Abstract
Purpose
The primary purpose of this study was to determine how flooding and draining cycles affect the redox chemistry of metal (hydr)oxides and organic matter in paddy soils and how the pH influences these processes. Our secondary purpose was to determine to what extent a geochemical thermodynamic equilibrium model can be used to predict the solubility of Mn and Fe during flooding and draining cycles in paddy soils.
Material and methods
We performed a carefully designed column experiment with two paddy soils with similar soil properties but contrasting pH. We monitored the redox potential (Eh) continuously and took soil solution samples regularly at four depths along the soil profile during two successive flooding and drainage cycles. To determine dominant mineral phases of Mn and Fe under equilibrium conditions, stability diagrams of Mn and Fe were constructed as a function of Eh and pH. Geochemical equilibrium model calculations were performed to identify Mn and Fe solubility-controlling minerals and to compare predicted total dissolved concentrations with their measured values.
Results and discussion
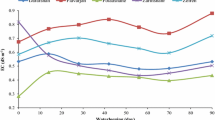
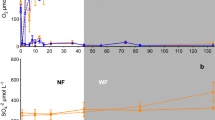
Flooding led to strong Eh gradients in the columns of both soils. In the acidic soil, pH increased with decreasing Eh and vice versa, whereas pH in the alkaline soil was buffered by CaCO3. In the acidic soil, Mn and Fe solubility increased during flooding due to reductive dissolution of their (hydr)oxides and decreased during drainage because of re-oxidation. In the alkaline soil, Mn and Fe solubility did not increase during flooding due to Mn(II) and Fe(II) precipitation as MnCO3, FeCO3, and FeS. The predicted levels of soluble Mn and Fe in the acidic soil were much higher than their measured values, but predictions and measurements were rather similar in the alkaline soil. This difference is likely due to kinetically limited reductive dissolution of Mn and Fe (hydr)oxides in the acidic soil. During flooding, the solubility of dissolved organic matter increased in both soils, probably because of reductive dissolution of Fe (hydr)oxides and the observed increase in pH.
Conclusions
Under alternating flooding and draining conditions, the pH greatly affected Mn and Fe solubility via influencing either reductive dissolution or carbonate formation. Comparison between measurements and geochemical equilibrium model predictions revealed that reductive dissolution of Mn and Fe (hydr)oxides was kinetically limited in the acidic soil. Therefore, when applying such models to systems with changing redox conditions, such rate-limiting reactions should be parameterized and implemented to enable more accurate predictions of Mn and Fe solubility.





Similar content being viewed by others
References
Allison JD, Brown DS, Novo-Gradac, KJ (1991) MINTEQA2/PRODEFA2, A geochemical assessment model for environmental systems: version 3.0 user’s manual. US EPA, Athens, GA. EPA/600/3-91/021
Bonten LTC, Groenenberg JE, Weng LP, van Riemsdijk WH (2008) Use of speciation and complexation models to estimate heavy metal sorption in soils. Geoderma 146(1–2):303–310
Brus DJ, Li ZB, Song J, Koopmans GF, Temminghoff EJM, Yin XB, Yao CX, Zhang HB, Luo YM, Japenga J (2009) Predictions of spatially averaged cadmium contents in rice grains in the Fuyang Valley, PR China. J Environ Qual 38(3):1126–1136
Chepkwony CK, Haynes RJ, Swift RS, Harrison R (2001) Mineralization of soil organic P induced by drying and rewetting as a source of plant-available P in limed and unlimed samples of an acid soil. Plant Soil 234:83–90
de Jonge M, Teuchies J, Meire P, Blust R, Bervoets L (2012) The impact of increased oxygen conditions on metal-contaminated sediments part I: effects on redox status, sediment geochemistry and metal bioavailability. Water Res 46(7):2205–2214
de Livera J, McLaughlin MJ, Hettiarachchi GM, Kirby JK, Beak DG (2011) Cadmium solubility in paddy soils: effects of soil oxidation, metal sulfides and competitive ions. Sci Total Environ 409(8):1489–1497
Dick WA, Tabatabai MA (1979) Ion chromatographic determination of sulfate and nitrate in soils. Soil Sci Soc Am J 43(5):899–904
Dijkstra JJ, Meeussen JCL, Comans RNJ (2009) Evaluation of a generic multisurface sorption model for inorganic soil contaminants. Environ Sci Technol 43(16):6196–6201
Du Laing G, Vanthuyne DRJ, Vandecasteele B, Tack FMG, Verloo MG (2007) Influence of hydrological regime on pore water metal concentrations in a contaminated sediment-derived soil. Environ Pollut 147:615–625
Du Laing G, Rinklebe J, Vandecasteele B, Meers E, Tack FMG (2009) Trace metal behaviour in estuarine and riverine floodplain soils and sediments: a review. Sci Total Environ 407:3972–3985
Dzombak DA, Morel FMM (1990) Surface complexation modeling: hydrous ferric oxide. Wiley, New York, p 393
Fiedler S, Kalbitz K (2003) Concentrations and properties of dissolved organic matter in forest soils as affected by the redox regime. Soil Sci 168(11):793–801
Frohne T, Rinklebe J, Diaz-Bone RA, Du Laing G (2011) Controlled variation of redox conditions in a floodplain soil: impact on metal mobilization and biomethylation of arsenic and antimony. Geoderma 160:414–424
Fulda B, Voegelin A, Kretzchmar R (2013) Redox-controlled changes in cadmium solubility and solid-phase speciation in a paddy soil as affected by reducible sulfate and copper. Environ Sci Technol 47(22):12775–12783
Gao XP, Schroeder TJ, Hoffland E, Zou CQ, Zhang FS, van der Zee SEATM (2010) Geochemical modeling of zinc bioavailability for rice. Soil Sci Soc Am J 74(1):301–309
Gotoh S, Patrick WH (1972) Transformation of manganese in a waterlogged soil as affected by redox potential and pH. Soil Sci Soc Am Pro 36(5):738–742
Grybos M, Davranche M, Gruau G, Petitjean P (2007) Is trace metal release in wetland soils controlled by organic matter mobility or Fe-oxyhydroxides reduction? J Colloid Interf Sci 314(2):490–501
Hagedorn F, Kaiser K, Feyen H, Schleppi P (2000) Effects of redox conditions and flow processes on the mobility of dissolved organic carbon and nitrogen in a forest soil. J Environ Qual 29(1):288–297
Hiemstra T, Antelo J, Rahnemaie R, van Riemsdijk WH (2010) Nanoparticles in natural systems I: the effective reactive surface area of the natural oxide fraction in field samples. Geo chim Cosmo chim Ac 74(1):41–58
Houba VJG, van der Lee JJG, Novozamsky I (1997) Soil and plant analysis. Part 1: soil analysis procedures. Wageningen University, Wageningen, The Netherlands
Houba VJG, Temminghoff EJM, Gaikhorst GA, van Vark W (2000) Soil analysis procedures using 0.01 M calcium chloride as extraction reagent. Commun Soil Sci Plant Anal 31:1299–1396
Jandl R, Sollins P (1997) Water extractable soil carbon in relation to the belowground carbon cycle. Biol Fert Soils 25(2):196–201
Kaiser K, Guggenberger G, Haumaier L, Zech W (1997) Dissolved organic matter sorption on subsoils and minerals studied by C-13-NMR and DRIFT spectroscopy. Eur J Soil Sci 48(2):301–310
Kalbitz K, Solinger S, Park JH, Michalzik B, Matzner E (2000) Controls on the dynamics of dissolved organic matter in soils: a review. Soil Sci 165(4):277–304
Khaokaew S, Chaney RL, Landrot G, Ginder-Vogel M, Sparks DL (2011) Speciation and release kinetics of cadmium in an alkaline paddy soil under various flooding periods and draining conditions. Environ Sci Technol 45(10):4249–4255
Kinniburgh DG, van Riemsdijk WH, Koopal LK, Borkovec M, Benedetti MF, Avena MJ (1999) Ion binding to natural organic matter: competition, heterogeneity, stoichiometry and thermodynamic consistency. Colloid Surface A 151(1–2):147–166
Kögel-Knabner I, Amelung W, Cao ZH, Fiedler S, Frenzel P, Jahn R, Kalbitz K, Kolbl A, Schloter M (2010) Biogeochemistry of paddy soils. Geoderma 157(1–2):1–14
Koopal LK, Saito T, Pinheiro JP, van Riemsdijk WH (2005) Ion binding to natural organic matter: general considerations and the NICA-Donnan model. Colloid Surface A 265(1–3):40–54
Koopmans GF, Groenenberg JE (2011) Effects of soil oven-drying on concentrations and speciation of trace metals and dissolved organic matter in soil solution extracts of sandy soils. Geoderma 161(3–4):47–158
Lindsay WL (1979) Chemical equilibria in soils. John Wiley & Sons, New York, p 449
Lindsay WL, Sadiq M (1983) Use of pe + pH to predict and interpret metal solubility relationships in soils. Sci Total Environ 28:169–178
Loeb R, Lamers LPM, Roelofs JGM (2008a) Effects of winter versus summer flooding and subsequent desiccation on soil chemistry in a riverine hay meadow. Geoderma 145(1–2):84–90
Loeb R, Lamers LPM, Roelofs JGM (2008b) Prediction of phosphorus mobilisation in inundated floodplain soils. Environ Pollut 156(2):325–331
Meeussen JCL (2003) ORCHESTRA: an object-oriented framework for implementing chemical equilibrium models. Environ Sci Technol 37(6):1175–1182
Meharg AA, Norton G, Deacon C, Williams P, Adomako EE, Price A, Zhu YG, Li G, Zhao FJ, McGrath S, Villada A, Sommella A, De Silva PMCS, Brammer H, Dasgupta T, Islam MR (2013) Variation in rice cadmium related to human exposure. Environ Sci Technol 47(11):5613–5618
Mulholland PJ, Dahm CN, David MB, Ditoro DM, Fisher TR, Hemond HF, Kögel-Knabner I, Meybeck MH, Meyer JL, Sedell JR (1990) What are the temporal and spatial variations of organic-acids at the ecosystems level. Life Sci R 48:315–329
Murase J, Kimura M (1997) Anaerobic reoxidation of Mn2+, Fe2+, S0 and S2- in submerged paddy soils. Biol Fert Soils 25(3):302–306
Novozamsky I, Houba VJG, Temminghoff EJM, van der Lee JJ (1984) Determination of ‘total’ N and ‘total’ P in a single soil digest. Neth J Agric Sci 32:322–324
Novozamsky I, van Eck R, Houba VJG, van der Lee JJ (1986) Use of ICP-AES for determination of iron, aluminium and phosphorus in Tamm’s soil extracts. Neth J Agric Sci 34:185–191
Patrick WH, Henderson RE (1981) Reduction and reoxidation cycles of manganese and iron in flooded soil and in water solution. Soil Sci Soc Am J 45(5):855–859
Patrick WH, Jugsujinda A (1992) Sequential reduction and oxidation of inorganic nitrogen, manganese, and iron in flooded soil. Soil Sci Soc Am J 56(4):1071–1073
Ponnamperuma FN (1972) The chemistry of submerged soils. Adv Agron 24:29–96
Postma D, Jakobsen R (1996) Redox zonation: equilibrium constraints on the Fe(III)/SO4-reduction interface. Geo chim Cosmo chim Ac 60:3169–3175
Regelink IC, Weng L, Koopmans GF, Van Riemsdijk WH (2013) Asymmetric flow field-flow fractionation as a new approach to analyse iron-(hydr)oxide nanoparticles in soil extracts. Geoderma 202:134–141
Ritvo G, Shitumbanuma V, Dixon JB (2004) Soil solution sulfide control by two iron-oxide minerals in a submerged microcosm. Aquaculture 239(1–4):217–235
Rupp H, Rinklebe J, Bolze S, Meissner R (2010) A scale-dependent approach to study pollution control processes in wetland soils using three different techniques. Ecol Eng 36:1439–1447
Sahrawat KL (2004) Organic matter accumulation in submerged soils. Adv Agron 81:169–201
Sahrawat KL (2005) Fertility and organic matter in submerged rice soils. Curr Sci India 88(5):735–739
Sedell JR, Dahm CN (1990) Spatial and temporal scales of dissolved organic-carbon in streams and rivers. Life Sci R 48:261–279
Shaheen SM, Rinklebe J, Rupp H, Meissner R (2014) Lysimeter trials to assess the impact of different flood-dry-cycles on the dynamics of pore water concentrations of As, Cr, Mo and V in a contaminated floodplain soil. Geoderma 228–229:5–13
Song J, Luo YM, Zhao QG, Christie P (2004) Microcosm studies on anaerobic phosphate flux and mineralization of lake sediment organic carbon. J Environ Qual 33(6):2353–2356
Tack FMG, Van Ranst E, Lievens C, Vandenberghe RE (2006) Soil solution Cd, Cu and Zn concentrations as affected by short-time drying or wetting: the role of hydrous oxides of Fe and Mn. Geoderma 137(1–2):83–89
Unger IM, Muzika RM, Motavalli PP, Kabrick J (2008) Evaluation of continuous in situ monitoring of soil changes with varying flooding regimes. Commun Soil Sci Plan 39(11–12):1600–1619
van der Geest HG, Paumen ML (2008) Dynamics of metal availability and toxicity in historically polluted floodplain sediments. Sci Total Environ 406(3):419–425
Vink JPM, Harmsen J, Rijnaarts H (2010) Delayed immobilization of heavy metals in soils and sediments under reducing and anaerobic conditions; consequences for flooding and storage. J Soils Sediments 10(8):1633–1645
Vorenhout M, van der Geest HG, van Marum D, Wattel K, Eijsackers HJP (2004) Automated and continuous redox potential measurements in soil. J Environ Qual 33(4):1562–1567
Vorenhout M, van der Geest HG, Hunting ER (2011) An improved datalogger and novel probes for continuous redox measurements in wetlands. Int J Environ An Che 91(7–8):801–810
Wang GQ, Koopmans GF, Song J, Temminghoff EJM, Luo YM, Zhao QG, Japenga J (2007) Mobilization of heavy metals from contaminated paddy soil by EDDS, EDTA, and elemental sulfur. Environ Geo Chem Hlth 29(3):221–235
Weng LP, Van Riemsdijk WH, Hiemstra T (2008) Cu2+ and Ca2+ adsorption to goethite in the presence of fulvic acids. Geo Chim Cosmo Chim Ac 72(24):5857–5870
Yu KW, Bohme F, Rinklebe J, Neue HU, DeLaune RD (2007) Major biogeochemical processes in soils—a microcosm incubation from reducing to oxidizing conditions. Soil Sci Soc Am J 71:1406–1417
Acknowledgments
This research was financially supported by the CAS-KNAW Joint PhD Training Programme (no. 5237829), the Key Project of the National Natural Science Foundation of China (no. 40971250), and the Key Project of the National Natural Science Foundation of China (no. 41230858). This research is also part of the strategic research program KBIV—“Sustainable spatial development of ecosystems, landscapes, seas and regions,” which is funded by the Dutch Ministry of Economic Affairs. Dr. Hans Meeussen is gratefully acknowledged for his advice in constructing the Eh-pH diagrams with the ORCHESTRA modeling framework.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Rongliang Qiu
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 385 kb)
Rights and permissions
About this article
Cite this article
Pan, Y., Koopmans, G.F., Bonten, L.T.C. et al. Influence of pH on the redox chemistry of metal (hydr)oxides and organic matter in paddy soils. J Soils Sediments 14, 1713–1726 (2014). https://doi.org/10.1007/s11368-014-0919-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-014-0919-z