Abstract
Purpose
Despite the great number of studies about methane uptake and its response to grazing in the Inner Mongolia grasslands, only a few focused on the methanotrophic composition. This study aimed to investigate the methanotrophic community structure and abundance, then to analyze the abiotic driving factors of methanotrophic community structure in different enclosed times in this area.
Materials and methods
In this study we chose typical grasslands in the Xilin River Basin of Inner Mongolia, China to investigate methanotrophic community structure and abundance under different enclosure treatments as follows: 79E (grassland enclosed since 1979), 99E (grassland enclosed since 1999), and G (freely grazed grassland). A clone library was used to reveal the methanotroph community structure, and their relationships with abiotic factors were analyzed by redundancy analysis. Methanotroph abundance was determined by real-time PCR.
Results and discussion
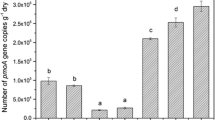
The OTUs of the three treatments mainly belonged to Type I methanotrophs, probably caused by the high pH value. Among all OTUs, only OTU1 belonged to upland soil cluster γ (USC-γ), whose abundance was the largest in all OTUs, indicating the USC-γ cluster was the main one to oxidize CH4 in the Inner Mongolia grasslands. Methanotrophic abundance (represented by the pmoA gene copies per gram of dry weight soil) decreased with the enclosure time as G (4.5 × 107) > 99E (2.8 × 107) > 79E (2.0 × 107), mainly caused by the lower soil moisture content in G. Lower soil moisture content facilitates more CH4 and O2 diffusive into soil thus leading to the proliferation of methanotrophs.
Conclusions
This study found a high abundance of methanotrophs in the soils of the Inner Mongolia grasslands, with the USC-γ cluster having the largest abundance, which may play a key role in oxidizing CH4 in the Inner Mongolia grasslands. Combined with those of previous studies, the results showed an obvious change of methanotrophic community composition with the increase of enclosure time.





Similar content being viewed by others
References
Abell GC, Stralis-Pavese N, Sessitsch A, Bodrossy L (2009) Grazing affects methanotroph activity and diversity in an alpine meadow soil. Environ Microbiol Rep 1:457–465
Adamsen APS, King GM (1993) Methane consumption in temperate and sub-arctic forest soils-rates, vertical zonation, and responses to water and nitrogen. Appl Environ Microbiol 59:485–490
Amaral JA, Ren T, Knowles R (1998) Atmospheric methane consumption by forest soils and extracted bacteria at different pH values. Appl Environ Microbiol 64:2397–2402
Belger L, Forsberg BR, Melack JM (2011) Carbon dioxide and methane emissions from interfluvial wetlands in the upper Negro River basin, Brazil. Biogeochemistry 105:171–183
Bhatia A, Ghosh A, Kumar V, Tomer R, Singh SD, Pathak H (2011) Effect of elevated tropospheric ozone on methane and nitrous oxide emission from rice soil in north India. Agric Ecosyst Environ 144:21–28
Bodrossy L, Murrell JC, Dalton H, Kalman M, Puskas LG, Kovacs KL (1995) Heat-tolerant methanotrophic bacteria from the hot water effluent of a natural gas field. Appl Environ Microbiol 61:3549–3555
Bodrossy L, Holmes EM, Holmes AJ, Kovács KL, Murrell JC (1997) Analysis of 16S rRNA and methane monooxygenase gene sequences reveals a novel group of thermotolerant and thermophilic methanotrophs, Methylocaldum gen. nov. Arch Microbiol 168:493–503
Boetius A et al (2000) A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 407:623–626
Castro MS, Steudler PA, Melillo JM, Aber JD, Bowden RD (1995) Factors controlling atmospheric methane consumption temperate forest soils. Glob Biogeochem Cycles 9:1–10
Chacon N, Silver WL, Dubinsky EA, Cusack DF (2006) Iron reduction and soil phosphorus solubilization in humid tropical forests soils: the roles of labile carbon pools and an electron shuttle compound. Biogeochemistry 78:67–84
Chen W et al (2010) Annual methane uptake by typical semiarid steppe in Inner Mongolia. J Geophys Res 115:1–10
Chen GC et al (2014) Rich soil carbon and nitrogen but low atmospheric greenhouse gas fluxes from North Sulawesi mangrove swamps in Indonesia. Sci Total Environ 487:91–96
Cicerone RJ, Oremland RS (1988) Biogeochemical aspects of atmospheric methane. Glob Biogeochem Cycles 2:299–327
Costello AM, Lidstrom ME (1999) Molecular characterization of functional and phylogenetic genes from natural populations of methanotrophs in lake sediments. Appl Environ Microbiol 65:5066–5074
Curry CL (2007) Modeling the soil consumption of atmospheric methane at the global scale. Glob Biogeochem Cyles 21. doi:10.1029/2006GB002818
Dalla Valle M, Codato E, Marcomini A (2007) Climate change influence on POPs distribution and fate: a case study. Chemosphere 67:1287–1295
Degelmann DM, Borken W, Drake HL, Kolb S (2010) Different atmospheric methane-oxidizing communities in European beech and Norway spruce soils. Appl Environ Microbiol 76:3228–3235
Del Grosso SJ et al (2000) General CH4 oxidation model and comparisons of CH4 oxidation in natural and managed systems. Glob Biogeochem Cyles 14:999–1019
Deng Y, Cui X, Luke C, Dumont MG (2013) Aerobic methanotroph diversity in Riganqiao peatlands on the Qinghai-Tibetan Plateau. Environ Microbiol Rep 5:566–574
Dubey SK, Singh JS (2001) Plant-induced spatial variations in the size of methanotrophic population in dryland and flooded rice agroecosystems. Nutr Cycl Agroecosyst 59:161–167
Dutaur L, Verchot LV (2007) A global inventory of the soil CH4 sink. Global Biogeochem Cy 21. doi:10.1029/2006GB002734
Geng YB, Luo GQ, Yuan GF (2010) CH4 uptake flux of Leymus chinensis steppe during rapid growth season in Inner Mongolia, China. Sci China-Earth Sci 53:977–983
Han B et al (2009) Diversity and activity of methanotrophs in alkaline soil from a Chinese coal mine. FEMS Microbiol Ecol 70:196–207
Hanson RS, Hanson TE (1996) Methanotrophic bacteria. Microbiol Rev 60:439–471
He PJ, Yang N, Fang WJ, Lu F, Shao LM (2011) Interaction and independence on methane oxidation of landfill cover soil among three impact factors: water, oxygen and ammonium. Front Environ Sci Eng China 5:175–185
Holmes AJ, Costello A, Lidstrom ME, Murrell JC (1995) Evidence that particulate methane monooxygenase may be evolutionarily related. FEMS Microbiol Lett 132:203–208
Holst J, Liu C, Yao Z, Brüggemann N, Zheng X, Giese M, Butterbach-Bahl K (2008) Fluxes of nitrous oxide, methane and carbon dioxide during freezing–thawing cycles in an Inner Mongolian steppe. Plant Soil 308:105–117
Horz HP, Rich V, Avrahami S, Bohannan BJM (2005) Methane-oxidizing bacteria in a California upland grassland soil: diversity and response to simulated global change. Appl Environ Microbiol 71:2642–2652
Hütsch BW (2001) Methane oxidation in non-flooded soils as affected by crop production—invited paper. Eur J Agron 14:237–260
Kirschke S et al (2013) Three decades of global methane sources and sinks. Nat Geosci 6:813–823
Kumaresan D, Hery M, Bodrossy L, Singer AC, Stralis-Pavese N, Thompson IP, Murrell JC (2011) Earthworm activity in a simulated landfill cover soil shifts the community composition of active methanotrophs. Res Microbiol 162:1027–1032
Lin JL, Radajewski S, Eshinimaev BT, Trotsenko YA, McDonald IR, Murrell JC (2004) Molecular diversity of methanotrophs in Transbaikal soda lake sediments and identification of potentially active populations by stable isotope probing. Environ Microbiol 6:1049–1060
Liu C et al (2007) Winter-grazing reduces methane uptake by soils of a typical semi-arid steppe in Inner Mongolia, China. Atmos Environ 41:5948–5958
Liu FC, Li HI, Dong Z, Zhang H (2012a) Advances in research on enclosure effects on vegetation restoration and soil physicochemical property of degraded grassland. Sci Soil Water Conserv 10:116–122
Liu GC et al (2012b) Microbial community composition controls the effects of climate change on methane emission from rice paddies. Environ Microbiol Rep 4:648–654
Ma X, Wang S, Wang Y, Jiang G, Nyren P (2006) Short‐term effects of sheep excrement on carbon dioxide, nitrous oxide and methane fluxes in typical grassland of Inner Mongolia. New Zeal J Agric Res 49:285–297
Moraes LE, Strathe AB, Fadel JG, Casper DP, Kebreab E (2014) Prediction of enteric methane emissions from cattle. Glob Chang Biol 20:2140–2148
Nercessian O, Noyes E, Kalyuzhnaya MG, Lidstrom ME, Chistoserdova L (2005) Bacterial populations active in metabolism of C1 compounds in the sediment of Lake Washington, a freshwater lake. Appl Environ Microbiol 71:6885–6899
Putkinen A, Larmola T, Tuomivirta T, Siljanen HM, Bodrossy L, Tuittila ES, Fritze H (2014) Peatland succession induces a shift in the community composition of Sphagnum-associated active methanotrophs. FEMS Microbiol Ecol 88:596–611
Raghoebarsing AA et al (2006) A microbial consortium couples anaerobic methane oxidation to denitrification. Nature 440:918–921
Sankaran M, Augustine DJ, (2004) Large herbivores suppress decomposer abundance in a semiarid grazing ecosystem. Eco 85:1052–1061
Sawakuchi HO, Bastviken D, Sawakuchi AO, Krusche AV, Ballester MVR, Richey JE (2014) Methane emissions from Amazonian Rivers and their contribution to the global methane budget. Glob Chang Biol 20:2829–2840
Sieburth JN, Johnson PW, Eberhardt MA, Sieracki ME, Lidstrom M, Laux D (1987) The first methane-oxidizing bacterium from the upper mixing layer of the deep ocean: Methylomonas pelagica sp. nov. Curr Microbiol 14:285–293
Singleton DR, Furlong MA, Rathbun SL, Whitman WB (2001) Quantitative comparisons of 16S rRNA gene sequence libraries from environmental samples. Appl Environ Microbiol 67:4374–4376
Steenbergh AK, Meima MM, Kamst M, Bodelier PLE (2010) Biphasic kinetics of a methanotrophic community is a combination of growth and increased activity per cell. FEMS Microbiol Ecol 71:12–22
Trotsenko YA, Khmelenina VN (2002) Biology of extremophilic and extremotolerant methanotrophs. Arch Microbiol 177:123–131
Van den Pol-van Dasselaar A, Oenema O (1999) Methane production and carbon mineralisation of size and density fractions of peat soils. Soil Biol Biochem 31:877–886
Vecherskaya M, Galchenko V, Sokolova E, Samarkin V (1993) Activity and species composition of aerobic methanotrophic communities in tundra soils. Curr Microbiol 27:181–184
Veraart AJ, Steenbergh AK, Ho A, Kim SY, Bodelier PLE (2015) Beyond nitrogen: the importance of phosphorus for CH4 oxidation in soils and sediments. Geoderma 259:337–346
Wang J, Song C, Zhang J, Wang L, Zhu X, Shi F (2014) Temperature sensitivity of soil carbon mineralization and nitrous oxide emission in different ecosystems along a mountain wetland-forest ecotone in the continuous permafrost of Northeast China. Catena 121:110–118
Yun J, Zhuang G, Ma A, Guo H, Wang Y, Zhang H (2012) Community structure, abundance, and activity of methanotrophs in the Zoige wetland of the Tibetan Plateau. Microb Ecol 63:835–843
Yun J, Ju Y, Deng Y, Zhang H (2014) Bacterial community structure in two permafrost wetlands on the Tibetan Plateau and Sanjiang Plain, China. Microb Ecol 68:360–369
Zhang X, Kong JY, Xia FF, Su Y, He R (2014) Effects of ammonium on the activity and community of methanotrophs in landfill biocover soils. Syst Appl Microbiol 37:296–304
Zheng Y, Yang W, Sun X, Wang SP, Rui YC, Luo CY, Guo LD (2012) Methanotrophic community structure and activity under warming and grazing of alpine meadow on the Tibetan Plateau. Appl Microbiol Biotechnol 93:2193–2203
Zheng Y, Zhang LM, Zheng YM, Di H, He JZ (2008) Abundance and community composition of methanotrophs in a Chinese paddy soil under long-term fertilization practices. J Soil Sediment 8:406–414
Zhou XQ, Wang YF, Huang XZ, Hao YB, Tian JQ, Wang JZ (2008a) Effects of grazing by sheep on the structure of methane-oxidizing bacterial community of steppe soil. Soil Biol Biochem 40:258–261
Zhou XQ, Wang YF, Huang XZ, Tian JQ, Hao YB (2008b) Effect of grazing intensities on the activity and community structure of methane-oxidizing bacteria of grassland soil in Inner Mongolia. Nutr Cycl Agroecosyst 80:145–152
Acknowledgments
This study was supported by the 100 Talents Program of The Chinese Academy of Sciences, 1000 Talents Program of Sichuan Province, the National Natural Science Foundation of China (No. 31570480) and Youth Science and Technology Innovation Team Support Foundation of Sichuan Province (Y5D2071100). The authors give special thanks to Ms. Wan Xiong for her edits and valuable comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Responsible editor: Zhihong Xu
Rights and permissions
About this article
Cite this article
Ma, T., Chen, H., Wang, Y. et al. Effects of enclosure time on the community composition of methanotrophs in the soils of the Inner Mongolia grasslands. J Soils Sediments 16, 1022–1031 (2016). https://doi.org/10.1007/s11368-015-1305-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-015-1305-1