Abstract
Purpose
Iodine and its compounds have several industrial uses, primarily in nutrition and healthcare. So far, no specific LCA data for industrial iodine production are available. The purpose of this study is to provide the first LCA using primary industry data for iodine production from caliche ore in Chile.
Methods
The study is a process-based, attributional LCA and follows the relevant ISO standards 14040/44. Primary data were collected from the world’s main producer representing their production for the years 2019 and 2020. Economic allocation was applied to deal with the by-product sodium nitrate. The impact assessment was performed with a set of CML 2001 indicators.
Results and discussion
Cradle-to-gate total LCIA results per 1000 kg of iodine prill product include a GWP100 of 1.48E+04 kg CO2 eq., an AP of 2.53E+02 kg SO2 eq., a POCP of 1.20E+01 kg C2H4 eq., an EP of 9.60E+00 kg PO43− eq., and an ADP fossil of 2.44E+05 MJ. The main contributor across process steps and for most impact categories is electricity consumption. Other hotspots include diesel combustion, hydrogen peroxide, sulfur, sulfuric acid, kerosene, and sodium hydroxide. A scenario analysis with renewable electricity revealed a reduction potential for all impact categories. As an example, the GWP could be reduced by 33–38%.
Conclusions
This study is the first LCA for iodine production from caliche ore based on primary industry data. The switch to renewable sources was identified as the main improvement potential for the hotspot electricity consumption, with a potential reduction of at least 25% for all impact categories except ODP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at 114 °C (237 °F), and boils to a violet gas at 184 °C (363 °F). The element was discovered by the French chemist Bernard Courtois in 1811. It is found in seaweed and brine extracted along with natural gas as well as Chilean caliche deposits. Iodine is a micronutrient element that is fundamental to a living body and is essential for the subsistence and growth of humans and animals (Kaiho 2015). It is on the World Health Organization’s List of Essential Medicines (World Health Organization 2021).
Iodine is used in a broad spectrum of products including medicated gargle, X-ray contrast media, antimicrobial agents, and catalyst and has many applications in the field of agriculture. Recently, iodine has found a wide range of applications in innovative materials, such as liquid–crystal display (LCD) polarizing film and electrolytes of dye-sensitized solar panels (Kaiho 2015).
The dominant producers of iodine today are in Chile and Japan. The global production volume of iodine is about 30,000 tons per year and almost 60% of this production comes from the north of Chile, where two thirds of the iodine reserves of the entire planet reside (SQM Iodine 2022).
LCA information of iodine production is scarce. To the knowledge of the authors, there is to date no primary industry data based LCA of iodine production available in the scientific literature or in LCA databases. Ecoinvent provides a dataset for iodine production from brine, but the documentation states that it is modeled based on bromine production as it is considered to be similar to iodine production (Sutter 2011). Therefore, this study represents the first industry data based LCA of iodine globally available.
This study was possible due to the collaboration with Sociedad Química y Minera de Chile (SQM), the main global producer of this product. The iodine produced by SQM has a purity of 99.9%. SQM is a Chilean chemical company and a supplier of plant nutrients, iodine, lithium, and industrial chemicals. SQM’s headquarters are in Santiago de Chile, but its natural resources and its main production facilities are located in the Atacama Desert in the regions of Tarapacá and Antofagasta. Today, SQM has the largest iodine plant in the world called Nueva Victoria and a second site in Pedro de Valdivia. For this study, comprehensive and detailed production data were collected at both sites.
Chile is the only country that produces iodine from the mineral caliche. The word caliche derives from a Quechua word, which means salt. The caliche mineral from northern Chile contains the largest known nitrate and iodine deposits in the world and is the world’s only source of commercial exploitation of natural nitrate (SQM Iodine 2022). As such the LCA presented here is only representative for the caliche route and not for the production from brine as the second most relevant production process for iodine.
2 Methods
This study was conducted in accordance with relevant ISO standards, ISO 14040 2006 and ISO 14044 2006 (International Organization for Standardization 2006a, b). This section documents the main elements and decisions of the goal and scope definition for the study. First, Sect. 2.1 introduces the goal of the study. Section 2.2 then goes into the different aspects of the scope definition.
2.1 Goal definition
The aim of this study was to quantify relevant resource inputs and emissions associated with the production of primary iodine from caliche ore to iodine prill product, to identify hotspots in the production of iodine, and to develop the first detailed LCA of iodine globally with primary data. The main target audience for the original study commissioned by SQM were internal company stakeholders and decision makers, while the communication to customers is seen as an option. For this paper, the main goal is to share the results with the scientific community.
2.2 Scope definition
This section documents the scope definition intended to achieve the goal described in Sect. 2.1. In the following, the product system and functional unit (Sect. 2.2.1), system boundaries (Sect. 2.2.2), allocation procedures (Sect. 2.2.3), life cycle inventory and data collection (Sect. 2.2.4), impact assessment (Sect. 2.2.5), and interpretation (Sect. 2.2.6) are presented.
2.2.1 Product system and functional unit
The studied product system is the production of iodine prill from caliche ore in Chile where it is produced with a minimum purity of 99.8% (SQM Iodine 2023). During this process, sodium nitrate is produced as a co-product. The co-product is included in the system boundary, but has been treated using the allocation approach described in Sect. 2.2.3. Iodine is an intermediate product with multiple uses. Consequently, 1000 kg of iodine prill product from caliche ore at the factory gate in Chile with a minimum purity of 99.8% has been defined as a mass-based functional unit and reference flow. This functional unit is also consistent with the goal of providing data to LCA practitioners and other stakeholders, as they typically connect their models on a mass basis to a cradle-to-gate system boundary.
The producing company, SQM, operates two production sites (Nueva Victoria and Pedro de Valdivia). Their contributions to the reported results per functional unit are scaled according to their respective production volumes.
2.2.2 System boundaries
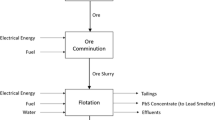
As iodine is an intermediate product, the study is a “cradle-to-gate” LCA covering all process steps from caliche ore mining (i.e., the cradle) to the finished iodine prill product (i.e., the gate). Figure 1 schematically shows the system boundary defined for the LCA.
The two production sites of Nueva Victoria and Pedro de Valdivia operate in a similar manner, but overall have different production routes. In Nueva Victoria, caliche ore is mined and then treated by heap leaching, while at Pedro de Valdivia, caliche ore is recovered from tailings of previously mined caliche deposits. From there, similar production routes for the resulting brine are taken with both sites including iodide production plants, in-process brine neutralization plants, and iodine production plants. They are also interconnected with a portion of the iodide produced at Nueva Victoria being transported by truck to Pedro de Valdivia for the iodine production step.
It is important to note that the in-process brine neutralization plant process step was allocated solely to the iodine prill product. If the iodide production process step was not occurring, the feeble brine would not need to be neutralized before proceeding to the next process step for sodium nitrate production; therefore, the inputs and outputs for the neutralization plant are considered to be within the iodine prill production system. It is modeled within the iodide production process step.
2.2.3 Allocation
The product system leads to two products, i.e., iodine prill and sodium nitrate (NaNO3). The processes of caliche ore mining and heap leaching serve the purpose of producing both products. The process step caliche ore mining is done as a joint process for both iodine and sodium nitrate production, while the heap leaching process step is conducted largely for the recovery of iodine and its economic value. In order to calculate the intended LCA for iodine prill only, the co-product sodium nitrate needs to be allocated. Avoiding allocation by subdivision of the processes is not possible. System expansion was not applied in order to keep the total results for both co-products consistent and to ensure that the sum of the allocated inputs and outputs are equal to the inputs and outputs of the unit processes before allocation. Furthermore, SQM is the only company producing both nitrates and iodine from caliche ore. SQM uses an allocation approach within their company and wanted to have an environmental profile for the co-product as well, necessitating allocation over system expansion.
Allocation by underlying physical relationships between the co-products, e.g., mass or energy, was not seen as preferred option as sodium nitrate is produced in a much larger quantity (differing two orders of magnitude in the 2019 and 2020 production quantities), whereas iodine has significantly higher economic value (earnings in 2019 and 2020 from iodine prill were two orders of magnitude higher per ton than that of nitrate derivatives: fertilizers and/or industrial chemicals (SQM 2020)). Consequently, the burdens have been allocated in proportion to their internal cost distribution for the mining and heap leaching processes with an economic allocation (73% to iodine and 27% to sodium nitrate). This allocation choice is in line with the harmonization of LCA methodologies for the metal and mining industry, where economic allocation is the preferred choice in cases where mass allocation would fail to capture the main purpose for the operations (Santero and Hendry 2016).
2.2.4 Life cycle inventory and data collection
The life cycle inventory was modeled in GaBi software (version 10.5.1.124) (Sphera Solutions GmbH 2021). The geographical scope was the production in Chile and the technological scope was the existing production processes of SQM in the years 2019 and 2020. For higher representativity and since the heap leaching process takes longer than one year, it was decided to use two years of data to calculate the average of the iodine prill production process at SQM.
A significant amount of primary data for all production steps operated by SQM was collected and validated. Table 1 shows the processes and flows collected as primary data. The last three columns in Table 1 show the secondary dataset which was used to model the inputs. For consistency reasons and coverage, preference was given to the GaBi database, whereas ecoinvent data were used in case no appropriate GaBi dataset was available.
In some instances, multiple datasets were combined to create the identified input material as shown in Table 1. This was the case for sulfonitric acid (combining sulfuric acid and nitric acid) and for the plastic packaging bags (e.g., Krealon bags and Maxibags). Additionally, a mix of ferrous metals was created for the ferrous metal waste output since the exact metals were unknown. In the case of sodium metabisulfite, there was no dataset available and a substitution was made using the dataset for sodium sulfite as a proxy since they have equivalent functions (Delgove et al. 2019). The impact of this replacement is discussed in the results (Sect. 3.3).
2.2.5 Impact assessment
The impact categories and indicators considered to be of relevance to the goals of the study are shown in Table 2. Various impact assessment methodologies are applicable for use in this context including, e.g., CML (Heijungs et al. 1992), ReCiPe (Goedkoop et al. 2009), and selected methods recommended by the ILCD as part of their EU Product Environmental Footprint (PEF) Initiative (European Commission 2013). For this study, the CML impact assessment methodology framework (CML 2001 update January 2016) was chosen (CML - Department of Industrial Ecology 2016). CML characterization factors are widely used and tested. Many companies and industry associations still prefer this method due to its robustness and for consistency with previous studies (PE International 2014). As demonstrated by Bach and Finkbeiner (2017) for selected impact categories, more recent impact assessment methods are not per se superior to established methods like CML.
Normalization and weighting are optional steps in LCA (International Organization for Standardization 2006a, b) and it was chosen not to apply them. No appropriate normalization data set was available and therefore, applying normalization would not provide added value.
2.2.6 Interpretation
The results of the LCI and LCIA were interpreted according to the procedure described in ISO 14044. First, significant issues such as the main process steps, inputs, and emissions with a high contribution to the overall results were identified. Second, the completeness and consistency of the data and model were checked. Third, sensitivity analysis for a mass-based allocation between iodine and sodium nitrate was performed. Finally, a scenario analysis for the use of alternative electricity sources (natural gas and renewables instead of the Chilean grid mix) was performed.
3 Results and discussion
This section presents and discusses the results of the study. First, selected results of the life cycle inventory are presented in Sect. 3.1. Then, Sect. 3.2 documents the life cycle impact assessment results and a contribution analysis before Sect. 3.3 goes into a more detailed hotspot analysis. A sensitivity analysis for the allocation choice is discussed in Sect. 3.4. Finally, the results of the electricity scenarios are discussed in Sect. 3.5.
3.1 Life cycle inventory
A full disclosure of the primary data and associated inventory is not possible due to confidentiality reasons. However, Table 3 shows selected, important inventory data in partly aggregated and rounded form.
Two thousand five hundred kilograms of caliche ore is required as input per 1000 kg of iodine prill product. Other key material inputs include kerosene, ammonium nitrate, sulfur, sulfuric acid, hydrogen peroxide, quick lime, sodium hydroxide, and sodium carbonate. The blue water consumed (the consumptive water use defined within GaBi as the ground and surface water that leaves the watershed, e.g., by evaporation and freshwater integration into products (Koehler and Thylmann 2016)) per 1000 kg of iodine prill product is 130 m3. Water is consumed in most process steps of iodine production, with the majority of blue water consumption occurring in the heap leaching process. Energy inputs consist mainly of electricity usage with 38,000 MJ per 1000 kg of iodine prill product and 63,000 MJ per 1000 kg of iodine prill product from diesel combustion during caliche ore mining and transport. Additionally, there are energy inputs from fuel oil combustion (2000 MJ/1000 kg iodine prill product) and thermal energy from natural gas (2 MJ/1000 kg iodine prill product).
3.2 Life cycle impact assessment results
This section summarizes the LCIA results in absolute terms and presents a relative contribution analysis of the various process steps. Table 4 presents the total results for the impact categories selected per 1000 kg of iodine prill.
Figure 2 presents a contribution analysis for the main process steps with regard to their share in the total results. Mining, heap leaching, and iodide production in general contribute to more than 80% of the impacts. Mining has the highest contribution to GWP and EP, whereas iodide production has the highest contribution to AP, POCP, and ADP fossil. Heap leaching dominates HTP. Operations at Pedro de Valdivia and iodine production are generally less relevant for the overall results. However, iodine production as an exception dominates the ODP result, but it has to be noted that the absolute values of ODP are very small.
With regard to the contribution of elementary flows, the GWP is clearly determined by CO2 emissions making up 92.6% of the total GWP, followed by methane with 4.9%, and then nitrous oxide with 2.5%. Sulfur dioxide with 89.3% is the largest contributor for AP, followed by nitrogen oxides (8.4%) and hydrogen chloride (1.4%). ODP is determined by carbon tetrachloride (66.9%) and halon 1301 (21.4%). POCP is contingent upon sulfur dioxide (75.1%), nitrogen oxides (9.9%), and carbon monoxide emissions (4.5%). Nitrogen oxides (57.4%) and nitrate (10.6%) emissions determine the EP. Crude oil (57.7%), natural gas (20.8%), and hard coal (18.7%) make up the largest contributions to ADP fossil. HTP is determined by hydrogen fluoride (28.6%), arsenic (+ V) (18.4%), selenium (13.1%), and nickel emissions (8.3%).
3.3 Hotspot analysis for individual process step
A hotspot analysis was carried out for the main contributing process steps. Over all process steps, the identified cross-cutting hotspots were electricity, diesel combustion, hydrogen peroxide, sulfur, sulfuric acid, kerosene, and sodium hydroxide.
As an example, Fig. 3 displays the detailed hotspot assessment for GWP for iodine production at Pedro de Valdivia. In this case, electricity based on the Chilean grid mix has the highest contribution with 44% (1% specific to water pumping and 43% from all other electricity used in the process step), followed closely by hydrogen peroxide with a contribution of 39%. Sulfuric acid is the third largest contributor with 6%. Sodium hydroxide, thermal energy, packaging, activated carbon, sodium sulfite, and waste treatment play minor roles with contributions of 2% or below. The potential impact of using sodium sulfite as a replacement for sodium metabisulfite is seen as negligible since it represents only a small contribution to the GWP (e.g., 1% in the iodine production process step).
3.4 Mass allocation sensitivity analysis
A sensitivity analysis for a mass-based allocation between iodine and sodium nitrate was performed to understand the impact of allocation choice. The results for mass-based allocation were consistently lower across all impact categories than for economic-based allocation. This is due to the significant mass difference of iodine and sodium nitrate that are produced which leads to the caliche ore mining and heap leaching process steps being almost fully allocated to the sodium nitrate when applying mass-based allocation. The results of the sensitivity analysis indicate that the economic-based allocation more accurately reflect the purpose of the operations. Table 5 displays the GWP per 1000 kg of iodine prill for the total, caliche ore mining, and heap leaching process steps using economic-based and mass-based allocation.
3.5 Electricity scenario analysis
Electricity was consistently identified as a hotspot for GWP, AP, EP, POCP, ADP fossil, and HTP across the various process steps. Therefore, an electricity scenarios analysis was conducted. In the baseline model underlying the results presented above, the Chilean electricity grid mix is used. The GaBi dataset for the Chilean electricity grid mix 1–60 kV (based on data from 2008 to 2017) includes hard coal (36.9%), hydropower (26.8%), natural gas (16.8%), biomass (7.6%), wind (4.4%), photovoltaics (4.3%), fuel oil (2.4%), biogas (0.7%), and geothermal (0.1%) (Sphera Solutions GmbH 2017). Despite the use of renewables, it is mainly the relatively high share of hard coal that leads to significant burdens for the grid mix in Chile. More recent data on the total energy supply in Chile shows that in 2020, the share of wind and solar power had increased but coal still makes up a significant share of the total supply (IEA 2022).
To assess the optimization potential for alternative electricity sources, electricity scenarios were conducted for electricity from solar power, wind power, hydropower, and natural gas by changing the electricity dataset from the Chilean grid mix to the alternative source for 100% of electricity usage in the foreground system (Fig. 4 and Table 5).
Figure 4 displays the results for the GWP impact category for the different electricity scenarios across the process steps. In total, the Chilean grid mix has the highest overall contribution (14,800 kg CO2 eq./1000 kg iodine prill), followed by natural gas (12,300 kg CO2 eq./1000 kg iodine prill), then hydropower (9900 kg CO2 eq./1000 kg iodine prill), solar power (9270 kg CO2 eq./1000 kg iodine prill), and wind power (9050 kg CO2 eq./1000 kg iodine prill). For the mining operations in Nueva Victoria, there is no significant reduction in GWP through using alternative electricity sources because the electricity is not the main hotspot in this process step. For all other process steps, a reduction in GWP is shown through using alternative electricity sources. The heap leaching process step shows the most significant reduction since electricity is the core contributor for GWP in this process step.
Table 6 shows the optimization potential of using alternative electricity sources in a quantitative overview. Replacing the Chilean grid mix with either wind power, solar power, hydropower, or electricity from natural gas leads to improvements in all studied impact categories with the exception of the ODP, which is not affected by the choice of the electricity source at all. The three different types of renewables lead to very similar results, which clearly shows that switching to renewable energy would reduce the environmental burden independent of the specific source.
The reduction of the GWP by renewables is in the range of 33–39%, whereas natural gas would lead to a reduction of 17%. When it comes to AP, POCP, and HTP, both the renewables and natural gas lead to similar reductions (23–28% for AP and POCP; 75–79% for HTP). For EP, the reduction potential with natural gas is, with 40%, slightly lower than for renewables (49–51%). For ADP fossil, the difference is obviously larger as natural gas barely leads to a reduction at all (just 6%), whereas the renewables lead to reductions between 26 and 27%.
4 Conclusions
This study is the first LCA for iodine production from caliche ore in Chile based on primary industry data. Cradle-to-gate total LCIA results per 1000 kg of iodine prill product include a GWP100 of 1.48E+04 kg CO2 eq., an AP of 2.53E+02 kg SO2 eq., a POCP of 1.20E+01 kg C2H4 eq., an EP of 9.60E+00 kg PO43− eq., and an ADP fossil of 2.44E+05 MJ. The main contributor across process steps and for most impact categories is electricity consumption. Other hotspots include diesel combustion, hydrogen peroxide, sulfur, sulfuric acid, kerosene, and sodium hydroxide. The switch to renewable sources was identified as the main improvement potential. A scenario analysis with renewable electricity revealed a reduction potential of at least 25% for all impact categories except for ODP. The GWP could be reduced by 33–38%.
References
Bach V, Finkbeiner M (2017) Approach to qualify decision support maturity of new versus established impact assessment methods—demonstrated for the categories acidification and eutrophication. Int J Life Cycle Assess 22:387–397. https://doi.org/10.1007/s11367-016-1164-z
CML - Department of Industrial Ecology (2016) CML-IA characterisation factors. https://www.universiteitleiden.nl/en/research/research-output/science/cml-ia-characterisation-factors. Accessed 18 Aug 2022
Delgove MAF, Laurent AB, Woodley JM et al (2019) A prospective life cycle assessment (LCA) of monomer synthesis: comparison of biocatalytic and oxidative chemistry. Chemsuschem 12:1349–1360. https://doi.org/10.1002/cssc.201900007
European Commission (2013) Recommendation 2013/179/EU on the use of common methods to measure and communicate the life cycle environmental performance of products and organisations. Official Journal of the European Union
Goedkoop M, Heijungs R, Huijbregts M et al (2009) ReCiPe 2008 a life cycle impact assessment method which comprises harmonised category indicators at the midpoint and the endpoint level report 1: characterisation. The Hague
Heijungs R, Guinée J, Huppes G et al (1992) Environmental life cycle assessment of products: Guide and backgrounds. Centre of Environmental Science (CML), Leiden University, Leiden
International Energy Agency (IEA) (2022) Chile. In: Total energy supply by source, Chile 1990–2020. https://www.iea.org/countries/chile. Accessed 19 Aug 2022
International Organization for Standardization (2006a) ISO 14040 (2006) Environmental management – life cycle assessment – principles and framework. International Organization for Standardization, Geneva
International Organization for Standardization (2006b) ISO 14044 (2006) Environmental management – life cycle assessment – requirements and guidelines. International Organization for Standardization, Geneva
Kaiho T (2015) Iodine chemistry and applications. John Wiley & Sons Inc, Hoboken, New Jersey
Koehler A, Thylmann D (2016) Introduction to water assessment in GaBi version 1.2. thinkstep AG, Leinfelden-Echterdingen
PE International (2014) Harmonization of LCA methodologies for metals. Canada, Ottawa
Santero N, Hendry J (2016) Harmonization of LCA methodologies for the metal and mining industry. Int J Life Cycle Assess 21:1543–1553. https://doi.org/10.1007/s11367-015-1022-4
Sphera Solutions GmbH (2021) GaBi software system and database for life cycle engineering (version 10.5.1.124). Sphera Solution GmbH, Leinfelden-Echterdingen
Sphera Solutions GmbH (2017) Process data set: electricity grid mix 1kV-60kV; AC, technology mix; consumption mix, to consumer; 1kV-60kV (en); location: CL. In: GaBi Doc. http://gabi-documentation-2022.gabi-software.com/xml-data/processes/a11e12a7-c803-4d82-8af2-c1ae390997ce.xml. Accessed 19 Aug 2022
SQM (2020) SQM S.A. 4Q20 earnings release. https://s25.q4cdn.com/757756353/files/doc_financials/2020/q4/PR_4Q20_ing_Final.pdf. Accessed 19 August 2022
SQM Iodine (2022) Sustainability: unique production from caliche. https://sqmiodine.com/en/sustainability/unique-production-from-caliche. Accessed 4 Jun 2022
SQM Iodine (2023) Iodine USP/ACS/Eur Ph grade prilled. https://sqmiodine.com/que-es-el-yodo/. Accessed 25 May 2023
Sutter J (2011) ecoinvent 3.8 dataset documentation “iodine production - GLO”. ecoinvent Association, Zurich. https://s25.q4cdn.com/757756353/files/doc_financials/2020/q4/PR_4Q20_ing_Final.pdf
World Health Organization (2021) World Health Organization Model List of Essential Medicines - 22nd List, 2021. WHO/MHP/HPS/EML/2021.02. Licence: CC BY-NC-SA 3.0 IGO. Geneva
Acknowledgements
We would like to thank Verónica Gautier, Beatriz Oelckers, Sebastian Franco, and others from SQM for their support in the data collection and for providing technical information.
Funding
Open Access funding enabled and organized by Projekt DEAL. Sociedad Química y Minera de Chile (SQM) provided funding for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Peter Rudolf Saling.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Roche, L., Muhl, M. & Finkbeiner, M. Cradle-to-gate life cycle assessment of iodine production from caliche ore in Chile. Int J Life Cycle Assess 28, 1132–1141 (2023). https://doi.org/10.1007/s11367-023-02200-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11367-023-02200-x