Abstract
Purpose
The critical coagulation concentration (CCC) is considered as one of the most important parameters to evaluate the particles aggregation and sedimentation behaviors in the environment. Even though there are a few methods for its measurement, each method has its limitations especially as those methods to be applied to polydisperse systems like soil. Thus, the purpose of this research is to establish a new and more reliable method for its determination.
Materials and methods
Two types of polydisperse colloidal materials were adopted: soil and humus in the experimental studies. The dynamic light scattering technique was employed to determine the effective hydrodynamic diameter of the particles or aggregates changing with time under different pH and electrolyte concentrations of CaCl2 or KCl. In addition, the fractal dimension of aggregates was also detected with the static light scattering technique.
Results and discussion
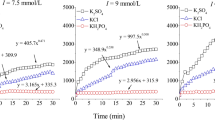
A new aggregation rate total average aggregation rate (TAA rate) was defined firstly. We found the defined TAA rate increased linearly with the increase of electrolyte concentration at electrolyte concentrations lower than the CCC value, and the TAA rate stayed approximately constant at electrolyte concentrations higher than the CCC value; an intersection point of the two straight lines therefore could be observed, and the electrolyte concentration at the intersection point will theoretically be the CCC value. The experimental results for the two materials under different pH conditions indeed meet those theoretical predictions, which imply that the CCC value can be determined through the measurement of the TAA rate under different electrolyte concentrations. The comparison of the CCC values obtained between our new method and the widely applied stability ratio method showed that the new method was much better than the stability ratio method for the two polydisperse materials. In addition, the static light scattering measurements also showed that the variations of the fractal dimensions of the aggregates with electrolyte concentrations could be well explained by the CCC values obtained by the new method, which once again verified the applicability of the new method.
Conclusions
A new theory and a corresponding new method for the CCC estimation, which can be applied to polydisperse colloidal suspensions, were developed, and the new method has been demonstrated to be much better than the widely applied method for polydisperse materials in environment.








Similar content being viewed by others
References
Adachi Y, Koga S, Kobayashi M, Inada M (2005) Study of colloidal stability of allophane dispersion by dynamic light scattering. Colloids Surf A Physicochem Eng Asp 265(1/3):149–154
Amal R, Coury JR, Raper JA, Walsh WP, Waite TD (1990) Structure and kinetics of aggregating colloidal hematite. Colloids Surf 46(1):1–19
Bergstrom L (1997) Hamaker constants of inorganic materials. Adv Colloid Interface 70:125–169
Berka M, Rice J (2004) Absolute aggregation rate constants in aggregation of kaolinite measured by simultaneous static and dynamic light scattering. Langmuir 20(15):6152–6157
Borda T, Celi L, Zavattaro L, Sacco D, Barberis E (2011) Effect of agronomic management on risk of suspended solids and phosphorus losses from soil to waters. J Soils Sediments 11:440–451
Broide ML, Cohen RJ (1992) Measurements of cluster-size distributions arising in salt-induced aggregation of polystyrene microspheres. J Colloid Interface Sci 153(2):493–508
Burns JL, Yan Y, Jameson GJ, Biggs S (1997) A light scattering study of the fractal aggregation behavior of a model colloidal system. Langmuir 13(24):6413–6420
Cahill J, Cummins PG, Staples EJ, Thompson L (1986) Aggregate size distribution in flocculating dispersions. Colloids Surf 18(2/4):189–205
Chen G (2012) S. typhimurium and E. coli O157:H7 retention and transport in agricultural soil during irrigation practices. Eur J Soil Sci 63:239–248
Chen KL, Mylon SE, Elimelech M (2006) Aggregation kinetics of alginate-coated hematite nanoparticles in monovalent and divalent electrolytes. Environ Sci Technol 40(5):1516–1523
Derrendinger L, Sposito G (2000) Flocculation kinetics and cluster morphology in illite/NaCl suspensions. J Colloid Interface Sci 222(1):1–11
Nadeu E, de Vente J, Martínez-Mena M, Boix-Fayos C (2011) Exploring particle size distribution and organic carbon pools mobilized by different erosion processes at the catchment scale. J Soils Sediments 11:667–678
Einarson MB, Berg JC (1993) Electrosteric stabilization of colloidal latex dispersions. J Colloid Interface Sci 155(1):165–172
Frenkel H, Fey MV, Levy GJ (1992) Organic and inorganic anion effects on reference and soil clay critical flocculation concentration. Soil Sci Soc Am J 56:1762–1766
García-García S, Wold S, Jonsson M (2007) Kinetic determination of critical coagulation concentrations for sodium- and calcium-montmorillonite colloids in NaCl and CaCl2 aqueous solutions. J Colloid Interface Sci 315(2):512–519
Gedan H, Lichtenfeld H, Sonntag H, Krug H (1984) Rapid coagulation of polystyrene particles investigated by single-particle laser-light scattering. J Colloid Surf 11(1/2):199–207
Giles D, Lips A (1978) Light-scattering method for study of close range structure in coagulating dispersions of equal sized spherical-particles. J Chem Soc Faraday Trans 1 74:733–744
Hanus LH, Hartzler RU, Wagner NJ (2001) Electrolyte-induced aggregation of acrylic latex. 1. Dilute particle concentrations. Langmuir 17(11):3136–3147
He YT, Wan JM, Tokunaga T (2008) Kinetic stability of hematite nanoparticles: the effect of particle size. J Nanopart Res 10:321–332
Heidmann I, Christl I, Kretzschmar R (2005) Aggregation kinetics of kaolinite-fulvic acid colloids as affected by the sorption of Cu and Pb. Environ Sci Technol 39(3):807–813
Hiemenz PC (1986) Principles of colloid and surface chemistry, 2nd edn. Marcel Dekker: CRC, New York
Hesterberg D, Page AL (1990) Flocculation series test yielding time-invariant critical coagulation concentrations of sodium illite. Soil Sci Soc Am J 54:729–735
Holthoff H, Egelhaaf SU, Borkovec M, Schurtenberger P, Sticher H (1996) Coagulation rate measurements of colloidal particles by simultaneous static and dynamic light scattering. Langmuir 12(23):5541–5549
Kuwatsuka S, Watanabe A, Itoh K, Arai S (1992) Comparison of 2 methods of preparation of humic and fulvic-acids, IHSS method and NAGOYA method. Soil Sci Plant Nutr 38(1):23–30
Liang XQ, Liu J, Chen YX, Li H, Ye YS, Nie Z, Su MM, Xu ZH (2010) Effect of pH on the release of soil colloidal phosphorus. J Soils Sediments 10:1548–1556
Lichtenbelt JW, Pathmama C, Wiersema PH (1974a) Rapid coagulation of polystyrene latex in a stopped-flow spectrophotometer. J Colloid Interface Sci 49(2):281–285
Lichtenbelt JW, Ras HJM, Wiersema PH (1974b) Turbidity of coagulating lyophobic sols. J Colloid Interface Sci 46(3):522–527
Lips A, Smart C, Willis E (1971) Light scattering studies on a coagulating polystyrene latex. J Chem Soc Faraday Trans 67(586):2979–2988
Lips A, Willis E (1973) Low-angle light-scattering technique for study of coagulation. J Chem Soc Faraday Trans 69(7):1226–1236
Mantegazza F, Giardini ME, Degiorgio V, Asnaghi D, Giglio M (1995) Electric birefringence study of reaction-limited colloidal aggregation. J Colloid Interface Sci 170(1):50–56
Matthews BA, Rhodes CT (1970) Studies of coagulation kinetics of mixed suspensions. J Colloid Interface Sci 32(2):332–338
Novich BE, Ring TA (1985) Photon-correlation spectroscopy of a coagulating suspension of illite platelets. J Chem Soc Faraday Trans 1 81:1455–1457
Ottewil RH, Shaw JN (1966) Stability of mono-disperse polystyrene latex dispersions of various sizes. Discuss Faraday Soc 42:154–163
Pelssers EGM, Cohen Stuart MA, Fleer GJ (1990) Single particle optical (SPOS): II. Hydrodynamic forces and application to aggregating dispersions. J Colloid Interface Sci 137(2):362–372
Reerink H, Th J, Overbeek G (1954) The rate of coagulation as a measure of the stability of silver iodide sols. Discuss Faraday Soc 18:74–84
Perdriala N, Perdriala JN, Delphinb JE, Elsassa F, Liewig N (2010) Temporal and spatial monitoring of mobile nanoparticles in a vineyard soil: evidence of nanoaggregate formation. Eur J Soil Sci 61:456–468
Rick AR, Arai Y (2010) Role of natural nanoparticles in phosphorus transport processes in ultisols. Soil Sci Soc Am J 75:335–347
Saejiew A, Grunberger O, Arunin S, Favre F, Tessier D, Boivin P (2004) Critical coagulation concentration of paddy soil clays in sodium–ferrous iron electrolyte. Soil Sci Soc Am J 68:789–794
Smith B, Wepasnick K, Schrote KE, Bertele AH, Ball WP, O’Melia C, Fairbrother DH (2009) Colloidal properties of aqueous suspensions of acid-treated, multi-walled carbon nanotubes. Environ Sci Technol 43(3):819–825
Virden JW, Berg JC (1992) The use photon-correlation spectroscopy for estimating the rate-constant for doublet formation in an aggregating colloidal dispersion. J Colloid Interface Sci 149(2):528–535
Wang DJ, Bradford CA, Paradelo M, Peijnenburg WJGM, Zhou DM (2012) Facilitated transport of copper with hydroxyapatite nanoparticles in saturated sand. Soil Sci Soc Am J 76:375–388
Weitz DA, Lin MY, Linday HM (1991) Universality laws in coagulation. Chemom Intell Lab 10(1/2):133–140
Xiong Y, Chen JF, Zhang JS (1985) Soil Colloid (Vol 2): methods for soil colloid research. Science, Beijing, pp 7–31, in Chinese
Zeichner GR, Schowalter WR (1979) Effects of hydrodynamic and colloidal forces on the coagulation of dispersions. J Colloid Interface Sci 71(2):237–253
Zhou DM, Wang DJ, Cang L, Hao XZ, Chu LY (2011) Transport and re-entrainment of soil colloids in saturated packed column: effects of pH and ionic strength. J Soils Sediments 11:491–503
Zhu HL, Li B, Xiong HL (2009) Dynamic light scattering study on the aggregation kinetics of soil colloidal particles in different electrolyte systems. Acta Phys Chim Sin 25:1225–1231, in Chinese
Acknowledgments
This work was supported by the National Natural Science Foundation of China (40971146), the National Basic Research Program of China (grant no. 2010CB134511), and the Natural Science Foundation Project of CQ CSTC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ying Ouyang
Rights and permissions
About this article
Cite this article
Jia, M., Li, H., Zhu, H. et al. An approach for the critical coagulation concentration estimation of polydisperse colloidal suspensions of soil and humus. J Soils Sediments 13, 325–335 (2013). https://doi.org/10.1007/s11368-012-0608-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-012-0608-8