Abstract
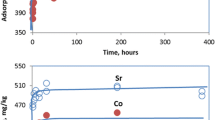
Sorption–desorption properties of cobalt(II) and strontium(II) ions were studied using a soil sample from the vicinity of the Serbian radioactive waste processing and interim storage facilities. The mobility of the cations in the soil was evaluated and compared with the intention to facilitate the selection of optimal remediation strategy in case of accidental soil contamination with radioactive cobalt-60 and strontium-90 isotopes. A systematic sorption study was performed through a series of batch experiments at different aging times, cation concentrations and pH. Kinetics experiments revealed that sorbed amounts of cobalt(II) continuously increased with contact time until quasi-equilibrium was reached, while initial fast strontium(II) sorption was followed by a desorption step. Based on the shapes of the sorption isotherms and calculated sorption parameters, it was concluded that cobalt(II) sorbed more selectively and strongly than strontium(II). Sequential extraction showed that, regardless of the initial content of contaminants in the soil and the aging time, high amounts of both cations were bonded to relatively mobile fractions: strontium(II) in the exchangeable, while cobalt(II) in the carbonate and ferromanganese oxide fraction. Strontium(II) was readily desorbed in acidic, calcium(II) and ethylenediaminetetraacetic acid-containing media, whereas complexing agents such as citric and tartaric acids at low pH were more effective reagents for cobalt(II) desorption. The results from the present study indicate that chemical extraction can be considered as remediation option for strontium(II)- and cobalt(II)-contaminated soil.






Similar content being viewed by others
References
Brown GE, Parks GA (2001) Sorption of trace elements on mineral surfaces: modern perspectives from spectroscopic studies and comments on sorption in the marine environment. Int Geol Rev 43:963–1074
Bunde RL, Rosentreter JJ, Liszewski MJ, Hemming CH, Welhan J (1997) Effects of calcium and magnesium on strontium distribution coefficients. Environ Geol 32:219–229
Cakara D, Kobayashi M, Skarba M, Borkovec M (2009) Protonation of silica particles in the presence of a strong cationic polyelectrolyte. Colloids Surf A 339:20–25
Dermont G, Bergeron M, Mercier G, Richer-Laflèche M (2008) Soil washing for metal removal: a review of physical/chemical technologies and field applications. J Hazard Mater 152:1–31
Dimović S, Smičiklas I, Šljivić-Ivanović M, Dojčinović B (2013) Speciation of 90Sr and other metal cations in artificially contaminated soils: the influence of bone sorbent addition. J Soils Sediment 13:383–393
Fuerstenau DW, Raghavan S (1980) In: Proceedings XII international mineral processing congress, vol II. Nacional Publicacoes & Pubicadado, Sao Paulo
Giles CH, D’Silva AP, Easton IA (1974) A general treatment and classification of the solute adsorption isotherm part. II. Experimental interpretation. J Colloid Interface Sci 47:766–778
Harmsen RW, Schulz WW (1998) Best-basis estimates of solubility of selected radionuclide in hanford single-shell tank sludge. HNF-3271. Lockheed Martin Hanford Corporation, Richland
Hsu C-N, Liu D-C, Chuang C-L (1994) Equilibrium and kinetic sorption behaviors of cesium and strontium in soils. Appl Radiat Isotopes 45:981–985
IAEA (1997) Technologies for in situ immobilization and isolation of radioactive wastes at disposal and contaminated sites. IAEA-TECDOC-972, Vienna
IAEA (1999) Technologies for remediation of radioactively contaminated sites. IAEA-TECDOC-1086, Vienna
Jinzhou D, Wenming D, Xiangke W, Zuyi T (1996) Sorption and desorption of radiostrontium on calcareous soil and its solid components. J Radioanal Nucl Chem 203:31–36
Kamel NHM (2010) Adsorption models of 137Cs radionuclide and Sr (II) on some Egyptian soils. J Environ Radioact 101:297–303
Kaplan DI, Serkiz SM (2001) Quantification of thorium and uranium sorption to contaminated sediments. J Radioanal Nucl Chem 248:529–535
Keppler J, Brümmer GW (1997) Schwermetall-Bindungsformen in Böden und ihre Veränderung über die Zeit - Ergebnisse eines Inkubationsversuches. Mitteilungen der Deutschen Bodenkundlichen Gesellschaft 85:255–258 (in German)
Kosmulski M (2011) The pH-dependent surface charging and points of zero charge V. Update. J Colloid Interface Sci 353:1–15
Kosmulski M (2014) The pH dependent surface charging and points of zero charge. VI. Update. J Colloid Interface Sci 426:209–212
Lavrentyeva GV (2014) Characteristic of pollution with groundwater in flow 90Sr natural waters and terrestrial ecosystems near a radioactive waste storage. J Environ Radioact 135:128–134
Lerouge C, Gaucher EC, Tournassat C, Negrel P, Crouzet C, Guerrot C, Gautier A, Michel P, Vinsot A, Buschaert S (2010) Strontium distribution and origins in a natural clayey formation (Callovian–Oxfordian, Paris Basin, France): a new sequential extraction procedure. Geochim Cosmochim Acta 74:2926–2942
Lim TT, Tay JH, Teh CI (2002) Contamination time effect on lead and cadmium fractionation in a tropical coastal clay. J Environ Qual 31:806–812
Lim TT, Chui PC, Goh KH (2005) Process evaluation for optimization of EDTA use and recovery for heavy metal removal from a contaminated soil. Chemosphere 58:1031–1040
Mulligan CN, Yong RN, Gibbs BF (2001) Remediation technologies for metal-contaminated soils and groundwater: an evaluation. Eng Geol 60:193–207
NCRP (National Council on Radiation Protection & Measurements) (1991) Some aspects of strontium radiobiology. NCRP report no. 110. Bethesda
Park CK, Hahn PS, Park HH (1994) Differentiation of sorptive bindings of some radionuclide with sequential chemical extractions in sendstones. J Korean Nucl Soc 26:461–470
Peters R (1999) Chelant extraction of heavy metals from contaminated soils. J Hazard Mater 66:151–210
Petrović D, Todorović M, Manojlović D, Krsmanović VD (2009) Speciations of trace metals in the accumulation Bogovina on the Crni Timok River. Pol J Environ Stud 18:873–884
Polić P, Pfendt P (1992) Iron and manganese oxides as dominant nickel substrates in the Novi Beograd aquifer. J Serb Chem Soc 57:697–703
Rigol A, Roig M, Vidal M, Rauret G (1999) Sequential extractions for the study of radiocesium and radiostrontium dynamics in mineral and organic soils from Western Europe and Chernobyl areas. Environ Sci Technol 33:887–895
Salbu B, Lind OC, Skipperud L (2004) Radionuclide speciation and its relevance in environmental impact assessments. J Environ Radioactiv 74:233–242
Singh A, Prasad SM (2014) Remediation of heavy metal contaminated ecosystem: an overview on technology advancement. Int J Environ Sci Technol. doi:10.1007/s13762-014-0542-y
Solecki J (2005) Investigation of 85Sr adsorption on selected-soils of different horizons. J Environ Radioactiv 82:303–320
Somasundaran P, Agar GE (1967) The zero point of charge of calcite. J Colloid Interface Sci 24:433–440
Sparks DL (2003) Environmental soil chemistry. Academic Press, San Diego
Sposito G (1984) The surface chemistry of soils. Oxford University Press, New York
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of articulate trace metals. Anal Chem 51:844–860
USDOE (1996) Evaluation of cobalt mobility in soils from thr Nevada test site. Department of Energy, Reno
Wallace SH, Shaw S, Morris K, Small JS, Fuller AJ, Burke IT (2012) Effect of groundwater pH and ionic strength on strontium sorption in aquifer sediments: implications for 90Sr mobility at contaminated nuclear sites. Appl Geochem 27:1482–1491
Wuana RA, Okieimen FE, Imborvungu JA (2010) Removal of heavy metals from a contaminated soil using organic chelating acids. Int J Environ Sci Tech 7:485–496
Xiongxin D, Zuy T (1999) Effect of carbonates on sorption and migration of radiostrontium in calcareous soil. J Radioanal Nucl Chem 242:727–730
Acknowledgments
This work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Project III43009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smičiklas, I., Dimović, S., Jović, M. et al. Evaluation study of cobalt(II) and strontium(II) sorption–desorption behavior for selection of soil remediation technology. Int. J. Environ. Sci. Technol. 12, 3853–3862 (2015). https://doi.org/10.1007/s13762-015-0817-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-015-0817-y