Abstract
Purpose
TiO2 photocatalytic degradation of 4-chlorobiphenyl (PCB3) in aqueous solution under UV irradiation was investigated as affected by different environmental factors, including initial PCB3 concentration, TiO2 content, UV intensity, H2O2 concentration, cosolvents, and surfactants.
Materials and methods
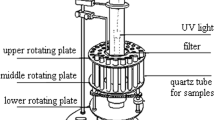
The solution of PCB3 with TiO2 was irradiated by medium mercury lamp. The concentration of PCB3 and intermediates was analyzed by GC-μECD and GC–MS. The values of point charge and bond length were also calculated with ChemOffice 2004 (Mopac unit).
Results and discussion
Photocatalysis was very effective for PCB3 removal, and the degradation kinetics were fitted with a pseudo-first order reaction model. PCB3 was efficiently degraded in the presence of acetone and acetonitrile, but was completely inhibited with other examined cosolvents. HPCD and Tween80 also inhibited the degradation rate of PCB3, while Brij35 slightly decreased the degradation rate of PCB3 at first, and then increased. The reaction mechanism occurred principally by hydroxyl radicals involving the participation of holes and superoxide anion oxidation. A possible photocatalytic degradation pathway of PCB3 was proposed based on the identified reaction intermediates as well as computer simulation.
Conclusions
The photocatalytic approach could be successfully applied to degrade PCB3, and cosolvents and surfactants significantly influenced its degradation kinetics.






Similar content being viewed by others
References
Augugliaro V, Palmisano L, Schiavello M, Sclafani A, Marchese L, Martra G, Miano F (1991) Photocatalytic degradation of nitrophenols in aqueous titanium dioxide dispersion. Appl Catal 69:323–340
Chen Y, Yang S, Wang K, Lou L (2005) Role of primary active species and TiO2 surface characteristic in UV-illuminated photodegradation of Acid Orange 7. J Photochem Photobiol A: Chem 172:47–54
Chiou C-H, Wu C-Y, Juang R-S (2008) Influence of operating parameters on photocatalytic degradation of phenol in UV/TiO2 process. Chem Eng J 139:322–329
Cho Y, Kyung H, Choi W (2004) Visible light activity of TiO2 for the photoreduction of CCl4 and Cr(VI) in the presence of nonionic surfactant (Brij). Appl Catal B: Environ 52:23–32
Chu W, Kwan CY (2002) The direct and indirect photolysis of 4,4'-dichlorobiphenyl in various surfactant/solvent-aided systems. Water Res 36:2187–2194
Chu W, Kwan CY (2003a) Remediation of contaminated soil by a solvent/surfactant system. Chemosphere 53:9–15
Chu W, Kwan CY (2003b) Reactor design and kinetics study of 4,4'-dichlorobiphenyl photodecay in surfactant solution by using a photosensitizer and hydrogen source. Water Res 37:2442–2448
Dai K, Peng T, Chen H, Zhang R, Zhang Y (2008) Photocatalytic degradation and mineralization of commercial methamidophos in aqueous titania suspension. Environ Sci Technol 42:1505–1510
Dai K, Peng T, Chen H, Liu J, Zan L (2009) Photocatalytic degradation of commercial phoxim over La-doped TiO2 nanoparticles in aqueous suspension. Environ Sci Technol 43:1540–1545
Davezza M, Fabbri D, Bianco Prevot A, Pramauro E (2011) Removal of alkylphenols from polluted sites using surfactant-assisted soil washing and photocatalysis. Environ Sci Pollut Res 18:783–789
Eng YY, Sharma VK, Ray AK (2010) Photocatalytic degradation of nonionic surfactant, Brij 35 in aqueous TiO2 suspensions. Chemosphere 79:205–209
Erdemoglu S, Aksu SK, SayIlkan F, Izgi B, Asiltürk M, SayIlkan H, Frimmel F, Güçer S (2008) Photocatalytic degradation of Congo Red by hydrothermally synthesized nanocrystalline TiO2 and identification of degradation products by LC–MS. J Hazard Mater 155:469–476
Fabbri D, Bianco Prevot A, Pramauro E (2004) Kinetic effects of SDS on the photocatalytic degradation of 2,4,5-trichlorophenol. Appl Catal B: Environ 49:233–238
Hidaka H, Zhao J, Pelizzetti E, Serpone N (1992) Photodegradation of surfactants. 8. Comparison of photocatalytic processes between anionic DBS and cationic BDDAC on the titania surface. J Phys Chem 96:2226–2230
Hidaka H, Nohara K, Ooishi K, Zhao J, Serpone N, Pelizzetti E (1994) Photodegradation of surfactants. XV: formation of SO 2−4 ions in the photooxidation of sulfur-containing surfactants. Chemosphere 29:2619–2624
Hoffmann MR, Martin ST, Choi W, Bahnemann DW (1995) Environmental applications of semiconductor photocatalysis. Chem Rev 95:69–96
Hong C-S, Wang Y, Bush B (1998) Kinetics and products of the TiO2, photocatalytic degradation of 2-chlorobiphenyl in water. Chemosphere 36:1653–1667
Huang Q, Hong C-S (2000) TiO2 photocatalytic degradation of PCBs in soil–water systems containing fluoro surfactant. Chemosphere 41:871–879
Jantunen APK, Koelmans AA, Jonker MTO (2010) Modeling polychlorinated biphenyl sorption isotherms for soot and coal. Environ Pollut 158:2672–2678
Jung KY, Park SB (2000) Enhanced photoactivity of silica-embedded titania particles prepared by sol–gel process for the decomposition of trichloroethylene. Appl Catal B: Environ 25:249–256
Kamiya M, Nakamura K, Sasaki C (1994) Inclusion effects of cyclodextrins on photodegradation rates of parathion and paraoxon in aquatic medium. Chemosphere 28:1961–1966
Kubátová A, Erbanová P, Eichlerová I, Homolka L, Nerud F, Šašek V (2001) PCB congener selective biodegradation by the white rot fungus Pleurotus ostreatus in contaminated soil. Chemosphere 43:207–215
Li G, Zhang L, Zhang Z (2008) Determination of polychlorinated biphenyls in water using dynamic hollow fiber liquid-phase microextraction and gas chromatography–mass spectrometry. J Chromatogr A 1204:119–122
Lin YJ, Chen YL, Huang CY, Wu MF (2006) Photocatalysis of 2,2',3,4,4',5'-hexachlorobiphenyl and its intermediates using various catalytical preparing methods. J Hazard Mater 136:902–910
Lu P, Wu F, Deng N (2004) Enhancement of TiO2 photocatalytic redox ability by [beta]-cyclodextrin in suspended solutions. Appl Catal B: Environ 53:87–93
Matsubara K, Abe K, Irie T, Uekama K (1995) Improvement of nasal bioavailability of luteinizing hormone-releasing hormone agonist, buserelin, by cyclodextrin derivatives in rats. J Pharm Sci 84:1295–1300
Minero C, Mariella G, Maurino V, Vione D, Pelizzetti E (2000) Photocatalytic transformation of organic compounds in the presence of inorganic ions. 2. Competitive reactions of phenol and alcohols on a titanium dioxide–fluoride system. Langmuir 16:8964–8972
Moreno-Cerezo JM, Córdoba-Díaz M, Córdoba-Díaz D, Córdoba-Borrego M (2001) A stability study of tetracycline and tetracycline cyclodextrins in tablets using a new HPLC method. J Pharm Biomed Anal 26:417–426
Nomiyama K, Tanizaki T, Ishibashi H, Arizono K, Shinohara R (2005) Production mechanism of hydroxylated PCBs by oxidative degradation of selected PCBs using TiO2 in water and estrogenic activity of their intermediates. Environ Sci Technol 39:8762–8769
Palominos RA, Mondaca MA, Giraldo A, Peñuela G, Pérez-Moya M, Mansilla HD (2009) Photocatalytic oxidation of the antibiotic tetracycline on TiO2 and ZnO suspensions. Catal Today 144:100–105
Parra S, Olivero J, Pulgarin C (2002) Relationships between physicochemical properties and photoreactivity of four biorecalcitrant phenylurea herbicides in aqueous TiO2 suspension. Appl Catal B: Environ 36:75–85
Pelizzetti E, Borgarello M, Minero C, Pramauro E, Borgarello E, Serpone N (1988) Photocatalytic degradation of polychlorinated dioxins and polychlorinated biphenyls in aqueous suspensions of semiconductors irradiated with simulated solar light. Chemosphere 17:499–510
Pelizzetti E, Minero C, Maurino V, Sclafani A, Hidaka H, Serpone N (1989) Photocatalytic degradation of nonylphenol ethoxylated surfactants. Environ Sci Technol 23:1380–1385
Prevot AB, Pramauro E, de la Guardian M (1999) Photocatalytic degradation of carbaryl in aqueous TiO2 suspensions containing surfactants. Chemosphere 39:493–502
Raja P, Bozzi A, Mansilla H, Kiwi J (2005) Evidence for superoxide-radical anion, singlet oxygen and OH-radical intervention during the degradation of the lignin model compound (3-methoxy-4-hydroxyphenylmethylcarbinol). J Photochem Photobiol A: Chem 169:271–278
Shirayama H, Tohezo Y, Taguchi S (2001) Photodegradation of chlorinated hydrocarbons in the presence and absence of dissolved oxygen in water. Water Res 35:1941–1950
Shown I, Ujihara M, Imae T (2010) Sensitizing of pyrene fluorescence by [beta]-cyclodextrin-modified TiO2 nanoparticles. J Colloid Interface Sci 352:232–237
Silva AMT, Nouli E, Xekoukoulotakis NP, Mantzavinos D (2007) Effect of key operating parameters on phenols degradation during H2O2-assisted TiO2 photocatalytic treatment of simulated and actual olive mill wastewaters. Appl Catal B: Environ 73:11–22
Szejtli J (1998) Introduction and general overview of cyclodextrin chemistry. Chem Rev 98:1743–1754
Turchi CS, Ollis DF (1990) Photocatalytic degradation of organic water contaminants: mechanisms involving hydroxyl radical attack. J Catal 122:178–192
Wang G, Wu F, Zhang X, Luo M, Deng N (2006) Enhanced TiO2 photocatalytic degradation of bisphenol A by [beta]-cyclodextrin in suspended solutions. J Photochem Photobiol A: Chem 179:49–56
Wang Y, Zhou D, Wang Y, Zhu X, Jin S (2011a) Humic acid and metal ions accelerating the dechlorination of 4-chlorobiphenyl by nanoscale zero-valent iron. J Environ Sci 23:1286–1292
Wang Y, Zhou DM, Wang YJ, Wang L, Cang L (2011b) Automatic pH control system enhance the dechlorination of 2,4,4'-trichlorobiphenyl and extracted PCBs from soil by nanoscale Fe0 and Pd/Fe0. Environ Sci Pollut Res. doi:10.1007/s11356-011-0587-0
Weber R, Takasuga T, Nagai K, Shiraishi H, Sakurai T, Matuda T, Hiraoka M (2002) Dechlorination and destruction of PCDD, PCDF and PCB on selected fly ash from municipal waste incineration. Chemosphere 46:1255–1262
Wong KH, Tao S, Dawson R, Wong PK (2004) Optimization of photocatalytic oxidation of 2,2',3,3'-tetrachlorobiphenyl. J Hazard Mater 109:149–155
Yang L, Yu LE, Ray MB (2008) Degradation of paracetamol in aqueous solutions by TiO2 photocatalysis. Water Res 42:3480–3488
Zhan M, Yang X, Xian Q, Kong L (2006) Photosensitized degradation of bisphenol A involving reactive oxygen species in the presence of humic substances. Chemosphere 63:378–386
Zhang H, Zong R, Zhao J, Zhu Y (2008) Dramatic visible photocatalytic degradation performances due to synergetic effect of TiO2 with PANI. Environ Sci Technol 42:3803–3807
Acknowledgements
This work was supported by the Public Welfare Project of Ministry of Environmental Protection of Peoples’ Republic of China (No. 201009009) and the National Basic Research and Development Program (No. 2007CB936604).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Huijun Zhao
Electronic supplementary material
Kinetic parameters for photocatalytic degradation of PCB3 with initial concentrations of PCB3 and Brij35/Tween80 (Table S1), the degradation intermediates identified from GC–EI–MS (Tables S2 and S3), charges of atoms and bond lengths of the PCB3 based on the AM1 level (Table S4), and the structure of 4-chlorobiphenyl (Fig. S1) as well as the GC–MS–EI spectrum of degradation products of PCB3 (Fig. S2) are included
ESM 1
(DOC 203 kb)
Rights and permissions
About this article
Cite this article
Zhu, X., Zhou, D., Cang, L. et al. TiO2 photocatalytic degradation of 4-chlorobiphenyl as affected by solvents and surfactants. J Soils Sediments 12, 376–385 (2012). https://doi.org/10.1007/s11368-011-0464-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-011-0464-y