Abstract
Purpose
The influence of bone sorbent addition onto distribution of 90Sr in artificially contaminated soil was preliminary studied to assess the possibility of biogenic apatite utilization for reduction of 90Sr mobility and availability. Simultaneously, the disruption of soil micro- (Cd, Zn, Co, Cu, Cr, and Ni,) and macroelements (Al, Fe, Mn, K, Mg, and Ca) upon Sr contamination and sorbent addition was monitored.
Materials and methods
The model soil was contaminated by inactive Sr, in the form of Sr(NO3)2 solution. As a soil additive, sorbent obtained by annealing bovine bones at 400 °C (B400) was applied. Both the uncontaminated and Sr-contaminated soils were mixed with 1, 3, 5, and 10 % of sorbent, suspended in distilled water (initial pH 5; solid/solution ratio, 1:2), and equilibrated for 15 days on a rotary shaker. Solid residues were subjected to modified Tessier five-step sequential extraction analysis, and the amounts of chosen metals in each fraction were determined by inductively coupled plasma–optical emission spectroscopy.
Results and discussion
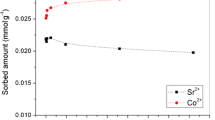
In the original soil, Sr was mainly found in exchangeable (61 %) and carbonate phase (16 %), whereas after contamination, the content of Sr in exchangeable phase raised to 94 %. With the addition of B400, the decrease in Sr amounts in exchangeable fraction was detected, whereas increase occurred mainly in operationally defined carbonate phase and in the residual. High level of Sr contamination caused the increase in Zn, Ni, Co, Cu, Cd, and Mn and decrease in Ca content in exchangeable phase. Sorbent addition resulted in a migration of these cations to less soluble fractions. This effect was observed even for major soil elements such as Fe, Al, and Mn, regardless of the excessive amounts of Sr in the soil.
Conclusions
Mixing the soil with B400 resulted in reduced Sr mobility and bioavailability. B400 acted as a stabilizing agent for heavy metals, as well. Apatite distinguished selectivity towards heavy metals may interfere with the Sr immobilization and disrupt original cation distribution. Further studies should include more realistic (lower) Sr concentrations in the soil, different soil types, pH, and longer incubation times.





Similar content being viewed by others
References
Admassu W, Breese T (1999) Feasibility of using natural fishbone apatite as a substitute for hydroxyapatite in remediating aqueous heavy metals. J Hazard Mater 69:187–196
Adriano DC (2001) Trace elements in terrestrial environments: biogeochemistry, bioavailability, and risks of metals. Springer, New York/Berlin/Heidelberg
Barona A, Romero F, Elejalde C (1995) Soil–metal interactions: associations of macroconstituent fractions in selected soils. J Hazard Mater 42:289–301
Boisson J, Ruttens A, Mench M, Vangronsveld J (1999) Evaluation of hydroxyapatite as a metal immobilizing soil additive for the remediation of polluted soils. Part 1. Influence of hydroxyapatite on metal exchangeability in soil, plant growth and plant metal accumulation. Environ Pollut 104:225–233
Bostick WD, Stevenson RJ, Harris LA, Peery D, Hall JR, Shoemaker JL, Jarabek RJ, Munday EB (2003) Use of apatite for chemical stabilization of subsurface contaminants. Final report (Work Performed Under Contract: DE-AC26-01NT41306). http://www.osti.gov/bridge/servlets/purl/820754-Fk9X7K/native/820754.pdf. Accessed 20 Dec 2011
Bradl HB (2004) Adsorption of heavy metal ions on soils and soils constituents. J Colloid Interf Sci 277:1–18
Cerling TE, Spalding BP (1982) Distribution and relationship of radionuclides to streambed gravels in a small watershed. Environ Geol 4:99–116
Chen W, Qian GR, Lim TT, Chui PC (2007) Speciation of heavy metals in surface sediments from Suzhou Creek. Journal of Shanghai University (English Edition) 11:415–425
Dimović S, Smičiklas I, Plećaš I, Antonović D, Mitrić M (2009) Comparative study of differently treated animal bones for Co2+ removal. J Hazard Mater 164:279–287
Egnér H, Riehm H, Domingo WR (1960) Untersuchungen über die chemische Bodenanalyse als Grundlage für die Beurteilung des Nährstoffzustandes der Böden. II. Chemische Extraktionsmethoden zur Phosphor- und Kaliumbestimmung. K Landbrukshögsk Annaler 26:199–215
Hashimoto Y, Taki T, Sato T (2009) Sorption of dissolved lead from shooting range soils using hydroxyapatite amendments synthesized from industrial byproducts as affected by varying pH conditions. J Environ Manage 90:1782–1789
Hickey MG, Kittrick JA (1984) Chemical partitioning of cadmium, copper, nickle, and zinc in soils and sediments containing high levels of heavy metals. J EnvironQual 13:372–376
Hodson ME, Valsami-Jones E, Cotter-Howells JD, Dubbin WE, Kemp AJ, Thornton I et al (2001) Effect of bone meal (calcium phosphate) amendments on metal release from contaminated soils: a leaching column study. Environ Pollut 112:233–243
IAEA (International Atomic Energy Agency) (2005) Remediation of sites with mixed contamination of radioactive and other hazardous substances, IAEA Technical Reports Series No. 442. International Atomic Energy Agency, Vienna
Kabata-Pendias A, Mukherjee AB (2007) Trace elements from soil to human. Springer, Berlin
Kamel NHM (2010) Adsorption models of 137Cs radionuclide and Sr(II) on some Egyptian soils. J Environ Radioact 101:297–303
Knox AS, Kaplan DI, Adriano DC, Hinton TG, Wilson MD (2003) Apatite and phillipsite as sequestering agents for metals and radionuclides. J Environ Qual 32:515–525
Lerouge C, Gaucher EC, Tournassat C, Negrel P, Crouzet C, Guerrot A et al (2010) Strontium distribution and origins in a natural clayey formation (Callovian–Oxfordian, Paris Basin, France): A new sequential extraction procedure. Geochim Cosmochim Acta 74:2926–2942
Li H, Shib W, Shaob H, Shaob M (2009) The remediation of the lead-polluted garden soil by natural zeolite. J Hazard Mater 169:1106–1111
Lukic D, Karadzic D, Radovanovic M, Milenkovic M, Gajic M, Milanovic S, Kovacevic-Majkic J (2012) The influence of chemical characteristics of precipitation on tree health in Banjica forest (Belgrade, Serbia). Arch Biol Sci Belgrade 64:1217–1225
Ma QY, Traina SJ, Logan TJ, Ryan JA (1993) In situ lead immobilization by apatite. Environ Sci Technol 27:1803–1810
Mason CFV, Lu N, Conca J (1999) Characterization, transport and remediation options for radioactive strontium and cesium contaminated sites. Los Alamos National Laboratory Technical Report LA-UR-99-3593. http://library.lanl.gov/cgi-bin/getfile?00796463.pdf. Accessed 10 Jan 2012
Melamed R, Cao X, Chen M, Ma LQ (2003) Field assessment of lead immobilization in a contaminated soil after phosphate application. Sci Total Environ 305:117–127
Mignardi S, Corami A, Ferrini V (2012) Evaluation of the effectiveness of phosphate treatment for the remediation of mine waste soils contaminated with Cd, Cu, Pb, and Zn. Chemosphere 86:354–360
Moody TE, Petersen SW, Torne EG, Vlcakova J, Higginbotham JF (1996) Laboratory scale stabilization of N-springs groundwater strontium-90 using phosphatic materials. Report BHI-00864. http://www.dtic.mil/cgi-bin/GetTRDoc?AD=ADA349254. Accessed 10 Jan 2012
Moody TE (1997) North plateau groundwater Sr-90 phosphate stabilization. Final report for the West Valley Demonstration Project, CH2MHILL
Moore RC, Sanchez C, Holt K, Zhang P, Xu H, Choppin GR (2004) Formation of hydroxyapatite in soils using calcium citrate and sodium phosphate for control of strontium migration. Radiochim Acta 92:719–723
Narasaraju TSB, Phebe DE (1996) Some physico-chemical aspects of hydroxylapatite. J Mater Sci 31:1–21
NCRP (National Council on Radiation Protection & Measurements) (1991) Some aspects of strontium radiobiology. NCRP report no. 110. National Council on Radiation Protection & Measurements, Bethesda
Nzihou A, Sharrock P (2010) Role of phosphate in the remediation and reuse of heavy metal polluted wastes and sites. Waste Biomass Valor 1:163–174
Pesic R, Kozmidis-Luburic U, Grujic S, Plecas I (2011) Radioactive waste management in Serbia, 2002–2010. Proceedings on the International Conference Nuclear Energy for New Europe 2011, Bovec, Slovenija. http://www.nss.si/nene2011/htm/abs/absNENE20112861.html. Accessed 20 Feb 2012
Petrović DJ, Todorović M, Manojlović D, Krsmanović VD (2009) Speciations of trace metals in the accumulation Bogovina on the Crni Timok River. Pol J Environ Stud 18:873–884
Scheckel KG, Ryan JA, Allen D, Lescano NV (2005) Determining speciation of Pb in phosphate-amended soils: method limitations. Sci Total Environ 350:261–272
Serne RJ, LeGore VL (1996) Strontium-90 adsorption–desorption properties and sediment characterization at the 100 N-area. Report no. PNL-10899. Pacific Northwest National Laboratory, Washington
Smičiklas I, Onjia A, Raičević S, Janaćković Ð, Mitrić M (2008) Factors influencing the removal of divalent cations by hydroxyapatite. J Hazard Mater 152:876–884
Smičiklas I, Dimović S, Šljivić M, Plećaš I, Lončar B, Mitrić M (2010) Resource recovery of animal bones: study on sorptive properties and mechanism for Sr2+ ions. J Nucl Mater 400:15–24
Solecki J (2005) Investigation of 85Sr adsorption on selected soils of different horizons. J Environ Radioact 82:303–320
Sumner ME, Miller WP (1996) Cation exchange capacity and exchange coefficients. In: Sparks DL (ed) Method of soil analysis. Part 3. Chemical methods. SSSA, Madison, pp 1201–1229
Suzuki T, Hatsushida T, Hayakawa Y (1981) Synthetic hydroxyapatites employed as inorganic cation-exchangers. J Chem Soc Farad T1(77):1059–1062
Szecsody JE (2006) Project Work Plan: Sequestration of strontium-90 subsurface contamination in the Hanford 100-N Area by surface infiltration of an apatite solution. (PNNL-SA-50071). Pacific Northwest National Laboratory, Washington
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–860
Valcke E, Cremers A (1998) The use of sapropels as amendments in radiocaesium and radiostrontium contaminated soils. Appl Geochem 13:155–164
Vermeul VR, Szecsody JE, Fritz BG, Williams MD, Fruchter JS (2010) 100-NR-2 Apatite treatability test: high-concentration calcium-citrate-phosphate solution injection for in situ strontium-90 immobilization. Final Report. Pacific Northwest National Laboratory. http://www.pnl.gov/main/publications/external/technical_reports/PNNL-SA-70033.pdf. Accessed 22 Dec 2011
Wright J, Conca JL, Rice KR, Murphy B (2004) “PIMS Using Apatite II™: How it works to remediate soil and water. http://www.pimsnw.com/files/1howapatiteiiworks30.pdf. Accessed 20 Jan 2012
Zhang Z, Li M, Chen W, Zhu S, Liu N, Zhu L (2010) Immobilization of lead and cadmium from aqueous solution and contaminated sediment using nano-hydroxyapatite. Environ Pollut 158:514–519
Acknowledgements
This work was supported by the Ministry of Science and Technological Development of the Republic of Serbia (project number III 43009).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jianming Xu
Rights and permissions
About this article
Cite this article
Dimović, S., Smičiklas, I., Šljivić-Ivanović, M. et al. Speciation of 90Sr and other metal cations in artificially contaminated soils: the influence of bone sorbent addition. J Soils Sediments 13, 383–393 (2013). https://doi.org/10.1007/s11368-012-0633-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-012-0633-7