Abstract
Purpose
The aims of the study were to investigate the interaction between fractions of organic matter and polycyclic aromatic hydrocarbons (PAHs) in bottom sediments and to use mussels as passive biomonitors and consensus-based sediment quality guidelines for ecological risk assessment in sediments.
Methods
Bottom sediment samples were taken from 46 points located in the Rożnów reservoir (Poland). The sediment organic matter (SOM) characteristics included total carbon (TC), total organic carbon (TOC), humic acid carbon (Cha), fulvic acid carbon (Cfa), non-hydrolysing carbon (Cnh), and dissolved organic carbon (DOC). The extraction procedure was carried out in bottom sediments as well as in freeze-dried mussel tissue samples to directly determine the accumulation potential of PAHs to the living organisms in their natural environment.
Results
The content of organic matter fractions was in the following order: Cfa (fulvic acid) > Cnh (non-hydrolysing carbon) > Cha (humic acid) > DOC (dissolved organic carbon). The mean ∑16PAHs (μg kg−1) concentration was 1755.2 ± 724 (total) and 256 ± 254 (bioavailable) in sediments and 1740 ± 72.2 in the mussel tissues. A significant positive correlation was found between the concentration of PAHs in the mussel tissues and the total and bioavailable concentration of PAHs in bottom sediments.
Conclusion
The PAH concentration in bottom sediments depended on the stabile carbon forms Cnh. Principal component analysis (PCA) suggests that the fine fraction can significantly increase the bioavailability of PAHs and can be an important factor in the distribution of PAHs in the sediments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Because of their widespread distribution in the environment, and toxic and carcinogenic properties, polycyclic aromatic hydrocarbons (PAHs) are included in lists of hazardous substances (Orecchio and Mannico 2010; Yang et al. 2016; Baran et al. 2017; Li et al. 2018; Ukalska-Jaruga et al. 2019). In the water ecosystem, PAHs, due to their hydrophobic properties, are quickly bound to organic particles suspended in water columns and deposited on bottom sediments (Qiao et al. 2006). Organic matter constitutes the main factor influencing the PAHs sorption, causing a decrease in their bioavailability, and reducing ecotoxicological risk for the living organisms (Wu et al. 2019; Honda and Suzuki 2020). The organic matter in water is present in a true solution, in colloidal solution, and as a suspension of organic detritus and live organisms. It naturally follows that each of these forms plays a different role in the supply of organic matter to sediments and undergoes various forming processes (Bordovskiy 1965). Therefore, the heterogeneity of organic matter causes different interactions with PAHs related to their diversified sorption affinity of these compounds. According to the literature, the highest sorption ability to PAHs is exhibited by non-hydrolysing humin fraction, a significantly lower by humic acids, and the lowest by fulvic acids (Klimkowicz-Pawlas 2009; Orecchio and Mannico 2010; Lubecki et al. 2019; Ukalska-Jaruga and Smreczak 2020). These relationships significantly contribute to the potentially toxic effect of PAHs in water as well as the environmental risks associated with the transmission of these compounds into the food chain of terrestrial organisms. Therefore, a key action of the Environmental Protection Agency (EPA 1994) outlined the objectives of the Contaminated Sediment Research Strategy included in the Sediment Quality Guideline (SQG) (Yang et al. 2016; Baran et al. 2017; Tarnawski and Baran 2018). In these documents, the sediment contaminant chemistry has been correlated with measured biological effects to develop several correlative-derived toxicity thresholds. Furthermore, a number of other studies have also indicated that the use of aquatic animals is a good tool to assess ecological risk connected with PAHs (Baumard et al. 1999; Kwok et al. 2013; Honda and Suzuki 2020). In the water environment, mussels, as benthic organisms, are a common and ecologically important resource. The mussels have several special properties, such as wide distribution, sedentary mode of life, bioaccumulation properties for pollutants, and easy sampling (Shulkin et al. 2003; Arias et al. 2009; González-Fernández et al. 2017; Honda and Suzuki 2020).
The aims of the study were to (1) investigate the concentration, sources, spatial distribution, and potential bioavailability of PAHs in sediments; (2) assess the effect of organic matter content on PAHs’ distribution in the bottom sediment; and (3) assess the contamination of sediments with PAHs using mussels as passive biomonitors and consensus-based sediment quality guidelines. Evaluations of the interaction between fractions of organic matter and PAHs in bottom sediments as well as the response of mussels are useful for a justified ecological risk assessment.
2 Materials and methods
2.1 Study area and sediment sampling
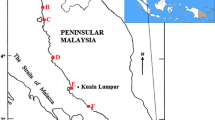
The Rożnów dam reservoir is located in the Lesser Poland Voivodeship in southern Poland (Fig. 1). The characteristics of this reservoir were described in more detail in the previous studies (Baran et al. 2019; Szara et al. 2020). This reservoir is susceptible to an intensive silting process, as the annual influx of sediments was even over 2 million m3 (in the period before the construction of additional relieving reservoirs in the upper part of the Dunajec river) (Szarek-Gwiazda 2014; Baran et al. 2019). Bottom sediment samples were taken from 46 points located throughout the entire bowl of the Rożnów reservoir using the Ekman sampler (Fig. 1). The top layer of the sediment was collected at a depth of 0–15 cm. The samples were stored in the dark under controlled conditions (temperature of 4 °C) pending the chemical analyses. The mussels (Anodonta anatine) were sampled from 10 sampling points (3, 7, 15, 23, 25, 33, 35, 36, 41, 45, and) (Fig. 1). Immediately after collection, mussels were transferred in the ice cool box to the laboratory. The mussels were kept for 24 h in filtered water under conditions corresponding to those at the sampling site to remove the gut content. Next, mussel samples were lyophilised and homogenised in a mortar.
2.2 Chemical analysis
2.2.1 Organic matter fractions
The sediment organic matter (SOM) characteristics included total carbon (TC), total organic carbon (TOC), humic acid carbon (Cha), fulvic acid carbon (Cfa), non-hydrolysing carbon (Cnh), and dissolved organic carbon (DOC). Different SOM fractions were analysed according to the methodology described in details in our previous study (Ukalska-Jaruga et al. 2019).
2.2.2 Extraction and analysis of PAHs
The PAH analysis involved determination of sixteen individual compounds according to the US EPA List. The extraction procedure was carried out in bottom sediments as well as in freeze-dried mussel tissue samples to directly determine the accumulation potential of PAHs to the living organisms in their natural environment. Moreover, the PAHs were determined in sediments in two forms, total and bioavailable, for better recognition of bioaccessibility and bioactivity processes of these compounds. Analysis of total PAH concentration was based on the extraction with dichloromethane in an ASE200 Accelerated Solvent Extractor (Dionex Co., Sunnyvale, CA, USA). Firstly, ground and homogenised samples (bottom sediments: grain size ≤ 0.10 mm, 5 g, and mussel 0.2 g) were mixed with 2 g of diatomaceous earth and spiked with 10 μl of a recovery standard containing five deuterated PAHs (PAH-31, Dr. Ehrenstorfer GmbH, Augsburg, Germany). After extraction, the concentrated solution was cleaned up on activated silica gel (16 h at 135 °C) and eluted by hexane. For the analysis of bioavailable PAH concentration, sediment sample (5 g) was homogenised for 40 min in an ultrasonic bath with 30 mL of 2-propanol and extracted for 60 min (ISO 17402: 2008; Riding et al. 2013; Cachada et al. 2014). The concentrations of PAHs were determined by gas chromatography triple mass spectrometry on an Agilent 7890B GC system (Agilent Tech., Santa Clara, CA, USA), equipped with an Agilent 7000C detector and an Agilent 7693 Autosampler (Table 1SM, Fig. 1SM). Sample analysis was performed in the multiple reactor monitoring (MRM) mode with three transitions for diagnostic ions. Quality control measures included analysis of a certified reference material (CRM 131, ANAB Accredited Tested Laboratory), duplicate matrix samples, and a solvent blank sample. The precision expressed as a relative standard deviation (RSD) determined for a single sample did not exceed 5%, and the recovery for individual compounds from CRM 131 was on average 87%. The limit of quantification (LoQ) for individual PAH compounds ranged 0.02–2.10 μg kg−1, while the limit of detection (LoD) was within the 0.01–0.81 μg kg−1 range. The analyses were performed in relation to the US EPA Method 8000C US EPA: 2003 as well as EN 16181: 2018.
2.3 Assessment of potential ecological risk
Risk assessment connected with PAH concentration in bottom sediments was carried out using biota-sediment accumulation factor (BSAF) and sediment quality guidelines (SQGs). The BSAF was calculated as the ratio between concentration of PAHs in mussels and concentration of PAHs in the bottom sediment. The PAH concentration was normalised to sediment with 1% (10 g kg−1) of TOC, as recommended (Macdonald et al. 2000; Kwok et al. 2013). The concentration of PAHs in bottom sediments was also compared with the consensus-based sediment quality guideline values referred to as TEC (threshold effect concentration) and PEC (probable effect concentration) (Macdonald et al. 2000). The hazard quotient (HQ) and the mean PEC quotient (PECq) for 16 PAHs were calculated (Tarnawski and Baran 2018). If HQ < 1, frequent adverse ecological effects are excepted. According to PECq values, bottom sediments were divided into four categories: non-adverse effect (PECq < 0.1), slightly adverse effect (0.1 < PECq < 0.5), moderate effect (0.5 < PECq < 1.0), and strong effect (PECq > 1.5) (Tavakoly Sany et al. 2014).
2.4 Statistical and graphical analysis
All statistical analyses (mean, standard deviation, minimum, maximum, the coefficient of variation (CV%), Pearson’s correlation matrix and principal component analysis (PCA)) were performed using the Statistica 13 and the Statgraphics Centurion (version XVII, Statpoint Technologies, The Plains, VA, USA) software package. Variability maps were created using the Surfer 8.0 software.
3 Results
3.1 Organic matter content
The total carbon (TC) content in bottom sediments ranged from 5.31 to 44.47 g kg−1 dw, with a mean content of 28.01 g kg−1 dw (Table 1). The average total organic carbon was 15.56 g kg−1 dw (Table 1). The determined TOC contents were, on average, 55.5% of the TC content, which indicates limited processes of organic matter formation in bottom sediments. Moreover, the TC and TOC were characterised by relatively small diversification of their content in the analysed sediments (CV = 24% and CV = 31%, respectively). Lower TOC concentrations were detected in the inlet zone (13.66 g kg−1 dw) and in the outlet zone (13.84 g kg−1 dw) of the reservoir compared to the middle zone (17.22 g kg−1 dw) (Fig. 2). A similar distribution was found for humic acid carbon (Cha), with contents ranging from 0.13 to 1.28 g kg−1 dw (Table 1, Fig. 2). The content of C extractable (Cext) (∑ Cha + Cfa) in the sediment samples ranged from 1.01 to 15.06 g kg−1 dw and Cnh (C non-hydrolysing) from 0.49 to 13.99 g kg−1 dw. The analysed individual carbon fractions accounted on average 58% of the TOC for Cext and 42% of the TOC for Cnh. This indicates that the humification process in sediments occurs slowly with the predominant content of extractable-labile fractions. Additionally, Cext fractions constitute the predominant part of TOC, and their concentration was the least heterogeneous (Cext = 39%) (Table 1). Moreover, the result showed that content of Cha was 18 times smaller than the content of carbon in the Cfa. The analysis of the distribution of the fulvic acid carbon (Cfa) content in the bottom sediment samples collected from the Rożnów reservoir revealed that the highest organic matter fraction was present in the reservoir outlet zone. Among the analysed organic matter fractions of sediments, the greatest variation was observed in the content of non-hydrolysing carbon Cnh (CV = 68%), and its highest value was noted in sediments from the reservoir middle zone. It should be highlighted that the Cnh contents shown in Fig. 2 clearly indicate the existence of different conditions in both reservoir zones. The DOC content was similar to Cha, with much less variation (CV = 28%) (Table 1).
3.2 Concentration of PAHs in bottom sediment
The total and bioavailable concentrations of ∑16 PAHs and individual compounds in the sediments are shown in Table 2, and spatial distribution of ∑16 PAHs in Fig. 2. The total ∑16PAH concentration in the surface sediments ranged 226–3980 μg kg−1 dw, with a mean of 1755.2 μg kg−1 dw. Phen was a predominant compound followed by Fln, PYR, BbF, CH, BaA, BaP, BkF, Flu, and Naph, whereas IPY, Bper, Anth, Acen, and Acyn were found at the lowest levels. The 16 US EPA PAHs were grouped into 2-ring (Naph), 3-ring (Acyn, Acen, Flu, Phen, and Anth), 4-ring (Fln, PYR, CH, and BaA), 5-ring (BbF, BkF, BaP, and DahA), and 6-ring (IPY and Bper) hydrocarbons. The mean concentration of lower molecular weight PAHs (2- and 3-ring) was 489.9 μg kg−1, accounting 27.9% of their total PAH content, while the mean concentration of higher molecular weight PAHs (4- to 6-ring) was 1265.3 μg kg−1, comprising around 72.1% of the ∑16 PAH (Table 2). The PAH profile was dominated by 4-ring hydrocarbons (47% of the total concentration of contaminants), with lower share of 3- and 5-ring compounds (25 and 20%, respectively). The 2- and 6-ring PAHs accounted only for 3 and 5%, respectively (Table 2). The highest variability in the concentration of PAHs was found for Bper, BaP, IPY, and Anth, while the lowest for Flu and Phen. However, the variability of their concentration, calculated at CV = 42%, suggests the medium variation in the total concentration of analysed hydrocarbons (Table 2, Fig. 2). In addition, the spatial distributions (Fig. 2) indicate the highest level of the total ∑16PAHs accumulation in the inlet zone (1937 μg kg−1 dw), while slightly less in the middle zone (1753 μg kg−1 dw) of the reservoir, and lowest in the outlet zone, close to the dam (1640 μg kg−1 dw).
The bioavailable concentrations of ∑16 PAHs in sediments ranged from 8.05 to 980 μg kg−1 dw, with a mean concentration of 256 ± 254 (μg kg−1 dw) (Table 2). The PYR was a predominant compound followed by Phen, Fln, BaA, BkF, BbF, and CH, whereas other PAHs were found at the lowest levels (< 10 μg kg−1 dw). Among all analysed hydrocarbons, 4-ring (66%) compounds dominated to the greatest extent, while other PAHs constituting 3- and 5-ring compounds (16%, respectively), as well as 2- and 6- ring compounds accounted for less than 2% of the total concentration (Table 2). The highest bioavailable concentration of ∑16 PAHs was recorded in the middle zone (355.3 μg kg−1 dw), slightly lower (283.5 μg kg−1 dw) in the inlet zone, while in the outlet zone the level of PAHs was almost 5 times lower (70.6 μg kg−1 dw) (Fig. 2), which accounted for 20%, 16%, and 4% of the total PAHs, respectively. The mean concentration of lower molecular weight PAHs (2- and 3-ring) was 44 μg kg−1 dw, making 17% of the total PAH mass, while the mean concentration of higher molecular weight PAHs (4- to 6-ring) was 212 μg kg−1 dw, comprising around 83% of the ∑ PAH mass (Table 2).
3.3 Sources of PAHs in bottom sediment
Different methods are used to identify the source of PAHs. These are proportion analysis, diagnostic PAHs ratios, and PCA analysis (Tobiszewski and Namieśnik 2012; Tavakoly Sany et al. 2014). It has been reported (Wu et al. 2019; Yunker et al. 2002;) that HMW PAHs (≥ 4-ring) are mainly generated by high-temperature combustion processes (e.g. vehicular exhaust and industrial coal combustion), while LMW PAHs are mainly generated by low- or moderate-temperature combustion processes (e.g. biomass combustion and domestic coal burning). The observed distribution of PAH in the studied sediments indicates the dominance of combustion over petrogenic sources (Tobiszewski and Namieśnik 2012; Zhao et al. 2017; Ugochukwu et al. 2019), and it is in line with earlier study of Baran et al. (2017). To better characterise possible PAH sources, several diagnostic molecular ratios (DMRs) were used in the study (Table 3). Due to their stability, the most commonly used PAH isomer ratios are Fln/(Fln+PYR), BaA/(BaA+CH), and IPY/(IPY+Bper) (Tavakoly Sany et al. 2014; Liu et al. 2017; Zhao et al. 2017; Klimkowicz-Pawlas et al. 2017; Shilla and Routh 2018; Pheiffer et al. 2018; Liang et al. 2019). A Fln/(Fln+PYR) ratio < 0.4, as well as BaA/(BaA+CH) and IPY/(IPY+Bper) < 0.2 indicate petroleum input (Zhao et al. 2017; Yunker et al. 2002). The combustion of coal and biomass is demonstrated if Fln/(Fln+PYR) and IPY/(IPY+Bper) is higher than 0.5 and BaA/(BaA+CH) is above 0.35 (Tobiszewski and Namieśnik 2012; Liang et al. 2019). The ratio of 0.2–0.35, 0.4–0.5, and 0.2–0.5 for BaA/(BaA+CH), Fln/(Fln+PYR), and IPY/(IPY+Bper), respectively, suggest mixed sources (especially liquid fossil fuel combustion and vehicle and crude oil spillage) (Jiao et al. 2015; Klimkowicz-Pawlas et al. 2017).
Some authors recommended other DMRs, e.g. LMW/HMW, Phen/Anth, Anth/(Anth+Phen), Fln/PYR or CH/BaA, as a reliable diagnostic tool to differentiate between pyrogenic and petrogenic origin. The values of LMW/HMW and CH/BaA < 1, Phen/Anth < 10, Anth/(Anth+Phen) > 0.1, and Fln/PYR > 1 suggest that PAHs originate from pyrogenic sources (Tavakoly Sany et al. 2014; Jiao et al. 2015; Shilla and Routh 2018; Ugochukwu et al. 2019). In this study, the Fln/(Fln+PYR) ratio ranged from 0.44 to 0.66 with a mean of 0.55, which indicates the prevalence of pyrogenic PAHs sources in 93% of sediment samples (Table 3, Fig. 3). This is confirmed by the BaA/(BaA+CH) values ranging from 0.20 to 0.80 with ratio above 0.35 in 89% of sediment samples, as well as the LMW/HMW ratio below 1 in all samples (0.23–0.91), and the Fln/PYR values above 1 in 93% of sediment samples. In the case of IPY/(IPY+Bper), values > 0.5 were reported in 64% of sediments, indicating combustion of coal, wood, or grass as a PAH source. However, in 29% and 7% of sediments, the IPY/(IPY+Bper) ratio was between 0.2–0.5 and < 0.2, respectively. This may suggest mixed PAH sources, liquid fossil fuel combustion, or a petroleum source. The possible origin of hydrocarbons from both pyrogenic and petrogenic sources is also confirmed by the Phen/Anth and CH/BaA ratios. The Phen/Anth ratio ranged from 3.98 to 91.98 and reached values above 15 (petrogenic origin) and below 10 (pyrogenic origin) in 31 and 56% of sediments, respectively. The CH/BaA ratio ranged between 0.26 and 3.91, and in 67% of sediments, it was > 1 (petrogenic source) and in 33% below 1 (values characteristic for pyrogenic PAHs origin).
In order to improve the accuracy of the PAH sources identification, the PCA analysis with varimax rotation was also applied to explore the sources fingerprint in sediments from the Rożnów reservoir (Table 4). The PCA analysis classified the dataset of PAHs into three principal components (PCs) that control 78% of the data variability. The first factor (43.4% of variance) had high loading values for 4-ring PAHs (Fln, PYR, CH, and BaA), and for 5-ring compounds (BbF, BkF, and BaP), suggesting the predominance of pyrogenic sources. The second factor corresponded to 23.1% of the total PAHs was dominated by Acen, Flu, Phen, Anth, Fln, and PYR. It is believed that these compounds are derived from petrogenic sources and are the tracers for volatilisation or spill of petroleum products (Yunker et al. 2002; Tavakoly Sany et al. 2014; Zhao et al. 2017). The third factor explained 11.5% of the total PAHs variance and was highly loaded on IPY, DahA, Bper, Naph, and Acyn (Table 4). Characteristic of PAHs related to PC3 indicates the mixed (petrogenic and pyrogenic) sources of these contaminants in sediment from the Rożnów reservoir. The Rożnów reservoir has been functioning for almost 80 years, and it encloses a mountainous catchment basin of an agricultural character, which is intensely subjected to intense siltation (Baran et al. 2019). Along with the flows and the sediment, impurities from the catchment area of more than 4800 km2 flow into the reservoir. The main sources of pollution are municipal sewage, craft plants including tanneries, agriculture, and products from the use of motor vehicles. Currently, the inflow of pollutants is significantly reduced as a result of the elevated construction of reservoirs, the operation of sewage treatment plants, and the reduction of illegal discharges from craft and industrial plants. Pollution may come from the reservoir’s own catchment area: tourist resorts, local pollution of tourist sites, and motorboat and sailing harbours. The higher content of PAHs in the reservoir middle zone (Fig. 2) is due to the location of marinas and motorboats in this area, which are the source of fuel leaks.
3.4 Organic matter impact on PAHs accumulation in bottom sediment
Results of correlations between total PAHs, potential bioavailable PAHs, and particular fractions of organic matter clearly indicate that the PAH concentration in bottom sediments depends on the stabile carbon forms Cnh. Generally, a significant positive correlation was found between the content of TOC (r = 0.331–0.504) and Cnh (r = 0.364–0.768) and the concentration of PAHs in bottom sediments (Table 5). The correlation analysis revealed only significant relationships between DOC and total ∑16PAHs and 3-ring compounds; Cfa and 2-ring PAHs; and Cha and 5- and 6-ring PAHs. Moreover, the highest correlation coefficient value with TOC and Cnh was observed for 4-ring PAHs, and it was higher for potentially bioavailable PAHs than for total PAHs. Principal component analysis (PCA) confirmed the above relation and also allowed to find interesting relationships between the content of organic matter fraction and PAHs in bottom sediments (Table 6). The other basic properties of bottom sediments (pH, granulometric fractions) were presented in our previous studies (Szara et al. 2020). The two PC factors described 66.6% of the variances. The first factor (PC1) accounted for 44.2% of the variance and was significantly positively correlated with the TOC, Cnh, total concentration of 3-, 4-, and 5-ring PAHs, ∑16PAHs, bioavailable concentration of all PAHs, and negatively to clay fraction. The second factor (PC2) explained only 17.4% of the variance with high positive loading on clay, TC, Cext, Cfa, DOC, total concentration of 2-ring PAHs, and negative correlation with sand fraction (Table 6). The first factor was found to be strongly significantly correlated with the PAHs and Cnh content, indicating that stable forms of organic matter play an important role in the accumulation, transport and sorption of total PAHs in bottom sediments, and hence, in the reduced bioavailability of these compounds. Moreover, PCA (PC2) and correlation analysis found significant correlations between labile forms of organic matter (Cext, Cfa, DOC) and total concentration of 2- and 3-ring PAHs. This implies that the Cfa and DOC can play an important role in the sorption and availability of these substances. The second factor suggests that the fine (silt and clay) fraction also has an important role in the concentration and distribution of PAHs in bottom sediments.
3.5 Ecological risk assessment for benthic fauna
3.5.1 Concentration of PAHs in mussels and BSAFs
The accumulation of PAHs in the mussel tissues showed variability with a concentration of the sum of 16 PAHs equal to 1740 ± 72.2 μg kg−1 (Table 2). The PAH profile in mussels was dominated by 4-ring hydrocarbons (37% of ∑16 PAHs), with lower share of 3- and 5-ring compounds (21% and 25%, respectively), while 2- and 6-ring PAHs accounted for only 8% and 9%, respectively (Table 2). Among the detected compounds, similarly as for bottom sediments, PYR and Phen were found at highest concentration, and the lowest concentration was determined for Bper. Moreover, the mussels contained nearly 3 times more HMW-PAHs relative to the LMW-PAHs. The BSAFs values of PAHs were in the range of 0.85 (Flu) to 6.61 (DahA) (Table 2). The highest mean values of BSAF were found for 2-ring PAHs (3.75), followed by 3-ring (3.06) > 5-ring (2.87) > 4-ring (2.07) > 6-ring (1.49) PAHs. The study also found a significant positive correlation between the concentration of PAHs in the mussel tissues and in the bottom sediments; correlation coefficient (at p ≤ 0.05) for total PAHs and for bioavailable PAHs was r = 0.98 and r = 0. 83, respectively. For comparison, in the study of Baumard et al. (1999), the total PAH concentration in the mussel tissues from the Western Baltic Sea was between 90 and 3900 μg kg−1 and the value of BSAFs ranged from 0.02 to 72. Other studies revealed that PAH concentration in mussels (Brachidontes sp. and Tagelus sp.) ranged from 348 to 1597 μg kg−1 and, in fish, showed a mean level of 1095 μg kg−1 in all tissues (Arias et al. 2009). Concentrations of LMW-PAHs in soft tissues of Mytilus spp. in different sampling sites: Grevelingen (the North Sea), Westerschelde (the North Sea), the Gulf of Gdańsk (the Baltic Sea) and Ile de Revv (Faxaflói Bay) were higher than HMW-PAHs. Additionally, Naph and Phen contributed most to total PAH concentrations in the mussel tissues with the percentage contribution of 36.1% to 90.9% (Olenycz et al. 2015).
3.5.2 Risk assessment based on SQGs
The sediment concentration of individual PAHs, such as Naph, Flu, Phen, Fln, CH, BaP, IPY, DahA, and Bper was ≤ TEC values and indicated a lower possibility of an adverse ecological effect. The concentrations of Acyn (69% of samples), Acen (95% of samples), PYR (20% of samples), and BaA (2% of samples) in sediments were between the TEC and PEC values. The highest values of hazard quotients (HQ) in sediments were found for 3-ring PAHs (0.27–1.43) and the lower for 6-ring PAHs (0.0–0.09). The HQ value > 1 was observed only for 3- and 4-ring PAHs in 13% and 4% of sediment samples, respectively. The mean PECq of PAHs ranged from 0.02 to 0.20. Significantly higher value of PECq was found for LMW PAHs than HMW PAHs in sediments. Generally, the ecological risk assessment revealed that the total concentration of PAHs was likely to cause a non-adverse effect (PECq < 0.1 in 58% of samples) or slightly adverse effect (0.1 < PECq <0.5 in 42% of samples). Our earlier studies showed that the ∑16PAH concentration in the surface sediments collected from ten reservoirs located in south-eastern Poland ranged from 150 to 33900 μg kg−1 dw (Baran et al. 2017; Tarnawski and Baran 2018). The total PAH concentration in bottom sediments was arranged in the following order: Rybnik > Rzeszów > Brzóza Królewska > Brzóza Stadnicka > Besko > Chechło > Ożanna > Głuchów > Narożniki. However, the PAH concentration in sediments depended on the location of the reservoir, i.e. the intensity of antopopression. Compared to the above PAHs level, their content in sediments of the Rożnów reservoir was rather low (Table 2).
4 Discussion
The determined values and differentiation of TC, TOC, and individual fractions of organic matter in samples of bottom sediments of the Rożnów reservoir resulted from the specific structure of the reservoir, including its coastline and, probably, also its depth, affecting shifting of sediments. In deeper zones of the reservoir, the shifting of bottom sediments and organic matter contained in them is lower than in shallower zones (Mielnik et al. 2009; Baran et al. 2019). The depth and the shifting of bottom sediments will directly determine not only the sediment content but also aeration and, consequently, transformation of organic matter deposited in them. This is confirmed by a much higher Chn content and a lower Cha content, especially Cfa in the reservoir middle zone, which clearly decreases in the outlet direction, indicating a more intense transformation of organic matter in this part of the reservoir. The relationship between the reservoir depth and the organic matter content in bottom sediments is also highlighted in the study of Rozpondek et al. (2017). It should be noted that there is a possibility of organic matter accumulation in shallower areas of the reservoir, and, as indicated by the studies, this is related to the distribution and death of aquatic vegetation (Rozpondek et al. 2017). According to Brzozowska et al. (2005) and Kowalczewska-Madura et al. (2011), in addition to the bottom sediment depth and shifting rate, temperature is an important factor affecting the accumulation and transformation of organic matter. Kowalczewska-Madura et al. (2011) demonstrated that decomposition is more intensive in bottom sediments coming from shallower areas with higher temperatures and better oxygenation.
Several studies found that TOC has an important role in the control of PAH concentration in sediments (Liu et al. 2008; Jiang et al. 2009; Chen and Chen 2011; Yang et al. 2011, 2016; Nascimento et al. 2017). However, other studies indicated no significant correlation between these parameters (Nudi et al. 2007; Mostafa et al. 2009). Yang et al. (2016) and Nascimento et al. (2017) found significant correlation only with the LMW-PAHs and TOC. The LMW-PAHs are connected with autochthonous TOC of plankton origin and allochthonous TOC of terrestrial origin. HMW-PAHs have affinity for black carbon (Nascimento et al. 2017; Lubecki et al. 2019). Some authors reported that non-hydrolysable carbon (Cnh) and black carbon (BC) are known as strong sorbent for PAHs and other organic pollutants in sediments (Feng et al. 2016; Lubecki et al. 2019; Baran et al. 2020). It was observed that the total and potential bioavailable concentration of PAHs had more often significant positive correlation with Cnh, then other humic substances (Cha and Cfa). In our opinion, Cnh can play an important role in the transport, distribution, and sorption of PAHs in the sediment of the Rożnów reservoir. Generally, the affinity of PAHs for Cnh is higher than that for Cha; however, in this study, the content of Cnh was the highest in organic matter, then Cha (Table 1). Thus, it can accumulate more hydrophobic PAHs. The affinity between PAHs and Cnh was present, because this fraction is more stable and stronger in the case of sorption of organic pollutants. Our results also indicated that Chn and 4- and 5-ring PAH concentrations in the bottom sediment are of the same origin. This is also indirectly visible in the results of the PCA analysis and a higher value of the PAHs correlation coefficient with Chn than with TOC. This indicates their deposition as a result of high-temperature combustion and co-emission from black carbon, shoot, charcoal, or external organic materials: sewage, agricultural leachate. Other authors also observed higher binding capacities for inorganic and organic pollutants by non-hydrolysing carbon than by humic and fulvic acids (Zhang et al. 2009; Yang et al. 2011; Bai et al. 2018). The study of Ukalska-Jaruga et al. (2019) demonstrated significant relation between Cnh and PAHs and no links between Cfa, Cha, and hydrocarbons in soil. Moreover, the above authors observed stronger links between Cnh and PAHs for LMW PAHs than for HMW PAHs. Zhang et al. (2009) clearly indicated that more aromatic carbon in Cnh fractions can result in higher Koc values of BaP and the dominance of Cnh in BaP sorption by sediments than other fractions of organic matter. Non-hydrolysable carbon is the insoluble fraction of organic matter, and, with comparison to other humic substances, it is relatively resistant to decomposition (Bai et al. 2018). Thus, we may expect a higher correlation of PAHs with Cnh than TOC. The Chn surface is covered with various functional groups: C(C–H/C–C), C–O(C–OH/C–O–C), C=O, and –COO, which may interact with PAHs possibly via multiple mechanisms, such as hydrophobic interactions including alkyl and aromatic carbon domains and π-π interactions with aromatic carbon domains (Ukalska-Jaruga et al. 2019; Ukalska-Jaruga and Smreczak 2020).
In an aquatic environment, the relation of PAHs with DOC (labile form of organic matter) is multi-threaded and can reduce the freely dissolved concentration, increase solubility or enhance diffusive mass transfer, and may have a large impact on their bioconcentration in the organisms (Tejeda-Agredano et al. 2014). The study of Tejeda-Agredano et al. (2014) revealed a strong correlation between 5- or more-ring PAHs and DOC and no significant relationships between 4- or fewer-ring PAHs. In contrast, significant relation between labile fraction of organic matter and 3-ring PAHs was observed in our study. However, it should be emphasised that significant relationship between Cfa, DOC, and PAHs was rarely observed, which indicates that these fractions of organic matter will not be important in the accumulation and distribution of PAHs in the bottom sediments of the Rożnów reservoir.
The sediment grain-size distribution is also an important agent governing PAH concentration, and it should be taken into consideration (Orecchio and Mannico 2010; Baran et al. 2017). In the study, a significant correlation was also observed between PAH concentration and the silt and clay fraction, but a significant negative correlation for PAHs and the sand fraction. The TOC content is commonly associated with the amount of silt and clay present in sediments. The finest fractions, such as silt and clay, have larger relative surface areas than sand particles and can adsorb colloidal and dissolved organic matter forming sedimentary complexes. Orecchio and Mannico (2010) reported that the interaction of PAHs with the mineral fraction of the sediment and their distribution in the organic matter are likely mechanisms reducing PAHs in sediments. Other authors found that the accumulation of PCDDs/Fs and PAHs were also affected by the proportion of the finest particles, containing organic matter as the important sorbent (Baran et al. 2020).
The bioaccumulation of PAHs in aquatic organisms is related to several factors, such as the concentration and Kow value of each PAHs, the lifestyle of organisms and their capacity to metabolise PAHs, and the content of organic matter in sediments (Arias et al. 2009; Al-Busaidi et al. 2013; Honda and Suzuki 2020). The mussels, as filter-feeding organisms, take up pollutants in two ways: the direct one is the absorption of substances from water phase through the gills, and the indirect one is the absorption of substances adsorbed on the small grain-size fraction of particles through the digestive system (Baumard et al. 1999). Therefore, mussels can directly absorb LMW PAHs, while HMW PAHs are mainly ingested through the digestive system. For instance, Phen and PYR are absorbed in 88% and 74%, respectively, while BaP is absorbed through particle ingestion (Al-Busaidi et al. 2013). In the present study, slightly higher values of the BSAF coefficient were observed for LMW PAHs than for HMW PAHs. In general, our results were expected, since LMW PAHs with low Kow values tend to be more mobile between sediments and water, causing higher bioaccumulation in the organisms. Moreover, lipophilic compounds, e.g. HMW PAHs, have a higher tendency to bind to the sediment organic matter and show less partitioning from sediments to water and therefore lower mobility and bioaccumulation in the organisms (Kwok et al. 2013; Honda and Suzuki 2020). Generally, it is believed that PAHs availability is reduced in sediments of high organic matter content. In addition, PAHs are strongly adsorbed on the sediment grains (fine fraction), and their bioavailability decreases compared to the sandy sediments (Orecchio and Mannico 2010). In sediments rich in fine fraction, the filtrating behaviour of the mussels can be related to the high PAH concentration in their tissues, because mussels take up from the finest fraction not only nutrients through filtering, but also pollutants. The mussels exposed to sandy sediments are therefore not highly exposed to fine fraction-adsorbed pollutants. The low organic carbon content is generally observed in sandy sediments; the binding of hydrophobic PAHs is reduced, making the dissolved fraction of hydrocarbons the major source of contamination with PAHs (Baumard et al. 1999; Orecchio and Mannico 2010). Therefore, suspended and deposited particular-bound forms and aqueous phases are the most bioavailable fractions of PAHs metabolised by filter-feeding organisms. We found positive correlation between PAHs and the silt and clay fraction and negative with sand. Therefore, the dominance of fine particles in bottom sediment from the Rożnów reservoir can significantly increase the bioavailability of PAHs and be an important factor in the distribution of PAHs in the analysed sediments.
5 Conclusions
The PAH profile in bottom sediments and mussels was dominated by 4-ring hydrocarbons. The mussels contained nearly 3 times more HMW PAHs relative to LMW PAHs. A significant positive correlation between the concentration of PAHs in the mussel tissues and the total (at p ≤ 0.05; r = 0.98) and bioavailable (at p ≤ 0.05; r = 0.83) concentration of PAHs in the bottom sediments was observed. The ecological risk assessment revealed that the total concentration of PAHs was likely to cause a non-adverse effect (58% of samples) or slightly adverse effect (42% of samples). Results of correlations and PCA indicate that the PAH concentration in bottom sediments depends on the stabile carbon forms Cnh. The study revealed that stable forms of organic matter play an important role in the accumulation, transport and sorption of total PAHs in bottom sediments, and hence, in the reduced bioavailability of these compounds. Moreover, the PCA suggests that the fine (silt and clay) fraction can significantly increase the bioavailability of PAHs and be an important factor in the distribution of PAHs in the analysed sediments.
Data availability
Additional data is available on request.
References
Al-Busaidi M, Yesudhason A, Al-Waili W et al (2013) Accumulation of some potentially toxic metals and polycyclic aromatic hydrocarbons (PAHs) in marine clam Liochoncha ornata collected from the Omani Sea. Int J Fish Aquac 5(9):238–247
Arias AH, Spetter C, Freije RH, Marcovecchio JE (2009) Polycyclic aromatic hydrocarbons in water, mussels (Brachidontes sp., Tagelus sp.) and fish (Odontesthes sp.) from Bahı´a Blanca Estuary, Argentina. Estuar Coast Mar Sci 85(1):67–81
Bai H, Jiang Z, He M, Ye B, Wei S (2018) Relating Cd2+ binding by humic acids to molecular weight: a modeling and spectroscopic study. J Environ Sci (China) 70:154–165
Baran A, Tarnawski M, Urbański K, Klimkowicz-Pawlas A, Spałek I (2017) Concentration, sources and risk assessment of PAHs in bottom sediments. Environ Sci Pollut Res 24:23180–23195
Baran A, Tarnawski M, Koniarz T, Szara M (2019) Content of nutrients, trace elements and ecotoxicity of sediment cores from Rożnów reservoir (Southern Poland). Environ Geochem Health 41:2929–2948
Baran A, Mierzwa-Hersztek M, Urbaniak M, Gondek K, Tarnawski M, Szara M, Zieliński M (2020) An assessment of the concentrations of PCDDs/Fs in contaminated bottom sediments and their sources and ecological risk. J Soils Sediments 20:2588–2597
Baumard P, Budzinski H, Garrigues P (1999) Polycyclic aromatic hydrocarbons in recent sediments and mussels (Mytilus edulis) from the Western Baltic Sea: occurrence, bioavailability and seasonal variation. Mar Environ Res 47:17–47
Bordovskiy OK (1965) Accumulation of organic matter in bottom sediments. Mar Geol 3(1–2):33–82
Brzozowska R, Dunalska J, Zdanowski B (2005) Preliminary characteristics of the chemical composition of the top-layer bottom deposits in Lake Dejguny (Mazurskie Lake District). Limnol Rev 5:11–16
Cachada A, Pereira R, Ferreira da Silva E, Duarte AC (2014) The prediction of PAHs bioavailability in soils using chemical methods: state of the art and future challenges. Sci Total Environ 472:463–480
Chen CW, Chen CF (2011) Distribution. origin. and potential toxicological significance of polycyclic aromatic hydrocarbons (PAHs) in sediments of Kaohsiung Harbor, Taiwan. Mar Pollut Bull 63:417–423
EN 16181 (2018) Soil, treated biowaste and sludge - Determination of polycyclic aromatic hydrocarbons (PAH) by gas chromatography (GC) and high performance liquid chromatography (HPLC). https://sklep.pkn.pl/pn-en-16181-2018-09p.html
Feng J, Xi N, Zhang F, Zhao J, Hu P, Sun J (2016) Distributions and potential sources of polycyclic aromatic hydrocarbons in surface sediments from an emerging industrial city (Xinxiang). Environ Monit Assess 188:61
González-Fernández MA, Albentosa M, Campillo JA et al (2017) Effect of mussel reproductive status on biomarker responses to PAHs: implications for large-scale monitoring programs. Aquat Toxicol 177:380–394
Honda M, Suzuki N (2020) Toxicities of polycyclic aromatic hydrocarbons for aquatic animals. Int J Environ Res Public Health 17:1363. https://doi.org/10.3390/ijerph17041363
ISO 17402 (2008) Requirements and guidance for the selection of methods for the assessment of bioavailability of contaminants in soil and soil materials. https://www.iso.org/standard/38349.html
Jiang JJ, Lee CL, Fang MD, Liu JT (2009) Polycyclic aromatic hydrocarbons in coastal sediments of southwest Taiwan: an appraisal of diagnostic ratios in source recognition. Mar Pollut Bull 58:752–760
Jiao H, Rui X, Wu S, Bai Z, Zhuang X, Huang Z (2015) Polycyclic aromatic hydrocarbons in the Dagag Oilfield (China): distribution, sources, and risk assessment. Int J Environ Res Public Health 12:57756–55791
Klimkowicz-Pawlas A (2009) Effect of polycyclic aromatic hydrocarbons on the soil habitat function, vol 22. Institute of Soil Science and Plant Cultivation – State Research Institute, p 92 (In Polish)
Klimkowicz-Pawlas A, Smreczak B, Ukalska-Jaruga A (2017) The impact of selected soil organic matter fractions on the PAH accumulation in the agricultural soils from areas of different anthropopressure. Environ Sci Pollut Res 24(12):10955–10965
Kowalczewska-Madura K, Dondajewska R, Gołdyn R (2011) Total phosphorus and organic matter content in bottom sediments of lake under restoration measures with iron treatment. Limnol Rev 10:139–145
Kwok CK, Liang Y, Leung SY, Wang H, Dong YH, Young L, Giesy JP, Wong MH (2013) Biota–sediment accumulation factor (BSAF), bioaccumulation factor (BAF), and contaminant levels in prey fish to indicate the extent of PAHs and OCPs contamination in eggs of waterbirds. Environ Sci Pollut Res 20:8425–8434
Li Q, Wu J, Zhao Z (2018) Spatial and temporal distribution of Polycyclic Aromatic Hydrocarbons (PAHs) in sediments from Poyang Lake, China. PLoS One 13(10):e0205484
Liang X, Junaid M, Wang Z, Li T, Xu N (2019) Spatiotemporal distribution. source apportionment and ecological risk assessment of PBDEs and PAHs in the Guanlan River from rapidly urbanizing areas of Shenzhen, China. Environ Pollut 250:695–707
Liu Y, Chen L, Jianfu Z, Qinghui H, Zhiliang Z, Hongwen G (2008) Distribution and sources of polycyclic aromatic hydrocarbons in surface sediments of rivers and an estuary in Shanghai. China. Environ Pollut 154:298–305
Liu Y-P, Wang Y-H, Ye C, Xie B, Yang H (2017) Sedimentary record of polycyclic aromatic hydrocarbons from the Shuanglong Catchment, Southwest China. Hindawi J Chem 4976574:1–10. https://doi.org/10.1155/2017/4976574
Lubecki L, Oen A, Breedveld G, Zamojska A (2019) Vertical profiles of sedimentary polycyclic aromatic hydrocarbons and black carbon in the Gulf of Gdańsk (Poland) and Oslofjord/ Drammensfjord (Norway), and their relation to regional energy transitions. Sci Total Environ 646:36–346
Macdonald DD, Ingersoll CG, Berger TA (2000) Development and evaluation of Consensus-Based Sediment Quality Guidelines for freshwater ecosystems. Arch Environ Contam Toxicol 39:20–31
Mielnik L, Piotrowicz R, Klimaszyk P (2009) Chemical properties of bottom sediments in throughflow lakes located in Drawieński National Park. Oceanol Hydrobio St 38(3):69–76. https://doi.org/10.2478/v10009-009-0033-5
Mostafa AR, Wade TL, Sweet ST, Al-Alimi AKA, Barakat AO (2009) Distribution and characteristics of polycyclic aromatic hydrocarbons (PAHs) in sediments of Hadhramout coastal area, Gulf of Aden, Yemen. J Mar Syst 78:1–8
Nascimento RA, de Almeida M, Escobar N et al (2017) Sources and distribution of polycyclic aromatic hydrocarbons (PAHs) and organic matter in surface sediments of an estuary under petroleum activity influence, Todos os Santos Bay, Brazil. Mar Pollut Bull 119:223–230
Nudi AH, Wagener ALR, Francioni E, Scofield AL, Sette CB, Veiga A (2007) Validation of Ucides cordatus as a bioindicator of oil contamination and bioavailability in mangroves by evaluating sediment and crab PAH records. Environ Int 33:315–327
Olenycz M, Sokołowski A, Niewińska A, Wołowicz M, Namieśnik J, Hummel H, Jansen J (2015) Comparison of PCBs and PAHs levels in European coastal waters using mussels from the Mytilus edulis complex as biomonitors. Oceanologia 57:196–211
Orecchio S, Mannico MR (2010) Chemical speciation of polycyclic aromatic hydrocarbons in sediments: partitioning and extraction of humic substances. Mar Pollut Bull 60:1175–1181
Pheiffer W, Quinn LP, Bouwman H, Smith NJ, Pieters R (2018) Polycyclic aromatic hydrocarbons (PAHs) in sediments from a typical urban impacted river: application of a comprehensive risk assessment. Ecotoxicology 27:336–351
Qiao M, Wang C, Huang S, Wang D, Wang Z (2006) Composition, sources, and potential toxicological significance of PAHs in the surface sediments of the Meiliang Bay, Taihu Lake, China. Environ Int 31(1):28–33
Riding M, Kieron J, Doick-Francis L et al (2013) Chemical measures of bioavailability/bioaccessibility of PAHs in soil: fundamentals to application. J Hazard Mater 261:687–700
Rozpondek K, Rozpondek R, Pachura P (2017) Characteristics of spatial distribution of phosphorus and nitrogen in the bottom sediments of the water reservoir Poraj. J Ecol Eng 18(4):178–184. https://doi.org/10.12911/22998993/74277
Shilla DJ, Routh J (2018) Distribution, behavior, and sources of polycyclic aromatic hydrocarbon in the water column, sediments and biota of the Rufiji Estuary, Tanzania. Front Earth Sci 6:70. https://doi.org/10.3389/feart.2018.00070
Shulkin VJ, Presley BJ, Kavun V (2003) Metal concentrations in mussel Crenomytilus grayanus and oyster Crassostrea gigas in relation to contamination of ambient sediments. Environ Int 29(4):493–502. https://doi.org/10.1016/s0160-4120(03)00004-7
Szara M, Baran A, Klimkowicz-Pawlas A, Tarnawski M (2020) Ecotoxicological characteristics and ecological risk assessment of trace elements in the bottom sediments of the Rożnów reservoir (Poland). Ecotoxicology 29(1):47–59
Szarek-Gwiazda E (2014) Potential effect of pH on the leaching of heavy metals from sediments of the Carpathian dam reservoirs. Geol Geophys Environ 40(4):349–358
Tarnawski M, Baran A (2018) Use of chemical indicators and bioassays in bottom sediment ecological risk assessment. Arch Environ Contam Toxicol 74:395–407
Tavakoly Sany SB, Hashim R, Salleh A, Rezayi M, Mehdinia A (2014) Polycyclic aromatic hydrocarbons in coastal sediment of Klag Strait. Malaysia: distribution pattern. risk assessment and sources. PLoS One 9(4):e94907
Tejeda-Agredano MT, Mayer P, Ortega-Calvo JJ (2014) The effect of humic acids on biodegradation of polycyclic aromatic hydrocarbons depends on the exposure regime. Environ Pollut 184:435–442
Tobiszewski M, Namieśnik J (2012) PAH diagnostic ratios for identification of pollution emission sources. Environ Pollut 162:110–119
Ugochukwu UC, Onuorah LA, Okwu-Delunzu VU, Odinkonigbo UL, Onuora OH (2019) Effects of power station and abattoir on PAH input into sediments of Oji River: ecological and human health exposure risks. Environ Monit Assess 191(12):775. https://doi.org/10.1007/s10661-019-7917-y
Ukalska-Jaruga A, Smreczak B (2020) The impact of organic matter on polycyclic aromatic hydrocarbon (PAH) availability and persistence in soils. Molecules 25:2470. https://doi.org/10.3390/molecules25112470
Ukalska-Jaruga A, Smreczak B, Klimkowicz-Pawlas A (2019) Soil organic matter composition as a factor affecting the accumulation of polycyclic aromatic hydrocarbons. J Soils Sediments 19:1890–1900
US EPA Method 8000C (2003) Determinative chromatographic separations. https://settek.com/EPA-Method-8000C/
USEPA (1994) Methods for measuring the toxicity and bioaccumulation of sediment-associated contaminants with fresh water invertebrates. EPA/600/R-94/024. US Environmental Protection Agency, Office of Research and Development, Washington, DC, USA
Wu H, Sun B, Li J (2019) Polycyclic aromatic hydrocarbons in sediments/soils of the rapidly urbanized lower reaches of the River Chaohu, China. Int J Environ Res Public Health 16:2302. https://doi.org/10.3390/ijerph16132302
Yang Y, Shu L, Wan X, Xing B, Tao S (2011) Impact of de-ashing humic acid and humin on organic matter structural properties and sorption mechanisms of phenanthrene. Environ Sci Technol 45(9):3996–4002
Yang X, Yu L, Chen Z, Xu M (2016) Bioavailability of polycyclic aromatic hydrocarbons and their potential application in eco-risk assessment and source apportionment in urban river sediment. Sci Rep 6:23134
Yunker MB, Macdonald RW, Vingarza R, Mitchell RH, Goyette D, Sylvestre S (2002) PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Org Geochem 33:489–515
Zhang J, He M, Shi Y (2009) Comparative sorption of benzo[α]pyrene to different humic acids and humin in sediments. J Hazard Mater 166(2–3):802–809
Zhao Z, Qin Z, Cao J, Xia L (2017) Source and ecological risk characteristics of PAHs in sediments from Qinhuai River and Xuanwu Lake, Nanjing, China. Hindawi J Chem:3510796 18. https://doi.org/10.1155/2017/3510796
Funding
Financial support for this research was provided by the University of Agriculture in Krakow through the financial support of the the National Science Centre, grant no. 2016/21/B/ST10/02127: “Assessment of the bottom sediment organic matter on bioavailability and toxicity of chemical compounds”
Author information
Authors and Affiliations
Contributions
Agnieszka Baran: Conceptualization, methodology, formal analysis, writing — original draft, supervision, funding acquisition
Agnieszka Klimkowicz-Pawlas: Investigation, conceptualization, formal analysis, writing — review and editing, visualization
Aleksandra Ukalska-Jaruga: Investigation, writing — review and editing
Monika Mierzwa-Hersztek: Investigation, writing — review and editing
Krzysztof Gondek: Writing — review and editing
Magdalena Szara-Bąk: Resources, investigation
Marek Tarnawski: Resources, visualization
Iwona Spałek: Investigation
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Additional information
Responsible Editor: Jan Schwarzbauer
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 138 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Baran, A., Klimkowicz-Pawlas, A., Ukalska-Jaruga, A. et al. Distribution of polycyclic aromatic hydrocarbons (PAHs) in the bottom sediments of a dam reservoir, their interaction with organic matter and risk to benthic fauna. J Soils Sediments 21, 2418–2431 (2021). https://doi.org/10.1007/s11368-021-02968-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-021-02968-1