Abstract
Purpose
This paper studies the carbon footprint and water scarcity footprint (WSF) of a milk protein, beta-lactoglobulin, produced by cellular agriculture and compares this to extracted dairy protein from milk. The calculations of the microbially produced proteins were based on a model of a hypothetical industrial-scale facility. The purpose of the study is to examine the role relative to dairy of microbially produced milk proteins in meeting future demand for more sustainably produced protein of high nutritional quality.
Methods
The evaluated process considers beta-lactoglobulin production in bioreactor cultivation with filamentous fungi T. reesei and downstream processing for product purification. The model considers four production scenarios in four different locations (New Zealand, Germany, US, and Australia) with a cradle-to-gate system boundary. The scenarios consider different sources of carbon (glucose and sucrose), different options for the fungal biomass treatment (waste or animal feed) and for the purification of the product. Allocation to biomass was avoided by considering it substituting the production of general protein feed. The carbon footprint and WSF (based on AWaRe factors) modelling is compared to calculations and actual data on extracted dairy protein production in NZ. The uncertainties of modelled process were addressed with a sensitivity analysis.
Results and discussion
The carbon footprint of microbially produced protein varied depending on the location (energy profile) and source of carbon used. The lowest carbon footprint (5.5 t CO2e/t protein) was found with sucrose-based production in NZ and the highest (17.6 t CO2e/t protein) in Australia with the glucose and chromatography step. The WSF results varied between 88–5030 m3 world eq./t protein, depending on the location, type of sugar and purification method used. The avoided feed production had a bigger impact on the WSF than on the carbon footprint. Both footprints were sensitive to process parameters of final titre and protein yield from sugar. The results for milk protein were of similar magnitude, c.10 t CO2e/t protein and 290–11,300 m3 world eq./t protein.
Conclusions
The environmental impacts of microbially produced milk protein were of the same magnitude as for extracted dairy protein. The main contributions were sugar and electricity production. The carbon footprints of proteins produced by cellular agriculture have potential for significant reduction when renewable energy and more sustainable carbon sources are used and combined with evolving knowledge and technology in microbial production. Similarly, the carbon footprint of milk proteins can potentially be reduced through methane reduction technologies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Putting aside protein demand and overconsumption of some protein-rich foods by those who can afford to do so (Hill et al. 2022), if it were ubiquitously distributed then global production of protein and essential amino acids is sufficient to meet not only the current requirements of the global population but the population for decades to come (Smith et al. 2021). However, overcoming the combined challenges of providing enough affordable, accessible, nutritionally balanced and sustainably sourced protein to meet the dietary requirements of an increasing population is an important problem to solve if we are to achieve the United Nations 2030 Agenda for Sustainable Development (United Nations 2015).
Henchion et al. (2017) examined global protein supply and demand and found that by 2050 with a population of 9.6 billion demand could vary between − 13% if protein was to be consumed at the average required for a sedentary adult to + 78% should the entire population consume protein at the maximum consumption levels of the developed world. Even at current levels of production, both animal- and plant-sourced protein-rich foods are associated with significant environmental impacts (Richie and Roser 2021). Producers and supply chains are experiencing increased pressures as a result of the mounting environmental concerns related to greenhouse gas (GHG) emissions and the use of local water and land resources, health concerns related to the use and overuse of antibiotics and ethical concerns over the raising and treatment of animals in food production (ECDC and EMA 2009). To meet future nutrition requirements and the growing demand for protein, alternative more efficient ways of protein production are likely to be needed, if not to replace, then at least to complement existing plant- and animal-sourced production systems, which must also improve their efficiency.
In recent years, dozens of new start-up companies (such as Perfect Day, ReMilk, New Culture, Shiru, and Clara Foods) have been launched to develop alternative ways to replace animal protein production, attracting billions of dollars of investment during the past 5 years often on the basis of claims of increased efficiency including lower environmental footprints. A growing number of such start-ups are focussed on the production of milk protein without the use of cows.
Milk protein has a significant role in nutrition (Boland and Hill 2020) and currently accounts for approximately 12% of all protein consumed by the global population (Smith et al. 2022). Perhaps more important than total protein are the essential amino acids (EAA), where the role of milk protein in global nutrition is even more striking (Smith et al. 2022). Milk is the highest contributor to lysine in the world (18% of global lysine), the number 2 contributor to leucine (17%) and threonine (15%), and the number 3 contributor to tryptophan (15%), methionine (14%), histidine (13%) and cystine (7%) (Smith et al. 2022). The major whey protein of milk—beta-lactoglobulin (BLG)—is important in this respect. It contains 17% more EAA, 33.5% more branched-chain amino acids and 74% more leucine than the average for dietary proteins (Etzel 2004).
The quality or bioavailability of protein and EAAs from different sources is important of course, and in this respect, milk proteins have high bioavailability coefficients and digestible indispensable amino acid scores (Smith et al. 2022). Milk is also an affordable source of nutrition. Modelling the minimum-cost purchase price of foods to create a nutritionally adequate diet in the United States (US), Chungchunlam et al. (2020) found that the price of milk would need to increase eightfold before it was no longer an affordable source of nutrition within the minimum-cost diet.
To create this milk protein and the other nutrients found in dairy, milk production utilises significant resources. Dairy farming occupies 7% of the world’s land, of which, 85% (or 850 million ha) is either pastures or rangeland (Hill 2017). Dairy cows consume 2.5 billion tonnes of dry matter, or approximately 40% of the global livestock feed intake (Hill 2017). Seventy-seven percent of this feed is human-inedible pasture or straw (Mottet et al. 2017). Milk production also creates 2.7% of global GHG emissions or 4.0% including meat from dairy animals (FAO 2010).
Cellular agriculture is a field in bio-based economy that focuses on the production of agriculture products, proteins, fats and meat tissue from cell cultures using a combination of molecular biology and biotechnology. Products such as cultured meat and microbial biomass, such as Quorn, are classified as cellular products, whereas proteins, lipids and food additives produced by microbes are classified as acellular products (Rischer et al. 2020). Cellular agriculture (also referred to as precision fermentation) offers one solution to the increasing demand for milk protein. Although the term cellular agriculture is relatively new, the concept of using genetically engineered microbes to produce food is not new. In 1990, the FDA-approved genetically engineered bacteria and 2007 genetically produced filamentus fungi to produce rennet, an enzyme mixture used in making cheese. The production of food supplements, e.g. vitamin B2 (riboflavin) and citric acid by the filamentous fungi Ashbya gossyppii and Aspergillus niger, are examples of the commercial use of microbes for food additive production (Revuelta et al. 2018; Behera 2020).
Trichoderma reesei (T. reesei) is an important and widely exploited protein production host with a high natural capacity to secrete enzymes. It has a long history of safe use in industrial enzyme production and has GRAS (generally regarded as safe) status (FDA; 21CFR §184.1250). Engineered T. reesei strains have been reported to produce over 100 g/L-secreted protein to the culture medium (Cherry and Fidantsef 2003). However, it is important to note that the proteins produced at these high titres are homologous and not heterologous or alien to the T. reesei genome.
Well-established molecular tools such as transformation, auxotrophic markers, strong endogenous and synthetic promoters, efficient targeting and the availability of the full genome sequence have expedited the use of T. reesei as a production host for a variety of different target proteins, such as enzymes for biomass hydrolysis, food and feed applications and therapeutic proteins. Examples of food-related enzymes commercially produced by T. reesei are amylase (A) to produce fermentable sugars from starch, glucoamylase for the production of high fructose corn syrup, beta-glucanase as a filtering aid in brewing and xylanase to increase bread volume in baking (Paloheimo et al. 2016). More recently, T. reesei has been reported as the production host for BLG (Perfect Day 2021) and ovalbumin (Järviö et al. 2021). The most commonly used protein expression systems in T. reesei are using native cellulase promoters, e.g. the cellobiohydrolase 1 (cbh1) promoter. The cbh1 promoter is strongly induced by the presence of inducing compounds, mainly cellulose, its derivatives (e.g. cellobiose and sophorose), or lactose. Recently, engineered T. reesei strains have been developed where the cellulase promoters are induced in the presence of glucose, thus allowing the use of an economically feasible and readily available carbon source in T. reesei cultivations (Derntl et al. 2013). In addition, the development of a synthetic expression system based on a synthetic promoter and expression of an artificial transcription factor enables the development of production strains producing mainly the target protein with low background into the culture medium (Rantasalo et al. 2018). Target proteins originating from a heterologous origin are often subjected to degradation by proteases secreted by T. reesei. This can be avoided by protease deletions that have been shown to improve the product yield without compromising the viability and growth of T. reesei (Landowski et al. 2015). With all the available tools and experience, T. reesei is an attractive production host for cellular agriculture products.
Some life cycle assessment (LCA) studies have been conducted and reported in recent years related to microbially produced proteins. For example, a study by Järviö et al. (2021) investigated the environmental impacts of microbial ovalbumin production with T. reesei and reported a carbon footprint of 7.5–17 kg CO2eq/kg ovalbumin, stating that the impact is lower than for chicken-egg-derived ovalbumin. A US-based start-up company, Perfect Day, received (GRAS) status in early 2020 for BLG produced by T. reesei. They claim to have produced whey protein using microbial production that has 85% to 97% lower GHG emissions (c. 2.7 kg CO2e/kg protein) than comparable milk proteins (Perfect Day 2021). They state that the primary driver of GHG emissions for Perfect Day whey protein is the utilities that contribute 40% to total GHG emissions. Utilities include the US average natural gas and electricity used in the protein production process. The protein development phase contributes 25% to total GHG emissions. However, the study uses mass-based allocation to the main product (protein) and by-product (biomass), assuming that the biomass is used in the pet food sector. With this approach, the by-product gets 78.3% of the environmental burden, leaving only 21.7% to protein. With a 100% allocation to the main product, i.e., protein, the carbon footprint would be 12 kg CO2e/kg protein. Tubb and Seba (2019) claim that through the use of technologies such as precision fermentation, ‘the cost of proteins will be five times cheaper by 2030 and 10 times cheaper by 2035 than existing animal proteins, before ultimately approaching the cost of sugar’. They also state that alternatives to animal-produced protein will be ‘up to 100 times more land-efficient, 10–25 times more feedstock-efficient, 20 times more time-efficient, and 10 times more water-efficient’ and ‘produce an order of magnitude less waste’. The motivation for this study was to examine the validity for such claims.
This paper studies the carbon footprint and water scarcity footprint (WSF) as the selected environmental impacts of the milk protein BLG produced by cellular agriculture (referred as recombinant BLG or rBLG in this study), compares this to milk protein produced by cows, referred to as dairy protein and examines the role that those microbially produced milk proteins could play in meeting future demand for protein. In this study, cell-cultured rBLG inventory data is based on a developed model to describe the hypothetical industrial-scale production, while dairy protein production is based on existing farms and processing sites.
2 Methods
2.1 Scope of the study
Carbon footprint and WSF were studied as the environmental sustainability indicators of the production of the recombinant proteins on a commercial scale. In addition to full life cycle assessments where several impact categories of milk production are assessed, these two impacts have also been assessed separately (e.g. Usva et al. 2019; Flysjö et al. 2011; Adom et al. 2012) and were thus found the most relevant categories to be studied. In addition, since this study is partially an ex-ante LCA (Cucurachi et al. 2018) and the recombinant production considers a future technological system, there is no actual data of the process emissions that would affect other possible impacts that would occur in reality. Thus, only two environmental impacts are considered, and a full LCA is suggested to be done when more data is available.
The carbon footprint and WSF results of recombinant protein (rBLG) were compared to those of dairy protein. These two indicators follow the main principles of the life cycle assessment method, ISO 14040 (ISO 2006), i.e. they consider the product’s full life cycle: raw material supply, fuel and energy production, transportation, processing, use stage and end-of-life options. In this study, however, the system boundaries were set as ‘cradle to gate’, i.e. the use stage and end of life of the proteins were not considered. This is appropriate for such a comparative study as any additional steps post-factory-gate and associated footprints should be the same for protein produced from milk or protein produced in a bioreactor. The functional unit of this study was one tonne of dry rBLG. In practice, the final product is of 90% purity in dry content and 95% dry matter in the product, which means that the rBLG protein content of the product is 85.8%. Therefore, the environmental impact for one tonne of the final product is 85.8% of the functional unit. For scenario 4 (with higher product purity requirements), the rBLG protein content of the final product is 90.3%. The comparison is done to one tonne of dairy protein.
Comparisons were made with dairy protein production in New Zealand (NZ) because the production system in NZ is an example of how billions of litres of milk and tens-of-thousands of tonnes of milk protein ingredients can be produced with relatively low, compared to global averages, environmental footprints (Payen et al. 2018; Ledgard et al. 2020). It assumes that rBLG will have similar properties in formulated food systems to the dairy protein it replaces noting that milk contains a variety of proteins with different properties, and it is unlikely that rBLG will be able to substitute for the properties of all protein ingredients used in all foods (see Boland and Singh 2020 for a comprehensive description of the properties of milk proteins and use in food systems). BLG was chosen because of its nutritional quality (Etzel 2004) but in theory cellular agriculture could be used to produce all milk proteins which could then be combined in ratios to reproduce most protein ingredients derived from milk.
A model for a hypothetical industrial-scale rBLG production process was developed to obtain mass and energy balances needed in the carbon footprint and WSF calculations of microbially produced protein.
2.2 Data sources
Model parametrization (yields) and design were defined based on data available from the literature and on experiences at VTT Technical Research Centre of Finland and Fonterra Research and Development Centre. VTT performed the data gathering with help from Fonterra. The energy use of the production was calculated applying specific energy consumption values for the different processing steps and according to methods described in ‘Sect. 2.4.’ The life cycle inventory data for raw materials, fuels and energy production and transportation modes were collected from the ecoinvent 3.7.1 database with the ‘Allocation, cut off by classification’ system model. The datasets used are presented in the Online Resource Data 2.
2.3 Scenarios and assessed locations
Scenario analysis included four scenarios to investigate the impacts affected by several types of raw materials and various product purity requirements. The assessed scenarios were:
-
Scenario 1 (base scenario—carbon source: glucose, fungal biomass applied as feed, 90% product purity
-
Scenario 2—carbon source: sucrose, fungal biomass applied as feed, 90% product purity
-
Scenario 3—carbon source: glucose, fungal biomass applied as waste, 90% product purity
-
Scenario 4—carbon source: glucose, fungal biomass applied as feed, 95% requirements for product purity achieved by additional chromatographic purification of the product
To examine how location can impact footprints, the environmental impacts of the system were studied in four different locations since the impacts are affected by local solutions, e.g. cultivation practices of the sugar (carbon source), the energy profile used in the process, and water availability in a certain location. The studied locations were NZ, Germany in Europe, Alabama in the US and Victoria in Australia. The locations were selected to provide an indication of the likely size of location-related impacts on the footprints for rBLG but were not intend to represent any upper or lower limits based on global location of the production. However, NZ was included because it is a highly efficient scale-producer of milk and milk protein with a high percentage of renewable electricity production, good rainfall and little to no sugar production. This is why NZ was also chosen as the reference for the milk protein from the dairy industry. Australia was chosen as a location because it has significant sugar and milk production, water stress issues and much lower levels of renewable energy than NZ, Germany and the US were selected as major economies (first and fourth respectively on the basis of GDP in 2020 (World Bank 2022)) and which already have start-up companies targeting the production of recombinant milk proteins using cellular agriculture.
The data for glucose is from the ecoinvent database and the used dataset is called ‘market for glucose’. It represents the production of glucose from maize starch. The same dataset is used for all locations, i.e. it represents the global production of glucose. However, glucose is not produced in NZ, and thus Australian glucose is assumed to be imported c. 3500 km by ship with additional truck transportation of 100 km. In Australia, the glucose production is assumed to be located in Queensland, and thus it needs to be transported also to Victoria, Australia, by ship for the assumed 2800 km in addition to the 100 km by truck. As with glucose, the same assumptions apply to corn steep liquor in Australia and NZ.
In scenario 2, sucrose can be produced from sugar beet or sugar cane. It has been assumed that sugar beet is the raw material of sucrose in Germany (Statista 2021), while sugar cane is the raw material in US and Australia (Shahbandeh 2021). Similarly to the glucose, Australian sucrose is assumed to be imported to NZ from Australia (Apeda 2021) since it is expected that the local sucrose availability would not be sufficient to fulfil the production capacity needs. The Australian sugar cane production is assumed to be produced in Queensland (Australian Government 2021) and transported to Victoria and NZ by ship. In addition, the US production of sugar cane is assumed to take place in Louisiana, from where it is transported to Alabama c. 600 km by truck.
For all other raw materials, it is assumed that production takes place in the same area, i.e. the location-specific water scarcity factors are used for each raw material, and 100 km truck transport is taking place. The transports are listed in Table 1. No transportation was assumed for the products or waste.
2.4 Process description
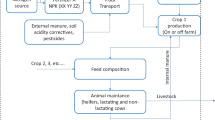
The evaluated and designed process considers rBLG production with filamentous fungi T. reesei and downstream processing (DSP) for product purification. A simplified flow diagram is shown in Fig. 1. The design of the production process is based on VTT’s and Fonterra’s know-how, and in addition, the bioreactor cultivation utilises the US’s Natural Renewable Energy Laboratory’s (NREL’s) cellulase bioreactor cultivation process and assumptions, which describes the production of cellulase by T. reesei in a fed-batch process (Humbird et al. 2011). The designed process describes an ex-ante study; however, the technologies used in system is assumed to be available after short-strain engineering and process development time and thus the data used, for example materials and energy are not prospected for the future but modelled as they would occur in the present.
The bioreactor cultivation consists of a series of pre-culture bioreactors having three stages for growing the fungal organism, T. reesei, and providing seed for the main production bioreactors in which most of the rBLG is secreted. There are multiple parallel pre-culture and production bioreactors to maintain the desired production of rBLG. In every new pre-culture step, 10% of the culture comes from the previous step. According to Humbird et al. (2017), the maximum industrial reactors with gas transfer limitations are less than 500 m3. Here, the size of the main bioreactor is estimated to be 300 m3, and the size of the pre-culture bioreactors are 0.3 m3, 3 m3 and 30 m3. Over the working period, each main bioreactor will see a cell growth period and a production period with a production time of 120 h and an offline period of 24 h for draining, cleaning and refilling. The reactors are temperature-controlled by chilled water flowing through internal coils, and production takes place at 28 °C. Cherry and Fidantsef (2003) have reported T. reesei to produce over 100 g/L-secreted protein mixture to the culture media, thus in this work it is estimated, that the final titre for single protein is 50 g/L. Furthermore, it is shown that the high purity of end products can be achieved in improved production systems (Rantasalo et al. 2019) and that the optimization of the strain and process would even enhance the purity. The rBLG fraction of the total secreted protein is estimated to be 90% in this work. The molar selectivity of sugar to CO2 is assumed to be 50% and 50% is assumed to be in protein and biomass. The final fungal biomass amount after bioreactor cultivation is estimated 30 g/l. Even lower biomass amounts might be reached due to T. reesei’s good ability to secrete protein; e.g. Humbird et al. (2011) estimates that only 10% of sugar is reacting through cell biomass reaction; however, 30 g/L is assumed reasonable for this study. From the process parametrization described above, one may calculate following selectivity and productivity figures for this study: the molar selectivity of sugar to protein 33%, the molar selectivity of sugar to fungal biomass 17%, the volumetric productivity of total proteins 0.46 g/l/h and the volumetric production of the rBLG target protein 0.42 g/l/h. This represents the yield of 0.223 g protein per gram of sugar for the base case, which is in line with the values found from literature (Ellilä et al. 2017; Humbird et al. 2011). In the microbial bioprocess, carbon from glucose or sucrose is converted to protein, biomass and CO2. Some unused carbon may stay in the broth, but generally, this should be rendered negligible through control of the process. The yield factor, given above, is therefore an important parameter for process performance, referring to the amount of protein formed (protein titre) from the amount of sugar fed to the process. The used elemental composition of BLG was calculated with Expasy’s ProtParam tool (Expasy 2022) to be C887H1440N224O266S12 and the T. reesei biomass elemental composition is CH1.645O0.445N0.205S0.005 (Humbird et al. 2011).
Glucose (in scenarios 1, 3 and 4) or sucrose (in scenario 2) is used as a carbon source for protein and fungal biomass production. The usage of main nutrients follows the study of Humbird et al. (2011)—NH3, SO2 and the carbon source are based on the stoichiometric need of carbon, hydrogen, nitrogen and sulphur for fungal biomass and protein production. Aeration requirements are based on the oxygen uptake rate. In addition, corn steep liquor and some additional nutrients are used in growth media. The use of these and an antifoam agent are adopted from Humbird et al. (2011) (see Table 2 for details). The electric energy consumption of bioreactor cultivation consists of agitation, a compressor providing air and cooling. According to the recent study by Humbird et al. (2017) on stirred tanks, the power required to deliver the required O2 is nearly constant when the system is optimised for agitation and aeration powers. In that analysis, the power needed to achieve a specified oxygen transfer rate (OTR) does not vary significantly with vessel size, being around 2 kWh/kg O2.
Cooling requirements for the bioreactor is estimated based on metabolic heat generation (combustion heat) and heat generated by agitation. Metabolic heat generation is estimated based on oxygen consumption rate according to Meyer et al. (2016).
where rw is the heat generation rate and rO2 is the oxygen consumption rate. A rule of thumb is used to estimate the electricity needed for cooling, being 0.259 kW per kW of removed heat (Couper et al. 2010).
Bioreactors are cleaned based on Fonterra’s cleaning-in-place (CIP) method using the chemical NaOH (75 °C, 1%, 100 l per m3 bioreactor) and rinsing with hot water (95 °C, 100 l per m3 bioreactor) after each bioreactor cultivation and HNO3 (75 °C, 1%, 100 l per m3 bioreactor) after every 3rd bioreactor cultivation.
After cultivation, the fungal cells are separated by a filter press from the proteins (filtrate), assuming a dry content of fungal biomass of 40% (Voutilainen et al. 2021).
Filtrate from the filter press continues to the downstream processing, including microfiltration assuming 100% fungus rejection and 97.5% permeate recovery, combined ultra- and diafiltration for product purification, and spray drying to produce protein powder with a dry matter content of 95%. See Online Resource Data 1 for details.
In the assessment, there are two options for how the fungal biomass is treated. It could be used as animal feed (scenarios 1, 2 and 4) or treated as waste and composted using industrial technology (scenario 3). In both options, fungal biomass and the reject from microfiltration are first sterilised at 140 °C. Heat recovery of 80% in the sterilisation is assumed (Niazi and Brown 2017). In addition, in the option where fungal biomass is used as animal feed, drying with a ring dryer up to 90% dry content is applied. The electricity consumption of 90 kWh/t of evaporated water and the fuel consumption of 747 kWh/t of evaporated water are estimated according to Beal et al. (2015).
In the scenario analysis, the effect of the increase in rBLG content to 95% is assessed by including a chromatographic separation using a simulated moving bed (SMB) unit (scenario 4, see Fig. 1B). After the chromatographic separation, an additional diafiltration unit is required to decrease the concentration of chemicals utilised in the chromatographic separation. The added equipment is located after the combined ultra- and diafiltration unit and prior to the spray dryer (see Online Resource Data 1 for details on added equipment in scenario 4).
2.5 Allocation
Different allocation options were considered when defining this study. The ISO standard (ISO 14040) recommends to avoid allocation as the first option. If allocation cannot be avoided, e.g. through more detailed data collection or system expansion, allocation should primarily be based on physical properties, and as the last option on some other properties, e.g. economic value. The ISO 14044 Amendment 2 (ISO 14044:2006/Amd.2:2020(E)) adds the possibility of using direct substitution as a way to avoid allocation. In the amendment, it is stated that ‘In practice, the co-products are compared to other substitutable products, and the environmental burdens associated with the substituted product(s) are subtracted from the product system under study’.
In addition to the rBLG, the production creates fungal biomass as a by-product, which needs to be treated and considered in some way. The actual use of fungal biomass side stream is not known, and thus its economic value cannot be defined in order to do an economic allocation. Allocation based on physical properties (mass or energy content) was not seen as the best option. The impact of mass-based allocation was also studied by Perfect Day, in which the by-product was assumed to be used in pet food sector and it got 78.3% of the environmental burden, leaving only 21.7% to protein (Perfect Day 2021). Thus, it was decided not to apply allocation for the production process in this study. All burdens were directed to the main product. The fungal biomass from the rBLG production was treated with direct substitution, where it was assumed to replace general protein animal feed (from the ecoinvent 3.7.1 database) in a 1:1 ratio in scenarios 1, 2 and 4. In scenario 3, the fungal biomass was treated as waste and therefore no allocation was needed.
Allocation of the secondary datasets is done using ecoinvent; the details can be found in their reports. The system model ‘Allocation, cut off by classification’ was used for the ecoinvent datasets.
2.6 Carbon footprint methodology
Carbon footprint is a standardised method, ISO 14067 (ISO 2018), which considers all emitted greenhouse gases during a product’s life cycle. The most typical GHGs released are carbon dioxide (CO2), methane (CH4) and dinitrogen monoxide (N2O). All GHGs are converted to carbon dioxide equivalents (CO2e) by using gas-specific factors by the Intergovernmental Panel on Climate Change (Myhre et al. 2013), which describe the global warming potential of each GHG. In addition to direct emissions, ISO 14067 also covers GHG emissions and removals occurring as a result of direct land use change (dLUC). However, internationally agreed-upon procedures to assess the indirect land use change (iLUC) are still under development and therefore are not included in the calculations. In addition, the biogenic carbon dioxide is considered to be zero in the calculations, and only biogenic methane is considered in the results.
The characterization factors used in the carbon footprint calculation were the global warming potential for 100 years (GWP100a) factors by IPCC 2013 (Myhre et al. 2013). The factors of the most important GHGs are reported in Table 3.
Since the system boundary of this study is set as cradle-to-gate instead of a full life cycle, the results of the carbon footprint are described as a partial carbon footprint.
2.7 Water scarcity footprint and the AWaRe method
Water footprint is also a standardised method, ISO 14046 (ISO 2014). While carbon footprint describes global impacts, the water footprint is a more local indicator, considering the local water availability and quality aspects. In this study, the water impacts with a midpoint indicator as a water scarcity footprint (WSF) were examined. It considers the water consumed during the life cycle of a product in each location in the value chain, i.e. the amount of water that is removed from a water source, e.g. by evaporation or within a product. For the NZ dairy farms, a water budget model (validated across NZ) was used to calculate average region-specific water consumption, accounting for rainfall (and irrigation for Canterbury) and evapotranspiration. The amounts of consumed water are multiplied by local water scarcity factors and added together to create the total WSF. The factors used for water scarcity are based on the AWaRe method (Available Water Remaining per area in a watershed, after the demand of humans and aquatic ecosystems has been met), which is provided by the WULCA working group (Boulay et al. 2018; WULCA 2019). The WSF results are expressed as ‘m3 world eq.’.
AWaRe gives annual water scarcity factors on the country level in an Excel file and on a watershed level as a Google Earth layer. While the country averages are not necessarily the most reasonable to use for large countries such as Australia or the US, the watershed level values are likely to be too specific for modelling of the kind used in this study, where the locations are assumptions instead of actual local data. Thus, it was decided to use an approach between these two values: The factors of relevant watersheds were collected by VTT from the Google Earth layer and averaged to describe relevant areas of production. The used factors are presented in Table 4, and the types of factors for each dataset are presented in the Online Resource Data 2. The average factors for the Australian Queensland coastline, Victoria coastline/Melbourne area, Alabama, and Louisiana are calculated from a Google Earth layer. More details are presented in the Online Resource Data 2. For the NZ dairy farms, regional factors were also determined using Google Earth, while for the Canterbury region a monthly weighted average was determined as described by Payen et al. (2018).
Since the ecoinvent database provides AWaRe results for the used datasets in rather large geographical areas (e.g. global, Europe, rest of world) and more specific locations were of interest in this study, the AWaRe results from ecoinvent were not used but instead the water consumption for each material and process was calculated based on the life cycle inventory (LCI) datasets. The sum of released (output) waters was subtracted from the sum of natural resource water inputs to the system, and the result was assumed to create the value for the consumed water as evaporated or removed water from the original watershed within a product. This value was then multiplied with a local water scarcity factor from AWaRe. This approach does not consider if the released waters are actually released to the same watershed from which the inputs come. In addition, only one AWaRe factor is used for each dataset, even though the LCI data would likely include water consumption in several geographical locations. These issues create some uncertainty to the calculations and must be remembered when interpreting the results.
2.8 Comparison to dairy protein
The results of the modelling of microbially produced protein are compared to dairy protein production, using detailed results from analyses for a range of dairy protein products (including caseinates, lactabumin, total milk protein and whey protein isolate) produced from average dairy cow milk production in NZ, which represents a highly efficient milk and milk protein producing country with a high percentage of renewable electricity production and good rainfall. This is presented as an example of dairy production, and alterations related to other locations are considered in the results, “Sect. 3.4.” Data for Fonterra’s NZ average for raw milk production in 2019/2020 was based on detailed farm surveys (Ledgard et al. 2020). This used data from the various regions of dairy production across NZ and weighted it for regional milk production to estimate an NZ average. The cradle-to-farm-gate carbon footprint was estimated using LCA (Ledgard et al. 2020) and the factors in Table 3. A similar approach was used to calculate the WSF, based on regional data and annual average AWARE factors, except for Canterbury, where monthly factors were used for irrigation (Payen et al. 2018).
Unlike the rBLG calculations done with MS Excel, the dairy protein analyses were carried out using SimaPro (version 9.2.0.1). However, the dairy protein assessment used the same data source, i.e. ecoinvent (version 3.7.1). For the cradle-to-farm-gate, the analyses used fat and protein corrected milk and biophysical allocation was applied between milk and live weight sold for meat (IDF 2015), with an average of 85% allocated to milk.
Primary data on milk collection and transport to NZ processing plants was used. Site-specific primary data from all milk processing plants across NZ was also used to calculate the carbon footprint of multiple products, including the milk protein products acid casein, rennet casein, caseinates, total milk protein and whey protein isolate (Fonterra 2020, 2021). The production of these involved concentrating the milk protein products from fluid milk by membrane processing, followed by spray drying for all except caseins, which involved a coagulation/separation step (Adamson 2015; Badem and Uçar 2017). Specific energy use applied for different products, and allocation methodology applied for other processing inputs based on dry mass (IDF 2015). Detailed water flow data for all plants was unavailable, and therefore the default WSF for the processing stage for dry whey powder processing from the European Product Environmental Footprint was used (EDA 2018).
Thus, the system boundary for the production of dairy protein (cradle-to-processor-gate) was the same as that for the recombinant protein production. The various dairy protein products had characteristics of 84–92% protein. The results were adjusted to a per-ton protein basis to match the functional unit of this study.
2.9 Limitations, uncertainty and sensitivity analysis
Since this study is an ex-ante LCA and the technology system for the recombinant protein (rBLG) production is only modelled, the production process for BLG and the calculations of the environmental impacts have several limitations and uncertainties. The LCI datasets used in the calculations are directly taken from the ecoinvent 3.7.1 database without further checking/editing, and thus some of them might be out-dated or too generic for the purpose described in this study. As an example, no production data was available for corn steep liquor in the ecoinvent database. Thus, it was modelled as ‘Distiller’s grains and solubles’ as the reference flow of the dataset, a by-product from ‘ethanol production from maize’. This as a maize based by-product should describe the corn steep liquor production as relatively reliable information, but still the environmental burden of corn steep liquor can be seen as uncertain. The datasets used in each location are defined in the Online Resource Data 2. The uncertainties related to the data used are not tested in this article with traditional uncertainty analysis such as Monte Carlo. In addition, even though this is an ex-ante LCA, no future predictions were made to describe the changed situations, e.g. on energy profiles or technologies used in the value chain.
However, the effect of key process parameters is evaluated using sensitivity analysis as is recommended for ex-ante LCAs (Cucurachi et al. 2018).The sensitivity analysis is performed by first moving one input variable to a more favourable and a less favourable value at a time and keeping others at their baseline value and then returning the variable to its baseline value. This is repeated for each of the variables that are selected for the sensitivity analysis. Scenario 1 and NZ were selected for the sensitivity analysis. The process parameters selected for the sensitivity analysis were final titre, final fungal biomass amount, carbon selectivity, power consumption and production time. A difference of 15% was considered significant in the sensitivity analysis.
3 Results
3.1 Carbon footprint results of rBLG production
The datasets used in the calculations are divided into 9 life cycle steps in the reporting. These are carbon source, nitrogen source, other materials, process water, electricity, thermal energy, transportation, waste treatment and avoided feed production. The results of the carbon footprint calculations are presented in Fig. 2 and in more detail in the Online Resource Data 3. The main sources of GHG emissions are sugar production (carbon source), especially in the case of glucose (scenarios 1, 3 and 4) and electricity production. More than a half of the climate impacts from both glucose and sucrose production relate to their raw materials’ production (maize or sugar cultivation) but also energy needed in the sugar processing plays a role in the GHG emissions, e.g. causing c.30% of the glucose’s climate impact. The avoided emissions from the animal feed production are shown in the results as negative emissions. The sum of the released and avoided emissions is described as a partial carbon footprint in the figures since the system boundary considered is cradle-to-gate instead of a full life cycle.
The carbon footprint results of all scenarios in all four locations. Scenario 1 describes the base case (i.e. glucose as the carbon source, biomass treated as feed, no chromatography); scenario 2 uses sucrose as the carbon source; scenario 3 describes the base case with biomass treated as waste; and scenario 4 describes the base case with chromatography. The sum of the released and avoided emissions is described as a partial carbon footprint since the system boundary considered is cradle-to-gate instead of a full life cycle
The carbon footprint results varied between 5.5 and17.6 t CO2e/t protein, being the lowest in NZ scenario 2 and the highest in Australia in scenario 4. In all locations, the scenario 2 with sucrose as the carbon source had the lowest carbon footprint (5.5, 7.7, 9.6 and 12.7 t CO2e/t protein in NZ, Germany, US and Australia, respectively), while the highest results were found in scenario 4 with chromatography (9.6, 13.4, 13.9 and 17.6 t CO2e/t protein in NZ, Germany, US and Australia, respectively).
3.2 Water scarcity footprint results of rBLG production
The same 9 life cycle steps as presented above for the carbon footprint are used in the WSF reporting. The results of the WSF calculations are presented in Fig. 3 and in more detail in the Online Resource Data 3. The WSF is mainly caused by the water consumed in sugar production (carbon source). In addition, the production process itself, nitrogen source production and electricity production play a role in WSF. In scenario 4, the process water and used chemicals contribute notably to the WSF. In addition, the biomass substituting average animal feed is creating avoided impacts that remarkably affect the results. It should be noted that the water consumption in each scenario is very similar in different locations, and the direct water consumption of the modelled process is the same in all locations. Also, the glucose production, which consumes the biggest share of the consumed water in scenarios 1, 2 and 4, is the same in all locations. The production of sucrose from sugarcane consumes more water in US, Australia and NZ than less-irrigated sugar beet production in Germany. Minor differences to the water consumption are caused by upstream processes describing different locations, e.g. the different energy profiles in different countries have a small impact on the water consumption amounts. However, the production location has a great impact on the results due to the differences in water availability and in the water needs of humans and ecosystems in different locations, which are reflected in the water scarcity factors that are used in the AWaRe method. Similarly to the carbon footprint, the sum of the WSF results is described as a partial water scarcity footprint in the figures since the system boundary considered is cradle to gate instead of a full life cycle.
The water scarcity footprint results of all scenarios in all four locations. Scenario 1 describes the base case (i.e. glucose as the carbon source, biomass treated as feed, no chromatography); scenario 2 uses sucrose as the carbon source; scenario 3 describes the base case with biomass treated as waste; and scenario 4 describes the base case with chromatography. The sum of the caused and avoided WSF results is described as a partial water scarcity footprint since the system boundary considered is cradle to gate instead of a full life cycle
The WSF results varied between 88 and5031 m3 world eq./t protein, being the lowest in US scenario 1 and the highest in Australia in scenario 2. In all locations, the scenario 1 had the lowest WSF (481, 183, 88 and 924 m3 world eq./t protein in NZ, Germany, US and Australia, respectively). The highest result for Germany was found in scenario 4 with chromatography (404 m3 world eq./t protein) while in the other locations the scenario 2 created the highest WSF (4587, 558 and 5031 m3 world eq./t protein in NZ, US and Australia, respectively).
3.3 Sensitivity analysis results of rBLG production
The sensitivity analysis was carried out for NZ scenario 1. The results in Fig. 4 show that the carbon footprint is affected by some of the process model parameters. The results above show that the carbon source (sugar production) is one of the key factors affecting both footprints. From the selected sensitivity parameters, the final titre, sugar selectivity to carbon dioxide and final fungal biomass amount all influence the yield of protein from sugar—the higher the final titre, the better the protein yield; and the lower the final biomass amount and sugar selectivity to carbon dioxide, the better the protein yield. In addition, when the yield is better, less sugar is needed to produce the same amount of secreted protein. Furthermore, if less sugar would be used, the environmental footprints of secreted protein would decrease.
Sensitivity results of carbon footprint (top) and water scarcity footprint (bottom) in NZ with different process parameters. Note that the effects of the final titre and final fungal biomass amount are contrasting in the carbon footprint and WSF. This is due to the production of fungal biomass per tonne of protein being higher than the baseline. Fungal biomass used as feed decreases the WSF significantly due to avoided emissions
The carbon footprint of the microbially produced milk protein could be decreased if the final titre could be increased or if the final fungal biomass amount would be decreased from the base scenario. On the other hand, the carbon footprint would be higher if the final titre would be lower, the sugar selectivity to carbon dioxide would be higher or the final biomass amount would be higher. Changes in the process power consumption increases or decreases the carbon footprint less significantly. The production time was not found to affect the carbon footprint of the protein within the used sensitivity ranges.
For the WSF, the effect of the final titre and final fungal biomass is the opposite of the carbon footprint as shown in Fig. 4. This means that the more favourable parameters for the process, i.e. the fungal biomass production of 10 g/L and final rBLG titre of 100 g/L, are less favourable for the WSF. Avoided impacts were applied to the side product (fungal biomass), and the sensitivity parameters’ final fungal biomass amount and final rBLG titre affect the ratio of fungal biomass and rBLG protein, thus influencing also the amount of avoided impacts. The production time and power consumption do not affect the WSF.
3.4 Comparison of the results of rBLG production to dairy protein production
For the range of milk protein products, the GHG emissions for the cradle-to-farm-gate stage and cradle-to-processing-gate were 8.5–8.8 and 9.6–10.4 t CO2e/t protein, respectively. Animal-related emissions dominated, with enteric methane emissions from animal feed digestion constituting 56% of the cradle-to-farm-gate total and excreta nitrous oxide emissions representing 9% of the total. The other main contributors were the production and use of brought-in feeds at 10% of the total and direct land-use change (accounting for feed source and NZ forest to pasture conversion; Ledgard et al. 2020) at 15% of the total. Direct energy use emissions were relatively minor at < 2% of the total. Transport of milk from farm to factory added 0.8% to the total cradle-to-farm-gate emissions, with milk processing adding a further 19–21%. Of the latter, 75% was from thermal energy emissions and 14% was from electricity emissions. The partial carbon footprint results per tonne of protein produced by cellular agriculture and dairy protein production in NZ are described in Fig. 5.
The WSF of the protein products for the cradle-to-farm-gate stage varied widely among the regions of production, ranging from 231 m3 world eq./t protein for the Waikato region (the largest milk supply region in NZ) to 385 m3 world eq./t protein for Otago/Southland and 11,238 m3 world eq./t protein for the Canterbury region with irrigated pastures. The corresponding cradle-to-processing-gate values were 288 to 484 and 11,337 m3 world eq./t protein, respectively. For the non-Canterbury regions, the main contributors to the cradle-to-farm-gate WSF were fertiliser production, production of brought-in feeds, irrigation evapotranspiration and output in milk at 21–48%, 8–29%, 5–54% and 2–4%, respectively. For Canterbury, the corresponding values were 1%, 8%, 89% and 1%, respectively. Transport of milk from farm to factory constituted 0.1–0.7% of the total cradle-to-processing-gate WSF, while milk processing contributed 1–34%. The partial WSF results per tonne of protein produced by cellular agriculture and dairy protein production in NZ are described in Fig. 6.
4 Discussion
4.1 Main findings of the calculations of protein production by cellular agriculture
The most important sources of GHG emissions in protein production by cellular agriculture are sugar production (carbon source) and electricity production in all four locations. Glucose production (scenarios 1, 3 and 4) has a higher carbon footprint than sucrose (scenario 2). Similarly, sucrose from sugar beet (Germany) has a lower footprint than sucrose from sugar cane (US, Australia, NZ). However, since the ecoinvent datasets were used for the sugar production, they may differ from the actual footprints of sugars available in each location. It might be even possible to choose a sugar producer with a low-carbon footprint further away. For example, in the case of NZ, sugars could be transported from the US by ship.
The GHG emissions of electricity production are dependent on electricity profile of a location. The electricity profile in NZ has a greater share of renewable energy than the profiles of Australia, Germany and the US (Alabama belonging to the South-East Regional Council, SERC), which is clearly visible in the impact shares from electricity in each location, with NZ having the lowest impacts from energy. However, renewable energy could be purchased in all locations, which would decrease the location-specific differences from electricity production.
The main contributor to the carbon footprint from the nitrogen source life cycle step is ammonia production. The carbon footprint of other materials increases its share in scenario 4 since the chromatography consumes chemicals more than the base scenario. Thermal energy production, waste treatment, and transportation have only minor impacts on carbon footprint. Finally, the avoided emissions from the replaced protein feed production have only a minor impact on GHG carbon emissions.
The WSF is strongly dependent on the location, especially on the location of the raw material production. Since the water availability in Australia is more limited than in Germany and the US (Alabama), the WSF of Australia is very high compared to the cases in Germany and the US. This is especially visible when sucrose is used as the carbon source since the sugar cane is assumed to be irrigated in the ecoinvent dataset. Scenario 4 consumes higher amounts of process water and chemicals than the other three scenarios, which are assumed to be produced nearby, and thus the local water scarcity factors play a key role in the WSF of chemicals. This could be avoided if chemicals would be imported from countries with higher water availability. However, the water scarcity factors used in the study are either country-specific or collected to a specific area (see Online Resource Data 2), and there is great variation within each area, especially in Australia. As an example, the AWaRe factors for agricultural activities vary between 0.3 and 39.6 in the Queensland coastline, and thus the actual production locations should be defined in order to do more specific WSF calculations.
The sensitivity analysis showed some possibilities for improving the environmental performance of the microbially produced protein. Especially the carbon footprint could be decreased if the final titre, and thus the protein yield from the carbon source, could be increased or if the final fungal biomass amount would be decreased. However, it is very likely that these improvements could not be achieved simultaneously. In contrast it is possible for the power consumption changes might be additive to the changes in the other parameters.
If the fungal biomass is treated as waste instead of feed, minor energy savings are achieved in the processing since there is no need to dry the biomass, while composting the biomass creates very small amounts of additional GHGs and water consumption. Thus, the differences between scenarios 1 and 3 are mainly caused by the (avoided) feed production. The protein feed used in the calculations was a dataset from ecoinvent describing the average global availability of market protein feed. There are 47 datasets for crude protein feed in ecoinvent with a wide variation in carbon footprint and water consumption. There were 33 datasets with a lower carbon footprint and 13 datasets with a higher carbon footprint than the market data used. The lowest carbon footprint was only 0.3% and the highest was 286% when compared to the market dataset (100%). Similarly, the water consumption was lower for 36 datasets and higher for 10 datasets than the market dataset used in the calculations. The lowest water consumption was only 0.01% and the highest water consumption was 2770% of the market dataset (100%). Thus, the avoided emission results are remarkably affected by the dataset selection and the protein feed that would actually be replaced with the biomass, especially when the WSF is considered. Despite the selected treatment option for the biomass, the biomass and purified wastewater must be directed somewhere, and this should be considered when the annual production capacity and location of the cellular agriculture facility are being defined.
When both of the studied indicators are considered, it can be stated that the sustainability of the microbially produced milk protein from climate and water scarcity perspectives is improved if a lower purity of the protein is acceptable and there is no need of chromatographic purification. For some of the other studied scenarios and parameters, the conclusions differ between the two indicators: the glucose as the source of carbon is a preferable choice from the water footprint perspective, but the sucrose is better when carbon footprint is considered, and similarly, if the final titre of the process is high, it reduces the carbon footprint but increases the WSF.
Cellular agriculture for producing food proteins is an emerging concept, and there is plenty of R&D going on. The mass and energy balance calculations for fictive production in this study were parametrized based on data gathering, and they were based on an MS Excel model which did not provide a platform for evaluating detailed data. In this study, chromatography separation had especially high uncertainty given the imprecision in the model used and limited data in the literature. In addition, since no specific data was available on production technologies or possible raw material and energy suppliers, all ecoinvent datasets used were secondary data and bring uncertainty to the results. Thus, due to these limitations of the modelling approach, the results of cell-cultured protein production have higher uncertainty than that of dairy protein. The assessment should be considered as an approximation of the rBLG production process. However, this is the case when assessing processes that are emerging and when the mass and energy balance data used is based on the modelling of hypothetical production instead of an existing production plant.
4.2 Comparison to dairy protein
Even though the dairy protein production was only considered for NZ in this study, the results outside of NZ can be estimated based on the milk footprints in each area since milk production is the main contributor to dairy protein footprints, especially if renewable energy is available for processing. For the US and the EU in general, the carbon footprints of fat and protein corrected milk are up to approximately 20% higher than for NZ, but the global average is much higher (around three times the NZ footprint), which is the result of relatively high emissions from developing dairy chains (FAO and GDP 2019; Ledgard et al. 2020). It is not likely, though, that the milk in the developing areas would be processed into proteins, and thus the magnitude of the carbon footprint results can be assumed to be similar or slightly higher in the US and the EU than in NZ, considering that the difference of carbon footprint of dairy proteins produced in NZ and other countries could be mainly dependent on the impacts of milk production (as described above), and the processing to protein could be done with renewable energy in all the locations.
In principle, a comparison of results between milk protein based on cellular agriculture and dairy protein production can be considered on two levels:
-
1.
Milk protein at the dairy farm-gate vs. milk protein by cellular agriculture at the production facility gate
For many dairy products such as fluid milk, yoghurts and cheeses, it is reasonable to use raw milk as a raw material instead of extracted milk proteins. For these products and in countries with developed dairy chains, dairy proteins within the raw milk are likely to create a smaller footprint than the use of rBLG would create. Comparison of rBLG and dairy proteins on this level is thus not very reasonable. It is also important to consider the very significant differences in the protein footprints of milk produced in different parts of the world and where milk proteins produced by precision fermentation will compete with dairy. It is very unlikely that rBLG will compete with milk produced in informal or less mature dairy markets where dairy footprints are highest. It is far more likely to compete with the milk produced in the more developed markets, which have lower milk protein footprints. Ultimately, to be totally disruptive, it will need to compete with what is possible for milk protein when produced at scale with the lowest footprint such as the pasture-based farming systems used in NZ.
-
2.
The extracted milk protein at the dairy factory gate vs. milk protein by cellular agriculture at the production facility gate
This is the most relevant comparison of footprints of different milk protein production options. This study shows that it is possible for the carbon footprint of dairy protein and the carbon footprint of cellular produced milk protein to be similar. But this is highly dependent on the raw materials and energy profile used in the microbial production process. The WSF results are more difficult to compare since location has such a major effect on both microbially produced and dairy protein production. But according to our study, the WSF can also be on a similar level for both protein production options.
However, it is important to note that the footprint of the final product using microbially produced milk protein or protein extracted from milk is the relevant comparison that should be made. This should also include the contribution from all other ingredients and the contribution from the manufacturing processes required. While, as above, microbially produced milk protein is unlikely to make a lower contribution than extracted milk protein to the footprint of the final food product, the footprint of such a product will also depend upon the contributions from all other ingredients in that product. Such analysis was beyond the scope of this study given the wide range of foods that could be formulated using milk protein and the wide range of other plant-sourced or animal-sourced ingredients that could be used. However, in such an instance, the use of microbially produced milk protein is a means of creating an animal-free product but not of itself a more sustainable product. In addition, care should be taken if the primary purpose of such foods is to provide dairy nutrition, and while the microbially produced milk protein can be a source of high-quality protein and essential amino acids, other food components may not be as nutritious or bioavailable (Clegg et al. 2021).
4.3 Microbial milk protein and future prospects
The microbial production might be able to utilise other sources of carbon and nitrogen. If by-products or even waste-based materials could be used, the footprints could decrease significantly. The use of other raw materials, such as straw as a source of carbon, has already been studied and found to also have economic potential (Voutilainen et al. 2021). In addition, other industrial side streams or agricultural by-products, such as distillers’ grains or molasses, could have a role as a carbon source in microbial production. However, it is important to note that the use of less refined production media may require additional DSP, which increases the footprints of microbially produced milk protein.
The carbon footprint results of the earlier LCAs related to microbially produced proteins by Järviö et al. (2021) and Perfect Day (2021) are relatively similar to the results of our study if allocation is avoided. Yet, the magnitude of environmental improvements provided by the use of precision fermentation technologies described by Tubb and Seba (2019) seem extremely unlikely based on our study. As described in the introduction, the carbon footprint of microbially produced milk protein by Perfect Day (2021) was c. 12 kg CO2e/kg protein when using 100% allocation to the main product, i.e. protein, which is on the same level as the result of this study (5.5–17.6 t CO2e/t protein). In contrast to the study of Perfect Day, this study avoided allocation and looked at the substitution of the feed as the means to consider the by-product biomass utilisation. In this study, the substitution approach of the base scenario reduced the carbon footprint by 7–10% and the WSF by 37–53% depending on the location, when compared to the scenario 3 which treated the biomass as a waste and considered 100% of the impacts on the rBLG. These reductions can be considered minor (for carbon footprint) or moderate (for WSF) when compared to the mass-based allocation approach with 78% impact reduction in the study of Perfect Day (2021). Other uses for the by-product biomass are also investigated in the world, e.g. the possibility of leather-like material production from fungi is currently studied (VTT 2019). If such innovations come to practice in the future, the assessment could be re-evaluated with other substitutable materials. Also, if the prices of the products and by-products would be known, economic allocation should be used as an additional allocation method.
Given the importance of milk protein to global nutrition and livelihoods (Hill 2017; Smith et al. 2022), such claims by Tubb and Seba (2019) and Perfect Day (2021) could have important consequences. Putting aside the fact that milk provides more nutrition and other benefits than just protein nutrition (Smith et al. 2022), the claims made by Perfect Day (2021) and Tubb and Seba (2019) would suggest that the valuable role that milk protein plays in global nutrition could be obtained with a much lower environmental impact. In this study, such dramatic changes were not visible for the carbon footprint and WSF. However, it should be acknowledged that microbial production technologies are undergoing constant development having the sustainability issues as key driver. Similarly, the major contributor of dairy protein production emissions from enteric fermentation of ruminants is the subject of intensive study, and they are decreasing per unit of production with gained knowledge and new/improved practices. Various technologies are also being developed to reduce methane emissions from enteric fermentation, enabling lower dairy footprints in the future (Sun et al. 2021).
5 Conclusions
This study showed that the assessed environmental impacts—namely, carbon footprint and WSF—of the microbially produced milk protein, rBLG, are in the same range as extracted dairy protein. The main contributions to the footprints are the sugar and electricity production. Thus, the footprints can be reduced if the share of renewable electricity is high (such is already the case in NZ.) Additionally, the possibility and availability of using food industry, or other carbohydrate rich side streams as a carbon source for recombinant protein production should be investigated because this could significantly reduce the footprints caused by sugar production. If specific purification technology (such as chromatography) is needed, it may significantly add environmental impacts. The WSF of both microbially produced milk protein and dairy protein production is very sensitive to the local conditions and water availability, and this can be considered as more uncertain than the carbon footprint.
Finally, it should be noted that the footprints of proteins and other food ingredients produced by cellular agriculture and traditional agriculture are not static; instead, there is potential for a significant reduction. The technology and knowledge about microbial production is evolving, and combined with renewable energy, the footprints could improve remarkably. In this respect, the carbon footprint of rBLG milk protein produced in NZ provides a possible picture of a future where 85% of electrical energy is renewable. Based on this analysis, improvements in sugar-to-protein conversion, thorough investigation of possible carbon sources with low footprints, and simple DSP should be a priority for improvements in milk protein produced by cellular agriculture.
As only two indicators were studied and since this assessment was based on process models for the microbially produced milk proteins instead of actual processing data, this analysis should be revisited in the coming years with a full LCA as new knowledge and improvements in technology emerge and when primary data of microbially produced milk proteins becomes available.
Data availability
Data generated or analysed during this study are described in this article and its supplementary information file. However, the data from ecoinvent database is available only through the vendors of this database.
References
Adamson N (2015) Whey processing. In: Bylund G (ed) Dairy processing handbook. Tetrapak Processing Systems, S-221 86 Lund, Sweden
Adom F, Maes A, Workman C et al (2012) Regional carbon footprint analysis of dairy feeds for milk production in the USA. Int J Life Cycle Assess 17:520–534. https://doi.org/10.1007/s11367-012-0386-y
Apeda (2021) AgriXchange, International trade, Importing country New Zealand, for Sugars. https://agriexchange.apeda.gov.in/inttrade/inttrade.aspx. Accessed 7 Dec 2021
Australian Government (2021) Australian sugar industry. In: Department of Agriculture, Water and the Environment. https://www.awe.gov.au/agriculture-land/farm-food-drought/crops/sugar. Accessed 7 Dec 2021
Badem A, Uçar G (2017) Production of caseins and their usages. Int J Food Sci Nutr 2:4–9
Beal CM, Gerber LN, Sills DL et al (2015) Algal biofuel production for fuels and feed in a 100-ha facility: a comprehensive techno-economic analysis and life cycle assessment. Algal Res 10:266–279. https://doi.org/10.1016/j.algal.2015.04.017
Behera BC (2020) Citric acid from Aspergillus niger: a comprehensive overview. Crit Rev Microbiol 46:727–749. https://doi.org/10.1080/1040841X.2020.1828815
Boland M, Hill J (2020) World supply of food and the role of dairy protein. In: Boland M, Singh H (eds) Milk proteins. From Expression to Food. Academic Press, 125 London Wall, London EC2Y 5AS, UK
Boland M, Singh H (2020) Milk proteins. From Expression to Food. Academic Press, 125 London Wall, London EC2Y 5AS, UK
Boulay AM, Bare J, Benini L et al (2018) The WULCA consensus characterization model for water scarcity footprints: assessing impacts of water consumption based on available water remaining (AWARE). Int J Life Cycle Assess 23:368–378. https://doi.org/10.1007/s11367-017-1333-8
Cherry JR, Fidantsef AL (2003) Directed evolution of industrial enzymes: an update. Curr Opin Biotechnol 14:438–443. https://doi.org/10.1016/S0958-1669(03)00099-5
Chungchunlam SMS, Moughan PJ, Garrick DP, Drewnowski A (2020) Animal-sourced foods are required for minimum-cost nutritionally adequate food patterns for the United States. Nature Food 1:376–381. https://doi.org/10.1038/s43016-020-0096-8
Clegg ME, Tarrado Ribes A, Reynolds R et al (2021) A comparative assessment of the nutritional composition of dairy and plant-based dairy alternatives available for sale in the UK and the implications for consumers’ dietary intakes. Food Res Int 148:110586. https://doi.org/10.1016/J.FOODRES.2021.110586
Couper JR, Penney WR, Fair JR, Walas SM (2010) Chemical process equipment revised: selection and design, 2nd edn. Butterworth-Heinemann
Cucurachi S, Van Der Giesen C, Guinée J (2018) Ex-ante LCA of emerging technologies. Procedia CIRP 69:463–468. https://doi.org/10.1016/j.procir.2017.11.005
Derntl C, Gudynaite-Savitch L, Calixte S et al (2013) Mutation of the Xylanase regulator 1 causes a glucose blind hydrolase expressing phenotype in industrially used Trichoderma strains. Biotechnol Biofuels 6:1–11. https://doi.org/10.1186/1754-6834-6-62
ECDC, EMA (2009) The bacterial challenge : time to react : a call to narrow the gap between multidrug-resistant bacteria in the EU and the development of new antibacterial agents, Publications Office, 2009. https://data.europa.eu/doi/10.2900/2518
EDA (2018) Product environmental footprint category rules for dairy products. https://ec.europa.eu/environment/eussd/smgp/pdf/PEFCR-DairyProducts_2018-04-25_V1.pdf. Accessed 17 Dec 2021
Ellilä S, Fonseca L, Uchima C et al (2017) Development of a low-cost cellulase production process using Trichoderma reesei for Brazilian biorefineries. Biotechnol Biofuels 10:30. https://doi.org/10.1186/s13068-017-0717-0
Etzel MR (2004) Manufacture and use of dairy protein fractions. J Nutr 134:996S-1002S. https://doi.org/10.1093/JN/134.4.996S
Expasy (2022) Protparam tool. Available at https://www.expasy.org/resources/protparam. Accessed 3 Jan 2022
FAO (2010) Greenhouse gas emissions from the dairy sector. A Life Cycle Assessment. https://www.fao.org/3/k7930e/k7930e00.pdf
FAO, GDP (2019) Climate change and the global dairy cattle sector – the role of the dairy sector in a low-carbon future. Rome. https://www.fao.org/3/ca2929en/ca2929en.pdf
Flysjö A, Cederberg C, Henriksson M et al (2011) How does co-product handling affect the carbon footprint of milk? Case study of milk production in New Zealand and Sweden. Int J Life Cycle Assess 16:420–430. https://doi.org/10.1007/s11367-011-0283-9
Fonterra (2021) Sustainability performance report 2021. https://www.fonterra.com/content/dam/fonterra-public-website/fonterra-new-zealand/documents/pdf/sustainability/2021/fonterra-sustainability-report-2021.pdf. Accessed 3 Jan 2022
Fonterra (2020) Environmental data reporting notes - Sustainability Report 2020. https://www.fonterra.com/content/dam/fonterra-public-website/fonterra-new-zealand/documents/pdf/sustainability/2020/2020-environmental-data-reporting-notes.pdf. Accessed 3 Jan 2022
Henchion M, Hayes M, Mullen AM et al (2017) Future protein supply and demand: strategies and factors influencing a sustainable equilibrium. Foods 6:1–21. https://doi.org/10.3390/FOODS6070053
Hill JP (2017) Assessing the overall impact of the dairy sector. In: van Belzen N (ed) Achieving sustainable production of milk. Volume 2. Burleigh Dodds Science Publishing, 82 High Street, Sawston, Cambridge, CB22 3HJ, UK. pp. 291–314. https://doi.org/10.19103/AS.2016.0005.43
Hill JP, McNabb WC, Smith NW, Fletcher AJ, van der Lee C (2022) Need or greed. Are the current plant-based and cellular agriculture alternatives to livestock derived foods targeting a hungry world or a greedy world?. World Climate Foundation. https://www.worldclimatefoundation.org/post/need-or-greed. Accessed 3 May 2022
Humbird D, Davis R, McMillan JD (2017) Aeration costs in stirred-tank and bubble column bioreactors. Biochem Eng J 127:161–166. https://doi.org/10.1016/J.BEJ.2017.08.006
Humbird D, Davis R, Tao L et al (2011) Process design and economics for biochemical conversion of lignocellulosic biomass to ethanol. United States. https://doi.org/10.2172/1013269
IDF (2015) A common carbon footprint approach for the dairy sector –– the IDF guide to standard life cycle assessment methodology. Bulletin of the International Dairy Federation 479. https://fil-idf.org/wp-content/uploads/2016/09/Bulletin479-2015_A-common-carbon-footprint-approach-for-the-dairy-sector.CAT.pdf. Accessed 3 Jan 2022
ISO (2006) Environmental management — life cycle assessment — principles and framework (ISO 14040:2006)
ISO (2020) Environmental management — life cycle assessment — requirements and guidelines AMENDMENT 2 (ISO 14044:2006/Amd.2:2020(E))
ISO (2018) Greenhouse gases. Carbon footprint of products. Requirements and guidelines for quantification (ISO 14067:2018)
ISO (2014) Environmental management — water footprint — principles, requirements and guidelines (ISO 14046:2014)
Järviö N, Parviainen T, Maljanen N-L et al (2021) Ovalbumin production using Trichoderma reesei culture and low-carbon energy could mitigate the environmental impacts of chicken-egg-derived ovalbumin. Nature Food 2:1005–1013. https://doi.org/10.1038/s43016-021-00418-2
Landowski CP, Huuskonen A, Wahl R et al (2015) Enabling low cost biopharmaceuticals: a systematic approach to delete proteases from a well-known protein production host Trichoderma reesei. PLoS ONE 10:e0134723. https://doi.org/10.1371/journal.pone.0134723
Ledgard SF, Falconer SJ, Abercrombie R et al (2020) Temporal, spatial, and management variability in the carbon footprint of New Zealand milk. J Dairy Sci 103:1031–1046. https://doi.org/10.3168/jds.2019-17182
Meyer H-PM, Minas W, Schmidhalter D (2016) Industrial-scale fermentation. In: Wittmann C, Liao JC (eds) Industrial biotechnology: products and processes. Wiley-VCH Verlag GmbH & Co, KGaA, Weinheim, Germany, pp 1–53
Mottet A, De Haan C, Falucci A et al (2017) Livestock: on our plates or eating at our table? A new analysis of the feed/food debate. Glob Food Sec 14:1–8
Myhre GD, Shindell D, Bréon F-M et al (2013) Anthropogenic and natural radiative forcing. In: Stocker TF, Qin D, Plattner GK et al (eds) Climate change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA. https://www.ipcc.ch/site/assets/uploads/2018/02/WG1AR5_Chapter08_FINAL.pdf
Niazi SK, Brown JL (2017) Fundamentals of modern bioprocessing. CRC Press Taylor & Francis Group
Paloheimo M, Haarmann T, Mäkinen S, Vehmaanperä J (2016) Production of industrial enzymes in Trichoderma reesei. In: Schmoll M., Dattenböck C. (eds) Gene expression systems in fungi: advancements and applications. Fungal Biology. Springer, Cham. https://doi.org/10.1007/978-3-319-27951-0_2
Payen S, Falconer S, Ledgard SF (2018) Water scarcity footprint of dairy milk production in New Zealand - a comparison of methods and spatio-temporal resolution. The Science of the Total Environment 639:504–515. https://doi.org/10.1016/J.SCITOTENV.2018.05.125
Perfect Day (2021) Comparative GHG emissions assessment of Perfect Day whey protein production to dairy protein. https://m4f6w9b2.rocketcdn.me/app/uploads/2022/01/Comparative-Perfect-Day-Whey-LCA-report-prepared-by-WSP_20AUG2021_Non-Confidential-1.pdf. Accessed 3 Jan 2022
Rantasalo A, Landowski CP, Kuivanen J et al (2018) A universal gene expression system for fungi. Nucleic Acids Res 46:e111. https://doi.org/10.1093/nar/gky558
Rantasalo A, Vitikainen M, Paasikallio T et al (2019) Novel genetic tools that enable highly pure protein production in Trichoderma reesei. Sci Rep. https://doi.org/10.1038/S41598-019-41573-8
Revuelta JL, Serrano-Amatriain C, Ledesma-Amaro R, Jiménez A (2018) Formation of folates by microorganisms: towards the biotechnological production of this vitamin. Appl Microbiol Biotechnol 102:8613–8620. https://doi.org/10.1007/s00253-018-9266-0
Richie H, Roser M (2021) Environmental impacts of food production. https://ourworldindata.org/environmental-impacts-of-food?utm_source=jeremycherfas&utm_medium=email&utm_campaign=eat-this-newsletter-132-underserved. Accessed 24 Apr 2022
Rischer H, Szilvay GR, Oksman-Caldentey KM (2020) Cellular agriculture — industrial biotechnology for food and materials. Curr Opin Biotechnol 61:128–134. https://doi.org/10.1016/j.copbio.2019.12.003
Shahbandeh M (2021) Leading sugar cane producers worldwide in 2019, based on production volume. Statista. https://www.statista.com/statistics/267865/principal-sugar-cane-producers-worldwide/. Accessed 7 Dec 2021
Smith NW, Fletcher AJ, Hill JP, Dave LA, McNabb WC (2021) Use of the DELTA model to understand the food system and global nutrition. J Nutr 151:3253–3261
Smith NW, Fletcher AJ, Hill JP, McNabb WC (2022) Modeling the contribution of milk to global nutrition. Front Nutr 8:1287. https://doi.org/10.3389/fnut.2021.716100
Statista (2021) Leading sugar beet producers worldwide in 2019, based on production volume. Statista. https://www.statista.com/statistics/264670/top-sugar-beet-producers-worldwide-by-volume/. Accessed 7 Dec 2021
Sun K, Liu H, Fan H et al (2021) Research progress on the application of feed additives in ruminal methane emission reduction: a review. PeerJ 9:e11151. https://doi.org/10.7717/PEERJ.11151
Tubb C, Seba T (2019) Rethinking food and agriculture 2020––2030 - the second domestication of plants and animals, the disruption of the cow, and the collapse of industrial livestock farming. https://static1.squarespace.com/static/585c3439be65942f022bbf9b/t/5d7fe0e83d119516bfc0017e/1568661791363/RethinkX+Food+and+Agriculture+Report.pdf. Accessed 7 Dec 2021
United Nations (2015) Transforming our world: the 2030 Agenda for Sustainable Development: REport A/RES/70/1. New York. https://sdgs.un.org/2030agenda. Accessed 7 Dec 2021
Usva K, Virtanen E, Hyvärinen H et al (2019) Applying water scarcity footprint methodologies to milk production in Finland. Int J Life Cycle Assess 24:351–361. https://doi.org/10.1007/s11367-018-1512-2
Voutilainen E, Pihlajaniemi V, Parviainen T (2021) Economic comparison of food protein production with single-cell organisms from lignocellulose side-streams. Bioresource Technology Reports 14:100683. https://doi.org/10.1016/j.biteb.2021.100683
VTT (2019) VTT develops alternative leather from fungi – aims at industrial-scale production. Press release. VTT Technical Research Centre of Finland Ltd. https://www.vttresearch.com/en/news-and-ideas/vtt-develops-alternative-leather-fungi-aims-industrial-scale-production . Accessed 3 May 2022
World Bank (2022) GDP (current US$) Data. https://data.worldbank.org/indicator/NY.GDP.MKTP.CD?most_recent_value_desc=true . Assessed 6 May 2022
WULCA (2019) What is AWARE (Available WAter REmaining)? https://wulca-waterlca.org/aware/what-is-aware/. Accessed 3 Jan 2022
Acknowledgements
Authors acknowledge Juha Tähtiharju, Dilek Ercili-Cura and Matias Alarotu for their contributions during the work.
Funding
Open Access funding provided by Technical Research Centre of Finland (VTT). This study was funded by Fonterra Research and Development Centre.
Author information
Authors and Affiliations
Contributions
Alan Welman, Marja Nappa, Nina Aro, Jeremy Hill contributed to the study conception and design. Marja Nappa and Marjut Suomalainen modelled the production process. Katri Behm carried out the investigation and calculations of environmental impacts. Stewart Ledgard and Jeremy Hill contributed the environmental impacts of dairy proteins. The first draft of manuscript was written by Katri Behm, Marja Nappa, Jeremy Hill, and Nina Aro and all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Author KB, MN, NA, SL and MS declare they have no financial interests. AW and JH are employees of Fonterra Cooperative Group Ltd.
Additional information
Communicated by Matthias Finkbeiner
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Behm, K., Nappa, M., Aro, N. et al. Comparison of carbon footprint and water scarcity footprint of milk protein produced by cellular agriculture and the dairy industry. Int J Life Cycle Assess 27, 1017–1034 (2022). https://doi.org/10.1007/s11367-022-02087-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11367-022-02087-0