accumulation rates, estimating sediment yield, and characterizing erosion vulnerability and resuspension (Mudroch et al. 1996; Avnimelech et al. 2001; Verstraeten and Poesen 2001). The evolution of sedimentation rate in water storages such as lakes can inform the relationship between anthropogenic changes in lakes and climate change (Mudroch et al. 1996). Hence, accurate and frequent sedimentation rate measurements are vital, particularly in reservoirs and lakes experiencing increased rate variability (Gonzalez Rodriguez et al. 2023). Dry bulk density, water content and porosity of aquatic sediments are interrelated properties. This means that knowing one property is sufficient to obtain the other two because there are only two phases present (solid and liquid) (Avnimelech et al. 2001). In aquatic sediments, density typically increases with depth, while porosity and water content decrease. Water content in sediment has been extensively used to determine DBD; similarly, porosity can be estimated from DBD and particle density (Boyd 1995; Mudroch et al. 1996).
Organic matter content (OM) serves as another significant property for sediment characterization and environmental assessment. Organic matter in aquatic sediments represents a substantial carbon sink, valuable for studying carbon sequestration rates (Gilbert et al. 2014). This property can also be used as an indicator of oxygen demand, and organic and pollution absorption capacity (Avnimelech et al. 2001) as well as a proxy to reconstruct paleoenvironments of lakes and infer the history of regional climate changes (Meyers and Teranes 2001). As organic matter is typically less dense than mineral particles, sediment bulk density tends to decrease as organic matter concentration increases (Avnimelech et al. 2001; Verstraeten and Poesen 2001). This property is also inversely proportional to thermal conductivity, given that organic matter possesses relatively low thermal conductivity compared to minerals (Abu-Hamdeh and Reeder 2000; Duque et al. 2016; Sebok and Müller 2019).
This study employs active DTS to explore the spatial variability of thermal conductivity along a pond channel. The integration of fibre optic measurements within a pond and subsequent analysis of sediment samples in the laboratory aimed to achieve several objectives: (a) investigating thermal propagation in sediment along a buried fibre optic cable at two different depths; (b) characterizing sediment samples concerning particle size, density, and organic matter content; and (c) proposing a methodology for spatially determining in situ thermal conductivity to provide accurate input for estimating groundwater-surface water flux exchange.
2 Materials and methods
2.1 DTS experimental setup
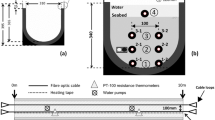
The experimental site was a constructed pond located at the University of the Sunshine Coast in Australia. The temperature sensing setup was deployed in a channel-shaped section of the pond (Fig. 1a). The channel was approximately 35 m long and had an average width of 1.8 m, with an average water column depth of 0.15 m during the testing period. The cable setting consisted of a 20-m heating tape with cross-sectional dimensions of 1.3 cm × 0.85 cm. Additionally, two 100-m fibre optic cables were attached to each face of the wider side of the heating cable using electrical tape. The FOC used was a multi-mode (OM3) and tight-buffered cable with an outer diameter of 2 mm, a 50/125 µm fibre core diameter, and a 20 mm critical bend radius (DiamondOptics 2021). To bury the cables in the pond, we used a trenching spade fabricated in-house to dig a trench approximately 2 cm wide along the channel at two specific depths. The cable setting was carefully placed into the trench, covered with sediments, and left undisturbed for a month prior to initiating measurements. This process aimed to achieve wall closure and restore original conditions of the pond.
We used one FOC to record temperature readings, while the other cable served as a spare in case of issues with the primary cable. A schematic of the FOC layout is shown in Fig. 1c. The first 7 m of the cable setting, referred to as Section 1, were buried at a mean depth of 16.7 cm (± 2.9 cm) from the water–sediment interface (WSI). This section included the FOC interval from 35 to 42 m of the total 100-m length. Another 7 m, known as Section 2, was buried at a mean depth of 30.9 cm (± 3.8 cm) from the WSI, corresponding to the FOC interval from 42 to 49 m. The remaining 5 m of the cable setting, designated as Section 3, was laid directly at the WSI, covering the FOC interval from 49 to 54 m. Additionally, at each end of the cable setting, 7 m of the FOC was coiled to monitor the water temperature. This coiling prevented significant temperature differences between the air and sediment near the cable setting, which could introduce uncertainty to the measurements. Depth markers, in the form of strings with known lengths, were attached to the cable setting every 2 m. These markers helped verify the sediment thickness above the FOC at each section. The variability in the cable depth (expressed as standard deviation) was associated with channel bed irregularities and the difficulties to guarantee a constant depth when burying the cable, particularly, in the presence of water.
Parallel to the main cable setting, a series of point-based sensors (Dallas Semiconductor Corp., model DS18B20) was deployed to monitor temperature at depths similar to those of the FOC (i.e. 16 and 30 cm) as well as at the WSI. These sensors were positioned approximately 30 cm away from the main cable setting to specifically monitor diurnal temperature change, excluding the induced temperature during heat cycles. Three sensors were placed in a sector close to the centre of each FOC section, while three additional sensors were located between the two sections (Fig. 1c). In each section, two sensors measured sediment temperature at the corresponding depth (to account for possible sensor failure), while one sensor recorded water temperature. The three sensors positioned between the two sections solely monitored water temperature. The deployment of water sensors at different locations aimed to identify any water temperature variance along the channel.
The FOC was connected to a DTS unit (Yokogawa Electronic Corp., model DTSX3000-2L) located 6 m away from the channel, where the operation site was established (Fig. 1b). To ensure uniform precision along the entire FOC, we used a double-ended connection setup for temperature measurement. This setup required connecting each cable end to the DTS so that measurements were taken at both ends (Tyler et al. 2009). The DTS unit was calibrated by introducing 20 m of coiled FOC into an ice bath at 0 °C and another 20 m into a water bath at a known temperature of 24.37 °C. The calibration parameter (wave number) was adjusted until the fibre optic matched the reference temperatures at both locations.
The heating tape was connected to an autotransformer with a 10 A current capacity and a maximum power of 2400 VA. This device allowed the injection of heat at different rates by adjusting the voltage. A Raspberry Pi (RPi) gathered diurnal temperature data from the nine point-based sensors. Power for the DTS, RPi, and laptop throughout the study duration was provided by two 100-W solar panels connected in parallel, along with a 12 V, 120 Ah battery. Additionally, a 240 V AC power outlet located 20 m from the operation site provided energy to the autotransformer during the heating periods.
2.2 Thermal conductivity estimation
Active DTS and the theory of heat conduction in an infinite line source (Rui et al. 2019; Sakaki et al. 2019) were used to determine in situ thermal conductivity (K) of sediment at different location along the OFC. The FOC monitored temperature changes in the sediment and water during the heating and cooling periods. Equation (1) was utilized to determine thermal conductivity, where K represents the thermal conductivity (W m−1 °C−1), Q is the power rate in W m−1, and the term “m” represents the slope determined from a plot of temperature increase as a function of the natural logarithm of time (ln(t)) during the heating period.
Heat was induced through the heating tape at a rate of 33.6 W m−1, with each heating cycle comprising a 4-h heating period followed by an overnight cooling period. This heating duration was established in an initial test by assessing the development of a straight-line region on the plot of temperature versus ln(t). In this plot, the slope changed gradually for the first 30 to 45 min followed by a constant slope. Consequently, a 4-h duration was deemed sufficient to accurately determine the constant slope’s value during the straight-line period.
To identify any variation in thermal conductivity estimation due to ambient temperature and boundary condition interference, induced temperature at 33.6 W m−1 was repeated on separate day. Similarly, two more cycles were conducted at different heat rates (i.e. 22.5, 40 W m−1) to verify the consistency of K estimates. DTS was configured to stack backscatter signals every 2 min to obtain an average temperature trace. Given that the Raman backscatter signal can be significantly weaker than the incident light, averaging multiple backscatter signals helped reduce signal noise and improve temperature resolution (Yokogawa 2014; Silixa 2022). As the DTS system generated a separate file for each trace, we developed a script in R (R Core Team 2023) to consolidate traces from a cycle into a single dataset. This script was also instrumental in generating plots of induced temperature versus ln(t), which were then used to conduct linear regressions for determining the slopes at various locations.
The FSA package in R (Ogle et al. 2023) was used to fit linear regression models to observations for the four heat pulses. In this package, function “lm” enabled to fit linear models and function “summary” provided the results of the regression such as residual standard error, coefficient of determination, and p-value. The initial 30–45 min of each test were excluded from the linear regression fitting because they did not follow a linear trend. The number of initial points following a curvilinear trend slightly varied from test to test, likely due to different ambient temperatures in each test and the induced heat rate applied. The coefficients of determination (R2) obtained for the linear regression models were used to adjust the number of points excluded in each test. However, a consistent cut-off was applied across all five locations within the same test.
2.3 Laboratory analysis and field data integration
Sediment samples were obtained from the channel by collecting sediment cores at five locations along the cable setting. The selection of these locations was based on the observed variation in temperature response during the heating periods as recorded by the DTS system. Sediment cores were collected using PVC pipes 50 cm long and 5 cm in diameter. The procedure described in NIWA (2016) was followed to collect the cores from the pond and prepare the sub-samples for analysis. Before proceeding with the core sampling, the pond was drained until the channel sediments were exposed to air to ease the sampling operation and minimize sediment disturbance during extraction. Core samples were cut at a distance of 10–15 cm from the FOC markers to ensure no damage to the FOC. One end of the pipes was sharpened to facilitate penetration into the sediment, reducing downward pressure and minimizing compression. For the same purpose, most part of the cores’ length was cut by slowly pushing the PVC down with our own weight. Only the last 8–10 cm were cut by placing a wood block on top of the pipe and carefully pushed the pipe down with rubber mallet. The lengths of the core samples ranged from 30 to 34 cm and they were carefully removed from the channel by digging around the pipe down to the bottom of the core. Once the samples were collected, the pipes were cut along their entire length on both sides using a circular saw mounted on a wooden base to ensure a consistent cut thickness, slightly less than the pipe wall thickness (approximately 1 mm). To prevent any damage to the core, a retractable knife was used to cut the remaining PVC filament. Each core was then sectioned at regular intervals of 2 cm, resulting in a total of 74 individual subsamples, which were carefully stored in labelled zip-lock bags. The subsamples were preserved in a refrigerator at 4 °C, following recommended practices (Mudroch et al. 1996; Batley and Simpson 2016).
To determine dry bulk density (i.e. dry mass per unit of sample bulk volume) saturated samples were weighed before and after drying in an oven at 105 °C for 24 h. The difference between these two weights represented the water mass. Sample bulk volume (solid volume “\({V}_{S}\)” + water volume “\({V}_{W}\)”) was obtained using Eq. (3) (Avnimelech et al. 2001). In this equation, sediment particle density was corrected for organic matter content (OM) using Eq. (4) (Avnimelech et al. 2001). For sub-samples with geometries closely approximating a perfect cylinder (diameter = 5 cm, height = 2 cm), DBD was also directly calculated by dividing the dry mass by the cylinder’s known bulk volume (38.33 cm3), an approach consistent with other studies such as Trabelsi et al. (2012). To assess the consistency of DBD estimations derived from Eq. (2) and this geometric method, we conducted a statistical comparison using R. Prior to this analysis, we performed a Shapiro–Wilk test in R (STHDA 2016) to assess the normality of the two paired datasets and select the appropriate comparison test. This inferential analysis was employed to evaluate whether there were significant similarities in the densities obtained by the two methods.
where DBD is the dry bulk density (g cm−3), \({V}_{T}\) the sample bulk volume (cm3), \({m}_{D}\) the dry sediment mass, \({\rho }_{Scorr}\) the corrected sediment grain density (assumed to be 2.65 g cm−3 before the correction for OM), \({m}_{W}\) the water mass, and \({\rho }_{W}\) the water density, which at ambient conditions is taken as 1 g cm−3.
OM was determined using the lost ignition method (Ahn et al. 2006; Yoswaty et al. 2021). Dried samples weighing 3–5 g were placed into a furnace at 550 °C for 4 h. After removal from the oven and furnace, the samples were cooled in a desiccator for 30–60 min to minimize moisture. OM was calculated using Eq. (5), where \({m}_{D}\) is the dry sediment mass (g), and \({m}_{A}\) is the burned sediment mass (ash mass) in grams.
The laboratory analysis was complemented with sediment particle size distribution (PSD) of one of the cores. PSD was conducted using a laser diffraction analyzer (Mastersizer3000 2023), which determines particle diameter based on the scattering intensity and angle produced when a laser light interacts with suspended sediment particles (Mudroch et al. 1996; Callesen et al. 2018). Samples were chosen based on three distinctive layers identified in most cores, characterized by colour and aspect. The samples were air-dried, and any agglomerated material was gently crushed to ensure that the particles are separated back into their primary, individual units (Šinkovičová et al. 2017; Callesen et al. 2018). Approximately 0.3 g of each sample was treated with 2–3 drops of a dispersant solution (10% sodium hexametaphosphate). The treated samples were gradually added to a 500 cm3 beaker (mounted on the wet dispersant unit) until the obscuration rate reached 3–4%. Prior to the measurement, 0.5 cm3 of dispersant solution was added to water in the beaker. To enhance sample dispersion, the sonication functionality available in the dispersant unit was performed for 30 s before initiating the measurement. Each sample was analyzed in duplicate, with five measurements repeated for each sample to obtain the mean PSD. The mean PSD values were used to classify sediment into clay, silt, and sand fractions according to diameter ranges established by the US Department of Agriculture, where particle diameters less than 2 µm were classified as clay, 2–50 µm as silt, and 50–2000 µm as sand (Boyd 1995; Brady and Weil 2002).
Scatter plots were used to investigate the relationships between K as estimated from DTS, and OM and DBD as determined in the laboratory. For each FOC section, the mean DBD and OM were calculated using data from sub-samples within the specified depth ranges (Section 1, 16.7 ± 2.9 cm; Section 2, 30.9 ± 3.8 cm). Cross plots were subsequently generated to examine the relationships between K and mean OM and DBD. Both linear and non-linear regression models were evaluated to determine the best fit, characterized by the highest coefficient of determination (R2), for predicting K from DBD and OM. The derived empirical models enabled the elaboration of thermal conductivity profiles for each core, which were used to study vertical and horizontal variability of thermal conductivity.
3 Results
3.1 DTS measurements and thermal conductivity estimation
Temperature profiles at the end of the 4-h heating period showed noticeable variations in induced temperatures along the buried FOC in the four heat cycles, even within the same sediment section (Fig. 2). Temperature peaks and valleys along the FOC observed at the end of the 4-h heating period occurred at the same locations in all four heat cycles. We attributed this variability in maximum temperatures to variations in sediment properties along the FOC. To validate this assumption, we collected and analyzed sediment samples (cores) at five locations where consistent differences in maximum temperatures were observed (core numbers indicating the location of samples along the FOC are shown in Fig. 2). Notably, the temperature in the water section (Section 3) did not demonstrate a consistent trend when compared to temperatures in the sediment sections across the different tests. For example, on day 1, temperatures in the water section were the lowest compared with all sediment locations, while on day 3, they were comparable to the highest temperatures recorded in sediment at locations 1 and 3. The variability in water temperatures is likely due to varying ambient conditions on each test day and the influence of heat dissipation mechanisms in water, such as conduction and convection.
Temperature versus distance along the FOC after 4 h of heating for the three heat rates and the replicate. The number in brakes indicated the five locations where thermal conductivity was determined. Each graph presents three consecutive temperature traces to validate the consistency of the measurement
The recorded temperature time series from the DTS at the five locations enabled the analysis of the FOC’s thermal behaviour during the heating and cooling periods, allowing for thermal conductivity estimation at each location. During the heating period, temperature rose abruptly during the first 15–20 min in all locations, then temperature increase slowed at different rates in every location, likely to variations in thermal properties along the FOC (example shown in Fig. 3a). During the cooling period, temperatures at different locations within each section converged to a similar value after approximately 10 h. Note, for instance, the profiles at locations 2 and 4 in Fig. 3a; during the heating period, they reached similar maximums, but during the cooling period, each profile followed the asymptotic temperature of its corresponding FOC section. According to the monitoring from point-based sensors, these asymptotic temperatures corresponded to the diurnal sediment temperature at each depth. The temperature profile at location 5 exhibited notable variance or noise in all tests, likely due to excessive fibre optic strain at the curvature between Sections 2 and 3.
a Temperature response over time for a 33.6 W m−1 heat pulse at five distinct locations along the FOC. b Determination of the slopes from the linear region of the temperature vs. natural logarithm of time (h) for each location. The distances mentioned in the legend of a represent the midpoint of each selected location. The shaded area in b delineates the range of data points that were excluded from the linear regression analysis
The slopes from the linear region of the temperature versus the logarithm of the time plot (Fig. 3b) were used as inputs in Eq. (1). The selected 4-h heating time allowed sufficient time to distinguish a linear relationship between temperature increase and the natural logarithm of time. In all tests, obtained R2 were higher than 0.9 for locations 1–4 and between 0.8 and 0.9 for location 5 with p-values < 0.05 for the predictors of the slopes, affirming the robustness of the linear models in describing the relationship between temperature increase and the natural logarithm of time. The lower R2 for location 5 in all tests was attributed to the higher datapoint dispersion observed at this location, presumably related to excessive fibre optic strain.
Table 1 displays the mean thermal conductivity (K) and standard errors derived from the four heat pulses. As anticipated, mean K at each location presented an inversed relationship with maximum temperatures shown in Fig. 2, meaning that locations 1 and 3 developed higher temperatures with respect to location 2, 4, and 5 due to their lower thermal conductivities. Calculated thermal conductivity differed substantially at very short distances (< 2 m) within each FOC section. For instance, the mean K at location 2 is around 35% higher than that of location 1, even though these two locations are at the same depth and are only ~ 1.5 m apart. In Section 1, mean K ranged between 1.22–2.03 W m−1 °C−1, while in Section 2 the range was 2.16–2.68 W m−1 °C−1.
3.2 Core description and particle size distribution
Upon visual inspection, most cores exhibited three distinct layers distinguished by their colour and texture. The uppermost layer, spanning approximately 10–12 cm, consisted of a soft flocculent material with a dark brown colouration (Fig. 4). Directly beneath this layer, there was a darker and fibric material extending around 7–10 cm, likely indicating a slower rate of organic degradation (Brady and Weil 2002; Gilbert et al. 2014). Cores 1, 3, and 5 showed a more pronounced presence of this layer. The lowermost part of the cores was characterized by a grey compacted layer, likely corresponding to the original pond bottom as reported in other studies (Gilbert et al. 2014).
Core 1 and subsamples used to determine particle size distributions for the three distinct layers. a Core sample after cutting and removing a half side of the PVC pipe. b Moist subsamples selected for the analysis. c Dried and crushed samples and replicates. Dashed lines on the core image indicate the transitions between the distinct sediment layers
The particle size distribution across the core’s three distinct layers revealed a low clay content, ranging from 1 to 2%, with silt and sand being the dominant fractions (refer to Table 2 for detailed distribution). The upper and middle layers were particularly rich in silt, constituting 52.6% and 61.3%, respectively. In contrast, the bottom layer was predominantly sandy, comprising 65.2% of the sediment. Median particle sizes (D50) for the top and middle layers were close, recorded at 41.8 µm and 36 µm, respectively. The bottom layer, however, had a considerably larger D50 at 107 µm, which was 2.6 and 2.9 times larger than that of the upper and middle layers, respectively. Detailed PSD frequency and cumulative percentage plots for samples of each layer are provided in the supplementary information (SI).
3.3 Density and organic matter content
The validation of Eq. (2) against the known or predefined volume method was carried out on a subset of 10 subsamples. The remaining samples were not included due to their less regular geometries, especially those from the uppermost layer, which exhibited a sticky and fluffy texture. The Shapiro–Wilk test yielded a p-value greater than 0.05, indicating a normal distribution. Consequently, a paired t-test was employed to evaluate the mean difference between the datasets. The null hypothesis assumed that there was no difference in density means between datasets (p > 0.05), which was consistent with the results (p-value = 0.11). This finding supports the suitability of Eq. (2) for determining the DBD of the remaining samples. The two data sets and the box plot for this analysis are included in the supplementary information (SI).
All three cores in Section 1 showed similar trends in DBD and OM in the upper and lower parts of the cores, while in the middle sector, only cores 1 and 3 exhibited similar profiles (Fig. 5a). Within the upper 12–14 cm of these cores, the OM ranged between 4 and 7% (average 5.61%). The DBD within this depth interval varied from 1.16–1.47 g cm−3 (average 1.32 g cm−3). Between 14 and 20 cm, cores 1 and 3 showed a significant increase in OM and a decrease in DBD, while core 2 exhibited a gradual decrease in OM and an increase in DBD. Interestingly, this depth interval enclosed the location of the FOC in Section 1 (16.7 ± 2.9 cm). The bottom layer of the cores in Section 1 (~ 23–31 cm) exhibited low variability in these properties, consistent with observations in other ponds worldwide, where it is associated with the original pond bottom (Avnimelech et al. 2001; Gilbert et al. 2014). The lowest OM and highest DBD for all cores were found in the lower layer, with averages of 0.29% and 2.02 g cm−3, respectively.
Organic matter content and dry bulk density as function of depth for cores in Sections 1 (a) and 2 (b) The 2-cm thickness samples are plotted at midpoint depths. Horizontal dotted lines indicate the average FOC depths in each section and the blue-shaded intervals correspond to the variability of the FOC depths measured by the standard deviation
In Section 2, OM slightly decreased and DBD increased in the first 10–11 cm of Cores 4 and 5 (Fig. 5b). Within this depth interval, the average OM and DBD were relatively similar for both cores (OMcore4 = 7.16%, OMcore5 = 7.20%, DBDcore4 = 1.22 g cm−3, DBDcore5 = 1.12 g cm−3). For Core 4, these trends continued almost to the bottom of the core, while Core 5 exhibited fluctuating OM contents and DBD between 12 and 20 cm. Core 4 showed a substantial increase in OM and a reduction in DBD towards the bottom, while Core 5 displayed relatively constant values for these properties in the lower portion of the core. At the depth where the FOC was buried in Section 2 (30.9 ± 3.8 cm), the average OM values were 1.18% and 0.29% for Cores 4 and 5, respectively. For the same depth interval, the average DBD values were 1.92 g cm−3 (Core 4) and 2.13 g cm−3 (Core 5).
3.4 Effects of density and organic matter on thermal conductivity
Clearly, the variations in DBD and OM in Section 1 (shaded region in Fig. 5a) and associated changes in the local thermal conductivity along the FOC caused differences in the maximum induced temperatures in this section despite a constant burial depth (Fig. 2). The increase of OM at locations 1 and 3 restricted heat dissipation, resulting in temperature excesses of 2–3 °C compared to that of location 2. For locations 2 (Section 1) and 4 (Section 2), the maximum temperatures at the culmination of the heat pulse were close. In contrast, the OM and DBD at the FOC depth in Section 2 (red profile within the shaded interval in Fig. 5b) were distinct from those in FOC Section 1 (orange profile within the shaded interval in Fig. 5a). This observation raises the prospect that at location 4 these properties may exhibit differences around the FOC and within the core sample, despite the relatively short distance between the FOC and the core (10–15 cm). Notably, the OM and DBD profiles in Fig. 5b reveal an abrupt change in properties towards the end of core 4, albeit evident in a sole subsample (24–26 cm). Hence, this abrupt change may occur near the FOC location, resulting in a more pronounced temperature increase compared to location 5.
Two to three sub-samples, located within the depth bands of Sections 1 and 2, were available and analyzed to determine mean DBD and OM at the five locations along the FOC (Table 3). Among various models, quadratic regression models (Eqs. 6 and 7) exhibited the best fit, capturing the influence of OM and DBD variability on K with coefficients of determination (R2) exceeding 0.9 (Fig. 6). Even more robust correlations would be achieved if the mean OM and DBD at location 4 were closer to those at location 2. This observation reinforces the possibility that these properties may be different within the core compared to those around the FOC at location 4.
The equations determined from quadratic regression models for OM and DBD, respectively, were as follows:
where K is in W m−1 °C−1, OM in fraction, and DBD in g cm−3.
Equations (6) and (7) were employed to generate two K profiles for the five cores (Fig. 7). In general, the K profiles generated by both equations exhibited similar trends. The average K per core determined using OM was 4–6% higher than that obtained using DBD. In Core 5, the subsample at a depth of 17 cm exhibited a DBD of 0.51 g cm−3 and an OM of 27%, which falls well outside the range used to create these models (DBD = 0.97–2.15 g cm−3; OM = 0.28–11.09%). Consequently, the calculated K values for this particular subsample differed significantly, resulting in the lowest value (0.76 W m−1 °C−1) derived from the K-DBD (Eq. (7)) and the highest value (2.88 W m−1 °C−1) derived from the K-OM (Eq. (6)).
Core thermal-conductivity profiles derived from dry bulk density and organic matter empirical correlations. Dashed lines correspond to average FOC depths in each section and the blue-shaped intervals indicated FOC depth variability expressed by the standard deviation of the marker depth measurements
Within the first 10–12 cm of each core (top layer), thermal conductivity profiles showed relatively similar trends, with an average K for this layer of 1.49 ± 0.18 W m−1 °C−1. More obvious differences in K were observed between the two models at the shallowest depths (top 3–5 cm), which can be attributed to very low values of DBD. This discrepancy may arise from the limitations of Eq. (2) to determine bulk density for unconsolidated material near the WSI. The K in the middle layer exhibited significant variation among the different cores. Cores 1 and 3 showed a considerable drop in K values within this layer, attributable to an increase in OM and a decline in DBD. The average K in this layer for cores 1 and 3 was 1.21 ± 0.07 W m−1 °C−1, while the mean OM and DBD were 11 ± 2% and 0.99 ± 0.09 g cm−3, respectively. Notably, during each heat pulse, the highest induced temperatures were observed in locations 1 and 3 (Fig. 2). In these two cores, calculated K profiles clearly demonstrate the association between these high temperatures and low K at the depth corresponding to the FOC (Fig. 7). In core 5, K was also expected to be affected at depth interval 16–18 cm due to the high content of OM (27%) and low DBD (0.51 g cm−3) determined in the corresponding subsample. However, the estimated K values for this subsample are not valid because the DBD and OM values fall well outside the range of application of the proposed empirical equations. In the bottom layer of all cores, the K profiles remained relatively constant. The higher K values in this layer can be ascribed to the presence of compacted sediments that facilitate thermal propagation through increased particle-to-particle contact (Brady and Weil 2002).
4 Discussion
The integration of active DTS measurements and core laboratory analysis has provided a comprehensive characterization of the vertical and horizontal variability of thermal conductivity (K). Validated under varying ambient conditions and heat rates, active DTS has proven to be a robust method for the field analysis of K. The observed differences in K within each section (of similar depth) and between sections (of different depths) were linked to the heterogeneity of sediment properties, specifically density and organic matter content. Notably, the standard error of K for the four tests (Table 1) was higher at locations 1, 2, and 3 (Section 1), where maximum temperatures were generally higher compared to those of Section 2. This variability may be caused by the effect of high temperatures on thermal dissipation (Briggs et al. 2016). Particularly at shallower depths with very high porosity (higher than 50%), the heat transfer by convection may be relevant (Avnimelech et al. 2001; Sayde et al. 2014).
The maximum temperatures in all heat pulses displayed repeated temperature peaks and valleys at specific locations along the FOC. This behaviour was attributed to significant heterogeneities in sediment properties even within similar sediment depths and at distances as short as two metres. This observation deviated from a previous study conducted in two large water containers where the FOC was covered with sand with homogeneous density and grain size distribution (Gonzalez Rodriguez et al. 2022). In that work, the maximum induced temperatures were similar along the FOC and independent of the burial depth in each tank. This comparison reveals the complexity of natural environments and highlights the suitability of the active DTS approach for high-resolution, in situ thermal conductivity determination.
The slightly difference in K profiles between Eqs. 6 and 7 may be the result of other factors affecting this property, such as mineral composition, grain sorting, and water content. Despite the similarity in median particle sizes for the top and middle layers of Core 1, their Ks differed markedly, suggesting that organic matter largely accounts for the observed variability in thermal conductivity. Conversely, the bottom layer’s larger median particle size and its dominance in sand content may be responsible for the higher thermal conductivity noted in that section. These observations show that not only DBD and OM but also sediment composition and texture could play an important role in the thermal behaviour of aquatic sediments; therefore, analysis such as PSD should also need to be considered in the laboratory analysis for a more holistic field-laboratory methodology.
Thermal conductivity profiles display substantial variability both vertically and horizontally, spanning from a minimum of 0.91 to a maximum of 2.68 W m−1 °C−1. This variability in thermal conductivity and its relationship with other sediment properties can significantly influence the modelling of groundwater-surface water interactions. A pertinent illustration of this can be found in the study by Duque et al. (2016), who identified differences ranging from 30 to 60% in the estimation of flux exchange across various sectors of a lagoon. These variations emerged when contrasting the use of a standard K value of 1.84 W m−1 °C−1 with average field measurements ranging between 0.72 and 1.82 W m−1 °C−1. This previous study and our findings highlight the substantial heterogeneity of organic matter at small scales in environments such as ponds, and its impact on thermal properties and flux exchange estimation. Therefore, a detailed characterization of thermal conductivity using DTS is expected to produced more accurate assessment of groundwater-surface water interaction and consequently better management of water resources. Our findings also have applications in other fields, including pollutant transport analysis and geothermal projects. In these areas, an understanding of sediment thermal properties can inform sustainable practices and efficient resource utilization, underscoring the broader relevance of our study.
This research has identified both limitations and opportunities for improvement that should be considered in future studies. Firstly, the DTS signal noise at location 5 was attributed to excessive strain at the curvature between Sections 2 and 3, likely affecting the accuracy of the slope fitting (R2) at this location in all tests. To mitigate this issue in future deployments, using a loose-tube FOC is recommended over a tight-buffered FOC. In a loose-tube FOC, the fibres are more freely situated within a protective tube, allowing greater movement and reducing strain. Secondly, K profiles derived from DBD (Eq. 6) and OM (Eq. 7) showed major differences for the top 3–5 cm of each core, likely due to limitations of Eq. (2) to accurately determine DBD for unconsolidated material close to the WSI. Instead, the syringe technique, recommended for determining bulk density in soft sediments, may yield more consistent results (Mudroch et al. 1996). Lastly, the empirical models developed here generated inconsistent values of K for DBD and OM outside the range used to create these models (DBD = 0.97–2.15 g cm−3; OM = 0.28–11.09%). Therefore, we advocate for the adoption of the field-laboratory integration approach detailed in this study to formulate site-specific models for aquatic systems with characteristic different to this case study.
5 Conclusions
Our study, utilizing active DTS in a natural sediment environment, has provided a detailed analysis into the spatial variability of sediment thermal conductivity and its dependence on other properties such as dry bulk density and organic matter content. The integration of field measurements with comprehensive laboratory analysis has enabled us to develop empirical models to characterize in situ thermal conductivity in both the vertical and horizontal domains. Key findings include the identification of substantial variability in thermal conductivity within short distances and across different sediment layers. This variability, predominantly influenced by organic matter content and density, significantly affects the heat transfer processes in sediment. Our study has demonstrated that even within similar depths and at metre scale, sediment properties can vary greatly, leading to notable differences in thermal conductivity. This heterogeneity is a critical factor in modelling groundwater-surface water interactions, as it can lead to substantial discrepancies in flux exchange estimations.
The research underscores the limitations of relying on standardized literature values for thermal conductivity in environmental and hydrological models. It highlights the necessity for site-specific, in situ measurements to obtain accurate data, which is paramount for effective water resource management and environmental assessment. The use of active DTS has proven to be a robust method for capturing these variations in the field, offering high-resolution and real-time data that can significantly enhance our understanding of sediment properties in aquatic environments.
Data availability
Data are included in the article and supplementary information, further inquiries can be made to the corresponding author.
Code availability
The R codes generated in the framework of this study are available from the corresponding author upon reasonable request.
References
Abu-Hamdeh NH, Reeder RC (2000) Soil thermal conductivity effects of density, moisture, salt concentration, and organic matter. Soil Sci Soc Am J 64:1285–1290. https://doi.org/10.2136/sssaj2000.6441285x
Ahn YS, Mizugaki S, Nakamura F, Nakamura Y (2006) Historical change in lake sedimentation in Lake Takkobu, Kushiro Mire, northern Japan over the last 300 years. Geomorphology 78:321–334. https://doi.org/10.1016/j.geomorph.2006.01.036
Avnimelech Y, Ritvo G, Meijer LE, Kochba M (2001) Water content, organic carbon and dry bulk density in flooded sediments. Aquac Eng 25:25–33. https://doi.org/10.1016/s0144-8609(01)00068-1
Bakx W, Doornenbal PJ, Van Weesep RJ, Bense VF, Essink GHPO, Bierkens MFP (2019) Determining the relation between groundwater flow velocities and measured temperature differences using active heating-distributed temperature sensing. Water 11:1619. https://doi.org/10.3390/w11081619
Barry-Macaulay D, Bouazza A, Singh RM, Wang B, Ranjith PG (2013) Thermal conductivity of soils and rocks from the Melbourne (Australia) region. Eng Geol 164:131–138. https://doi.org/10.1016/j.enggeo.2013.06.014
Batley GE, Simpson SL (2016) Sediment sampling, sample preparation and general analysis. Sediment quality assessment: a practical guide, 2nd edn. CSIRO Press, Clayton, Victoria, Australia, pp 21–23
Boyd CE (1995) Bottom soils, sediment, and pond aquaculture. Springer Science & Business Media, New York
Brady NC, Weil RR (2002) The nature and properties of soils. Prentice Hall, Upper Saddle River
Briggs MA, Buckley SF, Bagtzoglou AC, Werkema DD, Lane JW (2016) Actively heated high-resolution fiber-optic-distributed temperature sensing to quantify streambed flow dynamics in zones of strong groundwater upwelling. Water Resour Res 52:5179–5194. https://doi.org/10.1002/2015wr018219
Callesen I, Keck H, Andersen TJ (2018) Particle size distribution in soils and marine sediments by laser diffraction using Malvern Mastersizer 2000—method uncertainty including the effect of hydrogen peroxide pretreatment. J Soils Sediments 18:2500–2510. https://doi.org/10.1007/s11368-018-1965-8
Carslaw HS, Jaeger JC (1959) Conduction of heat in solids. Clarendon Press, Oxford
Ciocca F, Lunati I, Van De Giesen N, Parlange MB (2012) Heated optical fiber for distributed soil-moisture measurements: a lysimeter experiment. Vadose Zone J 11(vzj2011):0199. https://doi.org/10.2136/vzj2011.0199
De Lieto Vollaro R, Fontana L, Vallati A (2014) Experimental study of thermal field deriving from an underground electrical power cable buried in non-homogeneous soils. Appl Therm Eng 62:390–397. https://doi.org/10.1016/j.applthermaleng.2013.09.002
Del Val L, Carrera J, Pool M, Martínez L, Casanovas C, Bour O, Folch A (2021) Heat dissipation test with fiber‐optic distributed temperature sensing to estimate groundwater flux. Water Resour Res 57:e2020WR027228. https://doi.org/10.1029/2020wr027228
DiamondOptics (2021) Raw cables. https://diamond-optics.com/products/optical-cables-assemblies/raw-cables/. Accessed 13 Aug 2023
Duque C, Müller S, Sebok E, Haider K, Engesgaard P (2016) Estimating groundwater discharge to surface waters using heat as a tracer in low flux environments: the role of thermal conductivity: thermal conductivity estimating groundwater discharge. Hydrol Process 30:383–395. https://doi.org/10.1002/hyp.10568
Duque C, Haider K, Sebok E, Sonnenborg TO, Engesgaard P (2018) A conceptual model for groundwater discharge to a coastal brackish lagoon based on seepage measurements (Ringkøbing Fjord, Denmark). Hydrol Process 32:3352–3364. https://doi.org/10.1002/hyp.13264
Gilbert P, Taylor S, Cooke D, Deary M, Cooke M, Jeffries M (2014) Variations in sediment organic carbon between different types of small natural ponds along Druridge Bay, Northumberland, UK. Inland Waters 4:57–64. https://doi.org/10.5268/iw-4.1.618
Gonzalez Rodriguez L, McCallum A, Kent D, Rathnayaka C, Fairweather H (2023) A review of sedimentation rates in freshwater reservoirs: recent changes and causative factors. Aquat Sci 85:60. https://doi.org/10.1007/s00027-023-00960-0
Gonzalez Rodriguez L, McCallum A, Kent D, Rathnayaka C, Robins M, Fairweather H (2022) Detection of sedimentation in water storages using distributed temperature sensing (DTS). Hydrology & Water Resources Symposium 2022 (HWRS 2022): the past, the present, the future. Engineers Australia, Brisbane 812–823. https://doi.org/10.3316/informit.916065730908801
Goto S, Yamano M, Morita S, Kanamatsu T, Hachikubo A, Kataoka S, Tanahashi M, Matsumoto R (2017) Physical and thermal properties of mud-dominant sediment from the Joetsu Basin in the eastern margin of the Japan sea. Mar Geophys Res 38:393–407. https://doi.org/10.1007/s11001-017-9302-y
Haigh SK (2012) Thermal Conductivity of Sands Géotechnique 62:617–625. https://doi.org/10.1680/geot.11.P.043
Hartog AH (2017) An introduction to distributed optical fibre sensors. CRC Press, Boca Raton
He H, Dyck MF, Horton R, Ren T, Bristow KL, Lv J, Si B (2018) Development and application of the heat pulse method for soil physical measurements. Rev Geophys 56:567–620. https://doi.org/10.1029/2017rg000584
Karan S, Engesgaard P, Rasmussen J (2014) Dynamic streambed fluxes during rainfall-runoff events. Water Resour Res 50:2293–2311. https://doi.org/10.1002/2013WR014155
Kim TW, Cho YK, Dever EP (2007) An evaluation of the thermal properties and albedo of a macrotidal flat. J Geophys Res 112:C12009. https://doi.org/10.1029/2006jc004015
Mastersizer3000 (2023) Mastersizer 3000. https://www.malvernpanalytical.com/en/products/product-range/mastersizer-range/mastersizer-3000. Accessed 24 Jun 2023
Meyers PA, Teranes JL (2001) Sediment organic matter. In: Last WM, Smol JP (eds) Tracking environmental change using lake sediments: physical and geochemical methods. Springer, Dordrecht, pp 239–269
Mudroch A, Azcue JM, Mudroch P (1996) Manual of physico-chemical analysis of aquatic sediments. CRC Press, Boca Raton
NIWA (2016) Collecting sediment samples. https://niwa.co.nz/sites/niwa.co.nz/files/Sediment04_03Collecting_sediment_samples_0.pdf. Accessed 23 Jun 2023
Ogle DH, Doll JC, Wheeler AP, Dinno A (2023). FSA: simple fisheries stock assessment methods. R package version 0.9.5. https://CRAN.R-project.org/package=FSA. Accessed 16 Feb 2024
R Core Team (2023) R: a language and environment for statistical computing. Version 2023.06.2. R Foundation for Statistical Computing. https://www.R-project.org/. Accessed 13 Dec 2023
Rui Y, Hird R, Yin M, Soga K (2019) Detecting changes in sediment overburden using distributed temperature sensing: an experimental and numerical study. Mar Geophys Res 40:261–277. https://doi.org/10.1007/s11001-018-9365-4
Sakaki T, Lüthi BF, Vogt T, Uyama M, Niunoya S (2019) Heated fiber-optic cables for distributed dry density measurements of granulated bentonite mixtures: feasibility experiments. Geomech Energy Environ 17:57–65. https://doi.org/10.1016/j.gete.2018.09.006
Sayde C, Buelga JB, Rodriguez-Sinobas L, El Khoury L, English M, Van De Giesen N, Selker JS (2014) Mapping variability of soil water content and flux across 1–1000 m scales using the actively heated fiber optic method. Water Resour Res 50:7302–7317. https://doi.org/10.1002/2013wr014983
Sayde C, Gregory C, Gil-Rodriguez M, Tufillaro N, Tyler S, Van De Giesen N, English M, Cuenca R, Selker JS (2010) Feasibility of soil moisture monitoring with heated fiber optics. Water Resour Res 46:W06201. https://doi.org/10.1029/2009wr007846
Sebok E, Duque C, Engesgaard P, Boegh E (2015) Spatial variability in streambed hydraulic conductivity of contrasting stream morphologies: channel bend and straight channel. Hydrol Process 29:458–472. https://doi.org/10.1002/hyp.10170
Sebok E, Duque C, Kazmierczak J, Engesgaard P, Nilsson B, Karan S, Frandsen M (2013) High-resolution distributed temperature sensing to detect seasonal groundwater discharge into Lake Væng, Denmark. Water Resour Res 49:5355–5368. https://doi.org/10.1002/wrcr.20436
Sebok E, Müller S (2019) The effect of sediment thermal conductivity on vertical groundwater flux estimates. Hydrol Earth Syst Sci 23:3305–3317. https://doi.org/10.5194/hess-23-3305-2019
Selker JS, Thevenaz L, Huwald H, Mallet A, Luxemburg W, Van De Giesen N, Stejskal M, Zeman J, Westhoff M, Parlange MB (2006) Distributed fiber-optic temperature sensing for hydrologic systems. Water Resour Res 42:W12202. https://doi.org/10.1029/2006WR005326
Silixa (2022) Principles of distributed temperature sensing. https://silixa.com/principles-of-distributed-temperature-sensing/. Accessed 13 Aug 2023
Simon N, Bour O, Lavenant N, Porel G, Nauleau B, Pouladi B, Longuevergne L, Crave A (2021) Numerical and experimental validation of the applicability of active‐DTS experiments to estimate thermal conductivity and groundwater flux in porous media. Water Resour Res 57:e2020WR028078. https://doi.org/10.1029/2020wr028078
Šinkovičová M, Igaz D, Kondrlová E, Jarošová M (2017) Soil particle size analysis by laser diffractometry: result comparison with pipette method. IOP Conf Ser Mater Sci Eng 245. https://doi.org/10.1088/1757-899x/245/7/072025
Smits KM, Sakaki T, Limsuwat A, Illangasekare TH (2010) Thermal conductivity of sands under varying moisture and porosity in drainage–wetting cycles. Vadose Zone J 9:172–180. https://doi.org/10.2136/vzj2009.0095
STHDA (2016) Paired Samples T-test in R. http://www.sthda.com/english/wiki/paired-samples-t-test-in-r. Accessed 28 Dec 2023
Tirado-Conde J, Engesgaard P, Karan S, Müller S, Duque C (2019) Evaluation of temperature profiling and seepage meter methods for quantifying submarine groundwater discharge to coastal lagoons: impacts of saltwater intrusion and the associated thermal regime. Water 11:1648. https://doi.org/10.3390/w11081648
Trabelsi Y, Gharbi F, El Ghali A et al (2012) Recent sedimentation rates in Garaet El Ichkeul Lake, NW Tunisia, as affected by the construction of dams and a regulatory sluice. J Soils Sediments 12:784–796. https://doi.org/10.1007/s11368-012-0496-y
Tyler SW, Selker JS, Hausner MB, Hatch CE, Torgersen T, Thodal CE, Schladow SG (2009) Environmental temperature sensing using Raman spectra DTS fiber‐optic methods. Water Resour Res 45:W00D23. https://doi.org/10.1029/2008WR007052
Verstraeten G, Poesen J (2001) Variability of dry sediment bulk density between and within retention ponds and its impact on the calculation of sediment yields. Earth Surf Process Landf 26:375–394. https://doi.org/10.1002/esp.186
Vogt T, Schneider P, Hahn-Woernle L, Cirpka OA (2010) Estimation of seepage rates in a losing stream by means of fiber-optic high-resolution vertical temperature profiling. J Hydrol 380:154–164. https://doi.org/10.1016/j.jhydrol.2009.10.033
Yokogawa (2014) DTSXL distributed temperature sensor long range system guide. https://web-material3.yokogawa.com/IM39J06B40-01E_1.us.pdf. Accessed 15 Sept 2023
Yoswaty D, Amin B, Winanda H, Sianturi D, Lestari A (2021) Analysis of organic matter content in water and sediment in the coastal waters of Bengkalis Island, Riau Province. IOP Conf Ser Earth Environ Sci 934. https://doi.org/10.1088/1755-1315/934/1/012055
Acknowledgements
The authors thank the University of the Sunshine Coast (UniSC) for their support during the execution of this project. Special thanks to the Facility Management Department at UniSC for providing a research site and essential materials to carry out this study. We would also like to express our appreciation to Mark Robins from Yokogawa Australia for providing a DTS unit and technical support. LGR expresses gratitude to the Australian Government for financial support through the Research Training Program Scholarship.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions This research was sponsored by a Lake Macquarie Environmental Research Grant, which is funded by Lake Macquarie City Council and various sponsors. In the 2021/2022 funding cycle, the sponsors included Hunter Water Corporation, Delta Electricity, and Origin. LGR received financial support for the submitted work through the Research Training Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Alexander Koiter
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rodriguez, L.G., Kent, D., Rathnayaka, C. et al. Fibre optic sensing technology for field assessment of thermal conductivity of aquatic sediments. J Soils Sediments 24, 2144–2158 (2024). https://doi.org/10.1007/s11368-024-03772-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-024-03772-3