Abstract
Purpose
Although litter decomposition and nutrient release patterns have been studied in cocoa agroforestry systems in general, studies focusing on organic and conventional cocoa systems are lacking which is critical as organic farms are particularly dependent on nutrient returns from decomposing litter.
Materials and methods
Dynamics in leaf litter decomposition and the mineralisation of macro- and micro-nutrients in organic and conventional cocoa agroforestry systems were studied using the litterbag technique for 12 months.
Results
The average monthly mass loss was more than two times higher on organic farms (9.2–14.4 g month−1) compared to conventional farms (4.2–7.3 g month−1) in the first five months. The annual rate of decomposition (k) was higher on organic farms (1.9) compared to conventional systems (1.4). The time required for 50% (t50) and 99% (t99) decomposition of leaf litter was both lower on organic farms (t50 = 0.4 years, t99 = 2.6 years) than conventional farms (t50 = 0.5 years, t99 = 3.5 years). The estimated k values for macro- and micro-nutrients on organic cocoa systems ranged from 2.3 for calcium to 4.5 for potassium compared to 1.6 (Ca) to 2.8 (K) on conventional farms. The k values of all nutrients (except nitrogen and phosphorus) were significantly greater on organic farms than conventional systems. The estimated k values for both litter decomposition and nutrient mineralisation correlated with soil pH and moisture content, but not initial litter chemistry.
Conclusions
Organic management of smallholder cocoa agroforestry systems enhanced leaf litter decomposition and nutrient mineralisation through improved soil conditions. Thus, organic management of cocoa agroforestry systems may contribute to sustainable cocoa production in smallholder systems through enhanced nutrient return from litter decomposition.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
Cocoa production is worth over 12 billion US$ and provides livelihoods for 40–50 million people worldwide (Hütz-Adams et al. 2016). As the backbone of Ghana’s economy, cocoa production serves as the primary source of livelihood for 25–30% of Ghanaians (Kaba 2017). Although there is a growing demand for cocoa, its production is at cross-roads due to depletion of soil nutrients (ICCO 2014; Hütz-Adams et al. 2016; Kaba 2017). Depletion of soil nutrients and organic matter is a serious threat to sustaining cocoa production in West Africa and elsewhere (Daymond et al. 2017; Kaba 2017). Dwindling soil nutrients limit cocoa production in major cocoa-producing countries (Hütz-Adams et al. 2016; Daymond et al. 2017; Kaba 2017). Litter input from vegetation is a major pathway by which nutrients are returned to soils (Triadiati et al. 2011; Naik et al. 2018). Plant litter improves soil organic matter quality and quantity which in turn enhances soil quality through reducing bulk density and erosion, enhancing soil structure, increasing cation-exchange capacity, infiltration, water holding capacity, and the retention of soil nutrients (Murphy 2014; Bünemann et al. 2018). Additionally, plant litter enhances biodiversity and activity of soil microorganisms which underpins plant productivity (Barrios et al. 2018). Plant litterfall therefore plays a critical role in determining the physical, chemical and biological characteristics of soil as well as the productivity of an ecosystem.
Litter in general and leaf litter in particular is a central nutrient resource and litterfall is a critical link between plants and soils for the return and recycling of organic matter and nutrients (Hartemink 2005; Triadiati et al. 2011; van Vliet et al. 2015; Naik et al. 2018) and maintenance of soil fertility and ultimately contributes to the regulation of primary productivity in an ecosystem (Mamani-Pati et al. 2012; Fontes et al. 2014). Decomposition is a complex process that reduces dead organic matter or litter into mineral nutrients, water and carbon dioxide (Dawoe et al. 2010; Kaba 2017). The rate of litter decomposition in an ecosystem depends on the interaction of a variety of factors such as litter quantity and quality (e.g. concentration of nitrogen, phosphorus, lignin, polyphenols and their ratios), variety, composition and activities of decomposers, climatic conditions (particularly temperature and humidity), soil nutrient content and availability, age of vegetation or plantation, and vegetation and management types (Dawoe et al. 2010; Triadiati et al. 2011; Hasanuzzaman and Mahmood 2014; Kaba 2017; Naik et al. 2018).
Cocoa agroforestry is the practice of growing cocoa under a variety of shade species together with food crops (Dawoe et al. 2010; Asigbaase et al. 2019). The integration of trees into cocoa farms and its subsequent management can counteract the reduction of nutrient and organic matter content in soils through litter inputs from the shade species (Mamani-Pati et al. 2012; Fontes et al. 2014). Cocoa agroforestry systems in Ghana are either organically or conventionally managed. Conventional cocoa agroforestry systems rely on synthetic agrochemicals for nutrient replenishment and weed and pest control while the organic cocoa agroforestry systems rely on organic products as well as natural processes to supplement soil nutrients and control weeds and pests (Domínguez et al. 2014; Barrios et al. 2015; Lori et al. 2017). For example, organic farmers may opt to maintain shade species in their cocoa farms as a means to supplement soil organic matter and nutrients, reduce nutrient leakage and increase soil quality. Synthetic agrochemical-dependent cocoa systems (i.e. cocoa agroforestry systems under conventional management) pose threats to soil, animal and human health (Barrios et al. 2015) and their sustainability in the long run is questionable. Moreover, the use of synthetic agrochemicals can modify litter-soil biota and pose a threat to the decomposition processes (Domínguez et al. 2014; Barrios et al. 2015). In Ghana, it is common to remove shade trees in conventionally managed farms driven by the desire to increase yield (Asigbaase et al. 2019). Removal of shade trees pushes cocoa systems closer to monocultures thus reducing litter inputs from shade species.
According to Naik et al. (2018) and Kaba (2017), the rate of organic matter decomposition is critical to the functionality of any agroforestry system. Organic matter decomposition returns nutrients to the soil, reducing or possibly eliminating the need for chemical fertilizers, thus, contributing to their sustainability (Fontes et al. 2014; Costa et al. 2017; Asigbaase 2019). Additionally, decomposing organic matter enhances the physical and biological properties of soils (Domínguez et al. 2014; Bünemann et al. 2018). Although litter and nutrient decomposition has been studied in cocoa agroforestry systems in general, studies focusing on organic and conventional cocoa systems are rare. In agroforestry systems, nutrient supply rate and nutrient limitation are closely linked via management (Ofori-Frimpong et al. 2007; Kumar 2008; Mamani-Pati et al. 2012). Smallholder cocoa farmers rely heavily on natural nutrient recycling for soil fertility sustenance in their farms; it is therefore important to understand the dynamics of litter decomposition and nutrient release in organic and conventional cocoa systems as this will contribute to efficient management of these systems. We quantified and compared the rate of litter decomposition and nutrient mineralisation on organic cocoa agroforestry systems to conventional systems. We hypothesised that the rate of litter decomposition and nutrient return to the soil will be greater on organic cocoa systems than conventional systems due to improved soil conditions.
2 Materials and methods
2.1 Description of study site
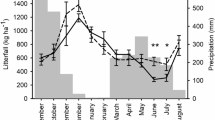
This study was conducted in two cocoa communities (Nsuta-wawase and Kuano) in Suhum Municipality (N 6° 5′ and W 0° 27′), Eastern Region, Ghana. The climate is tropical with a mean annual temperature and precipitation of 24–29 °C and 127–165 cm, respectively (Fig. 1). Relative humidity during the wet season (April to November) is 87–91% and the dry season (late November to March) is 48–52%. Cocoa production is the primary or sole source of livelihood for the population of Suhum. Certified organic cocoa production in Ghana begun in our study area and has spread to other parts of the country. The organic farmers are certified as a group by the Control Union, an international certification body active in over 70 countries. The conventional cocoa farmers rely on synthetic agrochemicals to replenish soil nutrients, control pests and supress weeds while the organic farmers rely on organic products and natural processes. In addition, organic farmers were encouraged to maintain/plant shade trees on their farms with the aim of exploiting the ecological benefits of shade trees, including contribution to nutrient return via litterfall and decomposition. Organic farmers pruned their cocoa trees once a year (i.e. March/April). Both the organic and conventional cocoa are produced under shade and details of the two systems in terms of species composition, yield and other biophysical characteristics are found in Asigbaase et al. (2019) and supplementary information (SI) (Appendix Tables 1 and 2). In brief, the cocoa trees were planted at 3 m × 3 m (i.e. approximately 1100 trees ha−1) under shade trees, meeting at least the recommended 12–18 emergent shade trees per ha and 40% shade cover (Ghana Cocoa Board 2018; Asigbaase et al. 2019).
Over 95% of the shade trees in the systems we evaluated were native tree species, with Citrus sinensis (L.) Osbeck, Holarrhena floribunda (G. Don) Dur and Schinz, Voacanga africana Stapf, Entandrophragma angolense (Welw.) C.DC., Milicia regia (A.Chev.) Berg and Persea americana Mill. as the dominant tree species (Asigbaase 2019). Since organic farming is an ecologically based production system, organic farmers were encouraged to exploit the ecological benefits of shade trees such as suppression of the growth of weeds, contribution to nutrient replenishment and provision of shade for the cocoa trees (Appendix Table 1). Organic farmers applied Elite organic fertilizer (N:P:K 3:4:4+9Ca+1Mg+0.04B+0.08Zn+11organic matter) at a rate of 988 kg ha−1 while conventional farmers used the cocoa fertilizer, ‘Asaasewura’ (N:P:K 0:22:18+9Ca+7S+6MgO), at the rate of 375 kg ha−1 (Ghana Cocoa Board 2018). Both organic and conventional systems had similar land use history, method of cocoa establishment and soil properties (Appendix Tables 2 and 3). The soils of the study area are well weathered and well drained, porous and loamy and are classified as forest ochrosols (FAO 1991).
2.2 Selection of cocoa farms
We randomly selected two cocoa villages from a list of organic and conventional cocoa-producing areas (provided by Ghana Cocoa Board, the regulator of the sector) in the Suhum Municipality of the Eastern Region of Ghana. Eight organic and eight conventional farms were randomly selected from separate lists of farmers involved in cocoa production in the two selected cocoa communities and 25 m × 25 m plots (one plot per farm) were established on their farms (Muoghalu and Odiwe 2011). The age of the cocoa farms ranged from 20 to 30 years on both farm types (four of the eight farms in each farm type were 20 years old). The research was conducted in private farms and the selected farmers agreed to participate in the research through oral consent.
2.3 Sample collecting and processing
We conducted the experiment using the litterbag technique over a 12-month period. Bulks of freshly fallen cocoa and shade tree leaf litter were collected from the floor of the selected cocoa farms in January 2017 (Muoghalu and Odiwe 2011; Hayashi et al. 2012). Leaf litter was air-dried for one week and thoroughly mixed (Muoghalu and Odiwe 2011; Naik et al. 2018). Seventy grams of leaf litter (ratio of cocoa leaves to shade tree species leaves was 1:1) from each farm was placed into 30 cm × 20 cm nylon netting litterbags with 2 mm mesh size, which is sufficient enough to prevent significant losses of leaves while large enough to support free entry of micro- and macro-organisms (Hayashi et al. 2012; Naik et al. 2018). Thirty-six litterbags were placed in each farm and three bags from each farm were oven dried at 70 °C for initial litter chemistry analysis (Guo et al. 2017; Naik et al. 2018). Three bags from each farm were subsequently retrieved monthly throughout the experimental period. Collected samples were gently and briefly washed under slowly running tap water, rinsed with distilled water, oven dried for 48 h at 70 °C and weighed to determine mass loss (Naik et al. 2018). Litter decomposition and nutrient mineralisation rates were expressed as loss of dry matter (DM) and percentage nutrient release per month (% mon−1), respectively. The litterbag experiment started on February 2017 and ended on March 2018.
2.4 Soil sampling and stand characteristics
Five soil samples were collected to the depth 0–15 cm in each plot using an Eijkelkamp soil auger (5 cm blade diameter). The soil samples for each plot were thoroughly mixed and subsampled for chemical analysis. Undisturbed soil samples (two per plot) were collected from the soil wall after digging for bulk density estimation using 139 cm3 bulk density cylinders. Both undisturbed soil samples were oven dried at 105 °C for 48 h. Soil subsamples were sieved with 2-mm mesh. All soil samples were collected on the same day (1 February 2017).
Data on stand characteristics (cocoa, fruit and shade tree species densities and basal areas, shade tree species richness and Shannon diversity, farm size, management practices) of the two farm types were obtained from the field data of Asigbaase (2019).
2.5 Data processing and chemical analysis
Oven-dried soil and litter samples were milled using an agate ball mill (Retch PM 400) at 290 rpm for 15 min and analysed for their chemical composition. Total C and N contents in the milled samples were obtained using CN analyser (Thermo Scientific™ Flash™ 2000 Organic Elemental Analyzer (OEA)). To determine the proportion of C and N, 5–6 mg of milled litter samples was combusted at 900 °C to produce nitrogen oxides, carbon dioxide and water which were eluted and detected. Six milliliters of concentrated HNO3 was added to 0.2 g of powered litter samples, microwave-digested and analysed for macro- and micro-nutrients (Online Resource 1) using ICP-MS (Thermo Scientific™ iCAP™ TQ). Chemical analyses for macro- and micro-nutrients were conducted every month, except C and N which were conducted every 3 months.
Attenuated total reflectance-Fourier transformed infrared (ATR-FTIR) spectroscopy was used to determine the organic composition of the powdered initial litter material. Specifically, we estimated 2919/1034, 2851/1034, 1617/1034, 1420/1034 and 1374/1034 ratios, following the procedure of Tonks et al. (2018). These wavelengths correspond to carbohydrates (1034), waxes, fats, lipids and lignin (2919/2851), lignin, and other aromatic/aliphatic carboxylates (1617), aliphatic structures (1420) and lignin and aliphatic structures (1374) (Artz et al. 2008; Lammers 2008; Tonks et al. 2018). Spectra were obtained using the FTIR spectrometer Bruker Tensor 27 FTIR equipped with N2 purge gas generator and an MCT detector. The scans (128) were obtained from the range 4000 to 400 cm−1 at a resolution of 4 cm−1 (Artz et al. 2008). Using a pressure applicator with a torque knob ensured that in pressing the powdered samples against the diamond crystal of the ATR device (DATR/KRS-5), the pressure applied was the same for all measurements. The background spectrum of the clear widow was obtained and subtracted from the spectrum of each sample before conversion into absorbance units. All spectra were normalised by subtraction of sample minimum followed by division by the average spectrum value for each sample (Artz et al. 2008). The peak heights of recalcitrant compounds (e.g. lignin and other aromatic or aliphatic structures) were divided by the peak heights of readily decomposable organic compounds to estimate the relative proportions of recalcitrant compounds in the initial litter material and their proportion every 3 months. In this instance, the lower the ratio, the lower the proportion of recalcitrant compounds to carbohydrates, and the faster the material will decompose.
The concentration of soil Al and exchangeable Ca, Mg, K and Na were determined using ICP-MS. Prior to the ICP-MS analysis, 2 g of each soil sample was extracted with 20 ml of 1 M NH4NO3, centrifuged at 3500 rpm for 30 min and filtering and diluting 1 ml of the supernatant with 9 ml of 2% HNO3. We determined soil pH in a 1:2.5 soil:solution slurry with a pH meter, calibrated with pH 4.01 and 7.00 buffer solutions. Soil moisture content (MC %) was calculated as the (S1/S2) × 100, where S1 is the fresh weight of soil minus oven-dried weight and S2 is the weight of oven-dried soil. We determined bulk density (BD g cm−3) as (W/V) × (100 − %CF)/100, where CF is coarse soil fraction, W is the weight of oven-dried soils and V is the volume of the bulk density cylinder. Laser ablation (Bechman Coulter LS 200) was used to evaluate soil particle size distribution and the soils were classified into textural classes using the USDA soil triangle (Soil Survey Division Staff 1993).
2.6 Data analysis
Annual leaf litter decay constants were estimated through regression analyses (Olson 1963) using the SigmaPlot (vs. 13). Decay constants of organic matter and macro- and micro-nutrients were obtained by using the model m = Ae−kt, where m is the % initial dry mass remaining at time t, A is a constant, k is the coefficient of the rate of decay per year and t is the time in years. The percentage of nutrients remaining was estimated as NR (%) = ((Ct × Mt)/(In × Im)) × 100, where NR = remaining nutrients (%), Ct = nutrient concentration at time t (mg/kg), Mt = oven-dry mass at time t (g), In = initial nutrient concentration (mg/kg) and Im = initial oven-dry mass (g). The time required for 50% (t50) and 99% (t99) decomposition of leaf litter and nutrients were computed as t50 = 0.693/k and t99 = 5/k (Olson 1963; Naik et al. 2018). We tested for normality for each variable using the Shapiro-Wilks W-test for homogeneity of variances; variables with variances which were not normally distributed were Box-Cox transformed. One-way ANOVA was used to assess mean differences in initial litter chemistry (including the ratios of recalcitrant compounds to carbohydrates), stand and soil characteristics, and decay constants of organic matter and nutrients. Repeated measures ANOVA was used to establish differences in mean values of nutrient remaining and litter chemistry; this analysis was restricted to the first 10 months of leaf litter installation because decomposition on organic farms was completed during this period. Spearman’s rank correlation and regression were used to relate decay constants with initial litter chemistry and soil physico-chemical properties. p values < 0.05 were considered significant.
3 Results
3.1 Initial litter chemistry and soil characteristics
Leaf litter from organic farms was 20 and 49% higher in S and Fe respectively compared to conventional farms (Table 1). The initial mean values for other plant macro- and micro-nutrients as well as the litter C to N ratio were similar between organic and conventional farms. The ratio of recalcitrant compounds (i.e. lignin, aromatic or aliphatic structures) to carbohydrates in the initial litter material was similar for both organic and conventional cocoa systems (Appendix Table 3). Organic farms had greater soil moisture content and pH than conventional farms (Appendix Table 4). Soil bulk density, effective cation-exchange capacity and the ratios, Ca:Mg, (Ca+Mg):K and (Ca+Mg):(K+Na), were similar in both organic and conventional farms. Organic and conventional farms had similar proportions of clay, silt and sand and their soils were classified as loam.
3.2 Mass loss of leaf litter on organic and conventional cocoa farms
The decomposition of leaf litter expressed as loss of dry matter (DM) followed a similar pattern on both farm types but was more rapid on organic farms than conventional farms (Fig. 2; Appendix Fig. 1–4). Compared to conventional farms, the average mass loss per month on organic farms was greater by up to 300% in the first and second months (i.e. March and April 2017) after litterbag installation, 200% from the third to fifth month (i.e. May–July 2017) and 20–50% from the sixth to ninth month (August–November 2017). The mass of dry matter in May 2017 was relatively lower than that of June 2017 (Fig. 2). The annual rate of litter decomposition (k) was 34% greater on organic farms than conventional farms (Table 2). The time required for 50% (t50) and 99% (t99) decomposition of leaf litter on organic farms was both 34% lower than on conventional farms. The annual rate of litter decomposition (k) was positively related to initial soil moisture content and pH, but negatively related to soil C:N ratio (Fig. 3). The ratios of recalcitrant compounds to carbohydrates were consistently lower on organic farms than conventional farms, but this depended on the month (Fig. 4; Table 3). The ratios 2919/1034, 2851/1034 and 1420/1034 were significantly lower in decomposing litter material on organic farms during August and November compared to conventional farms, but these ratios were similar during March and May. However, the ratios 1617/1034 and 1374/1034 differed between the farms during November, but were similar during March, May and August. Litter chemistry (ratio of recalcitrant compounds to carbohydrates) changed over time (months).
Changes in the ratio of recalcitrant compounds to carbohydrates over time (months) in decomposing leaf litter on organic and conventional farms at Suhum. Means ± SEM are shown. The panels a–e represents the wavelength ratios 2919/1034, 2851/1034, 1617/1034, 1420/1034 and 1374/1034 respectively. The darker line represents organic farms and the light dark line represents conventional farms. Number of replicates (n) is eight (8) per farm type
3.3 Macro-nutrients release dynamics
On both organic and conventional farms, the release of N from leaf litter followed the same pattern but the average release per month was 30, 20 and 9% greater in the third, sixth and ninth months, respectively, on organic compared to conventional systems (Fig. 5; Table 4; Appendix Fig. 1 and 3). At the end of the first three months of decomposition, more than 40% of the N content in leaf litter from both organic and conventional farms was released.
Decay pattern of macro-nutrients (mean ± SEM) of leaf litter on organic and conventional cocoa farms at Suhum. The panels a–f represent N, S, P, Mg, K and Ca, respectively. The darker lines represent organic farms and the light dark lines represent conventional farms. Number of replicates (n) is eight (8) per farm type
Primary (P and K) and secondary (S, Ca and Mg) macro-nutrients in leaf litter on organic farms were rapidly mineralised during the first to fourth months, with the most rapid mineralisation for K followed by S, P, Mg and Ca. Thereafter, these nutrients were gradually released over the rest of the experimental period (Fig. 5). Nutrient release was consistently higher on organic farms than conventional farms (Fig. 5; Table 4). On conventional farms, the mineralisation of these nutrients was gradual from the first to the twelfth month, except P which showed a rapid decrease in the first month. While the annual decomposition rate constant for K, Mg and Ca on organic farms was 44–59% higher than conventional farms, it was 92% higher for S (Tables 2 and 4). On organic farms, estimated t50 and t99 values for K, S, Mg and Ca were consistently 44–89% lower than conventional systems. While the rate of annual Mg, Ca and S mineralisation was positively correlated with initial soil moisture content and pH, the rate of annual K mineralisation was negatively associated with soil C:N ratio (Appendix Table 5).
3.4 Micro-nutrients release dynamics
The mineralisation of micro-nutrients was significantly higher on organic farms than conventional farms (Fig. 6a–g; Tables 2 and 4; Appendix Fig. 2 and 4). Specifically, from the first to the fourth month of decomposition, the micro-nutrients B, Mn, Mo, Cu, Fe, Ni and Zn were rapidly released on organic farms and then gradually released from the sixth to the twelfth month (Fig. 6a–g). On conventional farms, the aforementioned micro-nutrients were generally gradually released from the first month to the twelfth month, except during the third month where nutrient release was rapid for Mn, Mo, Fe, Ni and Zn. On conventional farms, the concentration of Fe (F1, 14 = 7.67, p = 0.015) and Zn (F1, 14 = 10.78, p = 0.005) increased during the fourth month. While the annual k values for Ni, Mo and Cu were 28–38% greater on organic farms than conventional farms; it was 46–69% greater for Fe, Mn, Zn and B (Table 2). The t50 and t99 values of the aforementioned micro-nutrients were 28–69% lower on organic farms compared to conventional farms. Micro-nutrients were released in the order Fe > B > Zn > Mo > Mn > Ni > Cu in organic farms and in the order Fe > Cu > Ni > Mo > Cu > Zn > Mn > B on conventional farms. The k values for B, Fe and Cu correlated positively with both soil moisture content and pH, Mo with moisture content, and Ni and Zn with pH.
Decay pattern of micro-nutrients in leaf litter on organic and conventional farms over 12 months. Mean ± SEM are shown. The panels, a–g, represent B, Mn, Mo, Cu, Fe, Ni and Zn, respectively. The darker lines represent organic farms and the light dark lines represent conventional farms. Number of replicates (n) is eight (8) per farm type
4 Discussion
4.1 Initial litter chemistry
Initial litter quality on both organic and conventional cocoa farms is comparable to and fall within the values reported by Kaba (2017) in Ghana and Rojas et al. (2017) in Colombia. The greater levels of S and Fe in leaf litter on organic farms compared to conventional farms are likely because organic farms maintained greater shade tree species diversity (Asigbaase et al. 2019; Appendix Table 2), and that leaf litter inputs from these trees accounted for the observed differences. Fontes et al. (2014) found that cocoa leaves served as a sink for nutrients while shade tree leaves served as a source. Moreover, Wood et al. (2006) asserted that for non-limiting nutrients such as Fe, there is a large degree of plant control over the amount of soluble nutrients that are resorbed before leaf abscission. The C to N ratios reported in this study for both farm types are similar to the ratio of 31.6 ± 2.7 reported for 30-year-old cocoa systems but lower than the 42.9 ± 1.5 reported for 15-year-old cocoa systems by Dawoe et al. (2010). The high C/N ratio (> 25) and low N content (< 2%) suggest that decomposition on both farms was partly regulated by leaf litter chemistry or quality; ‘high-quality litter’ (> 2% N and C/N ration below 25) generally decomposes more quickly due to leaching of readily soluble substances and non-lignified carbohydrates (Kaba 2017; Naik et al. 2018). For example, low-quality litter material (C/N > 25) releases N relatively more slowly than high-quality litter material (Naik et al. 2018).
4.2 Litter decomposition
Mass loss on both farms followed an exponential decay pattern where the rate of decomposition gradually decreases with increase in duration after litter installation, a pattern reported in several other studies (Majumder et al. 2010; Triadiati et al. 2011; Hayashi et al. 2012; Kaba 2017; Mohammed et al. 2019). The initial rapid mass loss is attributable to the leaching and breakdown of readily soluble substances, non-lignified carbohydrates and other labile fractions as reported in other studies (Issac and Nair 2005; Kumar 2008; Dawoe et al. 2010; Triadiati et al. 2011). The assertion that 30–50% of leaf biomass decomposes in the first 3–4 months in tropical agroforestry and plantation systems (Kumar 2008) was supported by our study as more than 30% of leaf biomass was lost within this period (Fig. 2). The more gradual mass loss in the latter stages is likely linked to the accumulation of recalcitrant fractions such as cellulose, lignin, fats, waxes and tannin in leaf litter as decomposition progresses (Kumar 2008; Fontes et al. 2014; Naik et al. 2018). Moreover, leaf litter in cocoa systems is predominantly cocoa leaves which are known to contain higher levels of lignin and polyphenol than forest trees (Dawoe et al. 2010). Litterbags retrieved between May 2017 and June 2017 contained termites (personal observation); it is possible their activities accelerated localised decomposition rates during these months accounting for the relatively lower mass of dry matter in June 2017 compared to July 2017. The rate of decomposition and mineralisation of leaf litter in cocoa systems is influenced by litter quality, soil organisms and physical environment (Kumar 2008; Fontes et al. 2014). Initial litter quality (e.g. C/N, C, N, P and K) was similar on both farm types but decomposition occurred at different rates suggesting that extrinsic factors, both environmental and biological and their interaction with leaf litter quality, possibly accounted for the differences in decomposition rates on the two farm types (Kumar 2008). This is partly confirmed by the fact that leaf litter k was related to soil moisture, pH and C:N (Fig. 3). The incorporation of pruned materials plus other organic management practices (Appendix Table 1) possibly enhanced soil condition in the organic farms (Appendix Table 4).
While the decay rate coefficient (k = 1.9) reported in this study for organic cocoa agroforestry systems is comparable to Indonesian natural forests during the wet period (k = 1.87) (Triadiati et al. 2011) and secondary forests in eastern Amazon (k = 1.2–1.9) (Hayashi et al. 2012), it is higher than the 0.46–1.11 for cocoa agroforestry systems in Brazil (Fontes et al. 2014), the 0.15 reported for secondary mixed deciduous forests in Northern Thailand (Podong et al. 2013) and the 0.35 for secondary forests in the Ashanti region of Ghana (Dawoe et al. 2010). The differences in k between our study and the aforementioned studies may be due to differences in climatic and soil conditions or litter chemistry. For example, in the study of Dawoe et al. (2010), the soil pH of the secondary forest was 5.7 compared to 6.8 in our organic farms. The rapid decomposition in organic systems accelerates nutrient return to the soils, thus enhancing their availability to cocoa and shade trees for growth and productivity.
The greater coefficient of decomposition observed on organic compared to conventional cocoa farms is attributable to greater moisture content and pH which influences the composition and activities of decomposer communities (Fig. 3; Domínguez et al. 2014; Lori et al. 2017). In addition to pH and soil moisture, other site conditions such as micro-climate, soil fertility and evapotranspiration reportedly moderate litter decomposition by influencing decomposer biomass, microbial and enzyme activities (Wood et al. 2006; Fontes et al. 2014). Microbes play an essential role in nutrient cycling in ecosystems and organic matter is a central source of energy for microbial life (Kumar 2008). Though we did not measure the impact of agrochemicals on decomposer communities, its use on conventional farms possibly altered decomposer communities thus accounting for lower decomposition and nutrient release rate whereas more adapted soil biota and local decomposers enhanced decomposition on the organic farms (Domínguez et al. 2014; Barrios et al. 2015; Lori et al. 2017). High nutrient resorption which characterises plants on nutrient-deficient soils leads to low litter quality and decomposition rates (Wood et al. 2006; Kumar 2008). The greater rate of leaf litter decomposition on organic farms enhances nutrient release, making them available in the soil for plant uptake.
4.3 Nutrient release dynamics
Our finding of greater rate of nutrient release on organic farms than conventional farms is in line with findings from agricultural systems (Fließbach et al. 2000; Vazquez et al. 2003; Domínguez et al. 2014). The chemical structure of leaf litter moderates the release or immobilisation of its nutrient contents (Kumar 2008). For example, non-structural elements such as K are rapidly lost from the organic material when the cell wall breaks down during decomposition and soluble P containing compounds are easily leached during decomposition compared to N which participates in the structural section of organic compounds; hence, it is slowly released during decomposition (Issac and Nair 2005; Hossain et al. 2011; Dawoe et al. 2010; Naik et al. 2018). Ordóñez-Fernández et al. (2015) indicated that K is highly soluble and mobile, that implies environmental conditions such as soil moisture or rainfall may possibly accelerate its release from decomposing matter compared to other nutrients. Additionally, preferential retention of N by microbes while K or P is being rapidly released from the substrate may account for the faster release of P and K than N (Singh and Sherhar 1989). Thus, the primary macro-nutrients mineralisation pattern (K > P > N) reported in our study may be related to whether they form part of the structural components of plants or not, preferential retention by microbes, their solubility or an interaction of these factors. The decomposition and nutrient mineralisation of Albizia procera leaf litter reportedly followed the pattern K > N > P in Central Indian agroforestry systems (Gupta et al. 2017). This disparity is possibly because differences in initial nutrient concentration, their ratios and the proportion of recalcitrant compounds (which exist among different tree species) influence the rate and pattern of nutrient release (Dawoe et al. 2010; Majumder et al. 2010; Hossain et al. 2011; Kaba 2017; Naik et al. 2018). For example, Dawoe et al. (2010) reported higher levels of recalcitrant compounds (e.g. lignin and polyphenols) in cocoa leaves than shade tree leaves. Furthermore, whereas mixed leaf litter from cocoa and diverse shade tree species were used in our study, the study of Gupta et al. (2017) used a single tree species; composition of litter material (mixed vs. single species) influence the pattern of decomposition. The concentration of N and P in the substrate decreased at every sampling point (Fig. 5), similar to the findings of Kaba (2017), but contrary to other workers (e.g. Lin et al. 2007; Majumder et al. 2010; Hossain et al. 2011; Hasanuzzaman and Mahmood 2014) who observed an increase and attributed it to microbial or non-microbial immobilisation.
The rapid release of secondary macro-nutrients and its release pattern (S > Mg > Ca) reported in this study is similar to Fontes et al. (2014) but contrary to the findings of Kaba (2017) possibly due to differences in physico-chemical properties of the litter material or environmental conditions. For example, Kaba (2017) reported a C:N ratio of 11–26 compared to the C:N ratio of 30–34 reported in our study; differences in initial nutrient concentration and their ratios influence the rate and pattern of decomposition and nutrient release. Ca is a structural component and generally non-mobile; thus, its release is slower than Mg which exists mainly in solution in plant cells hence quickly released during decomposition (Hayashi et al. 2012; Ranjbar and Jalali 2012). S is a component of plants protoplast and some amino acids that join together to form proteins; thus, it is easily leached during decomposition (Mahler 2004; Schroth et al. 2007). While in living tissues, S easily leaches out into the soil via roots during precipitation or irrigation (Mahler 2004); thus, the higher moisture content in the organic farms may explain its greater rapid mineralisation on the organic farms. Similarly, the rapid mineralisation of micro-nutrients (i.e. Mn, Fe, B, Ni, Cu, Zn and Mo) on organic farms compared to conventional systems (Table 2) may be due to enhanced soil moisture content, pH, changes in the ratio of recalcitrant materials to carbohydrates and their interaction (Fig. 4; Table 3; Appendix Table 5; Staaf 1980). Moreover, Lin et al. (2007) stated that leaf litter serves as a surface for fungi or heterotrophic organisms. The subsequent release of nutrients in the residual material after the period of immobilisation may be attributed to microbial oxidation of recalcitrant litter components and physical-biological fragmentation (Gupta et al. 2017; Naik et al. 2018). Different species have different nutrient release patterns, which are attributable to an interaction between litter quality and seasonal environmental factors (Hartemink 2005; Wang et al. 2014; Yang and Zhu 2014; Gupta et al. 2017; Lori et al. 2017). Furthermore, litter chemistry changes over time as decomposition progresses (Fig. 4), thus continuously affecting the rate of substrate decomposition and nutrient mineralisation (Kumar 2008; Domínguez et al. 2014; Lori et al. 2017).
5 Conclusion
The findings showed that mass loss and nutrient release follow the same pattern on both organic and conventional cocoa farms, but at a faster rate on organic farms. On an annual basis, litter was more readily decomposed, and its nutrients were more readily released on organic farms than conventional farms. The release of primary and secondary macro-nutrients was in the order K > S > Mg > Ca and it was faster on organic farms than conventional farms. This study demonstrates that organic management of smallholder cocoa agroforestry systems enhanced leaf litter decomposition and nutrient mineralisation and thereby make essential nutrients available to plants for growth and productivity. Thus, organic management of cocoa agroforestry systems may potentially contribute to sustainable cocoa production in smallholder systems through enhanced nutrient return from litter decomposition.
Data availability
All relevant data are within the paper and its Supporting Information file (Online Resource 1). All data are fully available without restriction.
References
Artz RRE, Chapman SJ, Robertson AHJ, Potts JM, Laggoun-Defarge F, Gogo S, Comont L, Disnar J-R, Francez A-J (2008) FTIR spectroscopy can predict organic matter quality in regenerating cutover peatlands. Soil Biol Biochem 40:515–527
Asigbaase M (2019) Contribution of organic cocoa agroforestry to sustainable land management. PhD thesis, School of Biosciences, University of Nottingham, UK
Asigbaase M, Sjogersten S, Lomax BH, Dawoe E (2019) Tree diversity and its ecological importance value in organic and conventional cocoa agroforests in Ghana. PLoS One 14(1):e0210557. https://doi.org/10.1371/journal.pone.0210557
Barrios E, Shepherd K, Sinclair F (2015) Agroecology for food security and nutrition; Proceedings of the FAO International Symposium on 18–19 September 2014, Rome, Italy. Food and Agriculture Organization of the United Nations, Rome
Barrios E, Valencia V, Jonsson M, Brauman A, Hairiah K, Mortimer PE, Okubo S (2018) Contribution of trees to the conservation of biodiversity and ecosystem services in agricultural landscapes. Int J Biodivers Sci Ecosyst Serv Manag 14:1–16. https://doi.org/10.1080/21513732.2017.1399167
Bünemann EK, Bongiorno G, Bai Z, Creamer RE, De Deyn G, de Goede R et al (2018) Soil quality – a critical review. Soil Biol Biochem 120:105–125
Costa PMO, de Araújo MAG, de Souza-Motta CM, Malosso E (2017) Dynamics of leaf litter and soil respiration in a complex multistrata agroforestry system, Pernambuco, Brazil. Environ Dev Sustain 19:1189–1203. https://doi.org/10.1007/s10668-016-9789-4
Dawoe EK, Isaac ME, Quashie-Sam J (2010) Litterfall and litter nutrient dynamics under cocoa ecosystems in lowland humid Ghana. Plant Soil 330:55–64
Daymond AJ, Acheampong K, Prawoto A, Abdoellah S, Addo G, Adu-Yeboah P et a (2017) Mapping cocoa productivity in Ghana, Indonesia and Côte d’Ivoire. International Symposium on Cocoa Research (ISCR), Lima, Peru, 13-17 November 2017
Domínguez A, Bedano CJ, Becker AR, Arolfo RV (2014) Organic farming fosters agroecosystem functioning in Argentinian temperate soils: evidence from litter decomposition and soil fauna. Appl Soil Ecol 83:170–176
FAO (1991) World soil resources; world soil resources report 66. FAO, Rome
Fließbach A, Mäder P, Niggli U (2000) Mineralization and microbial assimilation of 14C-labeled straw in soils of organic and conventional agricultural systems. Soil Biol Biochem 32:1131–1139
Fontes AG, Gama-Rodrigues AC, Gama-Rodrigues EF, Sales MVS, Costa MG, Machado RCR (2014) Nutrient stocks in litterfall and litter in cocoa agroforests in Brazil. Plant Soil 383:313–335. https://doi.org/10.1007/s11104-014-2175-9
Ghana Cocoa Board (2018) Manual for cocoa extension in Ghana. CCAFS manual. Ghana Cocoa Board, Ghana. Available at: https://ccafs.cgiar.org/publications/manual-cocoa-extension-ghana#.XkD8cyOnzIU. Accessed on: 29-02-2020
Guo J, Wang G, Geng Q, Wu Y, Cao F (2017) Decomposition of tree leaf litter and crop residues from ginkgo agroforestry systems in Eastern China: an in situ study. J Soils Sediments 18:1424–1431. https://doi.org/10.1007/s11368-017-1870-6
Gupta G, Yadav RS, Maurya M (2017) Decomposition of different litter fractions in agroforestry system of Central India. Int J Curr Microbiol App Sci 6:1089–1097
Hartemink AE (2005) Nutrient stocks, nutrient cycling, and soil changes in cocoa ecosystems: a review. Adv Agron 86:227–253
Hasanuzzaman M, Mahmood H (2014) Litter production and nutrient return through leaf litter of selected cropland agroforest tree species in southwestern Bangladesh. Agriculture and Forestry 60:221–233
Hayashi SN, Vieira ICG, Carvalho CJR, Davidson E (2012) Linking nitrogen and phosphorus dynamics in litter production and decomposition during secondary forest succession in the eastern Amazon. Boletim do Museu Paraense Emílio Goeldi. Ciências Naturais 7:283–295
Hossain M, Siddique MRH, Rahman MS, Hossain MZ, Hasan MM (2011) Nutrient dynamics associated with leaf litter decomposition of three agroforestry tree species (Azadirachta indica, Dalbergia sissoo, and Melia azedarach) of Bangladesh. J Forestry Res 22:577–582. https://doi.org/10.1007/s11676-011-0175-7
Hütz-Adams F, Huber C, Knoke I, Morazán P, Mürlebach M (2016) Strengthening the competitiveness of cocoa production and improving the income of cocoa producers in West and Central Africa. SÜDWIND e.V. Kaiserstr, Bonn
ICCO (2014): The world cocoa economy: current status, challenges and prospects; presentation at Multi-year expert meeting on commodities and development. Available at: http://unctad.org/meetings/en/Presentation/SUCMEM2014 09042014ICCO.pdf
Issac SR, Nair MA (2005) Biodegradation of leaf litter in the warm humid tropics of Kerala, India. Soil Biol Biochem 37:1656–1664
Kaba JS (2017) Nitrogen nutrition of cocoa (Theobroma cacao L.) in intercropping systems with gliricidia (Gliricidia sepium (Jacq.) Kunth ex Walp.). PhD thesis of The Free University of Bozen-Bolzano, Faculty of Science and Technology, Bolzano, Italy
Kumar BM (2008) Litter dynamics in plantation and agroforestry systems of the tropics—a review of observations and methods. In: Batish DR, Kohli RK, Jose S, Singh HP (eds) Ecological basis of agroforestry, 1st edn. CRC press, Boca Raton
Lammers K (2008) Infrared spectral and statistical analysis of leaf litter decomposition from the New Jersey pine barrens. Master of Science thesis, Camden Graduate School, Rutgers, The State University of New Jersey, US
Lin YM, Liu JW, Xiang P, Lin P, Ding ZH, Sternberg LDSL (2007) Tannins and nitrogen dynamics in mangrove leaves at different age and decay stages (Jiulong River Estuary, China). Hydrobiol. 583:285–295
Lori M, Symnaczik S, Mäder P, De Deyn G, Gattinger A (2017) Organic farming enhances soil microbial abundance and activity—a meta- analysis and meta-regression. PLoS One 12(7):e0180442. https://doi.org/10.1371/journal.pone.0180442
Mahler RL (2004) Nutrients plants require for growth. CIS 1124, College of Agriculture and Life Sciences, University of Idaho. Available at: https://www.extension.uidaho.edu/publishing/pdf/CIS/CIS1124.pdf. Last accessed on: 29-07-2020
Majumder M, Shukla AK, Arunachalam A (2010) Nutrient release and fungal succession during decomposition of crop residues in a shifting cultivation system. Commun Soil Sci Plant Anal 41:497–515. https://doi.org/10.1080/00103620903495571
Mamani-Pati F, Clay DE, Clay SA, Smeltekop H, Yujra-Callata MA (2012) The influence of strata on the nutrient recycling within a tropical certified organic coffee production system. ISRN Agronomy 2012:1–8. https://doi.org/10.5402/2012/389290
Mohammed AM, Robinson JS, Midmore DJ, Verhoef A (2019) Leaf litter decomposition and mitigation of CO2 emissions in cocoa ecosystems. https://doi.org/10.5772/intechopen.86520
Muoghalu JI, Odiwe AI (2011) Litter production and decomposition in cacao (Theobroma cacao Linn.) and kola nut (Cola nitida (Vent.) Schott & Endl.) plantations in Ile-Ife, southwestern Nigeria. Ecotropica 17:79–90
Murphy BW (2014) Soil organic matter and soil function – review of the literature and underlying data: effects of soil organic matter on functional soil properties. Grain Reseach and Development Corporation, Department of the Environtment Australia Goverment. pp 155
Naik SK, Maurya S, Mukherjee D, Singh AK, Bhatt BP (2018) Rates of decomposition and nutrient mineralization of leaf litter from different orchards under hot and dry sub-humid climate. Arch Agron Soil Sci 64:560–573. https://doi.org/10.1080/03650340.2017.1362104
Ofori-Frimpong K, Asase A, Mason J Danku L (2007) Shaded versus unshaded cocoa: implications on litter fall, decomposition, soil fertility and cocoa pod development. In Proc. 2nd Int. Symposium on Multistrata Agroforestry Systems with Perennial Crops: making ecosystem service count for farmers, consumers and the environment (17-21 September), CATIE Turrialba, Costa Rica (http: web.catie.ac.cr/AFS/Symposium)
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecol. 44:322–331
Ordóñez-Fernández R, Repullo-Ruibérriz de Torres M, Román-Vázque ZJ, González-Fernández P, Carbonel-Bojollo R (2015) Macronutrients released during the decomposition of pruning residues used as plant cover and their effect on soil fertility. J Agric Sci 153:615–630
Podong C, Poolsiri R, Katzensteiner K, Pengthamkeerati P, Thongdeenok P (2013) Species diversity and litter dynamics in secondary mixed deciduous forest, Thung Salaeng Lung National Park, Northern, Thailand. Folia Forestalia Polonica 55:196–204. https://doi.org/10.2478/ffp-2013-0022
Ranjbar F, Jalali M (2012) Calcium, magnesium, sodium, and potassium release during decomposition of some organic residues. Commun Soil Sci Plant Anal 43:645–659. https://doi.org/10.1080/00103624.2012.644005
Rojas MJ, Caicedo V, Jaimes Y (2017) Biomass decomposition dynamic in agroforestry systems with Theobroma cacao L. in Rionegro, Santander, Colombia. Agronomía Colombiana 35:182–189. https://doi.org/10.15446/agron.colomb.v35n2.60981
Schroth AW, Bostick BC, Graham M, Kaste JM, Mitchell MJ, Friedland AJ (2007) Sulfur species behavior in soil organic matter during decomposition. J Geophys Res 112:G04011. https://doi.org/10.1029/2007JG000538
Singh KP, Shekhar C (1989) Concentration and release patterns of nutrients (N, P and K) during decomposition of maize and wheat roots in a seasonally-dry tropical region. Soil Biol Biochem 21:81–85
Staaf H (1980) Release of plant nutrients from decomposing leaf litter in a South Swedish beech forest. Holarct Ecol 3:129–136
Tonks AJ, Aplin P, Beriro DJ, Cooper H, Evers S, Vane CH, Sjögersten S (2018) Impacts of conversion of tropical peat swamp forest to oil palm plantation on peat organic chemistry, physical properties and carbon stocks. Geoderma 289:36–45
Triadiati S, Tjitrosemito E, Sundarsono G, Qayim I, Leuschner C (2011) Litterfall production and leaf-litter decomposition at natural forest and cacao agroforestry in Central Sulawesi, Indonesia. Asian J Biol Sci 4:221–234. https://doi.org/10.3923/ajbs.2011.221.234
Van Vliet JA, Slingerland Maja, Giller Ken E (2015) Mineral nutrition of cocoa. A review. 57 pp. Wageningen University and Research Centre, Wageningen
Vazquez RI, Stinner BR, McCartney DA (2003) Corn and weed residue decomposition in northeast Ohio organic and conventional dairy farms. Agric Ecosyst Environ 95:559–565
Wang Y, Chang SX, Fang S, Tian Y (2014) Contrasting decomposition rates and nutrient release patterns in mixed vs singular species litter in agroforestry systems. J Soils Sediments 14:1071–1081. https://doi.org/10.1007/s11368-014-0853-0
Web 1 (n.d.) Past weather in Eastern Region, Ghana. Available at: https://www.timeanddate.com/weather/@2301360/historic?month=12&year=2018. Last Accessed on: 08-08-2019
Wood TE, Lawrence D, Clark AD (2006) Determinants of leaf litter nutrient cycling in a tropical rain forest: soil fertility versus topography. Ecosystems 9:700–710. https://doi.org/10.1007/s10021-005-0016-7
World Bank Group (2018) Climate change knowledge portal. Available at: https://climateknowledgeportal.worldbank.org/download-data. Last Accessed: 08-08-2019
Yang K, Zhu J-J (2014) Impact of tree litter decomposition on soil biochemical properties obtained from a temperate secondary forest in Northeast China. J Soils Sediments 15:13–23. https://doi.org/10.1007/s11368-014-0975-4
Acknowledgements
We are grateful to the cocoa farmers and field assistants who supported us during field data collection; special thanks to Mr. Kofi Mark and Mr. Eric Odei. We thank Saul Vazquez Reina and Li Dongfang for offering technical support on chemical analysis.
Funding
The study was fully funded by Ghana Education Trust Fund (GET Fund, http://www.getfund.gov.gh/) as part of a doctoral program. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
AM conceived the study, acquired the funding, designed the study, collected field data, carried out statistical analyses and drafted the manuscript; SS, BHL and DE participated in concept development, supervised study design, facilitated field data collection, participated in data analysis and critically revised the manuscript. All authors gave final approval for publication and agree to be held accountable for the work performed therein.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Field study was carried out on privately owned land and all selected farmers/landowners orally consented to participate in the study. The study did not involve endangered or protected species.
Consent for publication
Not applicable.
Code availability
Not applicable.
Additional information
Responsible editor: Jianming Xue
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Asigbaase, M., Dawoe, E., Sjogersten, S. et al. Decomposition and nutrient mineralisation of leaf litter in smallholder cocoa agroforests: a comparison of organic and conventional farms in Ghana. J Soils Sediments 21, 1010–1023 (2021). https://doi.org/10.1007/s11368-020-02844-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-020-02844-4