Abstract
Purpose
The study focuses on the use of surfactants as enhancing solutions in electrokinetic remediation trials on sediments, with the hypothesis that they will allow heavy metals to desorb from organic matter, and thus favour their removal to the solution.
Materials and methods
A total of 15 remediation trials were conducted. As enhancing solutions, four different non-ionic commercial surfactants were used, either alone or in combination with citric acid (CA) or ethylenediaminetetraacetic acid (EDTA) in both compartments. A comparison with distilled water was also performed. 30–40 VDC was applied between activated titanium electrodes. The pH, electroosmotic flow (EOF), mineralogy of the samples (before and after the electrokinetic tests), and the percentage of removal of Cr, Ni, Cu, Zn, As, Cd, Pb, and Hg were determined.
Results and discussion
Every test showed an increase in current intensity during the first hours, and in certain cases, additional intensity peaks were found during the trial, which were mostly attributed to the establishment of EOF episodes. Depending on the case, EOF was transferred to the anolyte or the catholyte. Reversal of EOF occurred in one case, but was not detected in the others. Cr was primarily removed when CA was used. In the catholite, Ni, Cu, Zn, and Pb were extracted preferentially with EDTA. Surfactant B was more effective at removing Zn and As. Only a few treatments removed Cd with CA and surfactant C extracting the most. Hg was detected in the electrolytes of some experiments, being extracted with surfactant A in the catholyte in all cases, and with surfactant B and surfactant C with EDTA. Cr, Ni, Cu, Zn, Cd, and Pb were preferentially collected in the anolyte. Cu and Zn were found in trace levels in the catholyte.
Conclusions
Surfactants have been shown to help with metal solubilisation to different degrees depending on the metal. Each metal has a unique optimal species combination in the enhancing electrolyte. The direction of the EOF is determined by the chemical conditions of the system as a whole, not by the type of surfactant. Surfactants in combination with CA and EDTA improve desorption in general, which has been attributed to an increase in charge density passed during the tests rather than a symbiotic enhancement between both types of enhancing solutions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Marine sediments can act as a reservoir of persistent and toxic organic pollutants that endanger the environment. These contaminants can accumulate in large quantities in marine sediments with high organic carbon content (Cameselle and Reddy 2019). Due to the growing concern regarding the negative effects of these pollutants, a large number of studies have been conducted in recent years to better understand their sources, effects on the aquatic environment, and the most effective technologies for their remediation. Various techniques have been used to remediate both metals and organic pollutants, primarily in soils and to a lesser extent in sediments. In particular, electrokinetic treatment, which involves applying a direct electric current between two electrodes to mobilise the contaminants in the sediment and concentrate them in an electrolyte (Reddy and Cameselle 2009). Decontamination by electrical fields proceeds mainly though two phenomena: electromigration and electroosmotic flux (EOF). The contaminated sediment is typically placed between electrodes connected to an external DC power supply. The current is carried between the anode and cathode by charged species in the sediment water, and charged contaminants are transported toward the electrode of opposite charge and collected in the corresponding electrolyte solution (Ribeiro et al. 2016). This process is known as electromigration. Reuss was the first to sense EOF (Reuss 1809), which is induced by the difference in potential that occur when two different phases are placed in contact, implying a separation of electric charges. As a result, on one of the phases, there is an excess of charge of one sign, which is balanced by the distribution of charges on the other phase. When an external field is applied to two phases, one of which is a stationary solid and the other moves in response to the applied electrical field, the phenomenon is known as electro-osmosis (Hunter 2013). It is also important to consider the effect of the electrode reactions, which assures the evolving system´s electroneutrality. In most cases, the electrode processes involve water electrolysis in which produces hydrogen gas and hydroxyl ions at the cathode, and oxygen and protons at the anode, leading alkalinisation and acidification in cathode and anode, respectively. Sorption and desorption processes in the liquid/solid interphase must also be considered in the overall process.
Concerning removal of metals, most of the treatments incorporate the use of acidic solutions, chelates, or mixed solutions to solubilize them (Reddy and Chinthamreddy 2004; Meers et al. 2005; Nystroem et al. 2006; Peng et al. 2009; Castellote and Botija 2011; Rozas and Castellote 2012; Pedersen et al. 2015; Song et al. 2016; Yuan et al. 2016; Garcia-Blas et al. 2020a; Ayyanar and Thatikonda 2021). Other treatments used redox agents (Chuan et al. 1996; Reddy and Chinthamreddy 1999; Nystroem et al. 2006; Cang et al. 2013; Pazos et al. 2013). The electrokinetic technology has also been used to remove organic contaminants including PAHs, PCBs, pesticides, and herbicides, and requires the use of surfactants, biosurfactants, cosolvents, or cyclodextrins to enhance their removal (Alcántara et al. 2008; Reddy and Cameselle 2009; Ribeiro et al. 2011; Huang et al. 2012; Guedes et al. 2014; Rozas and Castellote 2015; Cameselle and Gouveia 2018; Jing et al. 2018). Because organic pollutants are difficult to dissolve, these solubilising agents are required to improve the efficiency of electroosmosis, which transport pollutants to electrodes (Castellote et al. 2010; Lofrano et al. 2017; Gitipour et al. 2018).
There are not many studies that undertake the simultaneous removal of organic contaminants and heavy metals (Wang et al. 2007; Andreottola et al. 2010; Colacicco et al. 2010; Cang et al. 2013). When citric acid and a non-ionic surfactant were used together, the simultaneous removal of metals and PAHs was relatively good (Ammami et al. 2014). A combination of Tween 20 at higher concentrations and citric acid 0.1 M extending the treatment duration, as well as applying a periodic voltage, may produce better results (Ammami et al. 2015). The biosurfactant rhamnolipids and saponin combined with citric acid did not provide satisfactory metals or PAHs removal efficiencies (Tian et al. 2017). The combination of methylglycinediacetic acid (MGDA) and Tween® 80 resulted in effective Hg removal (Falciglia et al. 2017). The sequential treatment of citric acid and non-ionic surfactants enhanced the removal of Zn and As, but not other hazardous metals, whereas Tween 80 had no effect on metal removal percentages (Hahladakis et al. 2014). Using a non-ionic surfactant (Nonidet P40), high removal rates for metals and PAHs were observed (Hahladakis et al. 2016). The use of a combination of electroremediation and biological treatment, with the addition of EDTA and Tween 80, provided favourable results for decontaminating soil contaminated by heavy metals and organic compounds (Dong et al. 2013). The combination of the surfactant Tween 20 and citric acid in various concentrations was also effective in removing metals and PAHs from river sediments (Benamar et al. 2019). Metal extractions were improved by mixing rhamnolipid, saponin, and sophorolipid with contaminated sediment prior to electrokinetic experiments (Tang et al. 2018). The majority of these studies focused on the influence of surfactants on organic pollutant removal. However, the physicochemical changes introduced into the system should enhance heavy metals removal by lowering the interfacial surface tension and increasing solubility of hydrophobic organic compounds through micellar solubilisation (Saichek and Reddy 2005). This paper focuses on heavy metals removal using four different non-ionic surfactants alone and in combination with chelates (citric acid and EDTA), with the hypothesis that they will allow heavy metal desorption from the organic matter in the sediment and thus, favour their removal to solution.
2 Materials and methods
2.1 Characterisation of raw, natural sediment
Sediment samples were dredged from a European marine harbour in the south-east coast of Spain. Sampling and storage conditions and characterisation of the sediment are described in detail in (Garcia-Blas et al. 2020b). These included size particle distribution (67% of the sediment was ≤ 63 μm and around 30% was ≤ 2 μm); total organic carbon, following the ISO 10694 standard (2.1%); carbonate content according to NEN-EN-ISO 10693 (10.2 g kg−1); organotin compounds as specified by NEN-EN-ISO 23161 (tributyltin 57 µg kg−1); total petroleum hydrocarbons using gas chromatography–flame ionisation detection (TPH C10-C40:70 mg kg−1); polycyclic aromatic hydrocarbons (PAH-VROM, 10 < 0.30 mg kg−1), (total PAH (EPA, 16) < 0.48 mg kg−1); and polychlorinated biphenyls (PCB < 0.007 mg kg−1) by gas chromatography–mass spectrometry. The metals content (mg kg−1 d.w.) was 224.9, 1.35, 49, 2228.8, 317.5, 47.1, 2897.8, and 1.3 for As, Cd, Cr, Cu, Pb, Ni, Zn, and Hg, respectively. Equilibration of the sediment in distilled water at a solid/water ratio 1:3 led to a pH of the suspension of 7.5, indicating slightly basic character for the sediment.
2.2 Electrokinetic remediation tests
Fifteen electrokinetic remediation tests were performed using a U-shaped homemade electroosmotic cell device (ECD) described in detail in Garcia-Blas et al. (2020a). The electrolytes were placed in two 55 cm3 cylindrical chambers. Each chamber is made of two parts: the horizontal part, in PVC, in contact with one side of the sediment, and the vertical part, of translucent silicone to allow measuring the electroosmotic flux. Between the chambers, a PVC receptacle of 20 mm internal diameter and 60 mm length was filled with 25 ± 2 g of wet sediment and a Cintropur NW32 150 µm filter sleeve was taped to each side to allow the passage of the solutions and prevent sediment leakage. The electrodes consisted of wires of activated titanium separated 12 cm. The vertical part of the chambers was graduated to allow the measure of the electroosmotic flow (EOF) passing through the compartments. A picture of a test running is given in Fig. 1. Concerning enhancing solutions, four non-ionic different commercial surfactants: Tween 20 (surfactant A), Igepal (surfactant B), Surfsoil (surfactant C), and Soilteq (surfactant D), were diluted according to the technical specifications provided by their manufacturers, 2%, 5%, 5%, and 1% respectively. Citric acid (CA) and EDTA were also used as enhancing solutions.
The surfactant tests lasted 65–72 h because it has previously been demonstrated that the highest removal takes place during the first hours (Garcia-Blas et al. 2020a). The experimental parameters of the tests are detailed in Table 1. Tests 1 and 2 were performed with deionised water in the catholytes, and surfactants A or B in the anolytes, respectively. Tests 3–6 were conducted in the opposite order, with deionised water in the anolytes and surfactants A–D in the catholytes, respectively. Tests 7–10 and tests 11–14 combined each surfactant with 0.1 M citric acid and 0.1 M EDTA in the anolytes and catholytes, respectively. Test 15 was the reference with distilled water in both compartments.
Direct electric potential was applied with a PS3003 HQ-Power Power supply. 30 V was applied in Tests 1, 2, and 15, and 40 V was applied in the rest of tests. The difference in electric potential between the electrodes of each cell was recorded hourly with a 34972A Agilent data logger.
Liquid samples (40 ml) were extracted each 24 h from both anolyte and catholyte of every cell, and metal´s concentration was analysed using ICP-AES with a Varian 725-ES spectrometer. The liquid extracted was replaced by the initial solution of the corresponding compartment. At the end of the tests, the sediment samples were extracted from the PVC disks and they were divided into three parts: the portion closest to the anolyte, the portion closest to the catholyte, and the central portion. They were placed in ceramic crucibles and dried in a furnace at 100 °C for 24 h for X-ray diffraction (XRD) characterisation.
3 Results and discussion
3.1 Electrical properties, pH, and electroosmotic flow
Figure 2a shows the total amount of electricity that has passed through the sediment (charge density; Ccm−2), calculated as the area under the intensity (A) vs time (s) plot divided by the transversal area of the sediment, for the passage of current, at the end of the different tests. The addition of CA and/or EDTA increases the total amount of electrical current during the tests. Figure 2b shows the current intensity recorded for some of the tests, corresponding to various enhancing solutions (see Table 1).
Figure 2b demonstrates, as previously observed (Tang et al. 2018; Garcia-Blas et al. 2020a), a peak of current intensity that occurs in the first hours; afterwards, the intensity decreases exponentially, remaining relatively low (a few mA) and almost constant until the end in some of the tests (e.g. tests 1, 5, and 15). Others have intensity peaks at different points of the trial (e.g., tests 9, 12, and 14). Accordingly, the accumulated charge density passed at the end of the test differs for each of them, with lower values for tests 1–6 and 15, higher values in the experiments including CA (tests 7–10), and the highest values when EDTA is combined with the surfactants (tests 11–14).
The evolution of the pH in both compartments is shown in Fig. 3. The pH becomes acidic and alkaline in the anolyte and catholyte due to the water oxidation and reduction respectively, which establishes different pH fronts in the sediment. Despite the fact that the extracted liquid samples represent a significant proportion of the total volume of the compartment, and there is no doubt that this has an impact on the electrolytes’chemistry, their composition appears to be dominated by electrodic reactions and the EOF rather than the renovation effect, as evidenced by pH evolution.
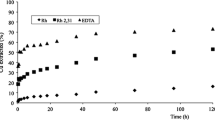
Figure 4 presents the electroosmotic flow (EOF) measured during the tests. The EOF moved towards the anolyte in tests 2, 5, 6, 8, 11, 12, and 15 while went towards the catholyte in 2, 3, 4, 7, 9, 10, 11, and 14. It is remarkable that trial 2 was the only one in which there was a reversal of EOF, going first to the anolyte and then to the catholyte, and the high values of EOF reached in trial 14. In trial 1, no EOF was detected.
It has to be pointed out that, as a general rule, the tests with sudden increases in current intensity correspond to those that with EOF occurring at different moments in the tests than the initial one (Castellote et al. 2003). Despite the fact that the establishment of the EOF is not related to the current passing through, when the EOF is produced, there is a movement of liquid carrying electrical charges, which usually leads to an increase in the electrical intensity. In order to illustrate this, the derivative of I t−1 vs t has been depicted for five different test (see Fig. 5). As previously stated, the EOF was evaluated each 24 h by making a liquid balance in both compartments, being the EOF detected that corresponded to the period’s average. These points of balance for the EOF have been indicated in the figure, as well as the presence of any EOF during each period. As a result, for tests 5, 12, and 14, a parallelism between both parameters can be found. However, in the case of test 15, no significant increase of the current intensity was detected during period identified as “2” even though some EOF was detected. It has to be pointed out that the surfactant does not determine the direction of the EOF, as EOF is not directly linked to the surfactant, which have a slight effect on the chemical conditions which drive the magnitude and the direction of EOF. As an example, trial 6 (towards +) and trials 10 and 14 (towards −), the three of them having surfactant D in the catholyte, which is attributed to the different external electrolytes, and consequently, different chemical conditions in the sediment. As an example, the initial pH in trials 6, 10, and 14 were (6.43–5.7), (2.23–2.16), and (4.53–4.49), respectively for (anolyte-catholyte) solutions.
3.2 Mineralogical changes after the trials
Some mineralogical changes have been observed in the samples tested after the electrokinetic tests. Figure 6 shows X-ray diffraction patterns for raw sediment before and after surfactant testing for the anodic side (+), cathodic side (−), and central part. In all experiments, the initial presence of NaCl (halite) and pyroxene have disappeared on the anodic sides of the samples (+). Muscovite and lizardite, a silicate of Fe, Mg and Al, remained in all of the samples except tests 1 and 2, where hematite, Fe2O3, was detected. Furthermore, browneite (S-Mg-Fe-Ca) and rockbridgeite (Fe and P) can be found in samples 2 and 1, respectively. Sample 10 deposits a Fe silico-aluminate. Samples from the central and cathode sides show similar patterns with minor variations of mineralogical composition, as the anodic sides. In some cases, small signals attributed to C-bearing phases are detected after the tests, and diffraction peaks more intense than in the untreated sediment can be seen, which can be attributed to oxidation/dissolution of existing phases and subsequent precipitation of other species, as well as the inherent heterogeneity of the sediment.
3.3 Removal of metals
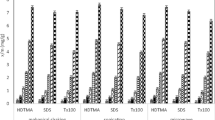
Figure 7 presents the percentages of metal removed during the tests, along with the maximum amounts found in the anolyte, catholyte, and total. In both compartments, the removal of Cr occurs primarily when CA is used, followed by water, with no significant favourable effect attributed to surfactants. In the catholyte, Ni, Cu, Zn, and Pb are extracted preferentially with EDTA. Surfactant B has higher efficiency for Zn. This is also true for higher As elimination. Cd was only removed in some of the trials, with a nearly 40% reduction in test 9 using CA and surfactant C. Hg was found in several of the trials’ electrolytes, and it was removed with surfactant A in the catholyte in all cases, with surfactant B without any other enhancing solution, and with surfactant C with EDTA. It is remarkable that it was extracted with water as well. Cr, Ni, Cu, Zn, Cd, and Pb were preferentially collected in the anolyte. Arsenic and small amounts of Cu and Zn were found in the catholyte. Speciation of these metals during the electrokinetic tests is described in a previous publication by our group (Garcia-Blas et al. 2020a). The maximum removal for each metal in function of the charge density passed (Ccm−2) in the anolyte, catholyte, and total at the end of the trial is shown in Fig. 8. As expected, removal of metals does not follow a linear trend, as other phenomena like EOF, electrodic reactions, precipitation, or desorption by the surfactants are involved. Most metals exhibit a significant influence of the enhancing agent used, as in the case of Cr, with higher percentages of extraction for CA at lower Ccm−2 than for EDTA. However, Ni, As, and Zn do not show such a clear distinction between the using one or the other chelate, presenting the removal a more like-linear dependence on the charge density passed.
During the electrokinetic tests, several interrelated phenomena occur, including sediment composition, type and quantity of contaminants, electrode material, pH of the electrolytes, and chemical agents in the solutions. All these factors determine the speciation of the contaminants in the matrix, as well as their ability to be driven by electromigration, EOF magnitude and direction. Metal retention mechanisms by organic matter involve the formation of inner-sphere complexes, ion exchange, and precipitation reactions (Stevenson 1994; Shaheen et al. 2013). In addition, the pH affects the stability of metals adsorbed by organic matter (Stevenson and Ardakani 1972; Stevenson 1994). Surfactants mobilize and/or solubilize organic matter by lowering interfacial tension at the soil–organic phase and water–organic phase interfaces, which must also affect the metal adsorption by the organic matter (Chu and Chan 2003). To try to discriminate the desorption induced by the surfactants in the global process, the percentages of removal have been normalized by the charge density passed considering that even they are not linearly related to the charge density passed, the significant differences in Ccm−2 for the different tests, and the positive trend between both parameters, allow a better comparison than normalising them by time (direct results). Most of the phenomena involved in the process are implicitly considered in this normalization as they are generally interrelated resulting in variations in the current intensity. Figure 9a–e shows the results separated by surfactant. Three general aspects can be pointed out from this graph: (1) surfactants as enhancing agents in electrokinetic tests contribute to a higher removal of metals; (2) there is no effect of desorption due to any of the surfactants for Cr; (3) combination of surfactants with other enhancing solutions (CA and EDTA) presents higher absolute values of extraction (Fig. 7) that with the exception of Pb and Hg are attributable to an increase in the charge density passed during the tests rather than a symbiotic enhancement of the effect of both enhancing solutions. Specifically, for different contaminants, surfactant D is the most effective for Ni, while surfactants A and B have no positive effect. Surfactants B, C, and D promote Cu and Zn desorption, with surfactant B and D having the highest removal rates, respectively. Surfactant D has a small influence on As, but surfactant B has a larger effect. Pb is desorbed by all the surfactants, with D having the highest values. Surfactant C helps to desorb Cd at the highest rate being removed in various trials, whereas it was not eliminated in the absence of surfactants. The normalized extraction was not higher when Hg was removed using surfactants than when water and CA were used.
The distribution of Cd and Cu in soils is primarily controlled by organic matter (Shaheen 2009), while Zn has a stronger association with the inorganic phase (Degryse et al. 2009). The relationships between metal distribution coefficient and soil properties were described in a previous review (Shaheen et al. 2013), including a list of references indicating — positive, negative, and non-significant — relationship for each of the properties. Concerning organic matter, they found for Cd (4,0,2); for Cr (0,0,1), for Cu (4, 0, 1), Ni (0,0,2), Pb (2,0,2), and Zn (1,0,2), being the numbers in the parenthesis the number of references of each type. Thus, with the exception of Cr, there are discrepancies in the literature for the majority of metals. This is in good agreement with the results of this study, which found no noticeable effect for Cr, while detecting Cd desorption with surfactants but no removal without surfactants (Test 15).
The comparison of raw and normalized percentages of extraction reveals that the efficiency of desorption of each surfactant is dependent on the specific metal for the same sediment in the same experimental electrokinetic configuration. For example, having the surfactants in the catholyte, D desorbs more Pb than B. Thus, the effect of acidic or complexing solutions adds its effect to the desorption of surfactants, improving the removal of metals (Ammami et al. 2014). For most of metals, the effect of electromigration, EOF, quaint effects, and pH fronts appears to be more important than the effect of desorption by surfactants, with the combination of surfactants with EDTA being the most efficient approach in this study.
4 Conclusions
The conclusions drawn from this research are as follows:
-
Surfactants, used as enhancing agents in electrokinetic tests, contribute to metal solubilisation to varying degrees depending on the metal. Each metal has a different optimum combination of species in the enhancing electrolyte.
-
Cd has only been removed using surfactants. On the contrary, no effect due to any of the surfactants on Cr desorption was observed.
-
Most metals exhibit a significant influence of the enhancing agent used, as in the case of Cr, with higher percentages of extraction for CA than for EDTA even at lower Ccm−2. However, Ni, As, and Zn do not show such a clear dependence or the type of chelate, presenting their removal a like-linear dependence on the charge density passed.
-
The direction of the EOF is not determined univocally by the surfactant.
-
Combining surfactants with enhancing solutions (CA and EDTA) results in higher absolute values of metal extraction, with the exception of Pb and Hg. Nevertheless, it seems to be due to an increase in the charge density passed during the tests rather than a symbiotic enhancement of the effect of both enhancing solutions. That means that for most metals, the effect of electromigration, EOF, chelates, and pH fronts appears to be more significant than the effect of surfactant desorption.
-
Surfactants combined with EDTA appear to be the most general effective combination in the conditions tested.
References
Alcántara M, Gómez J, Pazos M, Sanromán M (2008) Combined treatment of PAHs contaminated soils using the sequence extraction with surfactant–electrochemical degradation. Chemosphere 70:1438–1444
Ammami MT, Benamar A, Wang H, Bailleul C, Legras M, Le Derf F, Portet-Koltalo F (2014) Simultaneous electrokinetic removal of polycyclic aromatic hydrocarbons and metals from a sediment using mixed enhancing agents. Int J Environ Sci Technol 11:1801–1816
Ammami MT, Portet-Koltalo F, Benamar A, Duclairoir-Poc C, Wang H, Le Derf F (2015) Application of biosurfactants and periodic voltage gradient for enhanced electrokinetic remediation of metals and PAHs in dredged marine sediments. Chemosphere 125:1–8
Andreottola G, Bonomo L, De Gioannis G, Ferrarese E, Muntoni A, Polettini A, Pomi R, Saponaro S (2010) Lab-scale feasibility tests for sediment treatment using different physico-chemical techniques. J Soils Sediments 10:142–150
Ayyanar A, Thatikonda S (2021) Enhanced electrokinetic removal of heavy metals from a contaminated lake sediment for ecological risk reduction. Soil Sediment Contam 30:12–34
Benamar A, Tian Y, Portet-Koltalo F, Ammami M, Giusti-Petrucciani N, Song Y, Boulangé-Lecomte C (2019) Enhanced electrokinetic remediation of multi-contaminated dredged sediments and induced effect on their toxicity. Chemosphere 228:744–755
Cameselle C, Gouveia S (2018) Electrokinetic remediation for the removal of organic contaminants in soils. Curr Opin Electrochem 11:41–47
Cameselle C, Reddy K (2019) Electrochemical remediation for contaminated soils, sediments and groundwater, Engineering tools for environmental risk management. CRC Press, London, pp 447-465. ISBN: 9781315778754. https://doi.org/10.1201/b20405
Cang L, Fan G-P, Zhou D-M, Wang Q-Y (2013) Enhanced-electrokinetic remediation of copper–pyrene co-contaminated soil with different oxidants and pH control. Chemosphere 90:2326–2331
Castellote M, Llorente I, Andrade C (2003) Influence of the external solution in the electroosmotic flux induced by realkalinisation. Mater De Construccion 53(271–272):101–112
Castellote M, Botija S, Andrade C (2010) Assessment of electrophoresis and electroosmosis in construction materials: effect of enhancing electrolytes and heavy metals contamination. J Appl Electrochem 40:1195–1208
Castellote M, Botija S (2011) Electrokinetic decontamination of heavy metals in construction materials: contribution of the different parameters to the global efficiency. J Appl Electrochem 41:695–703
Chu W, Chan K (2003) The mechanism of the surfactant-aided soil washing system for hydrophobic and partial hydrophobic organics. Sci Total Environ 307:83–92
Chuan M, Shu G, Liu J (1996) Solubility of heavy metals in a contaminated soil: effects of redox potential and pH. Water Air Soil Pollut 90:543–556
Colacicco A, De Gioannis G, Muntoni A, Pettinao E, Polettini A, Pomi R (2010) Enhanced electrokinetic treatment of marine sediments contaminated by heavy metals and PAHs. Chemosphere 81:46–56
Degryse F, Smolders E, Parker D (2009) Partitioning of metals (Cd Co, Cu, Ni, Pb, Zn) in soils: concepts, methodologies, prediction and applications–a review. Eur J Soil Sci 60:590–612
Dong Z-Y, Huang W-H, Xing D-F, Zhang H-F (2013) Remediation of soil co-contaminated with petroleum and heavy metals by the integration of electrokinetics and biostimulation. J Hazard Mater 260:399–408
Falciglia PP, Malarbì D, Greco V, Vagliasindi FG (2017) Surfactant and MGDA enhanced–Electrokinetic treatment for the simultaneous removal of mercury and PAHs from marine sediments. Sep Purif Technol 175:330–339
Garcia-Blas N, Jimenez-Relinque E, Nevshupa R, Castellote M (2020a) Electrokinetic approach to assess the behaviour of a contaminated marine sediment. J Soils Sediments 20:2673–2684
Garcia-Blas N, Jimenez-Relinque E, Nevshupa R, Castellote M (2020b) Interaction dynamics between a contaminated dredged sediment and extracting solutions of different nature. J Soils Sediments 20:2664–2671
Gitipour S, Sorial GA, Ghasemi S, Bazyari M (2018) Treatment technologies for PAH-contaminated sites: a critical review. Environ Monit Assess 190:1–17
Guedes P, Mateus EP, Couto N, Rodríguez Y, Ribeiro AB (2014) Electrokinetic remediation of six emerging organic contaminants from soil. Chemosphere 117:124–131
Hahladakis JN, Lekkas N, Smponias A, Gidarakos E (2014) Sequential application of chelating agents and innovative surfactants for the enhanced electroremediation of real sediments from toxic metals and PAHs. Chemosphere 105:44–52
Hahladakis JN, Latsos A, Gidarakos E (2016) Performance of electroremediation in real contaminated sediments using a big cell, periodic voltage and innovative surfactants. J Hazard Mater 320:376–385
Huang D, Xu Q, Cheng J, Lu X, Zhang H (2012) Electrokinetic remediation and its combined technologies for removal of organic pollutants from contaminated soils. Int J Electrochem Sci 7:4528–4544
Hunter RJ (2013) Zeta potential in colloid science: principles and applications, 2. Academic Press
Jing R, Fusi S, Kjellerup BV (2018) Remediation of polychlorinated biphenyls (PCBs) in contaminated soils and sediment: state of knowledge and perspectives. Front Environ Sci 6:79
Lofrano G, Libralato G, Minetto D, De Gisi S, Todaro F, Conte B, Calabrò D, Quatraro L, Notarnicola M (2017) In situ remediation of contaminated marinesediment: an overview. Environ Sci Pollut Res 24:5189–5206
Meers E, Ruttens A, Hopgood M, Samson D, Tack F (2005) Comparison of EDTA and EDDS as potential soil amendments for enhanced phytoextraction of heavy metals. Chemosphere 58:1011–1022
Nystroem GM, Pedersen AJ, Ottosen LM, Villumsen A (2006) The use of desorbing agents in electrodialytic remediation of harbour sediment. Sci Total Environ 357:25–37
Pazos M, Iglesias O, Gómez J, Rosales E, Sanromán M (2013) Remediation of contaminated marine sediment using electrokinetic–Fenton technology. J Ind Eng Chem 19:932–937
Pedersen KB, Kirkelund GM, Ottosen LM, Jensen PE, Lejon T (2015) Multivariate methods for evaluating the efficiency of electrodialytic removal of heavy metals from polluted harbour sediments. J Hazard Mater 283:712–720
Peng J-f, Song Y-h, Yuan P, Cui X-y, G-lJJohm Q (2009) The remediation of heavy metals contaminated sediment. J Hazard Mater 161:633–640
Reddy KR, Chinthamreddy S (1999) Electrokinetic remediation of heavy metal-contaminated soils under reducing environments. J Waste Manag 19:269–282
Reddy KR, Chinthamreddy S (2004) Enhanced electrokinetic remediation of heavy metals in glacial till soils using different electrolyte solutions. J Environ Eng 130:442–455
Reddy KR, Cameselle C (2009) Electrochemical remediation technologies for polluted soils, sediments and groundwater. John Wiley & Sons
Reuss FF (1809) Sur un nouvel effet de l’électricité galvanique. Mem Soc Imp Natur Moscou 2:327–337
Ribeiro AB, Mateus EP, Rodríguez-Maroto J-M (2011) Removal of organic contaminants from soils by an electrokinetic process: the case of molinate and bentazone. Experiment Model Sep Purif Technol 79:193–203
Ribeiro AB, Mateus EP, Couto N (2016) Electrokinetics across disciplines and continents. Springer International Publishing, The Netherlands, New Strategies for Sustainable Development
Rozas F, Castellote M (2012) Electrokinetic remediation of dredged sediments polluted with heavy metals with different enhancing electrolytes. J Electrochim Acta 86:102–109
Rozas F, Castellote M (2015) Selecting enhancing solutions for electrokinetic remediation of dredged sediments polluted with fuel. J Environ Manag 151:153–159
Saichek RE, Reddy KR (2005) Electrokinetically enhanced remediation of hydrophobic organic compounds in soils: a review. Crit Rev Environ Sci Technol 35:115–192
Shaheen SM (2009) Sorption and lability of cadmium and lead in different soils from Egypt and Greece. Geoderma 153:61–68
Shaheen SM, Tsadilas CD, Rinklebe J (2013) A review of the distribution coefficients of trace elements in soils: Influence of sorption system, element characteristics, and soil colloidal properties. J Ad Colloid Interface Sci 201:43–56
Song Y, Ammami M-T, Benamar A, Mezazigh S, Wang H (2016) Effect of EDTA, EDDS, NTA and citric acid on electrokinetic remediation of As, Cd, Cr, Cu, Ni, Pb and Zn contaminated dredged marine sediment. Environ Sci Pollut Res 23:10577–10586
Stevenson F, Ardakani M (1972) Organic matter reactions involving micronutrients in soils. Micronutrients in Agriculture. Soil Sci Soc Am J
Stevenson FJ (1994) Humus chemistry: genesis, composition, reactions. John Wiley & Sons
Tang J, He J, Xin X, Hu H, Liu T (2018) Biosurfactants enhanced heavy metals removal from sludge in the electrokinetic treatment. Chem Eng J 334:2579–2592
Tian Y, Boulangé-Lecomte C, Benamar A, Giusti-Petrucciani N, Duflot A, Olivier S, Frederick C, Forget-Leray J, Portet-Koltalo F (2017) Application of a crustacean bioassay to evaluate a multi-contaminated (metal, PAH, PCB) harbor sediment before and after electrokinetic remediation using eco-friendly enhancing agents. Sci Tot Environ 607:944–953
Wang J-Y, Huang X-J, Kao JC, Stabnikova O (2007) Simultaneous removal of organic contaminants and heavy metals from kaolin using an upward electrokinetic soil remediation process. J Hazard Mater 144:292–299
Yuan L, Xu X, Li H, Wang N, Guo N, Yu H (2016) Development of novel assisting agents for the electrokinetic remediation of heavy metal-contaminated kaolin. Electrochim Acta 218:140–148
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This study was co-funded by the Region of Madrid’s “Garantía Juvenil” programme co-financing the contract of Nieves Garcia-Blas (grant PEJD-02017-PRE/AMB-4296).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Bruno Lemière
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Garcia-Blas, N., Jimenez-Relinque, E. & Castellote, M. Surfactants in electrokinetic remediation of sediments to enhance the removal of metals. J Soils Sediments 22, 2853–2864 (2022). https://doi.org/10.1007/s11368-022-03299-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-022-03299-5