Abstract
Purpose
Humic substances (HSs) and biochar (BC) are carbon-based soil amendments. These amendments improve soil health and fertility, enhance nutrient pools and carbon content, remove soil pollutants, and enhance plant performance. As a result, they contribute to agro-environmental sustainability and the development of a circular bioeconomy. However, there is a lack of research on the effects of HSs-aged BC or the co-application of BC and HSs on the agro-environmental system. Therefore, further studies are needed to understand the impacts of these amendments on the agro-environmental system.
Methods
This study utilizes a novel technique based on BC aging with HSs to investigate the BC-aging process, factors influencing it, as well as the impact of BC and HSs on soil physicochemical properties, nutrient pools, microbial communities, immobilization of metal ions in the soil, and plant performance. We gathered original research articles, meta-analysis papers, book chapters, conference proceedings, and technical notes from high-quality peer-reviewed journals and reputable websites.
Results and discussion
The extensive literature evaluation revealed that the potential benefits of BC are closely related to variations in the physicochemical composition of the BC and soil because microorganisms do not prefer fresh BC for colonization. In some studies, BC showed a detrimental impact on the soil microbiome. Therefore, the influence of BC on the soil microbiome, nutrient pool, pollutant removal, and plant growth strongly depends on the residence time of BC in the soil and its prior aging with HSs. Aging BC with HSs is more effective than using fresh BC as it enhances nutrient pools, accessibility to plants, pollutant amelioration capacity, microbial activities, and consequently, plant performance due to the presence of surface functional groups and the adsorbed nutrient-rich organic molecules.
Conclusions
The soil fertility traits and plant performance were impacted by aging or a combination of BC with HSs. However, detailed characterizations and continuous experiments are required to gain in-depth insights into the interaction mechanisms between the aging of BC with HSs via the liquid soaking technique and soil fertility traits.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
The long-term sustainability of agriculture and the environment depends on healthy soils (Tahat et al. 2020). However, with urbanization and economic growth, there has been a rise in global waste production, which threatens public health, the environment, and agriculture by piling up on land (Ukaogo et al. 2020). In response, researchers have recently embraced the concept of transforming waste materials into carbon-rich organic soil amendments, such as biochar, hydrochar, compost, humic substances, and others, as innovative, cost-effective, and environmentally friendly solutions (Elkhalifa et al. 2019; Van Nguyen et al. 2022). By converting these waste products into carbon-rich organic soil amendments, we can promote zero-waste practices, enhance agricultural and environmental sustainability, and foster the development of a circular bioeconomy (Ghodake et al. 2021).
Biochar (BC) is a solid carbon-rich material produced from various feedstocks through a pyrolysis temperature ranging from 250 to 800 °C and a limited oxygen supply (Verheijen et al. 2010). The multifunctional properties of BC, including its high specific surface area, surface activity, porous structure, oxygen-containing functional groups, and ion exchange capacity, make it a crucial organic soil amendment for research in agriculture and environmental science (Liu et al. 2020; Wang and Wang 2019).
BC application in soil has several advantages, including improved soil nutrient retention, enhanced soil structure, increased microbial activities, improved water holding capacity, decreased soil acidity, removal of contaminants, and creation of more favorable growing conditions (Biederman and Harpole 2013; Giagnoni and Renella 2022; Maienza et al. 2017; Nguyen et al. 2018; Rahim et al. 2022). However, due to the intricate interactions between BC, soils, and crops, substantial research on BC as a soil amendment has produced contradictory results (Lychuk et al. 2015).
Two fundamental hypotheses serve as a framework for investigating how BC affects soil health. The first idea presumes that BC could have an immediate or short-term effect on soil characteristics, such as improved nutrient accessibility in soil, microbial activity, higher organic carbon content, improved overall porosity and water-holding capacity, and reduced soil bulk density (Jinsheng et al. 2022; Lee et al. 2022). However, a 30-month field experiment predicted that BC with a diameter between 30 and 0.2 µm would increase the amount of water in the soil available to plants. They did not connect any of the observed soil changes to the porous interior of the BC. In this case, elevated earthworm activity was responsible for the improvements in near-saturated hydraulic conductivity and soil water content at 0.1 kPa (Hardie et al. 2014). It leads to the second hypothesis, which suggests that BC can have an indirect or aging effect on soil quality parameters. Incorporating BC into the soil changes its characteristics, such as specific surface area (SSA), surface morphology, acidity, elemental composition, ion exchange capacity, and aromaticity (Wang et al. 2020). These modifications can occur due to various natural forces, including temperature variations causing freeze-thaw cycles (Fu et al. 2019), rainfall events causing wetting and drying cycles (Meng et al. 2020), sunlight irradiation causing photochemical degradation (Quan et al. 2020a), and mild oxidation resulting from atmospheric oxygen, root exudates, or microorganisms (Hua et al. 2020; Liu et al. 2017). Over time, these changes in BC characteristics may enhance soil aggregate stability, improve soil structure and water-holding capacity, alter other soil physicochemical properties, and potentially create more suitable habitats for microorganisms, ultimately contributing to sustainable crop production (Van Zwieten et al. 2010).
The natural aging processes of BC in soil environments are slow and do not rapidly alter soil properties. Consequently, artificial aging methods involving physical (freeze-thaw, wetting-drying), chemical (chemical oxidation, organic acid modification), and biological (co-composting) approaches have been employed (Spokas 2010).
Studies have demonstrated the beneficial effects of using BC as a soil amendment with other organic substances on soil health (Hannet et al. 2021; Rahman et al. 2020). Among these co-amendments, natural organic matter (NOM), which aids in heat and water retention, prevents drainage and erosion, immobilizes contaminants, and promotes seed germination and root growth, can be valuable (Fernandes et al. 2007; Sierra et al. 2004). Approximately 80% of NOM comprises humic substances (HSs), such as humic acid, fulvic acid, and humin (Pettit 2004; Schnitzer and Khan 1975). These substances are formed spontaneously in the soil through the complex biogeochemical processes involving the breakdown and transformation of NOM by soil microbes (Li et al. 2019). The unique molecular composition of HSs, which includes diverse organic macro/micro-molecules and functional groups, contributes to their potential as soil biostimulants (Sutton and Sposito 2005). In soils amended with biochar, the enrichment of NOM appears to be influenced by HSs derived from BC. However, these BC-derived HSs differ from those naturally present in the soil as they exhibit more surface COOH groups and have a higher cation exchange capacity, potentially altering the characteristics of the BC surface (Jin et al. 2018). The HSs obtained from BC increase the aromaticity of low-temperature BC; however, their interaction with high-temperature BC can result in pore-blocking effects as the HSs extend into the pore spaces, artificially reducing the BC surface area (Pignatello et al. 2006).
Aligned with the Sustainable Development Goals of the 2030 Agenda, the aging or combination of BC with HSs can contribute to achieving a zero-waste society, agro-environmental sustainability, food security, a circular bio-economy, and human well-being. BC and HSs transform the exchangeable fraction of contaminants into the nonexchangeable fraction due to their large surface area and the abundance of organic functional groups (Zhou et al. 2018). However, HSs, such as humic acid, significantly influence the sorption of organic and inorganic pollutants by biochar (Park et al. 2017). While a few studies have documented the advantages of co-amending BC and HSs, their benefits in agriculture have not been thoroughly investigated.
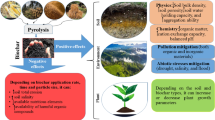
This study is divided into two main sections, following the preceding literature discussion. The first section shed light on the natural and artificial aging of BC and its effects on the soil. The second section focuses on the HSs-aged BC and its consequences for soil physicochemical properties, nutrient availability, and water holding capacity, microbial communities, and removal of hazardous substances. We present a novel approach to liquid aging of BC with HSs (HSs-aged BC). Additionally, due to the limited research on the liquid aging of BC with HSs, we investigate the combined effects of BC and HSs on soil physicochemical properties, nutrient cycling, water retention, microbial communities, and the removal of hazardous substances. The complete summary of this review article is illustrated in Fig. 1.
2 A brief overview of biochar (BC) aging process and factors
The apparent ability of BC to immobilize soil contaminants, store carbon in the soil environment, and boost soil fertility has garnered significant interest. However, it is crucial to consider how these processes work over time in the soil system. Several environmental conditions can influence BC’s physicochemical characteristics to vary when applied to soil. These factors include freeze-thaw cycles resulting from temperature changes, wetting-drying due to precipitation events, photochemical degradation caused by sunlight exposure, and mild oxidation induced by microbial activity and root exudates. These processes may cause BC to fragment, dissolve, or oxidize, significantly changing its characteristics. Extensive field testing is necessary to monitor the temporal changes in soils supplemented with biochar. Various artificial aging techniques, such as physical, chemical, and biological aging techniques, have been created to mimic natural aging processes (Fig. 2). This makes it possible for researchers to investigate the changes that BC experiences over time. The aging processes of BC, both natural and artificial, are briefly covered below.
2.1 Natural aging of BC in the soil environment
In recent years, research has concentrated on three main elements that affect BC’s aging process. These elements consist of (1) the effect of soil processes on BC aging; (2) the effect of temperature fluctuations, especially freeze-thaw cycles; and (3) the function of soil microorganisms in BC aging. Understanding these processes has advanced significantly, yielding important insights into soil type, classification, temperature effects, microbial involvement, and the fundamental mechanisms of BC aging in soil. Table 1 presents a systematic overview of the subject and the current breakthroughs and improvements. Here is a quick history of these procedures.
2.1.1 Soil-influenced aging of BC
The soil-influenced aging of BC happens gradually and usually proceeds steadily, stabilizing after a considerable amount of time. This process can be due to the gradual release of BCs more easily degradable elements, which leads to a majority of aromatic carbon that oxidizes or degrades more slowly (Hale et al. 2011; Liu et al. 2013; Mukherjee et al. 2014). In addition, harmonious reactions between BC and soil may affect BC’s surface chemical composition by microbe sorption and soil organic matter, resulting in an enhanced negative charge on the BC surface from a variety of functional groups, including carboxylic acid and others (Mukherjee et al. 2014). The physical barrier of soil interacting with mineral surfaces may also contribute to a slow aging process (Paetsch et al. 2018).
A study analyzing fresh and aged BC subjected to soil aging using SEM-EDS, XRD, and FTIR techniques observed that soil minerals deposited on the surface of BC during the aging process in the field. It resulted in the formation of organo-mineral complexes and the obstruction of cracks and channels within the BC. The hardness and compressive strength indicated a substantial increase in these properties for the aged BC compared to the fresh BC, which correlates with the soil minerals (Wang et al. 2021). Another research study revealed significant alterations in the physicochemical characteristics of BC during its aging process in the field. These modifications have an impact on the BC’s ability to absorb heavy metals (Xie et al. 2022).
In a recent study, BC modified with red mud and iron salt was synthesized and subjected to a 1-month soil aging process. After aging, the modified BC exhibited an increase in specific surface area and enhanced strength of oxygen-containing functional groups, accompanied by a decrease in Fe0 content. Additionally, the impurities in the raw material mixed with BC potentially improved its resistance to aging and protected Fe0 from oxidation. Following aging, both Fe/BC and RM/BC showed a decrease in their adsorption capacity for Cd(II) by approximately 20.76% and 18.03%, respectively (Wang et al. 2023). More detailed information on key parameters related to soil aging of BC is summarized in Table 1.
2.1.2 Temperature-influenced aging of BC
Environmental factors in the soil, such as moisture content, temperature, pH, aeration, and natural organic matter content, influence the surface chemistry of BC. For instance, high soil moisture and temperature conditions facilitate rapid biotic and abiotic aging of BC in soil (Fuertes et al. 2010). Temperature and moisture are among the crucial factors that influence the process of BC aging (Cheng and Lehmann 2009). Even while chemisorption between the surface of biochar and oxygen is endothermic, increasing the temperature above 70 °C has been shown to accelerate the aging process of BC (Cheng and Lehmann 2009; Cheng et al. 2006). Under natural conditions (Quan et al. 2020a) or particularly at very low temperatures like – 22 °C (Fan et al. 2018), the aging of BC continues to occur but at a comparatively slower rate. Studies have shown that the aging process of BC can take place in any terrestrial system, as a significant positive correlation between mean annual temperature and oxidation of aged BC was observed after 130 years of observation (Igalavithana et al. 2017; Kim et al. 2019). In a recent study, frequent freeze-thaw cycles in a laboratory experiment using simulated loam soil columns containing 0%, 1%, and 2% biochar resulted in a 19.69% increase in oxygen content and a 17.75% increase in nitrogen content while decreasing the carbon content. Biochar has the potential to enhance the soil-water environment, adsorb accessible nutrients, and reduce N2O emissions by 35.89 to 46.31% when applied at a rate of 12.39% (Yang et al. 2023).
2.1.3 Microbe-influenced aging of BC
BC application as an amendment to soil alters its pore structure and enhances its water-holding capacity, increasing the conditions for microorganisms to survive. The pores of BC might provide favorable environments for microbes to flourish. However, the mineralization process started by microbes is slow and could take several years because of BC’s resistant carbon (Singh et al. 2012). Microorganisms play a crucial role in the aging of BC. There has been a rising emphasis on the occurrence of ecologically persistent free radicals (EPFRs) in biochars. These EPFRs can produce reactive oxygen species, which aid in the remediation of pollutants. It is worth mentioning, however, that the presence of EPFRs might also cause oxidative stress in the microorganisms in the surrounding environment (Masiello et al. 2013; Ruan et al. 2019). For example, laboratory incubations of BC without microbial inoculation resulted in a 50–90% lower loss of carbon (C) compared with microbial activity (Zimmerman 2010).
In a long-term incubation study, analysis of carbon release revealed that nearly half of the aging observed in biochar was due to microbial aging mechanisms (Hale et al. 2011). Another study reported that biologically aged BC showed a decline in carbon content and stability compared to abiotic aging, especially when exposed to water-holding capacity. Additionally, the release of dissolved organic matter (DOM) from abiotically aged BC showed a linear increase over time, whereas, in biologically aged BC, the release of DOM followed a logarithmic pattern. These findings strongly suggest the crucial role of microbial activities in solubilizing BC (Quan et al. 2020b).
In a recent study by Qu and coworkers, the researchers investigated the impact of different aging methods BC derived from Auricularia auricular and its effects on soil properties and cadmium (Cd) passivation in chernozem soil with a pH value of 5.93. The three aging methods employed were freeze-thaw cycling aging (FB), acidified aging (AB), and microbial aging (MB). The Cd-contaminated soil (which initially contained 3 mg kg−1 of Cd) was amended with the three types of aged BC at a ratio of 4% (w:w) over a 56-day incubation period. The results showed that the application of FB and MB in the soil led to an increase in soil pH (0.82–1.04, 0.27–9.36), cation exchange capacity (1.06–2.53 cmol kg−1, 1.66–2.59 cmol kg−1), and organic matter content (2.28–4.67 g kg−1, 3.70–5.48 g kg−1). Among the aging methods, FB exhibited the best performance in stabilizing Cd concentrations (17.06–23.65%). On the other hand, AB resulted in a decrease in soil pH and CEC by 0.82–1.04 and 1.32–2.40 cmol kg−1, respectively, and activated Cd by 11.6–19.24% (Qu et al. 2022).
From the above discussion, it is concluded that BC aging through natural processes is slow and complex. It is impossible to fully comprehend how the physicochemical characteristics of BC change during its prolonged aging process. As a result, numerous artificial aging simulation techniques have been used to mimic the natural changes in BC characteristics. Here is a comprehensive discussion of several artificial aging techniques.
2.2 Artificial/chemical aging of BC
As discussed earlier, the process of BC aging is relatively slow due to the inert nature of BC. Consequently, the changes in BC composition are so small that they are difficult to detect within a practical experimental period (Kuzyakov et al. 2014). Artificial aging methods are used to simulate the natural aging of BC (Frišták et al. 2015; Jin et al. 2017). These methods include chemical oxidation with hydrogen peroxide (H2O2) (Huff and Lee 2016), treatment with HNO3 (Wang et al. 2019), treatment with H2SO4/HNO3 (Xia et al. 2023), K2Cr2O7 (Chen et al. 2016), H2O2/K2Cr2O7 (Han et al. 2018), H3PO4 (Chu et al. 2018), and others. Laboratory-simulated aging with these reagents can cause morphological changes in BC, replicating natural aging processes (Cross and Sohi 2013; Lawrinenko et al. 2016).
A study by Li et al. revealed that the oxidation/aging of BC with H2O2 promoted higher microbial abundance and resulted in different microflora with an increased presence of gram-positive bacteria, significantly altering the gram-positive/gram-negative bacteria ratios. Furthermore, H2O2-aged BC exhibited increased oxygen-containing functional groups and surface area, leading to enhanced adsorption of Cd (Li et al. 2020). In another study, rice straw-derived BC was oxidized or aged with H2SO4/HNO3, which introduced carboxylic functional groups to the BC surface. These groups acted as additional binding sites for heavy metals in contaminated farm soils and mitigated methane emissions from paddy soils (Nan et al. 2021; Qian et al. 2015). Similarly, coffee ground-derived BC aged with 15% H2O2 exhibited increased porous volume, specific surface area, and decreased pHpzc. Aged BC demonstrated stronger selective adsorption for Cd and zinc due to the dominant roles of ion exchange and complexation (Ke et al. 2022). In another study, Auricularia auricular spent substrate BC was oxidized with a 20% 4H2SO4/1HNO3 solution for 6 h, resulting in decreased soil pH and CEC by 0.82–1.04 and 1.32–2.40 cmol kg−1, respectively. Additionally, aged BC activated Cd by 11.6–19.24% (Qu et al. 2022). It has been reported that treating BC with H3PO4 dramatically improves its specific surface area and micropores through acid catalysis and crosslinking (Chu et al. 2018).
Although numerous studies have been conducted to systematically review either natural or artificial aging methods individually or as a whole, no single study critically evaluates and discusses the effects of humic substances (HSs) accelerated aging treatments on the performance of BC. In this regard, we aim to fill this gap in the following sections.
3 Routs of BC aging with HSs (HSs-aged BC)
Three main routes, namely, liquid aging of BC, homogenization (physical mixing in a specific ratio), and facile combinations, as illustrated in Fig. 3, have been employed to fabricate HS-aged BC. These routes are distinct in their mechanisms, leading to the development of remarkable properties in the resulting HS-aged BC.
3.1 Liquid aging of BC with HSs
A thorough literature search found that BC and HSs are widely used in soil applications. However, there has been little research on BC aged in liquid form with HSs before application to soil. Two synthesis pathways are used in liquid aging. The first route involves the synthesis of BC from various lignocellulosic feedstocks, such as agricultural residues (straw, husk, stalks, etc.), forest residues (roots, woodchips, sawdust, etc.), and herbaceous biomass (switchgrass, elephant grass, etc.), under different pyrolysis conditions. The HSs (humic acid, fulvic acid, and humin) are then extracted from lignocellulosic biomass or soil/peat. The second route involves the use of commercially available BC and HSs. The as-prepared/raw materials can undergo aging in a solution using standard procedures, as illustrated in Fig. 3a.
For example, in one study, aged/activated magnetic biochar (Fe3O4-γFe2O3@PBC) was prepared using artificially extracted humic acid (A-HA) from eucalyptus leaf powder with a particle size of 100 meshes. The materials were dispersed in ultrapure water in a three-necked flask, followed by the addition of A-HA dissolved in a 6% ammonia aqueous solution. After stirring, the materials were obtained through magnetic separation (Du et al. 2020).
In another study, commercially available biochar (prepared by Sonnenerd GmbH, Austria from agricultural waste, rich in cellulosic fibers and cereal husks at 600 °C) and humac rich in oxihumolite (Envi Proddukt, Czech Republic) were used. The characteristics of the products are enlisted in Table 2. The aging process involved placing the biochar, humac, and demineralized water in a gas-washing bottle and aerating the suspension for 7 days. The resulting product was homogenized and filtered (Hammerschmiedt et al. 2021).
Similarly, biochar powder (0.28 mm) and sodium humate (deep brown powder with 0.075 mm size, 52% humic acid, and 10.36) were mixed with a wood vinegar solution (pH, 3.36–3.96, and specific gravity, 1.086 g mL−1) at a ratio of 1.5 (42.8 wt %), 1.0 (28.6 wt %), and 1.0 (28.6 wt %). The mixture was then air dried and sieved to get the humic-acid-wood vinegar-aged biochar materials for further applications in contaminated soil remediation (Zhu et al. 2021).
In another study, a humic acid extracted from peat soil in Amherst, MA, was coated on biochar through a liquid mixing technique with a concentration of 2000 ppm. The ratio of biochar and humic acid was 1.5:1. The suspension was placed in an oven at a temperature of 70 °C for 5 days, and it was hand shaken once every day to make the solid particles uniformly contact the HA solution. Then the suspension was centrifuged, and the remaining solution was decanted. The residue was rinsed with deionized water to remove freely dissolved HA, freeze dried, and then re-suspended and washed with deionized water. The HA-coated biochar samples were freeze dried again and gently ground to pass through a 250 µm sieve (Zhang et al. 2015).
From the literature search, it can be concluded that research on the fabrication of liquid aging of BC with HSs is limited and requires more attention to gain in-depth insights.
3.2 Homogenization or facile combinations of BC and HSs
This technique primarily involves the physical treatment or mixing of BC and HSs in a specific ratio to achieve uniform aging before soil application (Fig. 3b). Hamzah and colleagues recently developed humic acid-coated biochar using an adhesive polymer. In their study, rice straw-derived biochar produced at a pyrolysis temperature of 400 °C was mixed with humic acid derived from organic matter in a balanced ratio of 1:1. Additionally, 1 g of polymer was added as an adhesive ingredient (Hamzah et al. 2022). However, compared to liquid aging, this process is less effective for BC functionalization, and only this study has utilized this aging technique.
The most common and widely used technique is the application of either BC or HSs alone or in combination (Fig. 3c). In this technique, HSs may accelerate the aging process of BC in the soil, but it cannot be considered an instant aging route for BC. For instance, Holatko and colleagues prepared combinations of low-BC (32 g kg−1) dose + HA (0.8 g kg−1) and high-BC (80 g kg−1) dose + HA (0.8 g kg−1). The results indicated that these combinations were not favorable for soil fertility and crop yield, possibly due to the interaction between BC and HSs over a short period (Holatko et al. 2020). In another study, Karimi and colleagues mixed BC with the soil at rates of 0, 20, and 40 g kg−1, while HA was applied through irrigation water at rates of 0, 250, and 500 mg L−1. They studied the growth and nutrient uptake of Calendula and found that the best results were obtained at 40 g kg−1 BC and 500 mg L−1 HA, indicating a significant interactive effect between BC and HA (Karimi et al. 2020).
4 Characterization of BC aged with HSs
The aging of BC with HSs alters their structure and surface attributes which are normally showed through different characterization techniques such as X-ray powder diffraction (XRD); Fourier transform infrared spectroscopy (FTIR); Brunauer, Emmett, and Teller (BET) specific surface area, scanning electron microscopy (SEM), energy dispersive X-ray analysis (EDX), X-ray photoelectron spectroscopy (XPS), and many others. It has been evaluated from the literature that the characteristics of BC, such as morphology, specific surface area, and chemical composition, can be directly influenced by HSs. For instance, Zhang et al. reported that the carbon and nitrogen contents at biochar surfaces, as determined by XPS, decreased, and their surface oxygen content and functional groups increased after humic acid coating. Moreover, FTIR analysis represented alicyclic C–H stretching, C–OH, and C = C vibrations in aromatic structures (Zhang et al. 2015). Du and co-workers reported that the BET surface area of magnetic biochar (Fe3O4 γFe2O3@PBC) treated with artificial humic acid was reduced to 744.7 from 1281.9 m2g−1 (0.02Fe3O4-γFe2O3@PBC) and 906.9 m2 g−1 (0.05Fe3O4 γFe2O3@PBC), that may be due to the blockage of biochar pores. Overall the material has well crystalline structure (confirmed by XRD), uniform morphology (showed by SEM and TEM), and diverse functional groups (showed by FTIR and XPS) (Du et al. 2020). In another work, humic acid (HA) and Fe–Mn oxides were loaded onto biochar to create a novel ternary material called HA/Fe–Mn oxide biochar (HFMB). FTIR analysis showed that the ternary material had more functional groups on the surface than the original biochar. Similarly, SEM showed the flower-like porous structure of HFMB (Fig. 4) (Guo et al. 2019). Overall, the aging/coating of biochar with humic substances imparts diverse functional groups, e.g., oxygen-containing functional groups, the stretching vibrations of the OH group, –C = O group, C–O group, stretching vibrations of the saturated C–H bond, the stretching vibrations of the aromatic C–H bond, and C–OH bond (Guo et al. 2019; Huang et al. 2021; Lu et al. 2021). The detail about FTIR-based functional groups with specific wavenumber cm−1 is presented in Table 3. These diverse functional groups could play a role in various processes in the soil environment, discussed in the following section.
SEM images of a pristine biochar and b HA/Fe–Mn oxide biochar (HFMB) (Guo et al. 2019)
5 Soil application of BC aged with HSs
In this section, we aim to discuss such impacts of BC aged/combined with HSs with different properties for soil applications in the context of agro-environmental sustainability, human wellbeing, and circular bioeconomy. The application of BC aged with HSs in the soil can cause several alterations in soil characteristics, including soil physicochemical (pH, EC, CEC, SOC, WHC, etc.), and biological properties (soil microbial communities dynamics), soil fertility and nutrient dynamics, soil pollution remediation, and plant growth. Each of the parameters is discussed in the following sections.
5.1 Effect of BC aged with HSs on soil physicochemical properties
The positive effects of BC and HSs on soil physicochemical properties have been reported in several studies. Reviews and meta-analysis showed that BC generally lowers soil acidity and increases buffering capacity; increases dissolved and total organic carbon, cation exchange capacity (CEC), available nutrients, water retention, and aggregate stability; and lowered bulk density (El-Naggar et al. 2019). Similarly, reviewed literature indicated that HSs positively affect soil physicochemical properties, including structure, water holding capacity, CEC, pH, EC, etc. (Ampong et al. 2022). In a recent study, it was reported that a leonardite-derived humic acid (HA) reduced soil pH from 5.88 to 5.33 but increased to 6.75 by bamboo willow-derived biochar in an incubation study for 180 days (Meng et al. 2022). However, the effect of BC aged by HSs on soil physicochemical properties has not been extensively investigated, although positive impact of co-amended BC and HA has been reported in a few studies and summarized in Table 4. In this regard, Zhang and co-workers reported that the integrated effect of BC and HA in optimum combination not only improved the particle-size distribution and adjusted the bulk density, porosity, and water-holding capacity (WHC) but also reduced pH and EC (Zhang et al. 2014). The improvement in the physical properties of soil could be due to the ability of BC and HA to optimize/reduce soil compaction (Jayasinghe et al. 2010; Rahim et al. 2019). In another study, it has been reported that the increase in porosity and water retention could be due to the action of the capillary pores of the BC and the hydrophilic group of the HA (Montoneri et al. 2008). The sole application of BC has been reported to increase the pH of growth media up to a level that is unfavorable for plant growth. However, the combined application of BC and HA reduced the pH and EC of growth media due to the presence of organic acid functional groups in humic substances and the ability of HA to increase the leaching of soluble salts (Montoneri et al. 2008; Varanini and Pinton 1995). Conversely, Holatko and colleagues reported that BC and HA amendments have a significant effect on soil pH (Holatko et al. 2020). Similarly, Haider et al. reported that the loading of HA on the surface of BC did not improve the efficiency of BC in soil for improving soil physicochemical properties and yield-related traits (Haider et al. 2015). According to the literature review, BC and humic compounds both have favorable effects on soil’s physical properties but differ in their impacts on soil’s chemical properties, nonetheless favoring plant growth and development (Fig. 5). Moreover, the work on liquid aging of BC with HA needs in-depth investigation regarding their effects and underlying mechanisms on soil physicochemical properties both under short-term control and long-term field conditions.
5.2 Effect of BC aged with HSs on soil nutrient availability
It has been observed that the co-application of BC and HSs, such as humic and fulvic acid, whether as a single, facile combination, or HSs-aged BC treatments, is a sustainable and environmentally benign method for increasing soil nutrient availability and supply for plants. According to reports, BC holds considerable promise as a source of readily available nutrients like nitrogen, phosphorus, potassium, carbon, sulfur, calcium, and magnesium for plants to absorb in soil. According to Azeem et al. (2023), biochar generated from cow bone at 500 and 800 °C improved nutrients such as NH4 up to 50%; NO3 up to 31%; Olsen P up to 48%; extractable K up to 18%, and dissolved organic carbon by up to 74% compared to control. Similarly, Wandansari et al. (2023) found that the concentration of organic carbon, nitrogen, and phosphorus was enhanced by 43.8%, 50.0%, and 97.3%, respectively, by commercial humic acid. However, the availability and supply of nutrients to plants may not always be accurately reflected by the sole application of BC and HSs in soil. Hence, co-applying or aging biochar with humic compounds may increase the availability of nutrients to plants. The key factors in this respect are compiled in Table 4. According to a review by Yang et al. (2021), the co-application of biochar and humic substances affects soil phosphorus mitigation and transformation by influencing the microbial community and enzymatic activity as well as by transforming the physicochemical properties of the soil. Karimi et al. (2020) indicated that the co-application of biochar and humic acid at the rate of 40 g kg−1 and 500 mg L−1 significantly (P < 0.05) enhanced the concentration of macro- (P, K, Ca, and Mg) and micronutrients (Fe, Zn, and Mn) in soil and their uptake by Calendula (Calendula officinalis L.). In a different study, it was found that adding biochar and humic acid (HA) collectively improved total ammonium nitrogen in the soil by − 5.37 to 21.37% compared to the control. However, HA decreased the soil’s ammonium nitrogen content. Similar to this, there was an increase in the concentration of available phosphorous, which was attributable to the release of phosphate that had been fixed in biochar by the reaction of humic acid. Potassium was not significantly improved with the co-application of biochar and humic acid (Ma et al. 2022). In contrast, Holatko et al. (2022) reported that the combined application of digestate, biochar, and humac did not show a significant effect on carbon, nitrogen, and phosphorus transformation in soil under controlled conditions. Similarly, Haider et al. (2015) indicated that BC, not HA, applied alone or in combination, increased the nitrogen use efficiency of plants.
The processes and mechanisms involved in the availability of nutrients in the soil with the application of BC and HSs are the following: (1) the application of BC with HA can improve the soil’s ability to store nutrients for plants while also supplying energy and nutrient sources for soil microbes, which in turn increases the availability of nutrients in the soil (El-Naggar et al. 2020, 2019); (2) the application of BC and HA in soil could transform ammonium nitrogen into sustained-released fertilizer through complex formation and surface adsorption (Hailegnaw et al. 2019); (3) HA, an organic macromolecule with several oxygen-containing functional groups, could create complexes with ammonium nitrogen to lower its losses from the soil (Aeschbacher et al. 2012); (4) the addition of humic compounds can increase the availability of phosphorus by reducing the Ca ions’ affinity for phosphorus in alkaline soil (Khan et al. 2018); (5) additionally, HA and BC can increase the amount of phosphorus readily available in soil by converting phosphorus fertilizer into slow-acting phosphorus (Ma et al. 2022). These processes and mechanisms are systematically illustrated in Fig. 5.
It is concluded that BC and HSs contain a significant concentration of nutrients. Their application in agricultural soils can supply the optimum concentration of nutrients for crops and microorganisms. Additionally, through complexation, surface adsorption, and release, BC and HSs added to the soil may transform nutrients therein into long-lasting nutrients. In this regard, the co-application of HSs-aged BC using a liquid-aging process has the potential to be an economical substitute for chemical fertilizers. However, more research is required.
5.3 Effect of BC aged with HSs on soil biological activities
Improving soil fertility and nutrient management frequently requires the application of exogenous organic matter, and such treatments can significantly increase microbial activity, root respiration, enzyme turnover, and many other biological activities in soil (Bamdad et al. 2022). Numerous studies have examined the effects of individual BC and humic compounds on the composition and activity of soil microbial communities (Pukalchik et al. 2019; Quan et al. 2020b). Existing research, however, consistently demonstrates that the facile combination of BC with HSs or aging BC with HSs can also boost soil biological activities (Al-Maliki et al. 2018), and the key parameters are summarized in Table 4. The high specific surface area and porosity of BC and HSs stimulate aeration and moisture retention, creating an ideal environment for microbial communities to flourish and function, illustrated in Fig. 5. To this end, it is reported that the soil’s microbial biomass carbon and respiration activity were higher when BC and humic compounds were applied together. The majority of enzyme activity (including β-glucosidase, arylsulfatase, N-acetyl-β-D-glucosaminidase, and phosphatase) was higher in soil that had been supplemented with humic compounds. In contrast to how aged biochar behaved, the application of humic substances and biochar with humic substances appeared to encourage microbial growth and activity, followed by the competition of microflora for nutrients with plants (Hammerschmiedt et al. 2021). However, previously it was indicated that the effect of non-treated biochar on basal respiration was found negative (Spokas et al. 2009), and the co-application of humic substances with biochar decreased the negative effect of non-treated biochar on basal respiration (Hammerschmiedt et al. 2021). In a related study, the combination of biochar and humac reduced microbial activities (Cmic, dehydrogenase activity, enzymes, substrate-induced respiration) in the amended soil at low biochar doses while attenuating the negative effects of high biochar doses on respiration and enzyme (phosphatase, arylsulphatase) activities (Holatko et al. 2020). The interaction between the substrate, enzyme, and biochar surface could be the cause of the decreased microbial activity, as well as the priming impact of biochar on native soil organic matter (Fang et al. 2015; Zimmerman et al. 2011). Similarly, the decrease in microbial activities could be assigned to the biochar surface properties that make the substrate unavailable for the enzyme to function (Allison 2006; Lammirato et al. 2011). In line with previous studies, Holatko et al. (2022) reported that the co-application of digestate with biochar and humac significantly enhanced short-term respiration activity and nitrification in soil.
6 Effect of BC aged with HS on soil pollution remediation
Anthropogenic activities leading to soil pollution with organic (xenobiotic organic compounds) and inorganic (heavy metals/metalloids) pollutants have severe impacts on crop yield, food quality, and human health (Rahim et al. 2021; Saha et al. 2017). The cleaning up of these pollutants from the soil is one of the main issues in the agro-environmental context. From the last decades, in situ immobilization based on stabilization/solidification (S/S) has proven to be one of the most effective methods for reducing the bioavailability of contaminants (particularly metal ions in soils) due to its affordability, speed of reaction, variety of amendments, and minimal environmental impact (Dhaliwal et al. 2020; Rahman et al. 2016). It can be performed by limiting metal ion flow to water, plants, and other environmental matrices via sorption, precipitation, complexation, and ion exchange (Rajendran et al. 2022). Lime and limestone, clay minerals, phosphates, metal oxides, hydroxides, and carbon-based adsorbents like compost and BC are some of the most often used amendments. Each adsorbent revealed a unique set of processes for removing pollutants from soil (Bolan et al. 2014; Idris et al. 2023). For instance, soil amendment with liming materials reduces the bioavailability of metal ions to plants by reducing H+ concentrations and increasing negatively charged ions to trigger metal ion precipitations of carbonate and hydroxides (Hale et al. 2012). Clay minerals used as soil amendments to immobilize metal ions by liming, precipitation, adsorption, and ion exchange reduced metal ion bioavailability in soil (Yi et al. 2017). Through surface adsorption and isomorphic substitution, hydroxyapatite-based soil amendments immobilized metal ions in soils by co-precipitating with Ca2+ (Xia et al. 2017). Inner-surface complex formation, co-precipitation, selective adsorption, newly formed secondary oxides, and co-precipitation were all assumed to be crucial metal oxide stability processes (Komárek et al. 2013).
Carbon-based soil additives like BC and compost have attracted remarkable interest recently for their ability to clean up contaminated soils (Fu et al. 2022). BC has unique physicochemical and biological characteristics, including a porous structure that retains moisture, abundant functional groups, a high CEC, and a high adsorptive capacity (Igalavithana et al. 2017). Additionally, BC can be considered a composite substance comprising phenol, alkane, and olefin compounds, together with cellulose, furan, pyran, dehydrosugar, carboxylic acid, and their derivatives. These elements provide an abundance of nutrition for microbial development. The structure, quantity, and types of functional groups on the surface of the biochar can alter how heavy metals are complex as it ages. This complexation process, in turn, decreases heavy metal mobility and bioavailability (Henryk et al. 2016; Li et al. 2001). Since pH affects the chemical forms of heavy metals in soil, a link between soil pH and the distribution of those forms has been identified. The pH influences surface stability, coordination properties, existing heavy metal forms, and adsorption sites. BC has alkaline qualities, and its use can efficiently increase soil pH. As a result, heavy metal mobility in the soil is reduced (Gul et al. 2015; Van Zwieten et al. 2010). The mechanisms of BC’s direct interaction with metal ions include electrostatic interaction with its negatively charged surface, complexation with oxygen-containing functional groups on its inner surface, precipitation with mineral oxides on its inner surface, and co-precipitation with additional mineral phases (Fu et al. 2022; Rahim et al. 2022). Similar indirect ways include changes in soil’s pH, CEC, mineral composition, and organic matter content (He et al. 2019).
The presence of functional groups such as COOH, phenolic-OH, alcoholic-OH, carbonyl groups, quinone, and oxygen-containing functional groups in the organic matter (humic substances) is responsible for contaminant sorption, metal chelation, redox mediation, the fate and transport of heavy metals and organic pollutants, as well as the stabilization of soil structure (Aeschbacher et al. 2012; Islam et al. 2020). According to research, humic compounds influence soil pH, which, in turn, slows down the decomposition of soil organic matter (SOM). Consequently, this reduction in SOM decomposition leads to a decrease in the bioavailability of heavy metals in the soil. This impact can be related to acidic functional groups in humic substances, namely, carboxyl groups, which make it easier for heavy metals to bind them and reduce their bioavailability. Additionally, humic substances have the ability to combine with soil minerals to produce complexes that immobilize humic acid and increase the number of heavy metal binding sites in the soil. Overall, these activities result in a fall in soil pH and a reduction in the amount of negative charge on the soil surface, which restricts the mobility of heavy metals (De Matos et al. 2001; Meng et al. 2017; Shi et al. 2018; Signa et al. 2015; Xiao et al. 2020).
According to previous reports, employing freshly generated BC for remediating contaminated soils involves challenges (Liu et al. 2022). Insufficient catalytic activity, regeneration, recycling from soil and water environments, and net negative surface charges that decreased its sorption capacity against metal ions are some of the issues with fresh BC (Chen et al. 2021, 2022; Zhang et al. 2019). A single humic material amendment has little effect on the immobilization of metal ions in soil (Hamzah et al. 2022). For example, in a recent comparative study, BC and HA were applied at the rate of 1 and 3% to remediate Cd and As in paddy soil in a 180-day incubation study. According to the findings, HA at a rate of 3% was more effective in removing both Cd and As. However, BC had a similar effect on Cd but a different influence on As. Additionally, HA decreased soil pH, whereas BC increased it, affecting the remediation of Cd and As in soil (Meng et al. 2022).
It has inspired scientists to reconsider their original plans and either create biochar aged with humic substances or employ both biochar and humic substances in facile combination in soil to immobilize metal ions. Table 5 provides a summary of the main parameters in this regard, and a further explanation is here.
A compound amendment comprised BC modified with chitosan, humic material produced from composted kitchen waste, and NPK compound fertilizer was developed and utilized to immobilize Pb in acidic soil. The results showed that humic material combined with biochar modified with chitosan efficiently immobilized Pb and controlled the pH of acidic soil (Liu et al. 2023). In their study, Meng et al. utilized leonardite-derived humic acid (L-HA) and bamboo willow-derived biochar (BWB) as amendments at doses of 1% and 3% in a paddy sandy soil (with an initial pH of 5.88) contaminated with various heavy metal(loid)s. The results showed that L-HA significantly reduced the soil pH, whereas BWB increased the soil pH. Furthermore, the L-HA amendment effectively decreased the bioavailability of cadmium (Cd) and arsenic (As) in the soil. On the other hand, the BWB amendment increased in the bioavailability of Cd, with no significant impact on the bioavailability of As, compared to the control group (Meng et al. 2023).
In a different research study, the effects of rice husk biochar (RBC) and humic acid (HA) were examined when introduced into cultivated land soil that was contaminated and had a high pH level (8.21). The objective was to explore how these additions would influence the availability of heavy metals and the overall quality of the soil. The findings from this study demonstrate that the inclusion of BC, HA, and their combination resulted in a decrease in the extractable Cd content as measured by DTPA. Furthermore, all levels of BC and HA enhance the electrical conductivity (EC) and cation exchange capacity (CEC) of the soil, promoting the immobilization of Cd and improving the nutritional condition (Ma et al. 2022). In a separate investigation, humic-acid-coated biochar was utilized to eliminate Pb and Cu from neutral soil with a pH value of 6.34. The outcomes indicated a reduction in the bioavailability of Pb by 40.04% to 87.28% and of Cu by 8.63% to 40.23%. This decline in the uptake of Pb and Cu by plants can be due to the immobilization process. Physisorption, chemisorption, and precipitation of these heavy metals occur due to the synergistic role of BC and HA. BC effectively hindered the activation of plants toward metals and reduced their uptake. Moreover, the immobilization of heavy metals by biochar resulted in the transformation of these metals into more stable and less toxic forms. However, the presence of humic acid facilitated the formation of water complexes between humic acid and Pb/Cu, preventing the formation of Pb/Cu hydroxide. As a result, this increased the availability and mobility of soil-bound Pb and Cu (Hamzah et al. 2022). Zhu and coworkers prepared a novel adsorbent (BHW) composed of biochar (BC), humic acid (HA), and wood vinegar (WV) for the immobilization of Ni2+ in soil. The efficiency of BHW was compared with fresh biochar (BC) and BC + HA composite (BH). The results showed that the immobilization of Ni2+ was in the order BHW > BH > BC. Additionally, the maximum adsorption capacity was raised in the same order of BHW > BH > BC (Zhu et al. 2021). Anton and colleagues used biochar in combination with three different fertirrigates, such as fertilizer solution, water, or commercial biostimulant derived from leonardite, to examine their impact on the immobilization of As, Cd, Pb, and Zn in sandy clay loam soil with slightly alkaline pH (7.34). Biochar application reduced the bioavailability of copper (Cu) and lead (Pb). Additionally, the amount of arsenic (As) and Pb in plants decreased when fertilizer and biochar were used together. When combined with a biostimulant, biochar lowered the amount of cadmium (Cd) bioavailable in the soil and the amount of Cd that pepper plants absorbed (Antón‐Herrero et al. 2022).
Keeping in view the above discussion, we can conclude that BC aged or combined with HSs can contribute to the reduction of heavy metal bioavailability in soil through several mechanisms. Firstly, the presence of HSs on the surface of BC increases its sorption capacity for heavy metals. HSs contain various functional groups, such as carboxyl, hydroxyl, and phenolic groups, which can be complex with heavy metal ions, effectively immobilizing them and reducing their availability for uptake by plants or leaching into groundwater.
Secondly, the addition of BC aged with HSs to soil can enhance soil pH. HSs are known to possess buffering properties, which can help maintain a more favorable pH range for plants and microorganisms. When the soil pH increases, it can lead to the precipitation or formation of less soluble forms of heavy metals, reducing their solubility and subsequent bioavailability.
Furthermore, the application of BC aged with HSs can improve soil structure and increase the cation exchange capacity (CEC). This enhanced CEC provides more sites for heavy metal adsorption and reduces their movement through the soil profile, thus reducing their bioavailability.
Overall, BC aged with HSs acts as a sorbent, pH modifier, and soil conditioner, all of which contribute to the remediation of heavy metal bioavailability in soil and mitigate potential environmental risks associated with heavy metal contamination. The discussed mechanisms and processes are systematically illustrated in Fig. 6.
However, it was discovered that while there are studies on the co-application of BC and HA, there are none on the liquid aging of BC with HSs and their impact on the immobilization of organic or inorganic contaminants in soil. In this regard, in-depth research is required.
7 Conclusions and future outlooks
Global action is required to ensure future food security for all people, given the current and expected conditions of expanding global demand for food, the intensification of climate change effects on agriculture, and the depletion and degradation of natural resources. Agricultural waste is produced in enormous amounts as a result of improper practices used across the food supply chain, from primary production to consumption. An important step in moving the global food system toward sustainability will be the development of a circular bioeconomy that reduces resource loss and feeds recovered materials back into the economy. The usage of biochar and the recently developed synthetic HSs generated from waste biomass can effectively minimize the problems caused by agricultural waste. The synthesis of carbon-based materials such as BC, compost, hydrochar, and HSs not only turns difficult-to-handle solid waste into valuable-added products but also serves as soil conditioners. Even more remarkable is that recycling nutrients from waste materials into carbon-based materials seems to lessen the overuse of chemical fertilizers and, in turn, the overexploitation of natural resources like farmlands and water. The aging of BC with HSs appears to be extraordinary and to have had a significant influence on the soil system. They have the potential to amend soil properties like pH, CEC, aggregate stability, and water-holding capacity. Similarly, they can bind metal ions and oxides in the soil using a variety of processes and mechanisms, including complexation, electrostatic interaction, and precipitation. They also impact the dynamics and activities of soil microbes, which in turn affect the biogeochemistry and the availability of nutrients for plant growth. The aforementioned discussion is explained in Fig. 7. BC and HS have a significant influence on sustainable agriculture; however, there are still areas of research that require investigation.
-
1.
The liquid aging technique for BC with HSs needs more attention and detailed characterization to gain in-depth insights into aged biochar morphology, functional groups, and new species formation.
-
2.
Choosing biomass with high levels of lignocellulosic substances for BC production and organic sources or soil with abundant functional organic acids or macro/micro-molecules for HSs extraction is important to enhance the liquid aging process and produce additional functional groups.
-
3.
Research should investigate aging time (ranging from hours to days), aging techniques, the use of sticky reagents like polymers, and other modifying chemicals such as zinc chloride to understand aged biochar morphology better.
-
4.
Studying the effect of HSs-aged BC on soil physicochemical parameters, nutrient dynamics, soil pollution remediation, and microbiological activities is crucial for comprehensive information gathering.
-
5.
There is a need to focus on the liquid aging of BC with HSs, extracting HS chemicals from soil or peat, improving aging processes, and understanding the impact of aged biochar on soil processes.
-
6.
Research on the aging of biochar should consider soil classification and type based on the World Reference Base for Soil Resources to provide more specific insights.
A hypothetical circular model showing the transformation of lignocellulosic feedstocks into value-added products, their application as soil amendments to get the maximum food and/or feed production, and again receiving lignocellulosic feedstocks. The cycle repeats, ensuring the agroecosystem’s sustainability, and is credited to circular bio-economy components
Data availability
Not applicable.
References
Aeschbacher M, Brunner SH, Schwarzenbach RP, Sander M (2012) Assessing the effect of humic acid redox state on organic pollutant sorption by combined electrochemical reduction and sorption experiments. Environ Sci Technol 46:3882–3890
Al-Maliki S, AL-Mammory H, Scullion J (2018) Interactions between humic substances and organic amendments affecting soil biological properties and growth of Zea mays L. in the arid land region. Arid Land Res Manag 32:455–470
Allison SD (2006) Soil minerals and humic acids alter enzyme stability: implications for ecosystem processes. Biogeochem 81:361–373
Ampong K, Thilakaranthna, MS, Gorim LY (2022) Understanding the role of humic acids on crop performance and soil health
Antón-Herrero R, Vega-Jara L, García-Delgado C, Mayans B, Camacho-Arévalo R, Moreno-Jiménez E, Plaza C, Eymar E (2022) Synergistic effects of biochar and biostimulants on nutrient and toxic element uptake by pepper in contaminated soils. J Sci Food Agric 102:167–174
Ascough PL, Bird MI, Francis SM, Thornton B, Midwood AJ, Scott AC, Apperley D (2011) Variability in oxidative degradation of charcoal: influence of production conditions and environmental exposure. Geochim Cosmochim Acta 75(9):2361–2378
Azeem M, Jeyasundar PGSA, Ali A, Riaz L, Khan KS, Hussain Q, Kareem HA, Abbas F, Latif A, Majrashi A (2023) Cow bone-derived biochar enhances microbial biomass and alters bacterial community composition and diversity in a smelter contaminated soil. Environ Res 216:114278
Bamdad H, Papari S, Lazarovits G, Berruti F (2022) Soil amendments for sustainable agriculture: microbial organic fertilizers. Soil Use Manag 38:94–120
Biederman LA, Harpole WS (2013) Biochar and its effects on plant productivity and nutrient cycling: a meta-analysis. GCB Bioenergy 5:202–214
Bolan N, Kunhikrishnan A, Thangarajan R, Kumpiene J, Park J, Makino T, Kirkham MB, Scheckel K (2014) Remediation of heavy metal (loid) s contaminated soils–to mobilize or to immobilize? J Hazard Mater 266:141–166
Chen D, Yu X, Song C, Pang X, Huang J, Li Y (2016) Effect of pyrolysis temperature on the chemical oxidation stability of bamboo biochar. Bioresour Technol 218:1303–1306
Chen H, Gao Y, El-Naggar A, Niazi NK, Sun C, Shaheen SM, Hou D, Yang X, Tang Z, Liu Z (2022) Enhanced sorption of trivalent antimony by chitosan-loaded biochar in aqueous solutions: characterization, performance and mechanisms. J Hazard Mater 425:127971
Chen J, Deng S, Jia W, Li X, Chang J (2021) Removal of multiple heavy metals from mining-impacted water by biochar-filled constructed wetlands: adsorption and biotic removal routes. Bioresour Technol 331:125061
Cheng C-H, Lehmann J (2009) Ageing of black carbon along a temperature gradient. Chemosphere 75:1021–1027
Cheng C-H, Lehmann J, Thies JE, Burton SD, Engelhard MH (2006) Oxidation of black carbon by biotic and abiotic processes. Org Geochem 37:1477–1488
Chu G, Zhao J, Huang Y, Zhou D, Liu Y, Wu M, Peng H, Zhao Q, Pan B, Steinberg CE (2018) Phosphoric acid pretreatment enhances the specific surface areas of biochars by generation of micropores. Environ Pollut 240:1–9
Cross A, Sohi SP (2013) A method for screening the relative long-term stability of biochar. GCB Bioenergy 5:215–220
De Matos A, Fontes MPF, Da Costa L, Martinez MA (2001) Mobility of heavy metals as related to soil chemical and mineralogical characteristics of Brazilian soils. Environ Pollut 111:429–435
Dhaliwal SS, Singh J, Taneja PK, Mandal A (2020) Remediation techniques for removal of heavy metals from the soil contaminated through different sources: a review. Environ Sci Pollut Res 27:1319–1333
Du Q, Zhang S, Song J, Zhao Y, Yang F (2020) Activation of porous magnetized biochar by artificial humic acid for effective removal of lead ions. J Hazard Mater 389:122115
El-Naggar A, Lee M-H, Hur J, Lee YH, Igalavithana AD, Shaheen SM, Ryu C, Rinklebe J, Tsang DC, Ok YS (2020) Biochar-induced metal immobilization and soil biogeochemical process: an integrated mechanistic approach. Sci Total Environ 698:134112
El-Naggar A, Lee SS, Rinklebe J, Farooq M, Song H, Sarmah AK, Zimmerman AR, Ahmad M, Shaheen SM, Ok YS (2019) Biochar application to low fertility soils: a review of current status, and future prospects. Geoderma 337:536–554
Elkhalifa S, Al-Ansari T, Mackey HR, McKay G (2019) Food waste to biochars through pyrolysis: a review. Resour Conserv Recycl 144:310–320
Fan Q, Sun J, Chu L, Cui L, Quan G, Yan J, Hussain Q, Iqbal M (2018) Effects of chemical oxidation on surface oxygen-containing functional groups and adsorption behavior of biochar. Chemosphere 207:33–40
Fang G, Zhu C, Dionysiou DD, Gao J, Zhou D (2015) Mechanism of hydroxyl radical generation from biochar suspensions: implications to diethyl phthalate degradation. Bioresour Technol 176:210–217
Fernandes A, Almeida C, Menezes C, Debacher N, Sierra M (2007) Removal of methylene blue from aqueous solution by peat. J Hazard Mater 144:412–419
Frišták V, Friesl-Hanl W, Wawra A, Pipíška M, Soja G (2015) Effect of biochar artificial ageing on Cd and Cu sorption characteristics. J Geochem Explor 159:178–184
Fu Q, Zhao H, Li T, Hou R, Liu D, Ji Y, Zhou Z, Yang L (2019) Effects of biochar addition on soil hydraulic properties before and after freezing-thawing. Catena 176:112–124
Fu T, Zhang B, Gao X, Cui S, Guan C-Y, Zhang Y, Zhang B, Peng Y (2022) Recent progresses, challenges, and opportunities of carbon-based materials applied in heavy metal polluted soil remediation. Sci Total Environ 158810
Fuertes A, Arbestain MC, Sevilla M, Maciá-Agulló JA, Fiol S, López R, Smernik R, Aitkenhead W, Arce F, Macìas F (2010) Chemical and structural properties of carbonaceous products obtained by pyrolysis and hydrothermal carbonisation of corn stover. Soil Res 48:618–626
Ghodake GS, Shinde SK, Kadam AA, Saratale RG, Saratale GD, Kumar M, Palem RR, AL-Shwaiman, H. A., Elgorban, AM, Syed A (2021) Review on biomass feedstocks, pyrolysis mechanism and physicochemical properties of biochar: state-of-the-art framework to speed up vision of circular bioeconomy. J Clean Prod 297:126645
Giagnoni L, Renella G (2022) Effects of biochar on the C use efficiency of soil microbial communities: components and mechanisms. Environ 9:138
Gul S, Whalen JK, Thomas BW, Sachdeva V, Deng H (2015) Physico-chemical properties and microbial responses in biochar-amended soils: mechanisms and future directions. Agric Ecosyst Environ 206:46–59
Guo J, Yan C, Luo Z, Fang H, Hu S, Cao Y (2019) Synthesis of a novel ternary HA/Fe-Mn oxides-loaded biochar composite and its application in cadmium (II) and arsenic (V) adsorption. J Environ Sci 85:168–176
Haider G, Koyro H-W, Azam F, Steffens D, Müller C, Kammann C (2015) Biochar but not humic acid product amendment affected maize yields via improving plant-soil moisture relations. Plant Soil 395:141–157
Hailegnaw NS, Mercl F, Pračke K, Száková J, Tlustoš P (2019) High temperature-produced biochar can be efficient in nitrate loss prevention and carbon sequestration. Geoderma 338:48–55
Hale B, Evans L, Lambert R (2012) Effects of cement or lime on Cd Co, Cu, Ni, Pb, Sb and Zn mobility in field-contaminated and aged soils. J Hazard Mater 199:119–127
Hale S, Hanley K, Lehmann J, Zimmerman A, Cornelissen G (2011) Effects of chemical, biological, and physical aging as well as soil addition on the sorption of pyrene to activated carbon and biochar. Environ Sci Technol 45:10445–10453
Hammerschmiedt T, Holatko J, Pecina V, Huska D, Latal O, Kintl A, Radziemska M, Muhammad S, Gusiatin ZM, Kolackova M (2021) Assessing the potential of biochar aged by humic substances to enhance plant growth and soil biological activity. Chem Biol Technol Agric 8:1–12
Hamzah A, Priyadarshini R, Astuti A (2022) The potential use of humic acid-coated biochar for reducing Pb and Cu in the soil to improve plant growth. J Degraded Mining Lands Manag 10:4001–4009
Han L, Ro KS, Wang Y, Sun K, Sun H, Libra JA, Xing B (2018) Oxidation resistance of biochars as a function of feedstock and pyrolysis condition. Sci Total Environ 616:335–344
Hannet G, Singh K, Fidelis C, Farrar MB, Muqaddas B, Bai SH (2021) Effects of biochar, compost, and biochar-compost on soil total nitrogen and available phosphorus concentrations in a corn field in Papua New Guinea. Environ Sci Pollut Res 28:27411–27419
Hardie M, Clothier B, Bound S, Oliver G, Close D (2014) Does biochar influence soil physical properties and soil water availability? Plant Soil 376:347–361
He L, Zhong H, Liu G, Dai Z, Brookes PC, Xu J (2019) Remediation of heavy metal contaminated soils by biochar: mechanisms, potential risks and applications in China. Environ Pollut 252:846–855
Henryk K, Jarosław C, Witold Ż (2016) Peat and coconut fiber as biofilters for chromium adsorption from contaminated wastewaters. Environ Sci Pollut Res 23:527–534
Holatko J, Hammerschmiedt T, Datta R, Baltazar T, Kintl A, Latal O, Pecina V, Sarec P, Novak P, Balakova L (2020) Humic acid mitigates the negative effects of high rates of biochar application on microbial activity. Sustainability 12:9524
Holatko J, Hammerschmiedt T, Latal O, Kintl A, Mustafa A, Baltazar T, Malicek O, Brtnicky M (2022) Deciphering the effectiveness of humic substances and biochar modified digestates on soil quality and plant biomass accumulation. Agronomy 12:1587
Hua Y, Zheng X, Xue L, Han L, He S, Mishra T, Feng Y, Yang L, Xing B (2020) Microbial aging of hydrochar as a way to increase cadmium ion adsorption capacity: Process and mechanism. Bioresour Technol 300:122708
Huang H, Guo T, Wang K, Li Y, Zhang G (2021) Efficient activation of persulfate by a magnetic recyclable rape straw biochar catalyst for the degradation of tetracycline hydrochloride in water. Sci Total Environ 758:143957
Huff MD, Lee JW (2016) Biochar-surface oxygenation with hydrogen peroxide. J Environ Manag 165:17–21
Idris MO, Yaqoob AA, Ibrahim MNM, Ahmad A, Alshammari MB (2023) Introduction of adsorption techniques for heavy metals remediation. In: Emerging techniques for treatment of toxic metals from wastewater. Elsevier, pp 1–18
Igalavithana AD, Mandal S, Niazi NK, Vithanage M, Parikh SJ, Mukome FN, Rizwan M, Oleszczuk P, Al-Wabel M, Bolan N (2017) Advances and future directions of biochar characterization methods and applications. Critical Reviews in Environ Sc Technol 47:2275–2330
Islam MA, Morton DW, Johnson BB, Angove MJ (2020) Adsorption of humic and fulvic acids onto a range of adsorbents in aqueous systems, and their effect on the adsorption of other species: a review. Sep Purif Technol 247:116949
Jayasinghe G, Tokashiki Y, Arachchi IL, Arakaki M (2010) Sewage sludge sugarcane trash based compost and synthetic aggregates as peat substitutes in containerized media for crop production. J Hazard Mater 174:700–706
Jin J, Sun K, Wang Z, Han L, Du P, Wang X, Xing B (2017) Effects of chemical oxidation on phenanthrene sorption by grass-and manure-derived biochars. Sci Total Environ 598:789–796
Jin J, Sun K, Yang Y, Wang Z, Han L, Wang X, Wu F, Xing B (2018) Comparison between soil-and biochar-derived humic acids: composition, conformation, and phenanthrene sorption. Environmen Sci Technol 52:1880–1888
Jinsheng L, Xinqing S, Huang D, Kesi L, Shang J, Zhang Q, Tianci Z, Xiaomeng Y (2022) Short-term biochar effect on soil physicochemical and microbiological properties of a degraded alpine grassland. Pedosphere 32:426–437
Karimi E, Shirmardi M, Dehestani Ardakani M, Gholamnezhad J, Zarebanadkouki M (2020) The effect of humic acid and biochar on growth and nutrients uptake of calendula (Calendula officinalis L.). Commun Soil Sci Plant Anal 51:1658–1669
Ke Y, Cui S, Fu Q, Hough R, Zhang Z, Li Y-F (2022) Effects of pyrolysis temperature and aging treatment on the adsorption of Cd2+ and Zn2+ by coffee grounds biochar. Chemosphere 296:134051
Khan R, Khan M, Khan A, Saba S, Hussain F, Jan I (2018) Effect of humic acid on growth and crop nutrient status of wheat on two different soils. J Plant Nutrit 41:453–460
Kim H-B, Kim J-G, Kim S-H, Kwon EE, Baek K (2019) Consecutive reduction of Cr (VI) by Fe (II) formed through photo-reaction of iron-dissolved organic matter originated from biochar. Environ Pollut 253:231–238
Komárek M, Vaněk A, Ettler V (2013) Chemical stabilization of metals and arsenic in contaminated soils using oxides–a review. Environ Pollut 172:9–22
Kuzyakov Y, Bogomolova I, Glaser B (2014) Biochar stability in soil: decomposition during eight years and transformation as assessed by compound-specific 14C analysis. Soil Biol Biochem 70:229–236
Lammirato C, Miltner A, Kaestner M (2011) Effects of wood char and activated carbon on the hydrolysis of cellobiose by β-glucosidase from Aspergillus niger. Soil Biol Biochem 43:1936–1942
Lawrinenko M, Laird DA, Johnson RL, Jing D (2016) Accelerated aging of biochars: Impact on anion exchange capacity. Carbon 103:217–227
Lee J-M, Park D-G, Kang S-S, Choi E-J, Gwon H-S, Lee H-S, Lee S-I (2022) Short-term effect of biochar on soil organic carbon improvement and nitrous oxide emission reduction according to different soil characteristics in agricultural land: a laboratory experiment. Agronomy 12:1879
Li K, Yin G, Xu Q, Yan J, Hseu Z-Y, Zhu L, Lin Q (2020) Influence of aged biochar modified by Cd2+ on soil properties and microbial community. Sustainability 12:4868
Li X, Pang Y, Zhang R (2001) Compositional changes of cottonseed hull substrate during P. ostreatus growth and the effects on the feeding value of the spent substrate. Bioresour Technol 80:157–161
Li Y, Fang F, Wei J, Wu X, Cui R, Li G, Zheng F, Tan D (2019) Humic acid fertilizer improved soil properties and soil microbial diversity of continuous cropping peanut: a three-year experiment. Sci Rep 9:1–9
Liu G, Chen L, Jiang Z, Zheng H, Dai Y, Luo X, Wang Z (2017) Aging impacts of low molecular weight organic acids (LMWOAs) on furfural production residue-derived biochars: porosity, functional properties, and inorganic minerals. Sci Total Environ 607:1428–1436
Liu J, Jiang J, Meng Y, Aihemaiti A, Xu Y, Xiang H, Gao Y, Chen X (2020) Preparation, environmental application and prospect of biochar-supported metal nanoparticles: a review. J Hazard Mater 388:122026
Liu M, Tan X, Zheng M, Yu D, Lin A, Liu J, Wang C, Gao Z, Cui J (2023) Modified biochar/humic substance/fertiliser compound soil conditioner for highly efficient improvement of soil fertility and heavy metals remediation in acidic soils. J Environ Manag 325:116614
Liu Z, Demisie W, Zhang M (2013) Simulated degradation of biochar and its potential environmental implications. Environ Pollut 179:146–152
Liu Z, Xu Z, Xu L, Buyong F, Chay TC, Li Z, Cai Y, Hu B, Zhu Y, Wang X (2022) Modified biochar: synthesis and mechanism for removal of environmental heavy metals. Carbon Res 1:1–21
Lu Y, Xie Q, Tang L, Yu J, Wang J, Yang Z, Fan C, Zhang S (2021) The reduction of nitrobenzene by extracellular electron transfer facilitated by Fe-bearing biochar derived from sewage sludge. J Hazard Mater 403:123682
Lychuk TE, Izaurralde RC, Hill RL, McGill WB, Williams JR (2015) Biochar as a global change adaptation: predicting biochar impacts on crop productivity and soil quality for a tropical soil with the Environmental Policy Integrated Climate (EPIC) model. Mitig Adapt Strat Glob Change 20:1437–1458
Ma X, Zhao Z, Li J, Han L, Sun G, Zheng X, Yue H (2022) Effect of co-application of biochar and humic acid on heavy metal contaminated arable soil quality in an arid area of Northwest China. Available at SSRN: https://ssrn.com/abstract=4075452 or https://doi.org/10.2139/ssrn.4075452
Maienza A, Baronti S, Cincinelli A, Martellini T, Grisolia A, Miglietta F, Renella G, Stazi SR, Vaccari FP, Genesio L (2017) Biochar improves the fertility of a Mediterranean vineyard without toxic impact on the microbial community. Agron Sustain Develop 37:1–9
Masiello CA, Chen Y, Gao X, Liu S, Cheng H-Y, Bennett MR, Rudgers JA, Wagner DS, Zygourakis K, Silberg JJ (2013) Biochar and microbial signaling: production conditions determine effects on microbial communication. Environ Sci Technol 47:11496–11503
Meng F, Huang Q, Cai Y, Xiao L, Wang T, Li X, Wu W, Yuan G (2022) A comparative assessment of humic acid and biochar altering cadmium and arsenic fractions in a paddy soil. J Soils Sedim 1–11
Meng F, Huang Q, Cai Y, Xiao L, Wang T, Li X, Wu W, Yuan G (2023) A comparative assessment of humic acid and biochar altering cadmium and arsenic fractions in a paddy soil. Journal Soils Sedime 23:845–855
Meng F, Yuan G, Wei J, Bi D, Wang H (2017) Leonardite-derived humic substances are great adsorbents for cadmium. Environ Sci Pollut Res 24:23006–23014
Meng Z, Huang S, Xu T, Deng Y, Lin Z, Wang X (2020) Transport and transformation of Cd between biochar and soil under combined dry-wet and freeze-thaw aging. Environ Pollut 263:114449
Montoneri E, Boffa V, Quagliotto P, Mendichi R, Chierotti M, Roberto G, Medana C (2008) Humic acid-like matter isolated from green urban wastes. Part I: structure and surfactant properties. Bioresour 3:123–141
Mukherjee A, Zimmerman A, Hamdan R, Cooper W (2014) Physicochemical changes in pyrogenic organic matter (biochar) after 15 months of field aging. Solid Earth 5:693–704
Nan Q, Hu S, Qin Y, Wu W (2021) Methane oxidation activity inhibition via high amount aged biochar application in paddy soil. Sci Total Environ 796:149050
Nguyen TTN, Wallace HM, Xu C-Y, Zwieten LV, Weng ZH, Xu Z, Che R, Tahmasbian I, Hu H-W, Bai SH (2018) The effects of short term, long term and reapplication of biochar on soil bacteria. Sci Total Environ 636:142–151
Paetsch L, Mueller CW, Kögel-Knabner I, Von Lützow M, Girardin C, Rumpel C (2018) Effect of in-situ aged and fresh biochar on soil hydraulic conditions and microbial C use under drought conditions. Sci Rep 8:1–11
Park CM, Han J, Chu KH, Al-Hamadani YA, Her N, Heo J, Yoon Y (2017) Influence of solution pH, ionic strength, and humic acid on cadmium adsorption onto activated biochar: experiment and modeling. J Indus Eng Chem 48:186–193
Pettit RE (2004) Organic matter, humus, humate, humic acid, fulvic acid and humin: their importance in soil fertility and plant health. CTI Res 10:1–7
Pignatello JJ, Kwon S, Lu Y (2006) Effect of natural organic substances on the surface and adsorptive properties of environmental black carbon (char): attenuation of surface activity by humic and fulvic acids. Environ Sci Technol 40:7757–7763
Pukalchik M, Kydralieva K, Yakimenko O, Fedoseeva E, Terekhova V (2019) Outlining the potential role of humic products in modifying biological properties of the soil—a review. Front Environ Sci 7:80
Qian L, Chen M, Chen B (2015) Competitive adsorption of cadmium and aluminum onto fresh and oxidized biochars during aging processes. J Soils Sedim 15:1130–1138
Qu J, Zhang X, Guan Q, Kong L, Yang R, Ma X (2022) Effects of biochar underwent different aging processes on soil properties and Cd passivation. Environ Sci Pollut Res 29:57885–57895
Quan G, Fan Q, Cui L, Zimmerman AR, Wang H, Zhu Z, Gao B, Wu L, Yan J (2020a) Simulated photocatalytic aging of biochar in soil ecosystem: insight into organic carbon release, surface physicochemical properties and cadmium sorption. Environ Res 183:109241
Quan G, Fan Q, Zimmerman AR, Sun J, Cui L, Wang H, Gao B, Yan J (2020b) Effects of laboratory biotic aging on the characteristics of biochar and its water-soluble organic products. J Hazard Mater 382:121071
Rahim HU, Akbar WA, Alatalo JM (2022) A comprehensive literature review on cadmium (Cd) status in the soil environment and its immobilization by biochar-based materials. Agronomy 12:877
Rahim HU, Mian IA, Arif M, Rahim ZU, Ahmad S, Khan Z, Zada L, Khan MA, Haris M (2019) Residual effect of biochar and summer legumes on soil physical properties and wheat growth. Pure Appl Biol 8:16–26
Rahim HU, Qaswar M, Wang M, Jing X, Cai X (2021) Environmental applications of reduced sulfur species and composites in transformation and detoxification of contaminants. J Environ Chem Engi 9:106696
Rahman G, Rahman MM, Alam MS, Kamal MZ, Mashuk H, Datta R, Meena RS (2020) Biochar and organic amendments for sustainable soil carbon and soil health. In: Carbon and nitrogen cycling in soil. Springer, pp 45–85
Rahman IM, Begum ZA, Sawai H (2016) Solidification/stabilization: a remedial option for metal-contaminated soils. In: Environmental remediation technologies for metal-contaminated soils. Springer, pp 125–146
Rajendran S, Priya T, Khoo KS, Hoang TK, Ng H-S, Munawaroh HSH, Karaman C, Orooji Y, Show PL (2022) A critical review on various remediation approaches for heavy metal contaminants removal from contaminated soils. Chemosphere 287:132369
Ruan X, Sun Y, Du W, Tang Y, Liu Q, Zhang Z, Doherty W, Frost RL, Qian G, Tsang DC (2019) Formation, characteristics, and applications of environmentally persistent free radicals in biochars: a review. Bioresour Technol 281:457–468
Saha JK, Selladurai R, Coumar MV, Dotaniya M, Kundu S, Patra AK (2017) Impacts of soil pollution and their assessment. In: Soil pollution-an emerging threat to agriculture. Springer, pp 37–73
Schnitzer M, Khan SU (1975) Soil organic matter. Elsevier
Shi W, Lü C, He J, En H, Gao M, Zhao B, Zhou B, Zhou H, Liu H, Zhang Y (2018) Nature differences of humic acids fractions induced by extracted sequence as explanatory factors for binding characteristics of heavy metals. Ecotoxicol Environ Safe 154:59–68
Sierra MM, Fernandes AN, Szpoganicz B (2004) Influence of amide linkages on acidity determinations of humic substances: testing with model-mixtures. Talanta 62:687–693
Signa G, Di Leonardo R, Vaccaro A, Tramati CD, Mazzola A, Vizzini S (2015) Lipid and fatty acid biomarkers as proxies for environmental contamination in caged mussels Mytilus galloprovincialis. Ecol Indic 57:384–394
Singh B, Fang Y, Johnston CT (2016) A fourier-transform infrared study of biochar aging in soils. Soil Sci Soc Am J 80(3):613–622
Singh BP, Cowie AL, Smernik RJ (2012) Biochar carbon stability in a clayey soil as a function of feedstock and pyrolysis temperature. Environ Sci Technol 46:11770–11778
Spokas K, Koskinen W, Baker J, Reicosky D (2009) Impacts of woodchip biochar additions on greenhouse gas production and sorption/degradation of two herbicides in a Minnesota soil. Chemosphere 77:574–581
Spokas KA (2010) Review of the stability of biochar in soils: predictability of O: C molar ratios. Carbon Manage 1:289–303
Sutton R, Sposito G (2005) Molecular structure in soil humic substances: the new view. Environ Sci Technol 39:9009–9015
Tahat MM, Alananbeh MK, Othman AY, Leskovar ID (2020) Soil health and sustainable agriculture. Sustainability 12:4859
Trompowsky PM, de Melo Benites V, Madari BE, Pimenta AS, Hockaday WC, Hatcher PG (2005) Characterization of humic like substances obtained by chemical oxidation of eucalyptus charcoal. Org Geochem 36(11):1480–1489
Ukaogo PO, Ewuzie U, Onwuka CV (2020) Environmental pollution: causes, effects, and the remedies. In: Microorganisms for sustainable environment and health. Elsevier, pp 419–429
Van Nguyen TT, Phan AN, Nguyen T-A, Nguyen TK, Nguyen ST, Pugazhendhi A, Phuong HHK (2022) Valorization of agriculture waste biomass as biochar: As first-rate biosorbent for remediation of contaminated soil. Chemosphere 135834
Van Zwieten L, Kimber S, Morris S, Chan K, Downie A, Rust J, Joseph S, Cowie A (2010) Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 327:235–246
Varanini Z, Pinton R (1995) Humic substances and plant nutrition. Prog Bot 97–117
Verheijen F, Jeffery S, Bastos A, Van der Velde M, Diafas I (2010) Biochar application to soils. A critical scientific review of effects on soil properties, processes, and functions. EUR 24099:162
Wandansari NR, Soemarno S, Suntari R, Kurniawan S (2023) The role of humic acid from various composts in improving degraded soil fertility and maize yield. J Degraded Mining Lands Manage 10:4245–4254
Wang H, Chen D, Wen Y, Zhang Y, Liu Y, Xu R (2023) Iron-rich red mud and iron oxide-modified biochars: a comparative study on the removal of Cd (II) and influence of natural aging processes. Chemosphere 330:138626
Wang J, Wang S (2019) Preparation, modification and environmental application of biochar: a review. J Clean Prod 227:1002–1022
Wang L, Gao C, Yang K, Sheng Y, Xu J, Zhao Y, Lou J, Sun R, Zhu L (2021) Effects of biochar aging in the soil on its mechanical property and performance for soil CO2 and N2O emissions. Sci Total Environ 782:146824
Wang L, O’Connor D, Rinklebe Jr, Ok YS, Tsang DC, Shen Z, Hou D (2020) Biochar aging: mechanisms, physicochemical changes, assessment, and implications for field applications. Environ Sci Technol 54:14797–14814
Wang Y, Zhang W, Shang J, Shen C, Joseph SD (2019) Chemical aging changed aggregation kinetics and transport of biochar colloids. Environ Sci Technol 53:8136–8146
Xia H, Riaz M, Ming C, Li Y, Wang X, Jiang C (2023) Assessing the difference of biochar and aged biochar to improve soil fertility and cabbage (Brassica oleracea var. capitata) productivity. J Soils Sedim 23:606–618
Xia W-Y, Feng Y-S, Jin F, Zhang L-M, Du Y-J (2017) Stabilization and solidification of a heavy metal contaminated site soil using a hydroxyapatite based binder. Construct Build Mater 156:199–207
Xiao L, Feng L, Yuan G, Wei J (2020) Low-cost field production of biochars and their properties. Environ Geochem Health 42:1569–1578
Xie Y, Zhou G, Huang X, Cao X, Ye A, Deng Y, Zhang J, Lin C, Zhang R (2022) Study on the physicochemical properties changes of field aging biochar and its effects on the immobilization mechanism for Cd2+ and Pb2+. Ecotoxicol Environ Safe 230:113107
Xing D, Cheng H, Ning Z, Liu Y, Lin S, Li Y, Wang X, Hill P, Chadwick D, Jones DL (2022) Field aging declines the regulatory effects of biochar on cadmium uptake by pepper in the soil. J Environ Manage 321:115832
Yang F, Sui L, Tang C, Li J, Cheng K, Xue Q (2021) Sustainable advances on phosphorus utilization in soil via addition of biochar and humic substances. Sci Total Environ 768:145106
Yang X, Hou R, Fu Q, Li T, Wang J, Su Z, Shen W, Zhou W, Wang Y (2023) Effect of freeze-thaw cycles and biochar coupling on the soil water-soil environment, nitrogen adsorption and N2O emissions in seasonally frozen regions. Sci Total Environ 164845
Yi X, Liang X, Yingming X, Xu Q, Huang Q, Lin W, Yuebing S (2017) Remediation of heavy metal-polluted agricultural soils using clay minerals: a review. Pedosphere 27:193–204
Zhang L, Sun X-Y, Tian Y, Gong X-q (2014) Biochar and humic acid amendments improve the quality of composted green waste as a growth medium for the ornamental plant Calathea insignis. Sci Horticul 176:70–78
Zhang M, Shu L, Guo X, Shen X, Zhang H, Shen G, Wang B, Yang Y, Tao S, Wang X (2015) Impact of humic acid coating on sorption of naphthalene by biochars. Carbon 94:946–954
Zhang S, Ji Y, Dang J, Zhao J, Chen S (2019) Magnetic apple pomace biochar: simple preparation, characterization, and application for enriching Ag (I) in effluents. Sci Total Environ 668:115–123
Zhou H, Meng H, Zhao L, Shen Y, Hou Y, Cheng H, Song L (2018) Effect of biochar and humic acid on the copper, lead, and cadmium passivation during composting. Bioresour Technol 258:279–286
Zhu J, Gao W, Ge L, Zhao W, Zhang G, Niu Y (2021) Immobilization properties and adsorption mechanism of nickel (II) in soil by biochar combined with humic acid-wood vinegar. Ecotoxicol Environ Safe 215:112159
Zimmerman AR (2010) Abiotic and microbial oxidation of laboratory-produced black carbon (biochar). Environmen Sci Technol 44:1295–1301
Zimmerman AR, Gao B, Ahn M-Y (2011) Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol Biochem 43:1169–1179
Funding
Open access funding provided by Università degli Studi di Ferrara within the CRUI-CARE Agreement. This study is funded by MUR 2020CXTLYS_003.
Author information
Authors and Affiliations
Contributions
Hafeez Ur Rahim: conceptualization, methodology, literature and data curation, formal analysis, writing original draft, review and editing, and visualization. Enrica Allevato: review and editing. Francesco Primo Vaccari: review and editing. Silvia Rita Stazi: supervision, review and editing, and funding acquisition.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Claudio Colombo
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rahim, H.U., Allevato, E., Vaccari, F.P. et al. Biochar aged or combined with humic substances: fabrication and implications for sustainable agriculture and environment-a review. J Soils Sediments 24, 139–162 (2024). https://doi.org/10.1007/s11368-023-03644-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-023-03644-2