Abstract
Purpose
The microbial turnover of sediment organic matter (OM) in ports and waterways impacts water quality, sonic depth finding and presumably also rheological properties as well as greenhouse gas emissions, especially if organic carbon is released as methane. As a consequence, sediment management practices as a whole are affected. This study aimed to discern spatial OM degradability patterns in the Port of Hamburg and investigated correlations with standard analytical properties as a basis for future predictive modelling.
Materials and methods
Sediments in the Port of Hamburg were repeatedly sampled at nine locations along an east-west transect using a 1-m corer. In a stratified sampling approach, layers of suspended particulate matter (SPM), fluid mud (FM), pre-consolidated sediment (PS) and consolidated sediment (CS) were identified and individually analysed for long-term aerobic and anaerobic degradation of organic matter, DNA concentration, stable carbon isotope signature, density fractions and standard solids and pore water properties.
Results and discussion
The investigation area was characterised by a distinct gradient with a 10-fold higher OM degradability in upstream areas and lower degradability in downstream areas. Concomitantly, upstream locations showed higher DNA concentrations and more negative δ13C values. The share of bulk sediment in the heavy density fraction as well as the proportion and absolute amount of organic carbon were significantly larger at downstream locations. A depth and hence age-related gradient was found at individual locations, showing higher degradability of the upper, younger material, concomitant with higher DNA concentration, and lower OM turnover in the deeper, older and more consolidated material. Deeper layers were also characterised by higher concentrations of pore water ammonium, indicative of anaerobic nitrogen mineralisation.
Conclusions
Organic matter lability is inversely linked to its stabilisation in organo-mineral complexes. The observed degradability gradient is likely due to the different OM quality in relation to its origin. Downstream OM enters the system with the tidal flood current from the direction of the North Sea whereas upstream locations receive OM originating from the catchment, containing more autochthonous, plankton-derived and more easily degradable components. At individual sampling points, depth-related degradability gradients reflect an age gradient, with easily degradable material in top layers and increasing stabilisation of OM in organo-mineral compounds with depth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Sources of organic matter in riverine sediments can be natural or anthropogenic. Examples of natural sources are eroded terrestrial topsoils and plant litter, supplying allochthonous organic matter, or planktonic and pelagic biomass, yielding autochthonous organic matter, whereas urban sewage and surface runoff reflect anthropogenic allochthonous sources. Under aerobic conditions, the microbial degradation of organic matter with oxygen as terminal electron acceptor releases carbon dioxide (CO2), whereas under anaerobic conditions organic carbon is additionally released as methane (CH4). Estuarine systems emit CO2 into the atmosphere at rates that are quantitatively significant for the global carbon cycle (Borges and Abril 2012). Streams and rivers are responsible for the dominant share of global CO2 emissions from inland waters (Raymond et al. 2013). Intense aerobic turnover of organic matter can cause oxygen minimum zones in the water body. Because of the poor solubility of methane in water, methanogenesis results in the formation of gas bubbles which are either entrapped within the sediment or released by ebullition through the overlaying water column. Gas bubbles produced from anaerobic degradation of organic matter in sediments are assumed to decrease sediment density, viscosity and shear strength and to delay sedimentation and consolidation. Gas bubbles may obstruct sonic depth finding and therefore maintenance of the navigable depth and increase dredging costs by requiring the operation of degassing units on trailer suction hopper dredgers (Wurpts and Torn 2005). Lastly, anaerobic degradation of organic matter in sediments leads to the release of the potent greenhouse gas methane. The magnitude and rate of anaerobic organic matter degradation depend on the organic matter content and degradability as well as the prevailing environmental (e.g. temperature) and geochemical (e.g. types of electron acceptors) conditions (Arndt et al. 2013).
The water depth in the Port of Hamburg, one of the biggest ports in Europe, is strongly affected by a tidal range of approximately 3.6 m. The port receives sediments, suspended and sediment-bound organic matter both from the upstream catchment and from the North Sea, the latter due to tidal pumping (Boehlich and Strotmann 2008). The resulting sediment load in combination with the presence of low-flow areas within port basins or the formation of eddies at turn-offs causes hotspots of sedimentation and hence respective dredging efforts to maintain the nautical depth. Due to the possible effects of sediment organic matter turnover highlighted above, quantification of the lability of organic matter at focal points of sedimentation plays an important role for optimising sediment management. In light of this, the aim of this study was to investigate the spatial variability of organic matter degradability in the Port of Hamburg and to examine possible correlations with standard analytical geochemical properties as prerequisite for the prediction of location-specific behaviour of organic matter, supporting spatially differentiated management of sediments. It was hypothesised that:
-
1.
Stabilisation of sediment organic matter in organo-mineral complexes increases with age and therefore with depth as well as along a transportation gradient.
-
2.
Ageing of sediments decreases organic matter lability and therefore changes biodegradation kinetics as well as the distribution of organic matter over different density fractions.
-
3.
An upstream-downstream decreasing gradient of organic matter degradability exists as labile organic matter enters the harbour mainly from upstream through algal biomass, adding a more rapidly degradable pool.
2 Investigation area and sampling strategy
2.1 Investigation area
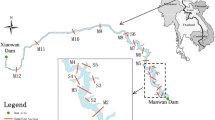
The Port of Hamburg (Fig. 1) is a tidal port located approximately 100 km inland from the North Sea. The sampling points (P1 to P9) mostly reflect key locations of maintenance of the nautical depth, with high sedimentation rates and hence increased dredging effort. Further, the sampling points represent a gradient from upstream to downstream. They feature different hydromorphological properties with respect to their distance to the main fairway, the geometry of the harbour basin and the extent of flow-through of water (see Table 1).
Investigation area (Port of Hamburg) with sampling locations (small dots) between river km 616 (P1, upstream) and 643 (P9, downstream); the big dots show the sampling location of the Hamburg Serviceportal (compare Sect. 4.2) (source: adapted from Hamburg Port Authority)
2.2 Sampling strategy
In 2018, sediment samples were taken with a core sampler (volume ca. 10 l) at nine locations in the Port of Hamburg (Fig. 1) in June, August, September and November. The goal of the sampling strategy was to include the most upstream and most downstream locations as well as the major sedimentation areas in the Port of Hamburg. Additionally, sampling aimed at analysing variability with depth (see Section 4.3) and time (not presented in this study). In a vertical cut, four layers were distinguished between the upper (water) and lower (riverbed) boundaries: suspended particulate matter (SPM), fluid mud (FM), pre-consolidated sediment (PS) and consolidated sediment (CS). While SPM layers still fully represent the aqueous phase, FM is the first layer below the lutocline, still showing fluidic but already non-Newtonian properties. This layer is not always present. The lutocline was identified visually, seconded by on-board measurements of density (Anton Paar density meter DMA 35). Below the FM layer follows pre-consolidated sediment (PS) which can be described as a non-fluidic sediment layer in the initial stages of consolidation, representing the topmost, freshly settled material. This layer is most pronounced in areas of high sedimentation rates. Below follows the consolidated sediment (CS) layer, defined as a firm, aged sediment layer of reduced water content. The described stratification was accompanied by a pronounced redox gradient with SPM still in an oxidised state but FM, PS and CS material usually in a reduced state with values decreasing with depth (see Fig. 7). In the core sampler, the depth of each layer was determined with reference to the lutocline (Wolanski et al. 1988), located between suspended particulate matter and fluid mud. In a stratified sampling approach, several cores were taken at each location, and layers were separated, homogenised and stored cool in air-tight containers until they were processed. Suspended particulate matter was pumped from the top of the core on the vessel and centrifuged in the laboratory.
3 Methods
3.1 Anaerobic degradation of organic matter
Depending on the available sample mass, the water content and the expected gas production rate, 20 g (SPM), 100 (FM) and 200 g fresh sample (PS and CS) were placed in triplicates into 100-ml (SPM), 250-ml (FM) or 500-ml glass bottles (PS and CS). The bottle headspace was flushed with 100% N2 to establish anaerobic conditions and incubated at 36 °C in dark. For quantification of gas production, an automated low gas flow measuring device (Gas Endeavour, Bioprocess Control, Sweden) was used for samples from PS and CS layers and a pressure gauge for samples from SPM and FM layers. After gas production had reached a level of below 2 ml day−1, considered as the lower limit for measurements with the Gas Endeavour device, the long-term gas production for pre-consolidated and consolidated layers were continued with the pressure gauge.
The anaerobic (anoxic) degradation of organic matter was quantified based on the release of carbon into the gas phase. According to the oxidation-reduction reactions taking place with decreasing redox potential and decreasing enthalpy, organic carbon is first released as CO2 from the reduction of nitrate, manganese, iron and sulphate. After the depletion of these terminal electron acceptors (TEAs), carbon is additionally released as CH4. Gas phase carbon was quantified applying the ideal gas law using the gas composition, the headspace volume, the incubation temperature and the pressure inside the bottle. The total amount of generated gas equals the sum of CO2 and CH4 produced and was expressed as mass unit of carbon released per mass unit of organic carbon (as present in the original sample), hence indicating the degradability of the organic carbon independent of its absolute amount. Gas chromatographic analyses did not detect any other gases. Values measured after 21 days (G21) and after 100 days (G100) were used as standard benchmarks.
3.2 Aerobic degradation of organic matter
To determine the degradation of organic matter under aerobic (oxic) conditions, in this paper also referred to as ‘respiration’, 15 g of homogenised fresh sample was incubated in 1-l glass bottles at 20 °C in the dark with a headspace consisting of atmospheric air. The sample was distributed on the bottom of the bottles in a layer of only a few millimetre thickness in order to minimise limitations to the diffusion of oxygen into the sample. To avoid the limitation of OM degradation by elevated CO2 levels, the bottle headspace was flushed with synthetic air after CO2 concentrations had reached three vol.% in the gas phase, then measurements were resumed. Applying the ideal gas law, the amount of degraded carbon was calculated using the concentration of CO2 as measured by gas-chromatographic analysis and the bottle headspace volume. Respiratory C-release was related to mass unit organic carbon present in the sample at the beginning of the incubation and calculated for the benchmark times of 21 days (R21) and 100 days (R100).
3.3 Carbon stable isotopes
The δ13C values of the sediment organic matter were determined with an isotope-ratio mass spectrometer (Delta V; Thermo Scientific, Dreieich, Germany) coupled to an elemental analyser (Flash 2000; Thermo Scientific). Prior to analysis, samples were treated with phosphoric acid (43%, 80 °C for 2 h) to release inorganic carbon. Values are expressed relative to Vienna Pee Dee Belemnite (VPDB) using the external standards IAEA IAEA-CH7 (− 31.62‰ vs. VPDB) and IVA soil 33,802,153 (27.46‰ vs. VPDB).
3.4 DNA concentration
Isolation of genomic DNA was performed using the ‘Quick-DNA Fungal/Bacterial Microprep’ kit (https://www.zymoresearch.eu/quick-dna-fungal-bacterial-kit) following the procedure indicated by the supplier, with the following minor modifications: for better cell lysis, 5 μl lysozyme (2000 μg ml−1) and 5 μl proteinase K (200 μg ml−1) were added to the sample. Genomic DNA was eluted in 27 μl distilled water into a 1.5-ml reaction tube. The eluate was passed through the same filter a second time. Isolated DNA was suspended overnight at 4 °C. DNA concentrations were measured using an Implen NanoPhotometer NP80 (Implen GmbH, München, Germany) at 260 nm (Cryer et al. 1975).
3.5 Density fractionation
Density fractionation was carried out using LUDOX® HS-40 colloidal silica suspension in water (Sigma-Aldrich) with a cut-off density of 1.4 g cm−3. The procedure is based on the method first presented by van den Pol-van Dasselaar and Oenema (1999), adapted from Meijboom et al. (1995), and also applied by Gebert et al. (2006) on riverine sediments in a previous study. In brief, fresh sediment samples were placed in glass beakers, mixed with an excess of Ludox® at a density of 1.4 g cm−3 and stirred thoroughly. Light material < 1.4 g cm−3 accumulated at the surface and was separated from heavy material > 1.4 g cm−3, sinking to the bottom, by decantation. The two fractions were washed with distilled water and oven-dried at 105 °C for subsequent physical and chemical analyses (see Section 3.6).
3.6 Standard properties of solids and pore water
The analysis of solid properties included total nitrogen (TN, DIN EN 16168 2012), total organic carbon (TOC, both DIN ISO 10694 1995), water content (WC, DIN ISO 11465 1993), redox potential (Eh, DIN 38404 2012), particle size distribution (DIN ISO 11277 2009), oxygen consumption after 3 h (AT3h, TV-W/I 1994), phosphorus (P) and sulphur (S, both DIN ISO 11885 2007), pH value (pH, DIN EN 15933 2012) and electrical conductivity (EC, DIN EN 27888 1993). The following parameters were measured in filtrated pore water: dissolved organic carbon (DOC, DIN EN 1484 1997), ammonium (NH4+, DIN ISO 11732 2005), phosphate (PO43−, DIN ISO 6878 2004) and sulphate (SO42−, DIN ISO 10304 2009).
3.7 Statistical evaluation of data
Linear regression analyses were carried out to detect possible interrelations between sediment properties and parameters of organic matter degradation. Statistical significance of Pearson’s coefficient r was accepted for a probability of error p < 0.01 for a two-sided test. The time course of carbon release from anaerobic and aerobic organic matter degradation was described by exponential fitting. The number of considered phases was increased until the coefficient of determination no longer improved and was highest for a two- or three-phase system, depending on the degradation state of the sample. For most of the samples, a three-phase exponential fit best described the data (Eq. 1).
with A1, A2, A3 = initial value in each phase
x = time in days
t1, t2, t3 = degradation rate constant
y0 = total cumulative anaerobically degraded organic matter
For the comparison between samples and for the correlation with sediment properties, benchmark values of carbon release under aerobic (R) and under anaerobic (G) conditions after 21 days (R21, G21) and 100 days (R100, G100) were used.
4 Results
4.1 Sediment properties
The thickness of the investigated pre-consolidated layer PS, the first freshly deposited material below the lutocline, was between 15 and 30 cm (Table 2). Except for the most downstream point P9, all samples showed a very high content of fines < 63 μm (sum of clay and silt) of up to 97% of the total mass. Sediment texture varied between clay (P4, most clay) to sandy clay loam (P9, most sand; FAO 2006). The sand-dominated texture at P9 and high sand contents at P5 reflect the fact that both are situated within the main flow channel (Table 1) where flow rates are higher, limiting sedimentation of fines. Correspondingly, these locations showed the lowest TOC contents. Concentrations of nitrogen (TN) and organic carbon (TOC) varied strongly between 0.2 and 1.0% (TN) and 2.0 and 7.6% (TOC) and were highest upstream and lowest downstream. The TOC-to-TN ratio was 10-fold higher at the downstream location (P9). The water content, phosphorus and electric conductivity showed similar patterns with increased values upstream and lower values downstream. NH4+ in the pore water was strongly increased at upstream point P1 (up to 70 times) compared with other locations; PO43− was increased two-fold. Inversely, the sulphate concentration was around five-fold higher at downstream location P9 compared with upstream location P1.
Organic matter degradation leads to transformation and stabilisation of the remaining organic matter in organo-mineral complexes with silt and clay, which causes a shift in the distribution of organic carbon towards higher density fractions. Compared with the bulk sediment (Table 2), the light fraction was enriched in organic matter, showing TOC contents of up to 14%. By contrast, TOC contents in the heavy fraction only reached up to 1.6% (data not shown). Between 40 and 50% of the total mass was found in the light fraction at upstream sampling point P1, comparing to only 5 to 20% at downstream sampling point P9 (Fig. 2, upper panel). In combination, between 80 and 90% of the organic carbon were found in the light density fraction at upstream point P1, whereas at downstream point P9 this share was 30–80% (Fig. 2, lower panel). Although the fractionated mass was often enriched in the heavy fraction, the share of TOC was always higher in the light fraction, with one exception for location P9 (CS layer from November 2018).
Share of total dry mass (DM, upper panel) and of total organic carbon (TOC, lower panel) in the light (< 1.4 g cm−3) and heavy (> 1.4 g cm−3) density fraction at points P1 (upstream, left) and P9 (downstream, right). FM = fluid mud, PS = pre-consolidated sediment, CS = consolidated sediment, samples taken in August, September and November
The contrast between upstream and downstream properties was also reflected by the stable isotope signature and the concentration of DNA. The organic matter at the downstream location P9 was enriched in 13C (Fig. 3, left panel); moreover, at all locations the heavy density fraction was enriched in 13C compared with the light density fraction (Fig. 3, right panel). Also, an increased concentration of DNA was found in the sediment at upstream point P1 (location RV at river-km 616) compared with downstream point P9 (location SW at river-km 643) (Fig. 4). While for the δ13C values no clear differentiation with depth could be identified, DNA concentrations showed a clear depth-related gradient with highest values in the youngest layers (fluid mud) and the lowest values in the oldest material (consolidated sediment).
δ13C signature of fluid mud (FM), pre-consolidated (PS) and consolidated sediment (CS) on the original sample (left panel) and on the density-fractionated samples (only PS and CS, right panel) over the sampling transect East (km 616)–West (km 643) for the locations P1, P2, P8 and P9, samples taken in November 2018. Left: bulk sample, right: light (< 1.4 g cm−3) and heavy (> 1.4 g cm−3) density fractions
4.2 Degradation of organic matter
An example of cumulated release of carbon from the anaerobic degradation of organic matter is shown in Fig. 5 for the most upstream (P1) and the most downstream (P9) location for pre-consolidated (PS) layers with averages of three parallel measurements. In spite of the significant differences in absolute levels of carbon release (about four times higher at P1 after 160 days), the time course for both the samples shown here and all other samples followed the same asymptotic pattern with decreasing rates over time. The time course of carbon release by aerobic respiration followed the same pattern over time: however, the amount of carbon released when oxygen was available as terminal electron acceptor was threefold compared with carbon release under anaerobic conditions (Fig. 6).
Both the degradation (carbon release normalised to dry mass) and the degradability (carbon release normalised to mass unit TOC) of organic matter followed a marked gradient with higher values upstream and lower rates downstream (Fig. 6). The following key differences were observed between the sampling locations regarding degradation and degradability of organic matter (Table 3):
-
10 to 30 times higher carbon release (per g DW) from anaerobic organic matter degradation (G21, G100) and up to 10-fold increased carbon release (per g DW) from aerobic degradation (R21, R100) related to dry mass at the upstream location P1 compared with the downstream location P9.
-
The consumption of oxygen within 3 h was higher at location P1 compared with P9 by the factor of seven (AT3h, P1: 2.0 g O2 kgDW−1, P9: 0.4 g O2 kgDW−1).
-
Higher degradability of organic matter at location P1, with a factor of 2.5 to 7 compared with location P9 for both aerobic (R21, R100) and anaerobic (G21, G100) conditions.
Besides the upstream-downstream gradient of organic matter degradability, a depth- and therefore age-related gradient was also observed at the individual sampling points (Fig. 7). Both anaerobic and aerobic (not shown) degradation of carbon per unit TOC decreased with increasing depth and hence age, coinciding with increased in situ concentrations of pore water ammonia (Fig. 8, left panel) and more negative redox potentials (Fig. 8, right panel) in deeper layers.
Cumulative organic matter degradation under anaerobic conditions (G) over depth for 21 days (G21) and 100 days (G100) with redox potential (meV) and ammonium concentration in pore water (mg l−1) at location P1 and P9 per layer (SPM - CS), depth below lutocline, samples taken in June 2018, values in brackets calculated from other campaigns
Depth profiles of redox potential and pore water concentration of ammonia (NH4+) at the time of sampling for selected sampling points, samples taken in June 2018. Square symbol = mean, line = median, box = 25th–75th percentile, whisker = 5th–95th percentile, cross = 1st–99th percentile, line symbol = minimum and maximum
4.3 Relationship between abiotic sediment properties and organic matter degradation
Correlation analyses showed strong relationships between parameters reflecting carbon release by aerobic and anaerobic degradation of organic matter (R and G) and individual sediment properties (Table 5). Both processes were strongly positively related to TN and TOC contents as well as to water content and inversely related to the ratio of TOC to TN. Further, organic matter degradation was positively related to clay content, total phosphorus, total sulphur, dissolved organic carbon and pore water ammonium, but negatively to sand content and pore water sulphate. Aerobic and anaerobic degradability of organic matter were correlated well with each other, with values for Pearson’s r of 0.85 or greater, both for short-term (after 21 days) and long-term (100 days) periods, indicating the same principal governing factors behind the two processes.
5 Discussion
5.1 Sediment properties and degradation of organic matter
5.1.1 Sediment properties
Both the abiotic sediment properties as well as organic matter degradation and degradability and DNA content in the investigation area were subject to a pronounced spatial gradient. In general, upstream sediments featured a higher organic matter content (reflected by higher TOC and TN contents) with a lower TOC-TN-ratio, higher total sulphur and phosphorus and higher concentrations in pore water of dissolved organic carbon, ammonium and phosphate. The higher solute concentration was reflected by a higher electric conductivity upstream, while downstream samples showed higher sulphate content, indicating increase of marine influence. The sulphate concentration in the sediment pore water can be influenced by two processes: the input of sulphate from marine origin through the stream water and the reduction of pore water sulphate under anaerobic conditions. Oxygen saturation of the water and hence higher redox potentials can be promoted by turbulence as induced by a stronger current (location P9 in the main fairway; Fig. 7). This can be seen from also higher turbidity values (Table 4, position IV), showing three-times higher turbidity values than at location P1 (compare position I). The water temperature, affecting organic matter decay by its influence on the biological activity, did not vary between the locations. The influence of tributaries (Alster: 6 m3 s−1 runoff, Bille: 4 m3 s−1) to the total flow (on average in 2017 around 500 m3 s−1) is negligible.
Concurrent with the gradient in sediment properties, both the mass-related release of carbon from the anaerobic and aerobic degradation of sediment organic matter and the degradability of the organic matter also decreased in the downstream direction. In terms of possible greenhouse gas emissions, this gradient means that sediments from the upstream location P1 can, within 21 days, produce up to 30 times the amount of methane and carbon dioxide per unit dry mass of dredged sediment than sediments from the downstream location P9. Carbon release under aerobic conditions was around three to five times higher compared with anaerobic conditions (Fig. 6), one reason being the higher energy gain when oxygen is available as terminal electron acceptor. Cumulative oxygen consumption values in this study, normalised to 20 °C, were in agreement with Spieckermann et al. (2018) who sampled at a similar location (near location P2) in 20 to 40 cm sediment depth.
5.1.2 Correlation between sediment properties and organic matter degradability
Statistically significant correlations were found between standard analytical sediment parameters and the degradation and degradability of organic matter (Table 5). Organic matter degradability (R and G are expressed in carbon release per unit TOC) correlated most strongly with total organic carbon, indicating that in the investigated system higher organic matter content also means a higher share of degradable organic matter. Degradability was inversely related to the ratio of TOC to TN, confirming the findings of Gebert et al. (2019) for degradation of organic matter in landfilled dredged sediment, suggesting lower degradability with decreasing contents of nitrogen per unit organic carbon. An inverse relationship can be expected because the nitrogen-to-carbon ratio in the decomposer biomass is higher than in soil (sediment) organic matter and litter, creating a constant demand for nitrogen (Berg and McClaugherty 2003). In terrestrial soils, decomposition rates for litter were thus also found to be positively linked to the TN/TOC ratio (Manzoni et al. 2008) and negatively to the TOC/TN ratio (e.g. van Dijk 1980). The strong correlation between sediment water content (WC) and TOC (r = 0.95) and TN (r = 0.93) contents reflects the fact that the youngest, most freshly settled material is the least consolidated one (highest water content) and at the same time the one with the least extent of organic matter decomposition, featured by (still) higher TOC and TN contents. From thermodynamic considerations (for a review see Arndt et al. 2013) it appears plausible that pore water sulphate correlated negatively with organic matter degradability, as the reduction of sulphate is energetically favourable compared with methanogenesis. In a study investigating gas production of sediments from ten federal German waterways, Gebert et al. (2006) found the lag phase until methane production commenced to be strongly correlated to the concentrations of inorganic electron acceptors such as sulphate in the pore water.
5.1.3 Organic matter quality
All systems with high organic carbon sedimentation rates, high internal primary production and anoxic bottom waters have a high CH4 emission potential (Grasset et al. 2018). Indeed, the strongest CH4 production potential was found for the sediments at the highly anoxic locations of this study (e.g. location P1; Fig. 7). Grasset et al. (2018) attributed low CH4 production from sediment to a low availability of labile compounds, comparable with downstream location P9 showing low concentrations in the light density fraction (Fig. 2) and high CH4 to the addition of fresh organic carbon to anoxic sediments. The same study showed that planktonic biomass (autochtonous organic carbon) was faster degradable than terrestrial biomass (allochtonous organic carbon). This suggests that, as also shown by Schoel et al. (2014, see Section 5.2), the organic carbon input at more upstream locations in the investigation area is dominated by autochthonous (phytoplankton, zooplankton) organic carbon, complementing the already mineral-phase bound and hence less degradable organic carbon pool. Other studies also related carbon release to organic matter quality and hence degradability, using different indicators. For example, White et al. (2002) described different classes of organic matter compounds that correlated positively (primary and secondary polysaccharides) or negatively (phenolic precursors, lignin, proteins and lipids) to the cumulative respiration. Further, Dai et al. (2002) could link carbon emission to the presence of easily degradable components such as polysaccharides. Straathof et al. (2014) found the concentration of the hydrophilic DOC fraction to correlate positively with respiration rates measured for compost. In this study, higher chlorophyll concentrations, indicative of higher phytoplankton biomass, are assumed to explain the higher degradability of organic matter seen at the downstream location P1.
5.2 Source and stabilisation of organic matter in sediments
Organic matter in soils and sediments is stabilised by formation of organo-mineral complexes which reduce its accessibility to biological degradation and hence to mineralisation (Baldock and Skjemstad 2000; Six and Paustian 2014; Gao et al. 2019). Conversely, non-complexed organic matter is more labile, i.e. prone to mineralisation, and has been found to be associated with microbial biomass C and N (Bu et al. 2012). The increased share of light fraction organic carbon upstream was seconded by a markedly higher concentration of DNA, indicative of a higher biomass content, as shown by Agnieszka et al. (2012) and Rehman et al. (2016). The gradient in DNA content supports the assumption a higher share of biomass-based degradable organic matter in the upstream area, as also suggested by the higher chlorophyll values in the water phase (Table 4). The chlorophyll concentration was nine times higher near the upstream location P1 (position I) compared with the more downstream location P8 (near position III; Hamburg Serviceportal 2019). It is assumed that at individual sampling locations the lower contents of DNA in deeper layers result from the mineralisation (decay) of the increasingly buried organisms without new biomass being introduced. The lower biomass levels are then maintained over time as the conditions such as scarcity of terminal electron acceptors (more negative redox potentials; Fig. 8) and poor rates of diffusive exchange of metabolites pose boundaries to biological activity.
A markedly higher share of light fraction organic carbon was found at the upstream location P1, while the downstream location P9 was dominated by heavy fraction organic carbon (Fig. 2). This suggests that the lower degradability of downstream organic matter results from a higher level of association with the mineral phase and that upstream organic matter was characterised by a higher share in light-fraction, easily accessible biogenic share of organic matter. The latter is corroborated by the aforementioned elevated levels of chlorophyll and therefore an increased share of biomass-based degradable organic matter in the upstream area. For landfilled Elbe sediment that had undergone several years to decades of organic matter degradation, Gebert et al. (2019) found over 93% of the bulk mass and over 80% of the organic carbon to be associated with the heavy fraction. Together, these data suggest a transition of organic carbon from the more accessible (light) to the less accessible (heavy) fraction and hence progressive stabilisation of organic matter in organo-mineral complexes with increasing mineralisation and hence age.
The findings were supported by the carbon isotopic signature, with a greater share of 12C in the bulk sample from upstream and an enrichment in 13C downstream. Organic matter components containing the lighter isotope 12C are degraded at a higher rate than those containing 13C, causing an enrichment of 13C and therefore an increase in the δ13C signature with age (overview in Wang et al. 2015) and hence an increased level of organic matter stabilisation. Together with the distribution of TOC over the light and heavy density fractions, this again points at aged, more stabilised organic matter at the downstream location. However, the isotopic signature is also influenced by the origin of the organic matter. For example, plant species following the C3-assimilation pathway have an average δ13C value of − 27‰, species assimilating via the C4 pathway an average δ13C value of − 14‰, whereas marine organic matter typically has δ13C values varying between −20 and − 22‰ (Schulz and Zabel 2006). Lammers et al. (2017) found δ13C values between 10.5 and 23.5‰ for eukaryotic phytoplankton in a shallow Dutch lake. The observed isotopic gradient can therefore reflect a higher age of the downstream sediment organic matter or a different origin. As point P9 (PS-layer − 26.6‰; Fig. 3) is characterised by input of downstream sediments carried in direction of the harbour with the flood tide and point P1 (PS-layer − 28.3‰; Fig. 3) is dominated by input from the upstream catchment with higher algal biomass in the water phase (see chlorophyll concentrations in Table 4), a source-based gradient over the investigation area is likely.
The δ13C signature was expected to increase with depth due to a preferred consumption of 12C by microorganisms. Likewise, it was expected that the share of carbon in the heavy density fraction would increase with depth. Both were only partly found, for example for the samples from November 2018 at location P9 (Fig. 2, lower right panel). It is assumed that the absence of a clear depth-related trend is due to the naturally dynamic hydro-morphological situation (variable current, tide, dredging activities, etc.), confounding depth gradients that would be visible if they reflected an age gradient of exactly the same original material. However, the assumption that heavy fraction organic carbon reflects the older, more stabilised and therefore less degradable pool was supported by the fact that heavy density fraction organic carbon showed an enrichment in 13C compared with the organic carbon found in the light density fraction (Fig. 3, right panel).
Schoel et al. (2014) showed that phytoplankton plays an important role for the concentration of dissolved oxygen in the Elbe. Also, Thorp and Delong (2002) described phytoplankton as the main driver of heterotrophic organic carbon cycling for eutrophic rivers. In line with the river continuum concept (Vannote et al. 1980), Schoel et al. (2014) demonstrated super-saturation with oxygen due to enhanced net primary production in the shallow and eutrophic upstream parts of the Elbe and light deficiency–induced decay of algal biomass by zooplankton grazing in the significantly deeper waters of the Hamburg Port area, inducing oxygen minimum zones. Concurrent to phytoplankton biomass, biological oxygen demand (BOD) and oxygen saturation plummeted within the Hamburg Port area, a trend is seen in the gradient of decreasing degradability of organic matter in downstream direction demonstrated in this study (Fig. 6). Within the harbour area, the river continuum concept is difficult to apply due to the strong tidal influence, additional urban afflux from streets, creeks, sewages or surface runoff as well as massive interventions like the removal of entire habitats (organisms, organic matter, etc.) by dredging.
Spatial variability of organic matter (degradation) with location and/or depth were also found in several other environments, for example in estuaries (Middelburg et al. 1996), mineral soils (Fang et al. 2005), intertidal sediments (Beck et al. 2008), lakes (Natchimuthu et al. 2015) and coastal wetlands (Yousefi Lalimi et al. 2018). Middelburg et al. (1996) found the spatial variability of organic matter mineralisation in intertidal sediments to be mainly caused by differences in the organic matter lability. As in our study, Yousefi Lalimi et al. (2018) and Fang et al. (2005) found a depth gradient of decreasing decomposition rates in (marsh) soil layers. The results from Fang et al. (2005) indicated that soil basal respiration rate is closely related to variations in available carbon pools occurring at different soil depths, also hypothesised in our study. Beck et al. (2008) described that products of organic matter remineralisation (e.g. DOC, NH4+, PO43−) increased with depth in pore waters of intertidal flat sediments, which can be also seen in this study (e.g. NH4+; Fig. 8).
The higher upstream degradability can be caused by a higher degradability of the organic matter itself, reflecting an intrinsic chemical property, or by more favourable environmental conditions such as increased availability of nutrients or both. In this study, the longitudinal gradient of organic matter degradability correlates with a gradient in decreasing nutrient concentrations, as seen from the contents in pore water phosphate and also total phosphorus (Table 2).
5.3 Transformation of nitrogen
Upstream locations showed stronger accumulation of NH4+ in the pore water (Table 2; Fig. 7), reflecting nitrogen mineralisation under anaerobic conditions, in line with higher degradability of upstream organic matter. The advanced state of organic matter degradation in deeper lying and therefore older layers was also seen by a depth gradient of increasing ammonium concentrations in the pore water (Fig. 8). This was most pronounced at point P1, where also both the highest organic matter turnover (related to dry weight of sample) and the highest degradability (related to mass unit organic carbon) were found.
Sanders et al. (2018) measured dissolved inorganic nitrogen in water samples of the stream along a gradient within the Elbe River in May 2013. Sampling locations included points near to points P1, P3, P6, P8 and P9 of this study. When comparing the two data sets, it is found that ammonium (NH4+) concentrations in the pore water (Table 2; layer PS) were 20 to 240 times higher compared with the concentrations in the water column, while nitrite concentrations (NO2−) were increased by only 2.5- to 12-fold and nitrate concentrations (NO3−) were partly even lower in the pore water compared with the water column (0.1- to 1.4-fold difference). Altogether, this indicates the dominance of anaerobic nitrogen mineralisation in the sediment, leading to the observed accumulation of ammonium, with denitrification being less relevant. Brockmann (1994) found decreased concentrations of dissolved organic nitrogen and dissolved organic phosphorus between Elbe river kilometres 630 and 770 towards the sea, mainly due to dilution with sea water. The decrease of the particulate organic matter concentration and the turbidity-organic matter-ratio in downstream direction was explained with organic matter degradation in the upper estuary and dilution with inorganic suspended particulate matter (SPM) from the lower estuary.
6 Conclusions and outlook
The time-course of organic matter degradation in long-term experiments showed the presence of differently available pools whose increasing depletion leads to an exponential decay of organic matter reactivity. The investigation area was characterised by a distinct gradient of sediment abiotic properties and organic matter lability, with higher degradability upstream and lower degradability downstream. Upstream locations were characterised by a higher concentration of DNA, a higher share of organic carbon in the light density fraction (i.e. not stabilised in organo-mineral complexes) and lower (more negative) δ13C values. This supports the hypothesis of sediment and organic matter properties being determined by a spatial stratification of the source organic matter and the conditions impacting sediment properties at any individual location along the stream. Data suggest that at downstream locations, organic matter is mainly of allochthonous origin (e.g. eroded soils, plant litter), entering the port mainly with the tidal flood current from the direction of the North Sea whereas upstream locations receive sediments and organic matter predominantly originating from the Elbe catchment, with an additional greater share of autochthonous organic matter (planktonic biomass), earmarked by a lighter organic carbon (less 13C). In between, sediments are of mixed origin, the magnitude of the individual upstream and downstream shares depending on the prevalent hydrodynamic conditions. Upstream, the suspended particulate matter feeding sedimentation contains more easily degradable components such as algal biomass, already suggested by higher chlorophyll concentrations in the water column, and reflected by a higher share of total mass in the sediment’s lighter density fraction. Downstream, a higher level of mineralisation and therefore of stabilisation of the input organic matter is evidenced by an increased share of organic carbon bound to the mineral phase and lower aerobic and anaerobic degradability. Whether the observed gradient is solely due to the assumed differences in input organic matter or whether an age gradient from upstream to downstream overlays the source pattern, cannot be determined at this moment. Ongoing clay mineralogical analyses of downstream and upstream sediments shall help to further elucidate the question.
Density fractionation appears to be a promising qualitative tool which can explain the presence of differently degradable organic matter pools in sediments. Future chemical fractionation will show whether these pools can be related to intrinsic organic matter chemical quality. While it became clear that degradability and density distribution are closely linked, the hypothesis that ageing (and hence increased mineralisation and stabilisation) leads to a shift towards increasing association of organic matter with the mineral phase could not be directly verified by the depth and hence age profiles at the individual sampling locations of this study. This is assumed to be due to depth profiles not representing a chronosequence of the very same settled material. Future experiments investigating the change in density distribution on one sample with ongoing incubation shall help to investigate this hypothesis.
The good correlation of organic matter degradability with standard analytical parameters such as TN and TOC may allow predictions of turnover using data on simple abiotic sediment properties. Additionally, the chlorophyll concentration might play an important role in the organic matter degradation. The significantly higher degradability of organic matter under aerobic compared with anaerobic conditions in combination with the marked spatial stratification of degradability provides the basis for the assessment of climate impact of dredging and relocation measures, but also for the quantification of potential greenhouse gas emissions from port areas without intervention.
Upcoming investigations will reveal the temporal variability of organic matter quality (fractions of dissolved organic carbon) and degradability in greater detail. All of the above will feed into the development of a process-based model predicting organic matter turnover in the Port of Hamburg in time and space. Benefits of this study will include increased process knowledge on organic matter degradation behaviour as a basis for implementing adaptive sediment management, positively impacting the water quality and possibly reducing greenhouse gas emissions.
References
Agnieszka W, Zofia S, Aleksandra B, Artur B (2012) Evaluation of factors influencing the biomass of soil microorganisms and DNA content. Open J Soil Sci 2:64–69
Arndt S, Jørgensen BB, LaRowe DE, Middelburg JJ, Pancost RD (2013) Quantifying the degradation of organic matter in marine sediments: a review and synthesis. Earth Sci Rev 123:53–86
Baldock JA, Skjemstad JO (2000) Role of the soil matrix and minerals in protecting natural organic materials against biological attack. Org Geochem 31:697–710
Beck M, Dellwig O, Liebezeit G, Schnetger B, Brumsack HJ (2008) Spatial and seasonal variations of sulphate, dissolved organic carbon, and nutrients in deep pore waters of intertidal flat sediments. Estuar Coast Shelf Sci 79:307–316
Berg B, McClaugherty CA (2003) Plant litter: decomposition, humus formation, carbon sequestration. Springer, Berlin
Boehlich MJ, Strotmann T (2008) The Elbe estuary. Die Küste, Arch Res Technol North Sea Baltic Coast 74:288–306
Borges AV, Abril G (2012) Carbon dioxide and methane dynamics in estuaries. Elsevier ESCO 00504
Brockmann UH (1994) Organic matter in the Elbe estuary. Aquat Ecol 28:371–381
Bu X, Ruan H, Wang L, Ma W, Ding J, Yu X (2012) Soil organic matter in density fractions as related to vegetation changes along an altitude gradient in the Wuyi Mountains, southeastern China. Appl Soil Ecol 52:42–47
Cryer D, Eccleshall R, Marmur J (1975) Isolation of yeast DNA. In: Prescott DM (ed) Method Cell. Biol., vol 12, pp 39–44
Dai XY, White D, Ping CL (2002) Comparing bioavailability in five Arctic soils by pyrolysis-gas chromatography/mass spectrometry. J Anal Appl Pyrolysis 62:249–258
DIN 38404 (2012) German standard methods for the examination of water, waste water and sludge - physical and physico-chemical parameters (group C) - calculation of the calcit saturation of water. Beuth, Berlin
DIN EN 1484 (1997) Water analysis - guidelines for the determination of total organic carbon (TOC) and dissolved organic carbon (DOC). Beuth, Berlin
DIN EN 15933 (2012) Sludge, treated biowaste and soil - determination of pH. Beuth, Berlin
DIN EN 16168 (2012) Sludge, treated biowaste and soil - determination of total nitrogen using dry combustion method. Beuth, Berlin
DIN EN 27888 (1993) Water quality; determination of electrical conductivity. Beuth, Berlin
DIN ISO 6878 (2004) Water quality - determination of phosphorus - ammonium molybdate spectrometric method. Beuth, Berlin
DIN ISO 11885 (2007) Water quality — Determination of selected elements by inductively coupled plasma optical emission spectrometry (ICP-OES). Beuth, Berlin, Germany
DIN ISO 10304 (2009) Water quality - determination of dissolved anions by liquid chromatography of ions - part 1: determination of bromide, chloride, fluoride, nitrate, nitrite, phosphate and sulfate. Beuth, Berlin
DIN ISO 10694 (1995) Soil quality - determination of organic and total carbon after dry combustion (elementary analysis). Beuth, Berlin
DIN ISO 11277 (2009) Soil quality - determination of particle size distribution in mineral soil material - method by sieving and sedimentation. Beuth, Berlin
DIN ISO 11465 (1993) Soil quality - determination of dry matter and water content on a mass basis - gravimetric method. Beuth, Berlin
DIN ISO 11732 (2005) Water quality - determination of ammonium nitrogen - method by flow analysis (CFA and FIA) and spectrometric detection. Beuth, Berlin
Fang C, Smith P, Moncrieff JB, Smith JU (2005) Similar response of labile and resistant soil organic matter pools to changes in temperature. Nature 433:57–59
Food and Agriculture Organization of the United Nations (FAO) (2006) Guidelines for soil description, 4th edn. United Nations, Rome
Gao J, Mikutta R, Jansen B, Guggenberger G, Vogel C, Kalbitz K (2019) The multilayer model of soil mineral-organic interfaces – a review. J Plant Nutr Soil Sci 000:1–15
Gebert J, Knoblauch C, Gröngröft A (2019) Gas production from dredged sediment. Waste Manag 85:82–89
Gebert J, Köthe H, Gröngröft A (2006) Methane formation by dredged sediment. J Soils Sediments 6:75–83
Grasset C, Mendonca R, Saucedo GV, Bastviken D, Roland F, Sobek S (2018) Large but variable methane production in anoxic freshwater sediment upon addition of allochthonous and autochthonous organic matter. Limnol Oceanogr 63:1488–1501
Hamburg Serviceportal (2019) HamburgService – Wassergüte-Auskunft https://gateway.hamburg.de/hamburggateway/fvp/fv/BSU/wasserguete/wfWassergueteAnfrageListe.aspx?Sid=37#. Accessed:2 May 2019
Lammers JM, Reichart GJ, Middelburg JJ (2017) Seasonal variability in phytoplankton stable carbon isotope ratios and bacterial carbon sources in a shallow Dutch lake. Limnol Oceanogr 62:2773–2787
Manzoni S, Jackson RB, Trofymow JA, Porporato A (2008) The global stoichiometry of litter nitrogen mineralization. Science 321:684–686
Meijboom FW, Hassink J, van Noordwijk M (1995) Density fractionation of soil macroorganic matter using silica suspensions. Soil Biol Biochem 27:1109–1111
Middelburg J, Klaver G, Nieuwenhuize J, Wielemaker A, de Haas W, Vlug T, van der Nat JFWA (1996) Organic matter mineralization in intertidal sediments along an estuarine gradient. Mar Ecol Prog Ser 132:157–168
Natchimuthu S, Sundgren I, Galfalk M, Klemedtsson L, Crill P, Danielsson A, Bastviken D (2015) Spatio-temporal variability of lake CH4 fluxes and its influence on annual whole lake emission estimates. Limnol Oceanogr 61:13–26
Raymond P, Hartmann J, Lauerwald R, Sobek S, McDonald C, Hoover M, Butman D, Striegl R, Mayorga E, Humborg C, Kortelainen P, Dürr H, Meybeck M, Ciais P, Guth P (2013) Global carbon dioxide emissions from inland waters. Nature 503:355–359
Rehman K, Ying Z, Andleeb S, Jiang Z, Olajide EK (2016) Short term influence of organic and inorganic fertilizer on soil microbial biomass and DNA in summer and spring. J Northeast Agric Univ 23:20–27
Schoel A, Hein B, Wyrwa J, Kirchesch V (2014) Modelling water quality in the Elbe and its estuary –Large Scale and Long Term Applications with Focus on the Oxygen Budget of the Estuary. Die Kueste 81:203–232
Schulz HD, Zabel M (2006) Marine Geochemistry. Springer, Berlin ISBN-10 3–540–32143-8
Sanders T, Schoel A, Dähnke K (2018) Hot spots of nitrification in the Elbe estuary and their impact on nitrate regeneration. Estuar Coasts 41:128–138
Six J, Paustian K (2014) Aggregate-associated soil organic matter as an ecosystem property and a measurement tool. Soil Biol Biochem 68:A4–A9
Spieckermann MJ, Gröngröft A, Eschenbach A (2018) Zeitliche Änderung des O2-Zehrungspotentials von Sedimenten aus dem Hamburger Hafen. Rostocker Baggergutseminar https://www2.auf.uni-rostockde/ll/baggergut/rbs10/pdf/Spieckermann.pdf Accessed:30 May 2019
Straathof AL, Chincarini R, Comans RNJ, Hoffland E (2014) Dynamics of soil dissolved organic carbon pools reveal both hydrophobic and hydrophilic compounds sustain microbial respiration. Soil Biol Biochem 79:109–116
Thorp JM, Delong MD (2002) Dominance of autochthonous autotrophic carbon in food webs of heterotrophic rivers. Oikos 96(3):543–550
Van den Pol-van Dasselaar A, Oenema O (1999) Methane production and carbon mineralisation of size and density fractions of peat soils. Soil Biol Biochem 31:877–886
Van Dijk H (1980) Survey of Dutch organic matter research with regard to humification and degradation rates in arable land. Land Use Seminar on Soil Degradation, Wageningen, 13–15
Vannote RL, Minshall WG, Cummins KW, Sedell JR, Cushing CE (1980) The river continuum concept. Can J Fish Aquat Sci 37:130–137
Wang G, Jia Y, Li W (2015) Effects of environmental and biotic factors on carbon isotopic fractionation during decomposition of soil organic matter. Sci Rep 5:11043
White DM, Garland DS, Dai X, Ping CL (2002) Fingerprinting soil organic matter in the arctic to help predict CO2 flux. Cold Reg Sci Technol 35:185–194
Wolanski E, Chappell J, Ridd P, Vertessy R (1988) Fluidization of mud in estuaries. J Geophys Res 93:2351–2361
Wurpts R, Torn P (2005) 15 years experience with fluid mud: definition of the nautical bottom with rheological parameters. Terra et Aqua 99:22–32
Yousefi Lalimi F, Silvestri S, D’ Alpaos A, Roner M, Marani M (2018) The spatial variability of organic matter and decomposition processes at the marsh scale. J Geophys Res-Biogeo 123:3713–3727
Acknowledgements
Aerobic respiration, organic carbon contents in density fractions and stable isotopes were measured by the Institute of Soil Science (Prof. Dr. Annette Eschenbach, Dr. Alexander Gröngröft and Dr. Christian Knoblauch, respectively), and DNA concentrations were measured by the Institute of Botany (Dr. Ines Krohn), both University of Hamburg.
Funding
This study was funded by Hamburg Port Authority and carried out within the project BIOMUD, a member of the MUDNET academic network www.tudelft.nl/mudnet/.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Susanne Heise
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zander, F., Heimovaara, T. & Gebert, J. Spatial variability of organic matter degradability in tidal Elbe sediments. J Soils Sediments 20, 2573–2587 (2020). https://doi.org/10.1007/s11368-020-02569-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-020-02569-4