Abstract
Purpose
Digital closed-loop medication administration (CLMA) is a prime example of how digitalization in healthcare can reduce time and costs while simultaneously increasing patient safety. However, in contrast to its social and economic benefits, associated environmental impacts have never been investigated. Since the required amount of packaging material is seen as a major subject of criticism, we assess associated environmental burdens using life cycle assessment (LCA) methodology to identify hotspots and provide adaptation recommendations.
Methods
Digital, as well as conventional paper-based medication management (PBMM), involve two types of packaging: pharmaceutical packaging (blisters or bottles), containing purchased medication, and clinical packaging (unit doses or medical dispensers) for medication dispensation. Therefore, a cradle-to-grave life cycle model was established comparing impacts at pharmaceutical packaging level, clinical packaging level, and a combined level. While PBMM requires small, blistered boxes, CLMA requires large, bottled packages. Therefore, the functional unit allows to compare different box sizes but inhibits comparing medication from different manufacturers. Primary data is obtained from University Medical Centre Hamburg-Eppendorf which practices state-of-the-art CLMA. PBMM data is obtained in three dispenser scenarios, varying the reusability of medical dispensers. The life cycle impact assessment was performed for five impact categories using CML 2001(2016) method.
Results and discussion
All compared blistered pharmaceutical packaging options yield higher impacts than their bottled counterparts. Cardboard and package inserts result in up to 40 times higher impacts for blisters than for bottles, which, however, is attributed to the methodology of comparing different box sizes. Yet, this does not influence the required amount of blister laminate. All blister materials were found to result in higher environmental burdens, peaking in nearly 240 times higher ozone depletion impacts for polyvinylchloride/polychlorotrifluoroethylene blisters. In terms of clinical packaging, two of three dispenser scenarios result in higher environmental burdens than the respective unit dose scenario. Only if reused, 7.2 times this trend is reversed. When combining selected impacts from both pharmaceutical and clinical impacts, all three dispenser scenarios perform worse than the unit dose scenario.
Conclusions
Packaging impacts are lowest if unit dose dispensation is combined with bottled pharmaceutical packaging. Given a specific pharmaceutical packaging, dispensation via medical dispensers may perform better, if they are comprehensively reused. With this study, the authors disproved that packaging impacts are generally higher for CLMA than for PBMM. However, both systems certainly comprise other processes and materials like consumed paper or energy to run required digital infrastructure, which should be subject to future studies.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
Digitalization in the healthcare sector is seen as a major driver to make processes more time- and cost-efficient by simultaneously increasing patient safety (Flott et al. 2021; Kiliç 2016). A prime example to reach these goals is the digitalization of medication management. The term medication management refers to all process steps required from medication prescription by a physician to medication administration by a patient. While conventional paper-based medication management (PBMM) is associated with a high degree of avoidable medication errors, studies clearly show that the implementation of digital medication management, such as closed-loop medication administration (CLMA), significantly reduces the prevalence of medication errors (Ball and Douglas 2002; Kozer 2009; Krähenbühl-Melcher et al. 2007; Schütze and Schneemann 2007). Medication errors may lead to adverse drug effects, decreased patient outcomes, and ultimately death (Schnurrer and Frölich 2003) resulting in annual costs of up to 2.5 billion pounds in the UK, 4.2 billion dollars in the USA, and 1.2 billion euros in Germany (Aly et al. 2015; Pan et al. 2015; Torjesen 2014). During PBMM, 37–67% of all medication errors occur either during medication dispensation or administration (Baehr 2018). CLMA significantly reduces these medication errors by introducing the concept of automatically packed single-dosed plastic pouches, the so-called unit doses (UD) (Fig. 1). Their major advantage lies in the fact that UD can be personalized with relevant patient data like patient name, date, and time of administration. In contrast, during PBMM, medication is dispensed manually (by the human workforce) into multi-dose dispensers (MDs) with four cavities (morning-midday-evening-night) (Fig. 1).
While the above-cited literature indicates the advantageous social and economic effects of UD over manual medication dispensation (Ball and Douglas 2002; Kozer 2009; Krähenbühl-Melcher et al. 2007; Schütze and Schneemann 2007), the third pillar of sustainability—the environmental pillar—was never substantially investigated. To this day, there is no study evaluating the environmental impacts of UD using the life cycle assessment (LCA) methodology. Anecdotal evidence from industrial, academic, and political experts in the field depicts that the considerable amount of packaging waste produced within CLMA—and the environmental impacts associated with it—is a growing concern to stakeholders. Indeed, with a total of about 1.7 billion tablets being dispensed in German hospitals each year, the packaging material required is considerable (IQVIA Commercial GmbH & Co. OHG 2021). In contrast, MD used during PBMM could theoretically be washed and reused—thus, stakeholders hypothesize lower environmental burdens. However, to quantitatively assess the environmental impacts of required packaging, it must be considered that both processes require a repackaging step. Tablets must be transferred from their pharmaceutical packaging (blister packs (B) or bottles (Bo)) into the respective clinical packaging (UD or MD). Thus, both pharmaceutical and clinical packaging should be considered. Literature on the impacts of pharmaceutical packaging is scarce but recent. Bassani et al. (2022) performed a cradle-to-gate LCA comparing the environmental impacts of 23 different medication packages commonly distributed in community pharmacies (Bassani et al. 2022). While sachets resulted in six times higher environmental impacts than B, the comparison of Bo and B leads to less clear results. Compared to B, impacts of Bo were found to result in lower (up to − 80%) and higher (up to + 57%) environmental impacts, depending on the compared package. Generally, material production accounted for the highest life cycle impacts (55–98%). The comparison of thermoformed blister laminates and cold-formed blister laminates resulted in lower impacts for thermoformed laminates. This was also found by Raju et al. who performed a cradle-to-gate LCA, finding that the production of a cold-formed B consumed 63% more energy and have a 70% higher global warming potential (GWP) than its thermoformed alternative (Raju et al. 2016).
To quantify the hypothesis that packaging material required for CLMA leads to higher environmental burdens than packaging material required for PBMM, pharmaceutical and clinical packaging of both processes were assessed using LCA methodology. Accordingly, this work aims to answer the following research question: Is packaging material required to perform CLMA associated with higher environmental burdens than packaging required to perform PBMM?
By answering the research question, this work aims to contribute to the literature as the first study in the field to assess the environmental burdens of packaging required to perform PBMM and CLMA, respectively. Furthermore, we advance the literature by considering end-of-life (EoL) in the system boundaries, which has not yet been addressed in previous pharmaceutical packaging studies. Thus, we intend to provide clarity for fact-driven discussions and provide further recommendations on how to implement mitigation measures in the future.
2 Methods
This study used the current state-of-the-art LCA methodology in line with ISO 14040 and ISO 14044 standards.
2.1 Goal and scope
Generally, pharmaceutical packaging is classified into primary, secondary, and tertiary packaging. While primary packaging includes all materials that are in direct contact with the dosage form, secondary packaging refers to consecutive coverage around the primary package, especially used for identification, labeling, and marketing. Tertiary packaging provides bulk handling for transportation (Siegert et al. 2018). In the LCA context, the technical words primary and secondary are often related to the virgin or recycled content of the material, respectively (Hauschild et al. 2018). Therefore, this study distinguished primary/secondary raw materials (referring to the respective life cycle stage) and primary/secondary packaging (referring to the type of packaging). Furthermore, the term tablets was used as a synonym for all other solid orally administrated drugs (e.g., capsules) which can be dispensed by the two evaluated processes.
A cradle-to-grave life cycle model was established to obtain the environmental impacts of packaging material required to perform PBMM and CLMA. The functional unit (FU) was defined as a “packaging required for correct dispensation and administration of one tablet – with the same active pharmaceutical ingredient, dosage, weight, volume, and form, produced by the same pharmaceutical manufacturer – each time a physician prescribes a tablet in a German inpatient facility.” Accordingly, the reference flow is set at 1 tablet. This definition implies impacts resulting from both pharmaceutical and clinical packaging. In contrast to prior studies (Bassani et al. 2022), the FU limits comparisons to tablets being produced by the same pharmaceutical manufacturer, having the same weight and volume. In the contrary, however, the FU used in this study allows comparing different box sizes since the purchase of small B is favorable for PBMM, while large Bo are favorable for CLMA.
To evaluate the packaging required during all process steps of medication management, results were compared on the level of pharmaceutical packaging (B/Bo comparison), clinical packaging (UD/MD comparison), and a combined level. On the combined level, principally four combinations were possible (B or Bo dispensed either in UD or MD). However, a peculiar difference between the two medication management processes leads to different pharmaceutical packaging requirements and thus purchasing patterns. While the repackaging step into MD is conventionally performed directly at the respective hospital unit (decentralized), where only limited storage space is available, conventionally small (mostly blistered) boxes are purchased and refilled regularly. In contrast, UD dispensation is performed in the hospital’s pharmacy (centralized). Here, preferably large bulk containers (e.g., Bo) are required to facilitate the refilling of UD dispensing machines. These store different tablet types in separate bulk containers before repackaging into UD. Therefore, refilling storage containers is simplified by purchasing large Bo instead of B, which needs to be deblistered manually. To acknowledge that both medication management processes favor different formats of pharmaceutical packaging, this work only compared the results of the two likeliest combinations (Bo–UD/B–MD) (Fig. 2).
The full UD inventory was obtained at the University Medical Centre Hamburg-Eppendorf in Germany (UKE). As one of the first hospitals to introduce CLMA in Germany, UKE practices state-of-the-art medication management and serves as a prime example for CLMA-related data collection. In contrast, inventory for MD was obtained from national statistics and literature (Klauber et al. 2021). This was done due to the following consideration: MD may be washed and reused; however since the reusability of dispensers is not being prospected in current legislation, each hospital reuses dispensers to a different extent. While some hospitals may not reuse dispensers at all and dispose of them directly after a single use, others might reuse dispensers once, twice, or even at a greater rate. Therefore, the dispenser demand per FU might vary extensively. Collecting inventory at a certain hospital would therefore only indicate reusability practices at the evaluated hospital but not give a comprehensive global picture of different hospitals. Therefore, three MD scenarios with varying dispenser demand per inpatient were established (MD S1–MD S3) (Fig. 2). In MD S1, all MD were disposed each day leading to a dispenser demand of one dispenser per inpatient per day. In MD S2, MD were reused for the whole stay of the inpatient. Thus, one dispenser was required per inpatient per stay. In MD S3, MD were disposed every second day. Consequentially, results from all three MD scenarios were compared to a UD scenario.
To analyze the impacts of pharmaceutical packaging before dispensation, the top 100 purchased tablets at UKE were selected, accounting for about one sixth of the total amount of purchased tablets. From the top 100, 25 are purchasable from the same manufacturer in B and Bo, respectively. These 25 blister-bottle pairs were included in the analysis, always including the largest purchasable bulk container and the smallest purchasable blister box traded by a specific pharmaceutical manufacturer.
If not other stated, the life cycle inventory for blister and bottle comparison was obtained following the methodological approach and assumptions established by Bassani et al. (2022). This, among others, includes neglecting tertiary packaging, transformation processes of packaging materials (e.g., extrusion of thermoformed forming foils, lamination of cold-formed forming foils), and average electricity consumption and production losses in the packing process. Deviation from their methodology was only done regarding transportation and EoL. As Bassani et al. (2022) showed, the pharmaceutical market is highly globalized and transportation routes differ widely. Therefore, transportation impacts vary drastically, depending on the respective weight, size, distance, and transportation type. However, they conclude that if tablets are transported solely by train or truck, 99% and 98% of the cradle-to-gate impacts, respectively, are associated with production. Similarly, neglectable are container ship transportation impacts in four out of five impact categories. Only in acidification, the selected packaging options led to impacts of up to 24%. In contrast, if tablets are transported by plane, transportation impacts increase drastically in all impact categories, peaking in 94% of the impacts for ozone depletion. Since Bassani et al. (2022) already performed an in-depth analysis of transportation scenarios showing that truck, train, and ship transportation does not lead to significant impacts, we excluded transportation of pharmaceutical packaging from the pharmaceutical plant gate to the hospital from the system boundaries. The reason for that is twofold: First, aircraft transportation is unlikely since all considered tablets have long expiry periods, must not be cooled, and do not need urgent transportation for other therapeutical or pharmaceutical reasons; second, in contrast to Bassani et al. (2022), this work does not compare packaging from varying manufacturers, which might be located in different continents. Since all B/Bo pairs are produced by the same respective manufacturer, transportation routes were considered to be similar, if not the same.
Differently, however, this work considered the transportation of clinical packaging. While UD pouches are specialized products for a niche market, they are produced and imported from Japan, with a transportation distance of 21,000 km by trans-oceanic ship and an additional 100 km lorry transport from Hamburg harbor to the hospital. In contrast, MDs are day-to-day products considered to be produced in Germany with a transportation distance of 100 km by lorry. Since transportation distances vary significantly within the compared packaging types, these distances were included in the system boundaries, and impacts were calculated based on transported mass. Acknowledging production facilities being located in Japan and Germany, respectively, global (GLO) and European (RER) datasets are considered for production.
Further amendments from Bassani et al.’s (2022) system boundaries are done regarding EoL treatment. While Bassani et al. (2022) performed a cradle-to-gate LCA, this study includes an EoL scenario that was considered representative of generic packaging waste management for all hospitals in Germany. As proposed in the Product Category Rule for future LCAs on pharmaceutical products and processes by Siegert et al. (2018), all primary packaging was considered to be incinerated in a municipal incineration plant while secondary packaging was considered to be recycled (Siegert et al. 2018). This EoL approach is in alignment with current practice at UKE. Siegert et al. (2020) indicate that packaging disposal in healthcare facilities is considered regular (via residual or commercial waste, in contrast to irregular disposal via sinks and toilets), and thus, 100% incineration was assumed, modeled as non-hazardous waste (Siegert et al. 2020). The EoL treatment of all materials is located in Germany, and a generic distance of 200 km is added for all materials to consider transportation from the hospital to the EoL facility. In line with previous studies (Bassani et al. 2022; Belboom et al. 2011), EoL multifunctionality was resolved by system expansion and accordingly credits for avoided energy and heat provision were given for the incineration of plastic waste, and credits for avoided virgin production were given for the recycling of paper.
All foreground data was measured either by the authors or provided by suppliers. Background data (including production and transformation processes, electricity, transportation as well as all recycling processes such as waste collection, sorting, processing, or incineration) were obtained from the ecoinvent 3.8 database (Wernet et al. 2016) (refer to Online Resource 1 for detailed overview of utilized ecoinvent processes).The life cycle of the packaged tablets itself was excluded since it is independent of the packaging. The system was modeled using Umberto 11 LCA software.
Figure 3 shows the Sankey diagram indicating the system boundaries.
2.2 Life cycle inventory
2.2.1 Inventory of pharmaceutical packaging
Twenty-five out of the top 100 purchased tablets were purchasable in both blistered (B) and bottled (Bo) packaging. Accordingly, 50 different packaging samples were included in the study. Packaging material composition was accessed from the manufacturers’ advanced prescribing information datasheet which is accessible online for healthcare professionals (Gelbe Liste 2022). In total, one cold-formed forming foil comprising oriented polyamide/aluminum/polyvinylchloride (oPA/Alu/PVC) and five thermoformed forming foils comprising polyvinylchloride (PVC), polyvinylchloride/polyvinylidenchloride (PVC/PVDC), polyvinylchloride/polyethylene/polyvinylidenchloride (PVC/PE/PVDC), polypropylene (PP), and polyvinylchloride/polychlorotrifluoroethylene (PVC/PCTFE) were assessed. Forming foils were modeled using the respective ecoinvent 3.8 processes. However, in the lack of a more appropriate process for PCTFE, tetrachloroethylene (PCE)—an intermediate product within PCTFE synthesis—was used to estimate its environmental impacts. Lidding foils most commonly consist of hard and inductile aluminum foils of 20.32-µm thickness, a double coat of a heat seal lacquer (HSL), and a heat-sealing print primer of similar composition (Mühlfeld et al. 2012; Pilchik 2000). Commonly used HSL and print primers are vinyl-acrylate-based adhesives. The applied HSL was assumed to be Vinnol® H40/43, a commonly used HSL for a variety of cold-formed and thermoformed blister composites. The exact composition was obtained from the literature (Wacker Chemie AG 2021). HSL was modeled by combining vinyl chloride and vinyl acetate in quantities of 65.7% and 34.3%, respectively. Vinnol® H15/45 M, also commonly used for different blister materials, was assumed as a primer. It was modeled by combining vinyl chloride, vinyl acetate, and dicarboxylic acid in quantities of 84%, 14%, and 1%, respectively (Wacker Chemie AG 2021). In the lack of a more suitable dicarboxylic acid ecoinvent process, maleic anhydride was used. As double-coat grammages vary from 6 to 9 g/m2 (Mühlfeld et al. 2012), a grammage of 7 g/m2 with a 90:10 ratio of HSL and print primer was assumed. Bo comprised a high-density polyethylene (HDPE) bottle and either an HDPE or PP cap. Box sizes utilized for data acquisition varied between 14 and 1000 tablets per box.
The inventory was obtained by measuring and calculating the average weight of three tablets in a box. According to the European Pharmacopoeia, the weights of tablets must lie within certain limits depending on their overall weight (7.5% for tablets of 80–250 mg and 5% for tablets > 250 mg) (van Vooren et al. 2002). These limits were considered appropriate to calculate the weight of empty blister cards as indicated in Eq. 1.
where:
Aiming to control the progress of data acquisition and to avoid typing errors, the weight of the total package was not only measured but also calculated by adding all individually measured parts (cartonnage, insert, blister cards, etc.). Furthermore, this procedure indicated systematic device-dependent weighing errors. The weight of each blister layer per FU was calculated as indicated in Eq. 2. The material selection databank CES EduPack 2015 was used to obtain the densities of all packaging materials.
where:
To validate the calculated material inputs, values obtained from Eqs. 1 and 2 were compared to the measured weights of the blister cards. A deviation of more than ± 0.099 g (or ± 5%) of the blister weight was considered unacceptable. Any error below that value was considered a reasonable systematic device-dependent weighing error. Likewise, the measured and calculated weights were compared to obtain the bottle material inputs. Some bottles contained desiccants to control humidity within the packaging. Silica gel desiccants were either packed in small sachets or HDPE containers which were attached to the inner side of the bottle’s cap. Table 1 summarizes the full inventory of all 25 compared B/Bo pairs per FU.
Specific production-related parameters like transformation processes, production losses, and electricity demand for the packing process are obtained from Bassani et al. (2022). However, to represent global production, global datasets were used for production and EoL of production losses. Transportation of raw materials to the packing location and transportation of production losses from the packing location to the EoL facility are also obtained from Bassani et al. (2022) and are considered representative of global production.
2.2.2 Inventory of clinical packaging
Literature data was used to obtain the three different MD scenarios (Table 2). This data is sufficient to calculate the material demand required to dispense medication via PBMM, varying the number of MD required per inpatient per day (Table 3). In MD S1, each inpatient received a new MD every day. With a total of about 140 million inpatient days spent in German hospitals and a total of about 1.7 billion tablets being dispensed in the same reference timeframe, each inpatient received an average of 12.3 tablets per day, which refers to 0.081 MD per tablet. MD S2 hypothesized that the MD is washed and reused for the full stay of the inpatient (average of 7.2 days). Accordingly, an average of 89.2 tablets is dispensed per MD, equaling 0.011 dispensers per FU. MD S3 hypothesized that each MD is washed once and disposed of the next day. Thus, 24.8 tablets are dispensed per MD, equaling 0.04 MD per FU.
According to the hygiene plan for manual repackaging of medicine published by the German federal pharmacist association (Bundesapothekerkammer), reusing MD requires appropriate cleaning before each reuse which may be performed with an industrial dishwasher or by hand (Bundesapothekerkammer 2017). As handwashing requires a considerable amount of scarce caregivers’ time, washing is more likely to be performed with a dishwasher. The life cycle inventory for dishwashing is obtained from Goulet et al. (2017) who collected dishwasher inventory to clean the mouthpiece of a medical inhaler (Goulet et al. 2017). As both the inhaler and MD are medical products with comparable hygienic requirements, the inventory was not further altered. Only the take-up factor was increased from 1 to 2% of the dishwasher’s volume to consider respective size differences. Reused MD was assumed to be washed once a day; hence, materials and energy were allocated accordingly. No further impacts, such as transportation impacts, are considered for reuse of MD since use, washing, and reuse are carried out within the same hospital unit.
Generally, MD may be purchased in multiple variations with minor differences in composition and weight; however, the chosen MD is considered representative since it is purchasable via wholesale in palleted quantities and has been used at UKE before the implementation of CLMA. MDs are purchasable in packs of 200 dispensers, and their data was obtained by supplier information (AMPri 2022). The “Bill” of Materials (BOM) for all three MD scenarios is indicated in Table 4. To model the transformation of the MD body, the extrusion process and thermoforming with 6% production losses were used; for the MD lid, an extrusion process with 3.1% production losses was used (Hischier 2007). The raw materials and transformation processes were modeled using European average values available in the ecoinvent database.
UD data was either measured directly or obtained by the packaging foil supplier. UD comprises a packaging foil which is printed using a printing ribbon foil (Table 4). Packaging and printing ribbon foils have a total of 420- and 400-m length, respectively, and each is packaged in a separate plastic pouch. Six packaging foils and ten printing ribbon foils, respectively, are packaged in a cardboard box for transport. Transformation processes for foil production were modeled using extrusion processes considering the respective production losses (Hischier 2007) and since they are manufactured in Japan, a global energy mix is assumed. In addition, within the UD-packing process, an additional scrap rate of 22.28% was considered, which was established from current practices at UKE. While the key concept of UD is to deliver each medication dose in a separate pouch, a dose usually consists of one tablet. However, it may also consist of two (or more) tablets to result in the appropriate therapeutical dosage. Based on primary data, 20% of all UD comprise more than one tablet. Therefore, the average number of drugs per pouch (1.2) was considered in the inventory compilation.
While tablet dispensing in the PBMM process is manual work, tablet dispensing in the CLMA process is performed automatically. Therefore, the additional energy demand must be considered for the dispensing and control machines. Table 5 indicates the respective specifications and inventory. In total, 12,000 UD pouches are produced per day (8-h working day). Electricity was modeled using the German grid mix.
If all UD were produced at once, without process-related breaks, the daily demand could be produced in 200 min. However, this does not reflect the reality as packaging foil rolls and bulk medication need to be refilled time by time. Furthermore, medication is supplied twice a day—once in the morning and once in the afternoon. To consider additional energy during standby, an energy demand of 30% was assumed.
2.3 Life cycle impact assessment
As proposed by Siegert et al. (2018) and following previous studies (Bassani et al. 2022; Raju et al. 2016), impacts were measured in the following impact categories using CML 2001(2016) method: global warming potential—100 years (GWP), abiotic depletion-fossil fuels (ADP fossil), acidification potential (AcP), eutrophication potential (EuP), and stratospheric ozone depletion potential (ODP) (Guinee 2002). CML 2001(2016) method was chosen to enable comparison of our results with previous data reported in the cited literature.
3 Results and discussion
The following section presents results in three subsections, following the study design indicated in Fig. 2. While Sect. 3.1. presents the results of the 25 assessed pharmaceutical packaging pairs, Sect. 3.3. presents the results of all four clinical packaging scenarios. Section 3.3. combines the results from the first two sections indicating the overall impact results.
3.1 Pharmaceutical packaging
This section presents the results of eight cold-formed B packages and 17 thermoformed B packages in comparison to their respective Bo packaging options in five impact categories (Fig. 4). All graphs are organized in the same manner: Bar graphs compare B and Bo, indicating the respective ID (Table 1). For a better comparison of the blister materials, bar graphs are grouped by blister laminate. Furthermore, the total box size (e.g., “B-20” for 20 blistered tablets per box or “Bo-100” for 100 bottled tablets per box) is indicated. Different box sizes were explicitly used for data collection to reflect real-life differences in purchasing patterns resulting from the abovementioned processual reasons. These lead to small B boxes being favored to perform PBMM, while large Bo boxes being favored to perform CLMA. This difference in purchase patterns had a significant impact on the overall demand for secondary packaging since fewer tablets per box result in higher secondary packaging demand. This can especially be observed for ID 13 where secondary packaging impacts are about 40 times higher for the blistered option with 14 tablets per box than the corresponding bottled option with 100 tablets per box (3977% for GWP and 4750% for EuP). Similar results can be obtained for all other analyzed packages, with secondary packaging impacts of blistered options exceeding those from bottled options by ranging from + 120% for AcP (ID11) to + 2011% for EuP (ID12). Especially noteworthy are the high absolute impact values of thermoformed ID 25 which are to a magnitude of three higher than all other assessed packages. These high impacts result from the fact that the only purchasable blistered packaging option for this medication is a 100-tablet containing bundle package, consisting of ten separate cardboard boxes containing ten tablets each.
Although purchasing small packages of blistered tablets highly increases the required secondary packaging demand, the primary packaging demand for small and large blistered boxes remains constant. Since the total number of tablets per box is determined by the number of identical blister cards within a box, the amount of blister material required stays constant. Exemplarily, a box with ten tablets may contain only one blister card, while a box with 100 tablets may contain ten identical blister cards. In contrast, primary packaging demand for bottled medication can be reduced when increasing the number of tablets in a bottle. This can especially be observed for ID 6, which contains 1000 tablets per bottle and in all evaluated impact categories results in the lowest absolute impacts. However, also all other evaluated bottled packages resulted in fewer environmental primary packaging impacts than their respective blistered counterparts. Cold-formed B performed especially worse. Here, impacts from production and EoL of primary packaging resulted in 494% higher impacts for GWP and up to 1,020% higher impacts for AcP compared to the respective bottled packages. Although cold-formed blister packages also performed worse in the impact category ODP (up to 490% higher impacts), extraordinarily high impacts in this impact category were obtained for PVC/PCTFE forming foils. Here, impacts for blistered packaging were nearly 240 times higher than for respective bottled packaging and should thus be avoided. These impacts result from PCE production which is an intermediate product within the PCTFE synthesis. To obtain PCTFE, PCE reacts with trichlorotrifluoroethane (CFC-113) to chlorotrifluoroethylene (CTFE), which is then polymerized to PCTFE (Siegemund et al. 2016). Since both the CFC-113 and CTFE are highly ozone-depleting chlorofluorocarbons, real PCTFE impacts are expected to be even higher as indicated.
Concerning primary packaging impacts, the findings of this work deviate from prior studies (Bassani et al. 2022). While in the present work, higher impacts for all evaluated blistered packages than for respective bottled packages were found, Bassani et al. (2022)’s analysis showed that bottles resulted in up to 57% higher impacts than the respective blistered package. This, however, may be a result of comparing non-identical tablets. While they compared tablets containing the same active pharmaceutical ingredient and dosage, their FU allowed comparing tablets from varying manufacturers with tablets differing in size, weight, and volume. Since the definition of the FU selected for the current work was explicitly limited to identical tablets, differences in impact results may be explained.
3.2 Clinical packaging
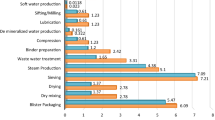
In a second step, environmental impacts arising from clinical packaging by defining one UD scenario and three MD scenarios were analyzed (Fig. 5). Here, similar trends were observed for all five examined environmental impact categories. Primary packaging production and EoL were the most important impact contributors for both the MD and UD scenarios. Only for MD S2, which assumed an average MD reusability of 7.2 times, washing impacts result in higher burdens than MD production and EoL. As intended, the results show that MD washing and reuse can lead to reduced environmental impacts in comparison to a single use. While MD S1 (single use) resulted in the highest environmental burdens, MD S2 (reuse for a whole hospital stay) and MD S3 (disposal every 2 days) resulted in significantly lower environmental impacts. Compared to MD S1, impacts in MD S2 are reduced by 49–82% depending on the impact category, while impacts in MD S3 were reduced by 25–41%. Regarding the UD scenario, it performed better than MD S1 and MD S3, but worse than MD S2 in all evaluated environmental impact categories. For the impact category GWP, the UD scenario resulted in 40, 61, and 119% of the impacts compared to MD S1, 3, and 2, respectively.
In contrast to the evaluated pharmaceutical packaging, secondary packaging of MD and UD was not a significant impact contributor. UD packaging foil is shipped in boxes containing 2520 m of UD packaging foil. This relates to more than 35,000 FUs which are packaged in one secondary packaging box. Similarly, MDs are shipped in boxes containing 200 MDs, which relates to 2460–17,840 dispensed FU, depending on the dispenser scenario. Likewise, transportation impacts are of minor significance for all scenarios. Only in the impact category, AcP UD transportation impacts are significant, relating to 21.2% of the overall impacts.
3.3 Combined results
When evaluating the environmental impacts of packaging required to perform medication management, impacts from both pharmaceutical and clinical packaging need to be taken into consideration. By performing the above-presented comparisons, it is possible to calculate the combined impacts of purchasing a specific tablet in either B or Bo which is then dispensed using either MD or UD. While all four combinations are principally possible, we acknowledge that both medication management processes favor different formats of pharmaceutical packaging. Therefore, we suppose that a tablet that is available in both, blistered and bottled packaging format, is most likely to be purchased in a blistered format when a dispensation is performed using MD, and in a bottled format when UD is used for dispensation. Thus, in the latter, results are only discussed for these two combinations (Fig. 6). Combined impacts can be obtained for all 25 evaluated medications being dispensed in all discussed dispensing scenarios. Since similar trends are observed within all environmental impact categories, we only present combined impacts for the impact category GWP.
Although results from Sect. 3.3 indicated that the clinical packaging impact of MD S2 yielded the lowest impacts, the combined pharmaceutical and clinical packaging impacts yielded different results. Here, the UD scenario resulted in the lowest impacts. While clinical packaging impacts stayed constant, the overall result of each scenario changed due to the added pharmaceutical packaging impacts. Since impacts from blistered pharmaceutical packaging are, in most cases, significantly higher than impacts from bottled pharmaceutical packaging, combined impacts of all three MD scenarios performed worse than the UD scenario.
4 Conclusions and recommendations for future work
The overall aim of this study was to analyze if the packaging material required to perform digital CLMA is associated with higher environmental impacts than the packaging material required to perform conventional PBMM. Therefore, results were obtained at the level of pharmaceutical packaging, clinical packaging, and combined level. Regarding pharmaceutical packaging, the results show that in all evaluated cases, primary as well as secondary packaging impacts of blisters are associated with higher impacts than the respective bottled packaging option. Results obtained for clinical packaging show that if both, MD and UD, are disposed of after single use, MD performs worse in all impact categories (e.g., UD results in 40% of MD impacts for GWP). MD impacts can be reduced by comprehensive reuse (at least 7.2 times), which requires adequate cleaning. However, comprehensive MD collection, cleaning, and reuse would consume a considerable additional amount of scarce caregivers’ time, and therefore, disposal seems to be the faster and more practical approach. Combined environmental impacts of pharmaceutical and clinical packaging show that, irrespective of the MD reusability, the choice of pharmaceutical packaging determined the overall impact balance. Due to the utilization of blistered pharmaceutical packaging, all three MD scenarios performed worse than the UD scenario using bottled pharmaceutical packaging. Thus, pharmaceutical packaging should always be included when assessing the environmental impacts of packaging required for medication management. All in all, it is possible to conclude that packaging impacts resulting from medication management are the lowest if UD dispensation is combined with bottled pharmaceutical packaging. Given a specific pharmaceutical packaging, MD dispensation may perform better, depending on the extent of its reusability. However, given that the proposed MD scenarios studied in this work are based on literature data only, obtained absolute values might differ from reality, which depicts a major limitation of our study. Therefore, future studies should develop a comprehensive and reliable MD scenario that is representative of all PBMM-performing hospitals.
Although this study is based on data obtained in Germany, its findings are applicable to hospitals elsewhere. Independent of the geographic location, hospitals purchase medication in pharmaceutical packaging and dispense it using clinical packaging. Therefore, stakeholders from any country could implement the following recommendations to further decrease environmental impacts from medication management practices: (a) PBMM-performing hospitals could on the one hand re-evaluate if purchasing larger blistered medication boxes is possible to reduce secondary pharmaceutical packaging impacts. On the other hand, clinical packaging impacts could be reduced by purchasing reusable MD and simultaneous implementation of a comprehensive collection and cleaning workflow, which does not contradict other caregivers’ responsibilities; (b) CLMA-performing hospitals could not only demand more bottled medication from the pharmaceutical industry but furthermore also demand bottles containing larger tablet quantities. Since medication, once opened, stays within the controlled conditions of the hospital pharmacy and is handled by educated staff, no technical or pharmaceutical reasons would contradict the provision of bottles containing thousand or even ten thousand tablets. This way, the environmental impacts of pharmaceutical packaging could be significantly decreased. UD impacts could only be decreased by a reduction of respective material demand or usage of secondary raw materials.
With this study, we are the first in the field to evaluate the environmental impacts of different medication management processes. Given that, we were able to answer the proposed research question, disproving that the packaging material required to perform CLMA generally leads to significantly higher environmental impacts than the packaging material required to perform conventional PBMM. However, certainly digital and conventional medication management comprises many other processes and materials than just packaging. Therefore, future studies should comprehensively evaluate PBMM-related parameters like consumed paper and required medication storage space, as well as CLMA-related parameters like energy to run required digital infrastructure.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AcP:
-
Acidification potential
- ADP fossil:
-
Abiotic depletion-fossil fuels
- B:
-
Blister pack
- Bo:
-
Bottle
- BOM:
-
Bill of Materials
- CFC-113:
-
Trichlorotrifluoroethane
- CLMA:
-
Closed-loop medication administration
- CTFE:
-
Chlorotrifluoroethylene
- EoL:
-
End-of-life
- EuP:
-
Eutrophication potential
- FU:
-
Functional unit
- GLO:
-
Global
- GWP:
-
Global warming potential—100 years
- HDPE:
-
High-density polyethylene
- HSL:
-
Heat seal lacquer
- ID:
-
Identifier
- LCA:
-
Life cycle assessment
- MD:
-
Multi-dose dispenser
- MD S1:
-
Multi-dose dispensing scenario 1
- ODP:
-
Stratospheric ozone depletion potential
- oPA/Alu/PVC:
-
Oriented polyamide/aluminum/polyvinylchloride
- PBMM:
-
Paper-based medication management
- PCE:
-
Tetrachloroethylene
- PP:
-
Polypropylene
- PVC:
-
Polyvinylchloride
- PVC/PCTFE:
-
Polyvinylchloride/polychlorotrifluoroethylene
- PVC/PE/PVDC:
-
Polyvinylchloride/Polyethylene/polyvinylidenchloride
- PVC/PVDC:
-
Polyvinylchloride/polyvinylidenchloride
- RER:
-
Europe
- UD:
-
Unit dose
- UKE:
-
University Medical Centre Hamburg-Eppendorf
References
Aly AF, Köberle U, Stammschulte T, Bräutigam K (2015) Zentrale Erfassung und Bewertung von Medikationsfehlern innerhalb des Spontanmeldesystems der Arzneimittelkommission der deutschen Ärzteschaft (AkdÄ). In Arzneiverordnung in der Praxis 42(3):105–110. Available online at http://www.akdae.de/Arzneimitteltherapie/AVP/Artikel/201503/105.pdf
AMPri (2022) PP Einweg Medikamentendispenser, 4 Fächer, 22,2 x 6 x 1,5 cm, weiß, Med-Comfort: Polypropylen Schiebeblister mit aufgedruckten Tageszeiten zur Aufbewahrung des Tagesbedarfs von Medikamenten als Stations- und Patientenbedarf kaufen. | L-09180-W. Available online at https://www.ampri.de/PP-Einweg-Medikamentendispenser-4-Faecher-22-2-x-6-x-1-5-cm-weiss-Med-Comfort-Polypropylen-Schiebeblister-mit-aufgedruckten-Tageszeiten-zur-Aufbewahrung-des-Tagesbedarfs-von-Medikamenten-als-Stations-und-Patientenbedarf-kaufen./L-09180-W, updated on 12/22/2022, checked on 12/22/2022
Baehr M (2018) Closed loop medication management. Arzneimitteltherapiesicherheit im Krankenhaus. With assistance of Simone Melzer. 1st ed. Berlin: Medizinisch Wissenschaftliche Verlagsgesellschaft. Available online at https://ebookcentral.proquest.com/lib/kxp/detail.action?docID=5332596
Ball MJ, Douglas JV (2002) Redefining and improving patient safety. In Methods of Information in Medicine 41(4):271–276. https://doi.org/10.1055/s-0038-1634487
Bassani F, Rodrigues C, Marques P, Freire F (2022) Life cycle assessment of pharmaceutical packaging. In Int J Life Cycle Assess 27(7):978–992. https://doi.org/10.1007/s11367-022-02062-9
Belboom S, Renzoni R, Verjans B, Léonard A, Germain A (2011) A life cycle assessment of injectable drug primary packaging: comparing the traditional process in glass vials with the closed vial technology (polymer vials). In Int J Life Cycle Assess 16(2):159–167. https://doi.org/10.1007/s11367-011-0248-z
Bundesapothekerkammer (2017) Hygieneplan für die manuelle Neuverpackung von Fertigarzneimitteln. Available online at https://www.abda.de/, checked on 2/6/2023
Flott K, Maguire J, Phillips N (2021) Digital safety: the next frontier for patient safety. In Future Healthcare Journal 8(3):e598–e601. https://doi.org/10.7861/fhj.2021-0152
Gelbe Liste (2022) Gelbe Liste Pharmindex Online | Gelbe Liste. Available online at https://www.gelbe-liste.de/, updated on 12/22/2022, checked on 12/22/2022
Goulet B, Olson L, Mayer BK (2017) A comparative life cycle assessment between a metered dose inhaler and electric nebulizer. In Sustainability (switzerland) 9(10):1–13. https://doi.org/10.3390/su9101725
Guinee JB (2002) Handbook on life cycle assessment operational guide to the ISO standards. In Int J LCA 7(5):311–313. https://doi.org/10.1007/BF02978897
Hauschild MZ, Rosenbaum RK, Olsen SI (2018) Life Cycle Assessment. Theory and Practice /edited by Michael Z. Hauschild, Ralph K. Rosenbaum, Stig Irving Olsen. Cham: Springer, checked on 12/14/2022
Hischier R (2007) Life Cycle Inventories of packaging and graphical papers (Part 2). ecoinvent report No.8. Swiss centre for Life cycle inventories. Dübendorf. Available online at https://db.ecoinvent.org/reports/08_Chemicals.pdf, checked on 12/14/2022
IQVIA Commercial GmbH & Co. OHG (2021) IMS Dataview\
Kiliç T (2016) Digital hospital. an example of best practice. Int J Health Serv Res Policy. https://doi.org/10.23884/ijhsrp.2016.1.2.04
Klauber J, Wasem J, Beivers A, Mostert C (2021) Krankenhaus-Report 2021 - Versorgungsketten – Der Patient im Mittelpunkt. In 14394413. Available online at https://doi.org/10.1007/978-3-662-62708-2
Kozer E (2009) Medication errors in children. In Pediatric Drugs 11(1):52–54. https://doi.org/10.2165/0148581-200911010-00017
Krähenbühl-Melcher A, Schlienger R, Lampert M, Haschke M, Drewe J, Krähenbühl S (2007) Drug-related problems in hospitals: a review of the recent literature. In Drug Safety 30(5):379–407. https://doi.org/10.2165/00002018-200730050-00003
Mühlfeld L, Langguth P, Häusler H, Hagels H (2012) Influence of blister package design on usability among older adults. In International Journal of Clinical Pharmacy 34(4):553–560. https://doi.org/10.1007/s11096-012-9643-1
Pan J, Mays R, Gill S, Albert NM, Patel D, Stephens J et al (2015) Published costs of Medication errors leading to preventable adverse drug events in US hospitals. In Value in Health 18(3):A83. https://doi.org/10.1016/j.jval.2015.03.483
Pilchik R (2000) Pharmaceutical blister packaging. Part 1 Rationale and Materials. Pharm Tech 24(11):68, checked on 2/6/2023
Raju G, Sarkar P, Singla E, Singh H, Sharma RK (2016) Comparison of environmental sustainability of pharmaceutical packaging. In Perspectives in Science 8:683–685. https://doi.org/10.1016/j.pisc.2016.06.058
Schnurrer JU, Frölich JC (2003) Zur Häufigkeit und Vermeidbarkeit von tödlichen unerwünschten Arzneimittelwirkungen. In Der Internist 44(7):889–895. https://doi.org/10.1007/s00108-003-0988-3
Schütze L, Schneemann H (2007) Computerized prescription in hospitals—computerized physician order entry (CPOE) systems by comparison. In Krankenhauspharmazie 28(8):328–342
Siegemund G, Schwertfeger W, Feiring A, Smart B, Behr F, Vogel H et al (2016) Fluorine compounds, Organic. In : Ullmann’s Encyclopedia of Industrial Chemistry: John Wiley & Sons, Ltd, pp. 1–56
Siegert M-W, Lehmann A, Emara Y, Finkbeiner M (2018) Harmonized rules for future LCAs on pharmaceutical products and processes. In Int J Life Cycle Assess 24(6):1040–1057. https://doi.org/10.1007/s11367-018-1549-2
Siegert M-W, Lehmann A, Emara Y, Finkbeiner M (2020) Addressing the use and end-of-life phase of pharmaceutical products in life cycle assessment. In Int J Life Cycle Assess 25(8):1436–1454. https://doi.org/10.1007/s11367-019-01722-7
Torjesen I (2014) Medication errors cost the NHS up to £2.5bn a year. Pharm J 293(7834):450. https://doi.org/10.1211/pj.2014.20066893
van Vooren L, Spiegeleer B, de; Thonissen, Thomas; Joye, Philippe; van Joost, Durme; Slegers, Guido, (2002) Statistical analysis of tablet breakability methods. In Journal of Pharmacy and Pharmaceutical Sciences 5(2):190–198
Wacker Chemie AG (2021) Heat-Sealable Coatings I Printing Inks I Industrial Coatings I Vinnol Resins Product Overview. Available online at https://www.wacker.com/h/medias/7529-EN.pdf, checked on 2/6/2023
Wernet G, Bauer C, Steubing B, Reinhard J, Moreno-Ruiz E, Weidema Bo (2016) The ecoinvent database version 3 (part I): overview and methodology. In Int J Life Cycle Assess 21(9):1218–1230. https://doi.org/10.1007/s11367-016-1087-8
Acknowledgements
The idea to perform this study evolved in close cooperation between Technical University of Darmstadt and NOVA School of Science and Technology (Lisbon) within the scope of the EIT RawMaterials–labeled international double-degree master program “Advanced Materials: Innovative Recycling” and was finalized after graduation. Thus, this study was conducted without funding. Therefore, the authors thank iPoint-systems GmbH for granting free access to Umberto 11 software.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Conceptualization: Julian Baehr. Methodology: Julian Baehr and Laura Göllner-Völker. Formal analysis and investigation: Julian Baehr. Data curation: Julian Baehr and Michael Baehr. Writing—original draft preparation: Julian Baehr. Visualization: Julian Baehr and Hariharan Muthukumar. Writing—review and editing: Laura Göllner-Völker, Michael Baehr, Hariharan Muthukumar, Nuno Lapa, and Liselotte Schebek. Supervision: Nuno Lapa and Liselotte Schebek.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Melissa Bilec
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Baehr, J., Göllner-Völker, L., Baehr, M. et al. Life cycle assessment of pharmaceutical and clinical packaging required for medication administration practices. Int J Life Cycle Assess 29, 416–432 (2024). https://doi.org/10.1007/s11367-023-02270-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11367-023-02270-x