Abstract
The aging population in Europe faces a substantial burden from dementia, with vascular cognitive impairment and dementia (VCID) being a preventable cause. Atrial fibrillation (AF), a common cardiac arrhythmia, increases the risk of VCID through mechanisms such as thromboembolism, cerebral hypoperfusion, and inflammation. This review explores the epidemiology, pathophysiology, and preventive strategies for AF-related VCID. Epidemiological data indicate that AF prevalence rises with age, affecting up to 12% of individuals over 80. Neuroimaging studies reveal chronic brain changes in AF patients, including strokes, lacunar strokes, white matter hyperintensities (WMHs), and cerebral microbleeds (CMHs), while cognitive assessments show impairments in memory, executive function, and attention. The COVID-19 pandemic has exacerbated the underdiagnosis of AF, leading to an increase in undiagnosed strokes and cognitive impairment. Many elderly individuals did not seek medical care due to fear of exposure, resulting in delayed diagnoses. Additionally, reduced family supervision during the pandemic contributed to missed opportunities for early detection of AF and related complications. Emerging evidence suggests that long COVID may also elevate the risk of AF, further complicating the management of this condition. This review underscores the importance of early detection and comprehensive management of AF to mitigate cognitive decline. Preventive measures, including public awareness campaigns, patient education, and the use of smart devices for early detection, are crucial. Anticoagulation therapy, rate and rhythm control, and addressing comorbid conditions are essential therapeutic strategies. Recognizing and addressing the cardiovascular and cognitive impacts of AF, especially in the context of the COVID-19 pandemic, is essential for advancing public health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Europe is aging at an unprecedented rate, presenting significant challenges for public health systems [1]. Among the various age-related diseases, dementia stands out as a particularly burdensome condition [2,3,4,5,6]. Dementia is broadly categorized into two main types: Alzheimer’s disease (AD) and vascular cognitive impairment and dementia (VCID) [5, 7]. AD is a neurodegenerative disorder characterized by the progressive loss of cognitive functions, including memory, language, and executive functions. AD accounts for half of dementia cases and currently affects millions worldwide, with the incidence sharply increasing with age. Unfortunately, AD remains incurable and unpreventable, posing a significant challenge for healthcare systems.
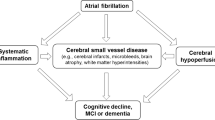
VCID refers to cognitive impairment and dementia resulting from macrovascular and microvascular pathologies, such as stroke and cerebral small vessel disease (CSVD) [8,9,10,11]. VCID is a leading cause of dementia and is preventable, which sets it apart from AD [5, 7]. The incidence of VCID increases significantly with age [5, 7]. In Europe, where nearly one-fifth of the population is aged 65 or older, the prevalence of VCID is rising [2]. Understanding the epidemiological trends of VCID is crucial for developing effective preventive and therapeutic strategies. Given that AD remains incurable, it is critical to focus on preventable causes of dementia, such as VCID. Identifying and managing these preventable factors can significantly reduce the burden of dementia on individuals and healthcare systems [6].
Atrial fibrillation (AF) emerges as a significant preventable cause of VCID [12,13,14]. AF is a common cardiac arrhythmia characterized by an irregular and often rapid heart rate. This arrhythmia can lead to various complications, including thromboembolism and reduced cerebral perfusion, which are directly linked to the development of dementia [12, 14,15,16,17,18,19].
The goal of this review is to overview the current evidence on the relationship between atrial fibrillation and vascular contributions to cognitive impairment and dementia. We aim to explore the pathophysiological mechanisms linking AF to VCID, examine clinical evidence supporting this connection, and discuss therapeutic implications and future directions for research and treatment. By understanding and addressing AF as a preventable cause of VCID, we can develop more effective strategies to mitigate cognitive decline and improve the quality of life for the aging population.
Epidemiology of atrial fibrillation
AF is a major concern for the aging population due to its increasing prevalence with advancing age [15,16,17,18,19]. Approximately 2% of the general population is affected by AF, but this figure rises dramatically to 10–12% among those aged 80 and older [20,21,22,23,24,25,26,27]. The condition is more prevalent in men than in women, although the risk for women increases with age [28,29,30]. Geographical differences in the prevalence of AF also exist [27, 30], with higher rates observed in North America and Europe compared to Asia and Africa. These variations can be attributed to differences in lifestyle, healthcare access, and genetic factors. Socioeconomic factors further influence the prevalence and management of AF. Differences in health literacy, patient preferences, and healthcare demands likely contribute to socioeconomic variations in health outcomes [31]. In wealthier regions, better access to healthcare and early diagnosis contribute to more effective management of AF. In contrast, lower socioeconomic status is associated with higher rates of undiagnosed and untreated AF, exacerbating the risk of complications such as VCID [32,33,34].
Underdiagnosis of AF is a significant problem, particularly among the elderly [35]. Many cases of AF remain asymptomatic or present with non-specific symptoms, leading to delayed diagnosis and treatment [35,36,37,38,39]. Studies suggest that a substantial proportion of AF cases go undetected, particularly in older adults who are at higher risk for complications. This underdiagnosis is a critical issue as it prevents timely intervention that could mitigate the risk of stroke and cognitive impairment. Addressing the underdiagnosis of AF requires increased awareness and proactive screening, especially in high-risk populations. Utilizing technologies such as wearable devices and implementing regular ECG screenings in routine check-ups can help detect AF early [40,41,42,43]. Increased utiliztion of wearable devices may also help clarify the burden of paroxysmal AF that meets the threshold for benefit from therapeutic interventions such as anticoagulation or ablation therapy, and allow for individualized care of AF [44,45,46]. By improving the detection and management of AF, we can reduce its impact on cognitive health and overall quality of life for the aging population.
AF, cognitive decline, and dementia: clinical evidence
Numerous epidemiological studies have established a link between AF and an increased risk of cognitive impairment and dementia [15,16,17,18,19, 47]. These studies suggest that AF independently increases the risk of VCID beyond traditional stroke pathways. The presence of AF has been shown to correlate with a higher incidence of dementia, even in the absence of clinically apparent strokes [15,16,17,18,19]. Studies suggest that older female AF patients have a higher risk of developing dementia compared to their male counterparts [48].
Neuroimaging findings
Neuroimaging studies have provided valuable insights into the brain changes associated with AF [49,50,51,52,53,54]. Findings such as white matter hyperintensities (WMH), cerebral microhemorrhages (CMHs; also known as cerebral microbleeds), lacunar infarcts, and cortical atrophy are more prevalent in patients with AF [49,50,51,52,53,54,55]. WMHs indicate areas of chronic ischemia, while CMHs are signs of microvascular damage. Cortical atrophy, or the thinning of the brain’s cortex, further illustrates the structural brain damage associated with AF [56, 57]. These neuroimaging findings are indicative of the chronic brain damage that contributes to cognitive decline in individuals with AF.
Cognitive function assessments
Cognitive function tests in patients with AF often reveal impairments in various domains, including memory, executive function, and attention [56,57,58,59,60,61]. These impairments highlight the multifaceted impact of AF on cognitive health. For instance, memory tests may show deficits in recalling recent events or learning new information, while executive function tests may reveal difficulties in planning, organizing, and completing tasks [56,57,58,59,60,61]. Attention assessments might indicate problems with maintaining focus or processing information quickly. These cognitive deficits underscore the need for comprehensive management strategies to address the cognitive aspects of AF. By recognizing and addressing these impairments early, healthcare providers can develop targeted interventions to help mitigate the cognitive decline associated with AF.
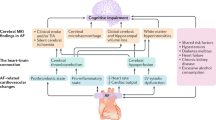
Pathophysiology linking AF and VCID
Thromboembolism
Atrial fibrillation can lead to the formation of blood clots within the hypomotile recesses of the atria. These small clots may travel to the brain, causing ischemic strokes [33, 62,63,64,65], which are causally linked to VCID [66]. Of particular concern are multiple microstrokes, which may not produce immediate clinical symptoms but cumulatively cause significant brain damage over time. The individual microemboli causing these microstrokes are likely to be clinically occult; however, in high-risk patients, microemboli might be detectable by transcranial doppler ultrasound as high-intensity transient signals, and the connection between ultrasound identification of microemboli, microemboli burden, and cognitive decline is an area in need of further investigation [67]. These recurrent microstrokes can disrupt brain networks and lead to rapid cognitive decline. The cumulative effect of these microstrokes can result in substantial brain damage, contributing to the progression of cognitive impairment and dementia.
Cerebral hypoperfusion
Atrial fibrillation may cause fluctuating or reduced blood flow to the brain [68]. This reduced blood flow can lead to the development of white matter lesions and other types of brain damage associated with VCID [68]. Chronic hypoperfusion affects the brain’s ability to function optimally, leading to gradual cognitive decline. One critical concept in understanding cerebral hypoperfusion is the vulnerability of watershed areas. These are regions of the brain located at the border zones between major arterial territories and are particularly susceptible to ischemic injury. In aging, due to structural and functional alterations in the cerebral microcirculation, such as capillary rarefaction, impaired endothelial vasodilation, and neurovascular impairment, there is a significant area at risk for ischemic injury [69,70,71,72]. These changes exacerbate the effects of reduced cerebral blood flow in patients with AF, increasing the risk of cognitive decline.
Inflammation and endothelial dysfunction
Both AF and VCID involve significant inflammatory processes and endothelial dysfunction [73,74,75,76]. Systemic inflammation associated with AF can exacerbate vascular damage, accelerating the progression of cognitive impairment. Inflammatory markers are often elevated in patients with AF, contributing to a pro-thrombotic state and promoting vascular injury.
Endothelial dysfunction, a hallmark of AF, can lead to impaired blood–brain barrier integrity [77]. This dysfunction allows for the infiltration of inflammatory cells and molecules into the brain, further exacerbating neuronal injury and cognitive decline [78, 79]. The impaired endothelial function reduces the brain’s ability to regulate blood flow effectively, increasing the susceptibility to ischemic damage and contributing to the pathogenesis of VCID [74,75,76].
Taking together, the pathophysiological mechanisms linking AF to VCID are multifaceted, involving thromboembolism, cerebral hypoperfusion, inflammation, and endothelial dysfunction. Understanding these mechanisms is crucial for developing targeted interventions to mitigate cognitive decline in patients with AF.
Preventive and therapeutic implications
Early detection and prevention of VCID
Early detection of AF is critical in preventing AF-related VCID [80, 81]. Timely identification of AF allows for interventions that can mitigate the risk of stroke and cognitive decline. It is estimated that offering a rhythm check to every 5000 people aged ≥ 65 years will prevent one stroke in the first year [82]. Raising awareness through public health campaigns can play a pivotal role in educating the general population about the importance of early detection.
Awareness campaigns
Public health initiatives should focus on increasing awareness of AF and its association with cognitive decline. These campaigns can emphasize the importance of routine check-ups and encourage individuals to monitor their heart health actively [83,84,85,86,87,88].
Encouraging self-diagnosis techniques, such as regular pulse taking, can help individuals identify irregular heartbeats that may indicate AF [89, 90]. Educational materials and resources should be made widely available to teach people how to check their pulse accurately and recognize potential warning signs of AF.
General practicioners play a crucial role in the early detection of AF [91,92,93,94]. Training and continuous education for primary care providers can enhance their ability to recognize the signs and symptoms of AF, conduct appropriate diagnostic tests, and refer patients for specialist care when necessary.
Patient education
Comprehensive patient education programs are essential. These programs should inform patients about the risks associated with AF, the importance of medication adherence, and lifestyle modifications that can reduce the risk of VCID [95,96,97,98,99].
Smart devices
The use of smart devices, such as fitness trackers and smartwatches equipped with heart rate monitors, can facilitate the early detection of AF [46, 100,101,102,103]. These devices can alert users to irregular heart rhythms, prompting timely medical consultation and intervention [104].
Diagnosis of paroxysmal AF
Paroxysmal AF, which occurs intermittently, presents unique diagnostic challenges [105]. Traditional 24-h Holter monitoring may not capture these sporadic episodes. Longer-term monitoring using wearable devices or implantable loop recorders can improve the detection rates of paroxysmal AF, ensuring timely diagnosis and treatment. Moreover, in high-risk patients, implantable loop recorders appear to have a significantly greater rate of AF detection that external loop recorders (15% versus 5%) [106].
Anticoagulation therapy
Anticoagulants, such as warfarin and direct oral anticoagulants (DOACs), play a crucial role in reducing stroke risk in AF patients. By preventing the formation of blood clots, these medications can mitigate cognitive decline [18, 107,108,109,110,111,112,113]. However, the benefits of anticoagulation must be weighed against the risk of bleeding, particularly in elderly patients. Individualized treatment plans that consider the patient’s overall health, comorbid conditions, and risk factors for bleeding are essential for optimizing outcomes.
In addition, atrial appendage closure procedures may be considered as an adjuct or alternative AF therapy to anticoagulation [114]. In particular, atrial appendage closure to reduce the risk of stroke and thromboembolism in AF may be a consideration in patients at high risk of hemorrhagic complications from anticoagulation, such as patients with cerebral amyloid angiopathy. Cerebral amyloid angiopathy, characterized by cerebrovascular deposition of amyloid β, is a common age-related small vessel pathology associated with intracerebral hemorrhage and independely associated with cognitive decline [115,116,117,118]. In its early stages, cerebral amyloid angiopathy is characterized by alteration of cerebrovascular physiology and non-hemorrhagic brain injury that then gives way to hemorrhagic brain injury in later stages [119]. The presence of higher risk cerebral amyloid angiopathy phenotypes, such as transient focal neurologic episodes, disseminated and focal cortical superficial siderosis, and lober hemorrhage with cerebral microbleeds, may inform an individualized approach to AF management that includes atrial appendage closure as a means to avoid long-term anticoagulation exposure [117].
Rate and rhythm control
Strategies to control heart rate or rhythm in AF patients can impact cognitive outcomes [120,121,122]. Rate control medications aim to regulate the heart rate, while rhythm control strategies, which include medications and arrhythmia ablation procedures [123], attempt to restore and maintain normal sinus rhythm. The choice between rate and rhythm control should be based on individual patient characteristics and the potential benefits of each approach.
Multifactorial approaches
Managing comorbid conditions that contribute to both AF and VCID, such as hypertension [124] and diabetes [125, 126], is vital. A multifactorial approach that includes lifestyle modifications is essential for reducing the risk of cognitive decline.
Adopting a heart-healthy diet, such as the Mediterranean diet, can reduce the risk of cardiovascular diseases and cognitive decline [127, 128]. Regular physical activity improves cardiovascular health and can help manage risk factors such as hypertension and diabetes [129]. Quitting smoking reduces the risk of cardiovascular diseases, stroke, and microvascular dysfunction and associated cognitive decline. Limiting alcohol intake can decrease the risk of AF and its complications.
A comprehensive approach that addresses all contributing factors is necessary for optimal patient outcomes. Collaborative care models involving cardiologists, neurologists, primary care providers, and other healthcare professionals can ensure that patients receive holistic and effective treatment plans tailored to their specific needs. Additionally, novel diagnostic tests exploring the interplay between the heart and brain may provide potential tools to identify patients at high risk for cerebral ischemia [130, 131].
Impact of the COVID-19 pandemic on AF and cognitive decline
The COVID-19 pandemic has had a profound impact on healthcare delivery, particularly for the elderly population [132]. During the pandemic, many older adults did not receive adequate care due to several factors, including lockdowns, fear of contracting the virus, and overwhelmed healthcare systems [133, 134]. This section explores how these challenges affected the diagnosis and management of AF and subsequently contributed to cognitive decline and dementia.
Reduced access to healthcare
During the pandemic, general practitioners and cardiologists were often unable to see patients regularly. Routine check-ups and elective procedures were postponed or canceled, leading to delays in diagnosing and managing chronic conditions like AF. Telehealth services, although beneficial, were not universally accessible or utilized by older adults, further limiting their access to necessary medical care.
Patient reluctance to seek care
Many elderly individuals avoided seeking medical care due to fear of exposure to COVID-19. In fact, some studies suggest that reluctance to seek medical care and stringent public health measures during the pandemic may have contributed to regional decreases in patients presenting for acute ischemic stroke [132, 135]. This reluctance also likely led to a significant number of AF cases going undiagnosed for extended periods, often for years. Without regular monitoring and timely intervention, these patients were at a higher risk of developing complications, including strokes and microstrokes.
Lax family supervision
The pandemic also led to a reduction in family supervision for many elderly individuals. Restrictions on travel and social gatherings meant that family members visited their older relatives less frequently. This lack of regular contact and oversight resulted in missed opportunities to observe changes in health status that could indicate underlying conditions such as AF. Family members often play a critical role in recognizing symptoms and encouraging medical consultations, and their reduced involvement during the pandemic further exacerbated the problem of undiagnosed AF and its complications.
Undiagnosed strokes and microstrokes
The delay in diagnosing AF during the pandemic contributed to an increase in undiagnosed strokes and microstrokes. These cerebrovascular events often went unnoticed without the routine diagnostic measures usually in place. Over time, the accumulation of these undetected strokes led to significant brain damage and an accelerated decline in cognitive function in many patients.
Increased cognitive decline
The combination of undiagnosed AF and subsequent strokes contributed to a significant increase in cognitive decline among the elderly during the pandemic [136,137,138,139]. Many patients who might have otherwise been diagnosed and treated early experienced more severe cognitive impairments due to the prolonged lack of medical intervention.
Specific challenges faced
The pandemic strained healthcare resources, making it challenging for patients to access specialist care. GPs and cardiologists were often redeployed to COVID-19 care units, further reducing the availability of routine cardiovascular care. Hospitals and clinics were overwhelmed with COVID-19 cases, leading to the postponement of non-emergency procedures and consultations. This shift in focus resulted in the neglect of chronic conditions like AF. While telehealth emerged as an alternative to in-person visits, many older adults faced barriers in accessing and using these technologies. Lack of familiarity with digital tools and limited internet access contributed to reduced utilization of telehealth services.
Long COVID and AF
COVID-19 can lead to a range of cardiovascular complications, including AF [140, 141]. Understanding the relationship between long COVID [142, 143], a condition characterized by persistent symptoms following an acute COVID-19 infection, and AF is crucial for developing effective management strategies for patients experiencing prolonged symptoms.
Long COVID is associated with a sustained inflammatory response, which can affect the heart and vascular system [144,145,146]. Chronic inflammation, which also include autoimune reactions [147], can damage cardiac tissues, potentially leading to arrhythmias such as AF. Additionally, the SARS-CoV-2 virus can directly infect cardiac cells, causing myocarditis and other structural changes [148,149,150,151,152]. These changes can create a substrate for the development of AF. Damage to small blood vessels, resulting in microvascular dysfunction, has been observed in long COVID patients [145, 152,153,154,155,156,157,158]. This can lead to impaired perfusion of cardiac tissues and contribute to the onset of AF.
Vaccine side effects and AF
While COVID-19 vaccines have been crucial in controlling the pandemic and preventing severe illness, there have been reports of side effects, including potential cardiac complications such as AF [159, 160]. In rare cases, some individuals have experienced AF shortly after receiving a COVID-19 vaccine. These occurrences are relatively uncommon and typically mild, often resolving without long-term effects. The mechanisms behind vaccine-induced AF are not fully understood but may involve transient inflammatory responses. It is important for healthcare providers to monitor and manage any cardiac symptoms post-vaccination.
Conclusion
In conclusion, atrial fibrillation is intricately linked to vascular contributions to cognitive impairment and dementia. Early detection and comprehensive management of AF are crucial to mitigating the risk of cognitive decline. Public awareness campaigns, advanced diagnostic tools, patient education, and proactive screening are essential components in identifying AF early and preventing its complications. Effective management strategies, including anticoagulation therapy, rate and rhythm control, and addressing comorbid conditions through lifestyle modifications, can significantly reduce the risk of VCID.
By addressing both the cardiac and cognitive aspects of AF, healthcare providers can significantly improve patient outcomes and quality of life. Collaborative care models and individualized treatment plans tailored to patient needs are pivotal in managing AF and preventing cognitive decline. Continued research and innovation in treatment strategies will further enhance our ability to combat the dual burden of AF and dementia, ultimately improving the health and well-being of the aging population.
References
Eurostat: Aging Europe. https://ec.europa.eu/eurostat/cache/digpub/ageing/. Accessed 4 Nov 2022.
GBD. Dementia forecasting collaborators: estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2019;2022(7):e105–25. https://doi.org/10.1016/S2468-2667(21)00249-8.
Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol. 2015;11:157–65. https://doi.org/10.1038/nrneurol.2015.10.
Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413–46. https://doi.org/10.1016/S0140-6736(20)30367-6.
Iadecola C, Duering M, Hachinski V, Joutel A, Pendlebury ST, Schneider JA, Dichgans M. Vascular cognitive impairment and dementia: JACC scientific expert panel. J Am Coll Cardiol. 2019;73:3326–44. https://doi.org/10.1016/j.jacc.2019.04.034.
Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368:1326–34. https://doi.org/10.1056/NEJMsa1204629.
Gorelick PB, Counts SE, Nyenhuis D. Vascular cognitive impairment and dementia. Biochim Biophys Acta. 2016;1862:860–8. https://doi.org/10.1016/j.bbadis.2015.12.015.
van den Brink H, Doubal FN, Duering M. Advanced MRI in cerebral small vessel disease. Int J Stroke. 2023;18:28–35. https://doi.org/10.1177/17474930221091879.
Elahi FM, Wang MM, Meschia JF. Cerebral small vessel disease-related dementia: more questions than answers. Stroke. 2023;54:648–60. https://doi.org/10.1161/STROKEAHA.122.038265.
Markus HS, van Der Flier WM, Smith EE, Bath P, Biessels GJ, Briceno E, Brodtman A, Chabriat H, Chen C, de Leeuw FE, et al. Framework for clinical trials in cerebral small vessel disease (FINESSE): a review. JAMA Neurol. 2022;79:1187–98. https://doi.org/10.1001/jamaneurol.2022.2262.
De Silva TM, Faraci FM. Contributions of aging to cerebral small vessel disease. Annu Rev Physiol. 2020;82:275–95. https://doi.org/10.1146/annurev-physiol-021119-034338.
Silva R, Miranda CM, Liu T, Tse G, Roever L. Atrial fibrillation and risk of dementia: epidemiology, mechanisms, and effect of anticoagulation. Front Neurosci. 2019;13:18. https://doi.org/10.3389/fnins.2019.00018.
Wandell P, Carlsson AC, Sundquist J, Sundquist K. The association between relevant comorbidities and dementia in patients with atrial fibrillation. Geroscience. 2018. https://doi.org/10.1007/s11357-018-0029-8.
Friberg L, Rosenqvist M. Less dementia with oral anticoagulation in atrial fibrillation. Eur Heart J. 2018;39:453–60. https://doi.org/10.1093/eurheartj/ehx579.
Zhai Y, Hu F, Yuan L, Ye X, Shi W, Yang R, Cao Y, Sun J, He J, Xu F. Atrial fibrillation increases the risk of all-cause dementia, Alzheimer’s disease, and vascular dementia: a cohort study of 373, 415 participants in the UK Biobank. J Affect Disord. 2024;351:323–30. https://doi.org/10.1016/j.jad.2024.01.224.
Brooks K, Yoshimura H, Gonzalez-Izquierdo A, Zakkak N, Kukendra-Rajah K, Lip GYH, Providencia R. The association between atrial fibrillation and dementia: a UK linked electronic health records cohort study. Eur J Clin Invest. 2024;54:e14154. https://doi.org/10.1111/eci.14154.
Bansal N, Zelnick LR, An J, Harrison TN, Lee MS, Singer DE, Fan D, Go AS. Incident atrial fibrillation and risk of dementia in a diverse, community-based population. J Am Heart Assoc. 2023;12:e028290. https://doi.org/10.1161/JAHA.122.028290.
Rivard L, Friberg L, Conen D, Healey JS, Berge T, Boriani G, Brandes A, Calkins H, Camm AJ, Yee Chen L, et al. Atrial fibrillation and dementia: a report from the AF-SCREEN international collaboration. Circulation. 2022;145:392–409. https://doi.org/10.1161/CIRCULATIONAHA.121.055018.
Lai SW. Atrial fibrillation and the risk of dementia. Am J Geriatr Psychiatry. 2022;30:117. https://doi.org/10.1016/j.jagp.2021.07.001.
Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009;104:1534–9. https://doi.org/10.1016/j.amjcard.2009.07.022.
Spannella F, Giulietti F, Pimpini L, Lombardi FE, Re S, Schiavi P, Dragano G, Antonicelli R, Sarzani R. Prevalence and predictors of subclinical atrial fibrillation in hospitalized older adults. Aging (Albany NY). 2021;13:17024–37. https://doi.org/10.18632/aging.203270.
Shkolnikova MA, Jdanov DA, Ildarova RA, Shcherbakova NV, Polyakova EB, Mikhaylov EN, Shalnova SA, Shkolnikov VM. Atrial fibrillation among Russian men and women aged 55 years and older: prevalence, mortality, and associations with biomarkers in a population-based study. J Geriatr Cardiol. 2020;17:74–84. https://doi.org/10.11909/j.issn.1671-5411.2020.02.002.
Ritchie LA, Oke OB, Harrison SL, Rodgers SE, Lip GYH, Lane DA. Prevalence of atrial fibrillation and outcomes in older long-term care residents: a systematic review. Age Ageing. 2021;50:744–57. https://doi.org/10.1093/ageing/afaa268.
Ritchie LA, Harrison SL, Penson PE, Akbari A, Torabi F, Hollinghurst J, Harris D, Oke OB, Akpan A, Halcox JP, et al. Prevalence and outcomes of atrial fibrillation in older people living in care homes in Wales: a routine data linkage study 2003–2018. Age Ageing. 2022;51. https://doi.org/10.1093/ageing/afac252.
Lindberg T, Wimo A, Elmstahl S, Qiu C, Bohman DM, Sanmartin BJ. Prevalence and incidence of atrial fibrillation and other arrhythmias in the general older population: findings from the Swedish national study on aging and care. Gerontol Geriatr Med. 2019;5:2333721419859687. https://doi.org/10.1177/2333721419859687.
Kjerpeseth LJ, Igland J, Selmer R, Ellekjaer H, Tveit A, Berge T, Kalsto SM, Christophersen IE, Myrstad M, Skovlund E, et al. Prevalence and incidence rates of atrial fibrillation in Norway 2004–2014. Heart. 2021;107:201–7. https://doi.org/10.1136/heartjnl-2020-316624.
Joseph PG, Healey JS, Raina P, Connolly SJ, Ibrahim Q, Gupta R, Avezum A, Dans AL, Lopez-Jaramillo P, Yeates K, et al. Global variations in the prevalence, treatment, and impact of atrial fibrillation in a multi-national cohort of 153 152 middle-aged individuals. Cardiovasc Res. 2021;117:1523–31. https://doi.org/10.1093/cvr/cvaa241.
Siddiqi HK, Vinayagamoorthy M, Gencer B, Ng C, Pester J, Cook NR, Lee IM, Buring J, Manson JE, Albert CM. Sex differences in atrial fibrillation risk: the VITAL rhythm study. JAMA Cardiol. 2022;7:1027–35. https://doi.org/10.1001/jamacardio.2022.2825.
Chang YT, Chen YL, Kang HY. Revealing the influences of sex hormones and sex differences in atrial fibrillation and vascular cognitive impairment. Int J Mol Sci. 2021;22(16):8776. https://doi.org/10.3390/ijms22168776.
Bose A, O’Neal WT, Wu C, McClure LA, Judd SE, Howard VJ, Howard G, Soliman EZ. Sex differences in risk factors for incident atrial fibrillation (from the reasons for geographic and racial differences in stroke [REGARDS] study). Am J Cardiol. 2019;123:1453–7. https://doi.org/10.1016/j.amjcard.2019.01.056.
Olsen F, Uleberg B, Jacobsen BK, Heuch I, Tande PM, Bugge E, Balteskard L. Socioeconomic and geographic differences in ablation of atrial fibrillation in Norway - a national cohort study. BMC Public Health. 2022;22:303. https://doi.org/10.1186/s12889-022-12628-9.
Tanner RM, Baber U, Carson AP, Voeks J, Brown TM, Soliman EZ, Howard VJ, Muntner P. Association of the metabolic syndrome with atrial fibrillation among United States adults (from the REasons for Geographic and Racial Differences in Stroke [REGARDS] Study). Am J Cardiol. 2011;108:227–32. https://doi.org/10.1016/j.amjcard.2011.03.026.
Soliman EZ, Howard G, Meschia JF, Cushman M, Muntner P, Pullicino PM, McClure LA, Judd S, Howard VJ. Self-reported atrial fibrillation and risk of stroke in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Stroke. 2011;42:2950–3. https://doi.org/10.1161/STROKEAHA.111.621367.
Meschia JF, Merrill P, Soliman EZ, Howard VJ, Barrett KM, Zakai NA, Kleindorfer D, Safford M, Howard G. Racial disparities in awareness and treatment of atrial fibrillation: the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Stroke. 2010;41:581–7. https://doi.org/10.1161/STROKEAHA.109.573907.
Guenancia C, Garnier F, Fichot M, Sagnard A, Laurent G, Lorgis L. Silent atrial fibrillation: clinical management and perspectives. Future Cardiol. 2020;16:133–42. https://doi.org/10.2217/fca-2019-0066.
Mitrega K, Lip GYH, Sredniawa B, Sokal A, Streb W, Przyludzki K, Zdrojewski T, Wierucki L, Rutkowski M, Bandosz P, et al. Predicting silent atrial fibrillation in the elderly: a report from the NOMED-AF cross-sectional study. J Clin Med. 2021;10. https://doi.org/10.3390/jcm10112321.
Kashou AH, Adedinsewo DA, Noseworthy PA. Subclinical atrial fibrillation: a silent threat with uncertain implications. Annu Rev Med. 2022;73:355–62. https://doi.org/10.1146/annurev-med-042420-105906.
Hernandez-Pinilla A, Clua-Espuny JL, Satue-Gracia EM, Palleja-Millan M, Martin-Lujan FM, Study-Group P-T. Protocol for a multicentre and prospective follow-up cohort study of early detection of atrial fibrillation, silent stroke and cognitive impairment in high-risk primary care patients: the PREFA-TE study. BMJ Open. 2024;14:e080736. https://doi.org/10.1136/bmjopen-2023-080736.
Bretzman JP, Tseng AS, Graff-Radford J, Lee HC, Asirvatham SJ, Mielke MM, Knopman DS, Petersen RC, Jack CR Jr, Vemuri P, et al. Silent cerebral infarcts in patients with atrial fibrillation: clinical implications of an imaging-adjusted CHA2DS2-VASc score. Cardiol J. 2022;29:766–72. https://doi.org/10.5603/CJ.a2022.0055.
Park YJ, Bae MH. Screening and diagnosis of atrial fibrillation using wearable devices. Korean J Intern Med. 2024. https://doi.org/10.3904/kjim.2023.521.
Muller M, Hanssen TA, Johansen D, Jakobsen O, Pedersen JE, Aamot Aksetoy IL, Rasmussen TB, Hartvigsen G, Skogen V, Thrane G. Validity of a smartwatch for detecting atrial fibrillation in patients after heart valve surgery: a prospective observational study. Scand Cardiovasc J. 2024;58:2353069. https://doi.org/10.1080/14017431.2024.2353069.
Henson C, Rambaldini B, Freedman B, Carlson B, Parter C, Christie V, Skinner J, Meharg D, Kirwan M, Ward K, et al. Wearables for early detection of atrial fibrillation and timely referral for Indigenous people >/=55 years: mixed-methods protocol. BMJ Open. 2024;14:e077820. https://doi.org/10.1136/bmjopen-2023-077820.
Adasuriya G, Barsky A, Kralj-Hans I, Mohan S, Gill S, Chen Z, Jarman J, Jones D, Valli H, Gkoutos GV, et al. Remote monitoring of atrial fibrillation recurrence using mHealth technology (REMOTE-AF). Eur Heart J Digit Health. 2024;5:344–55. https://doi.org/10.1093/ehjdh/ztae011.
Chew DS, Li Z, Steinberg BA, O’Brien EC, Pritchard J, Bunch TJ, Mark DB, Patel MR, Nabutovsky Y, Greiner MA, Piccini JP. Arrhythmic burden and the risk of cardiovascular outcomes in patients with paroxysmal atrial fibrillation and cardiac implanted electronic devices. Circ Arrhythm Electrophysiol. 2022;15:e010304. https://doi.org/10.1161/CIRCEP.121.010304.
Chen LY, Chung MK, Allen LA, Ezekowitz M, Furie KL, McCabe P, Noseworthy PA, Perez MV, Turakhia MP, American Heart Association Council on Clinical C, et al. Atrial fibrillation burden: moving beyond atrial fibrillation as a binary entity: a scientific statement from the American Heart Association. Circulation. 2018;137:e623–44. https://doi.org/10.1161/CIR.0000000000000568.
Bashar SK, Han D, Hajeb-Mohammadalipour S, Ding E, Whitcomb C, McManus DD, Chon KH. Atrial fibrillation detection from wrist photoplethysmography signals using smartwatches. Sci Rep. 2019;9:15054. https://doi.org/10.1038/s41598-019-49092-2.
Ott A, Breteler MM, de Bruyne MC, van Harskamp F, Grobbee DE, Hofman A. Atrial fibrillation and dementia in a population-based study. The Rotterdam Study Stroke. 1997;28:316–21. https://doi.org/10.1161/01.str.28.2.316.
Chen YL, Chen J, Wang HT, Chang YT, Chong SZ, Hsueh S, Chung CM, Lin YS. Sex difference in the risk of dementia in patients with atrial fibrillation. Diagnostics (Basel). 2021;11(5):760. https://doi.org/10.3390/diagnostics11050760.
Shao IY, Power MC, Mosley T, Jack C Jr, Gottesman RF, Chen LY, Norby FL, Soliman EZ, Alonso A. Association of atrial fibrillation with white matter disease. Stroke. 2019;50:989–91. https://doi.org/10.1161/STROKEAHA.118.023386.
Schwennesen H, Browndyke JN, Wright MC, Fudim M, Daubert JP, Newman MF, Mathew JP, Piccini JP. A pilot study of longitudinal changes in neurocognition, white matter hyperintensities, and cortical thickness in atrial fibrillation patients following catheter ablation vs medical management. Heart Rhythm. 2024;O2(5):122–30. https://doi.org/10.1016/j.hroo.2024.01.002.
Kawada T. Risk assessment of cerebral microbleeds and white matter hyperintensities in patients with non-valvular atrial fibrillation. J Neurol Sci. 2017;373:249. https://doi.org/10.1016/j.jns.2017.01.017.
de Leeuw FE, de Groot JC, Oudkerk M, Kors JA, Hofman A, van Gijn J, Breteler MM. Atrial fibrillation and the risk of cerebral white matter lesions. Neurology. 2000;54:1795–801. https://doi.org/10.1212/wnl.54.9.1795.
D’Anna L, Filippidis FT, Harvey K, Marinescu M, Bentley P, Korompoki E, Veltkamp R. Extent of white matter lesion is associated with early hemorrhagic transformation in acute ischemic stroke related to atrial fibrillation. Brain Behav. 2021;11:e2250. https://doi.org/10.1002/brb3.2250.
Amberger U, Lippert J, Mujanovic A, Beyeler M, Siepen B, Vynckier J, Scutelnic A, Goeldlin M, Seiffge D, Jung S, et al. Association of chronic covert cerebral infarctions and white matter hyperintensities with atrial fibrillation detection on post-stroke cardiac rhythm monitoring: a cohort study. J Am Heart Assoc. 2022;11:e026962. https://doi.org/10.1161/JAHA.122.026962.
Zito M, Muscari A, Marini E, Di Iorio A, Puddu GM, Abate G. Silent lacunar infarcts in elderly patients with chronic non valvular atrial fibrillation. Aging (Milano). 1996;8:341–6. https://doi.org/10.1007/BF03339591.
Knecht S, Oelschlager C, Duning T, Lohmann H, Albers J, Stehling C, Heindel W, Breithardt G, Berger K, Ringelstein EB, et al. Atrial fibrillation in stroke-free patients is associated with memory impairment and hippocampal atrophy. Eur Heart J. 2008;29:2125–32. https://doi.org/10.1093/eurheartj/ehn341.
Goette A, Braun-Dullaeus RC. Atrial fibrillation is associated with impaired cognitive function and hippocampal atrophy: silent cerebral ischaemia vs. Alzheimer’s disease? Eur Heart J. 2008;29:2067–9. https://doi.org/10.1093/eurheartj/ehn343.
Stefansdottir H, Arnar DO, Aspelund T, Sigurdsson S, Jonsdottir MK, Hjaltason H, Launer LJ, Gudnason V. Atrial fibrillation is associated with reduced brain volume and cognitive function independent of cerebral infarcts. Stroke. 2013;44:1020–5. https://doi.org/10.1161/STROKEAHA.12.679381.
Silva DS, Coan AC, Avelar WM. Neuropsychological and neuroimaging evidences of cerebral dysfunction in stroke-free patients with atrial fibrillation: a review. J Neurol Sci. 2019;399:172–81. https://doi.org/10.1016/j.jns.2019.02.027.
Elias MF, Sullivan LM, Elias PK, Vasan RS, D’Agostino RB Sr, Seshadri S, Au R, Wolf PA, Benjamin EJ. Atrial fibrillation is associated with lower cognitive performance in the Framingham offspring men. J Stroke Cerebrovasc Dis. 2006;15:214–22. https://doi.org/10.1016/j.jstrokecerebrovasdis.2006.05.009.
Ball J, Carrington MJ, Stewart S, SAFETY investigators. Mild cognitive impairment in high-risk patients with chronic atrial fibrillation: a forgotten component of clinical management? Heart. 2013;99(8):542–7. https://doi.org/10.1136/heartjnl-2012-303182.
Seiffge DJ, Cancelloni V, Raber L, Paciaroni M, Metzner A, Kirchhof P, Fischer U, Werring DJ, Shoamanesh A, Caso V. Secondary stroke prevention in people with atrial fibrillation: treatments and trials. Lancet Neurol. 2024;23:404–17. https://doi.org/10.1016/S1474-4422(24)00037-1.
Nam KW, Kwon HM, Lee YS, Won SH, Moon HS, Park JH. Outcomes of non-vitamin K oral anticoagulants for secondary prevention in ischemic stroke with atrial fibrillation. Sci Rep. 2024;14:9838. https://doi.org/10.1038/s41598-024-60660-z.
Goeldlin MB, Hakim A, Branca M, Abend S, Kneihsl M, Valenzuela Pinilla W, Fenzl S, Rezny-Kasprzak B, Rohner R, Strbian D, et al. Early vs late anticoagulation in minor, moderate, and major ischemic stroke with atrial fibrillation: post hoc analysis of the ELAN randomized clinical trial. JAMA Neurol. 2024. https://doi.org/10.1001/jamaneurol.2024.1450.
Bruun-Jensen M, Winther S, Schmidt SE, Moller DC. DARE-ISC model for prediction of 1-year ischaemic stroke risk in the general population and atrial fibrillation patients: a Danish nationwide cohort study. BMJ Open. 2024;14:e076640. https://doi.org/10.1136/bmjopen-2023-076640.
Hachinski V, Einhaupl K, Ganten D, Alladi S, Brayne C, Stephan BCM, Sweeney MD, Zlokovic B, Iturria-Medina Y, Iadecola C, et al. Preventing dementia by preventing stroke: the Berlin Manifesto. Alzheimers Dement. 2019;15:961–84. https://doi.org/10.1016/j.jalz.2019.06.001.
Hudorovic N. Clinical significance of microembolus detection by transcranial Doppler sonography in cardiovascular clinical conditions. Int J Surg. 2006;4:232–41. https://doi.org/10.1016/j.ijsu.2005.12.001.
Anselmino M, Scarsoglio S, Saglietto A, Gaita F, Ridolfi L. Transient cerebral hypoperfusion and hypertensive events during atrial fibrillation: a plausible mechanism for cognitive impairment. Sci Rep. 2016;6:28635. https://doi.org/10.1038/srep28635.
Ungvari Z, Tarantini S, Kiss T, Wren JD, Giles CB, Griffin CT, Murfee WL, Pacher P, Csiszar A. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat Rev Cardiol. 2018;15:555–65. https://doi.org/10.1038/s41569-018-0030-z.
Ungvari Z, Tarantini S, Sorond F, Merkely B, Csiszar A. Mechanisms of vascular aging, a geroscience perspective: JACC focus seminar. J Am Coll Cardiol. 2020;75:931–41. https://doi.org/10.1016/j.jacc.2019.11.061.
Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of vascular aging. Circ Res. 2018;123:849–67. https://doi.org/10.1161/CIRCRESAHA.118.311378.
Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol. 2017;312:H1–20. https://doi.org/10.1152/ajpheart.00581.2016.
Mukai Y. Inflammation and atrial fibrillation. J Arrhythm. 2024;40:26–7. https://doi.org/10.1002/joa3.12984.
Okawa K, Sogo M, Morimoto T, Tsushima R, Sudo Y, Saito E, Ozaki M, Takahashi M. Relationship between endothelial dysfunction and the outcomes after atrial fibrillation ablation. J Am Heart Assoc. 2023;12:e028482. https://doi.org/10.1161/JAHA.122.028482.
Ke HH, Li J, Liang G, Li S, Wang S, Zhong G. Correlation of oxidative stress and vascular endothelial dysfunction with hippocampal perfusion in atrial fibrillation patients with cognitive impairment. SAGE Open Med. 2024;12:20503121241243250. https://doi.org/10.1177/20503121241243247.
Corban MT, Toya T, Ahmad A, Lerman LO, Lee HC, Lerman A. Atrial fibrillation and endothelial dysfunction: a potential link? Mayo Clin Proc. 2021;96:1609–21. https://doi.org/10.1016/j.mayocp.2020.11.005.
Aryal R, Patabendige A. Blood-brain barrier disruption in atrial fibrillation: a potential contributor to the increased risk of dementia and worsening of stroke outcomes? Open Biol. 2021;11:200396. https://doi.org/10.1098/rsob.200396.
Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-brain barrier: from physiology to disease and back. Physiol Rev. 2019;99:21–78. https://doi.org/10.1152/physrev.00050.2017.
Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018;14:133–50. https://doi.org/10.1038/nrneurol.2017.188.
Park SH, Lee SR, Choi EK, Lee H, Chung J, Choi J, Han M, Ahn HJ, Kwon S, Lee SW, et al. Low risk of dementia in patients with newly diagnosed atrial fibrillation and a clustering of healthy lifestyle behaviors: a nationwide population-based cohort study. J Am Heart Assoc. 2022;11:e023739. https://doi.org/10.1161/JAHA.121.023739.
Mant J, Modi RN, Dymond A, Armstrong N, Burt J, Calvert P, Cowie M, Ding WY, Edwards D, Freedman B, et al. Randomised controlled trial of population screening for atrial fibrillation in people aged 70 years and over to reduce stroke: protocol for the SAFER trial. BMJ Open. 2024;14:e082047. https://doi.org/10.1136/bmjopen-2023-082047.
Ford GA, Hargroves D, Lowe D, Hicks N, Lip GYH, Rooney G, Oatley H. Targeted atrial fibrillation detection in COVID-19 vaccination clinics. Eur Heart J Qual Care Clin Outcomes. 2021;7:526–8. https://doi.org/10.1093/ehjqcco/qcab061.
Wendelboe AM, Raskob GE, Angchaisuksiri P, Blanco AN, Buller H, Ddungu H, Dvorak JD, Hunt BJ, Hylek EM, Kakkar A, et al. Global public awareness about atrial fibrillation. Res Pract Thromb Haemost. 2018;2:49–57. https://doi.org/10.1002/rth2.12051.
Reading SR, Go AS, Fang MC, Singer DE, Liu IA, Black MH, Udaltsova N, Reynolds K. Anticoagulation, risk factors in atrial fibrillation-cardiovascular research network I. Health literacy and awareness of atrial fibrillation. J Am Heart Assoc. 2017;6(4):e005128. https://doi.org/10.1161/JAHA.116.005128.
McCabe PJ, Lloyd MPA, Balls-Berry J, Johnson J, LaScotte J, Lee HC. Atrial fibrillation: community screening events improve awareness in older adults. J Gerontol Nurs. 2019;45:31–8. https://doi.org/10.3928/00989134-20190813-04.
Lakkireddy D, Natale A. World atrial fibrillation awareness day: creating grassroots level awareness to combat a global scourge. J Atr Fibrillation. 2016;9:1482. https://doi.org/10.4022/jafib.1482.
Briggs R, Drumm B, Dwyer R, O’Neill D, Kennelly SP, Coughlan T, Collins R. Awareness of atrial fibrillation-effectiveness of a pilot national awareness campaign. Ir J Med Sci. 2020;189:149–53. https://doi.org/10.1007/s11845-019-02049-w.
Akiyama H, Hasegawa Y. Awareness of atrial fibrillation in Japan: a large-scale, nationwide Internet survey of 50 000 Japanese adults. Geriatr Gerontol Int. 2018;18:1100–7. https://doi.org/10.1111/ggi.13318.
Virtanen R, Kryssi V, Vasankari T, Salminen M, Kivela SL, Airaksinen KE. Self-detection of atrial fibrillation in an aged population: the LietoAF study. Eur J Prev Cardiol. 2014;21:1437–42. https://doi.org/10.1177/2047487313494041.
Jaakkola J, Virtanen R, Vasankari T, Salminen M, Airaksinen KEJ. Self-detection of atrial fibrillation in an aged population: three-year follow-up of the LietoAF intervention study. BMC Geriatr. 2017;17:218. https://doi.org/10.1186/s12877-017-0607-0.
Wong KC, Kok C, Marschner S, Usherwood T, Chow CK. Screening for atrial fibrillation and other arrhythmias in primary care. BMC Fam Pract. 2020;21:79. https://doi.org/10.1186/s12875-020-01151-8.
Theunissen L, Abdalrahim R, Dekker LRC, Thijssen EJM, de Jong S, Polak PE, van de Voort PH, Smits G, Scheele K, Lucas A, et al. Regional implementation of atrial fibrillation screening: benefits and pitfalls. Eur Heart J Digit Health. 2022;3:570–7. https://doi.org/10.1093/ehjdh/ztac055.
Suzuki A, Okamura T, Sasaki M, Matsuoka H, Ikeda Y, Takahashi A, Akiyama S, Ono F, Yoshihara N, Akita Study Group. Acceleration of opportunistic atrial fibrillation screening for elderly patients in routine primary care. PLoS One. 2020;15:e0244240. https://doi.org/10.1371/journal.pone.0244240.
Callanan A, Bayat F, Quinlan D, Kearney PM, Buckley CM, Smith SM, Bradley CP. Facilitators and barriers to atrial fibrillation screening in primary care: a qualitative descriptive study of GPs in primary care in the Republic of Ireland. BJGP Open. 2023;7. https://doi.org/10.3399/BJGPO.2022.0110
Qvist I, Lane DA, Risom SS, Hendriks JM, Hojen AA, Johnsen SP, Frost L. Implementation of patient education for patients with atrial fibrillation: nationwide cross-sectional survey and one-year follow-up. Eur J Cardiovasc Nurs. 2024;23:251–7. https://doi.org/10.1093/eurjcn/zvad066.
Mihas P, Rosman L, Armbruster T, Walker J, Deyo Z, Gehi A. Assessing a virtual education intervention for patients with atrial fibrillation: a qualitative study of patient perceptions. J Cardiovasc Nurs. 2024;39:E1–11. https://doi.org/10.1097/JCN.0000000000000984.
Luo C, Bian L, Jiang L, Liang W, Wu Z. Does YouTube provide qualified patient education videos about atrial fibrillation? Front Public Health. 2022;10:925691. https://doi.org/10.3389/fpubh.2022.925691.
Gagne M, Legault C, Boulet LP, Charbonneau L, Lemyre M, Giguere AMC, Poirier P. Impact of adding a video to patient education on quality of life among adults with atrial fibrillation: a randomized controlled trial. Patient Educ Couns. 2019;102:1490–8. https://doi.org/10.1016/j.pec.2019.03.015.
Antoniou P, Dafli E, Giannakoulas G, Igimbayeva G, Visternichan O, Kyselov S, Lykhasenko I, Lashkul D, Nadareishvili I, Tabagari S, Bamidis PD. Education of patients with atrial fibrillation and evaluation of the efficacy of a mobile virtual patient environment: protocol for a multicenter pseudorandomized controlled trial. JMIR Res Protoc. 2024;13:e45946. https://doi.org/10.2196/45946.
Ding EY, Tran KV, Lessard D, Wang Z, Han D, Mohagheghian F, Mensah Otabil E, Noorishirazi K, Mehawej J, Filippaios A, et al. Accuracy, usability, and adherence of smartwatches for atrial fibrillation detection in older adults after stroke: randomized controlled trial. JMIR Cardio. 2023;7:e45137. https://doi.org/10.2196/45137.
Dorr M, Nohturfft V, Brasier N, Bosshard E, Djurdjevic A, Gross S, Raichle CJ, Rhinisperger M, Stockli R, Eckstein J. The WATCH AF trial: SmartWATCHes for detection of atrial fibrillation. JACC Clin Electrophysiol. 2019;5:199–208. https://doi.org/10.1016/j.jacep.2018.10.006.
Liao MT, Yu CC, Lin LY, Pan KH, Tsai TH, Wu YC, Liu YB. Impact of recording length and other arrhythmias on atrial fibrillation detection from wrist photoplethysmogram using smartwatches. Sci Rep. 2022;12:5364. https://doi.org/10.1038/s41598-022-09181-1.
Mela T. Smartwatches in the fight against atrial fibrillation: the little watch that could. J Am Coll Cardiol. 2018;71:2389–91. https://doi.org/10.1016/j.jacc.2018.03.485.
Nuvvula S, Ding EY, Saleeba C, Shi Q, Wang Z, Kapoor A, Saczynski JS, Lubitz SA, Kovell LC, McKee MD, McManus DD. NExUS-Heart: novel examinations using smart technologies for heart health-data sharing from commercial wearable devices and telehealth engagement in participants with or at risk of atrial fibrillation. Cardiovasc Digit Health J. 2021;2:256–63. https://doi.org/10.1016/j.cvdhj.2021.08.001.
Klingenheben T, Israel CW. Use of telemedicine in the diagnosis of paroxysmal atrial fibrillation and to monitor the effect of antiarrhythmic drug therapy. Herzschrittmacherther Elektrophysiol. 2006;17:225–8. https://doi.org/10.1007/s00399-006-0539-4.
Buck BH, Hill MD, Quinn FR, Butcher KS, Menon BK, Gulamhusein S, Siddiqui M, Coutts SB, Jeerakathil T, Smith EE, et al. Effect of implantable vs prolonged external electrocardiographic monitoring on atrial fibrillation detection in patients with ischemic stroke: the PER DIEM randomized clinical trial. JAMA. 2021;325:2160–8. https://doi.org/10.1001/jama.2021.6128.
Sung SF, Yang HY, Tsai CF, Fang CW. Comparing the risk of dementia in patients with atrial fibrillation taking different oral anticoagulants. Eur J Intern Med. 2024;122:139–41. https://doi.org/10.1016/j.ejim.2024.02.008.
Latif F, Nasir MM, Meer KK, Farhan SH, Cheema HA, Khan AB, Umer M, Rehman WU, Ahmad A, Khan MA, et al. The effect of oral anticoagulants on the incidence of dementia in patients with atrial fibrillation: a systematic review and meta-analysis. Int J Cardiol Cardiovasc Risk Prev. 2024;21:200282. https://doi.org/10.1016/j.ijcrp.2024.200282.
Carbone G, Ercolano E, Bencivenga L, Palaia ME, Scognamiglio F, Rengo G, Femminella GD. Atrial fibrillation and dementia: focus on shared pathophysiological mechanisms and therapeutic implications. J Am Med Dir Assoc. 2024;25:465–9. https://doi.org/10.1016/j.jamda.2024.01.010.
Akerstrom F, Charitakis E, Paul-Nordin A, Braunschweig F, Friberg L, Tabrizi F, Jensen-Urstad M, Drca N. Reduced dementia risk in patients with optimized anticoagulation therapy undergoing atrial fibrillation ablation. Heart Rhythm. 2024. https://doi.org/10.1016/j.hrthm.2024.04.038.
Grymonprez M, Petrovic M, De Backer TL, Ikram MA, Steurbaut S, Lahousse L. Comparing the risk of dementia in subjects with atrial fibrillation using non-vitamin K antagonist oral anticoagulants versus vitamin K antagonists: a Belgian nationwide cohort study. Age Ageing. 2023;52(3):afad038. https://doi.org/10.1093/ageing/afad038.
Pendlebury ST. Direct oral anticoagulants and prevention of dementia in nonvalvular atrial fibrillation. Stroke. 2021;52:3469–71. https://doi.org/10.1161/STROKEAHA.121.035664.
Lee SR, Choi EK, Park SH, Jung JH, Han KD, Oh S, Lip GYH. Comparing warfarin and 4 direct oral anticoagulants for the risk of dementia in patients with atrial fibrillation. Stroke. 2021;52:3459–68. https://doi.org/10.1161/STROKEAHA.120.033338.
Connolly SJ, Healey JS, Belley-Cote EP, Balasubramanian K, Paparella D, Brady K, Reents W, Danner BC, Devereaux PJ, Sharma M, et al. Oral anticoagulation use and left atrial appendage occlusion in LAAOS III. Circulation. 2023;148:1298–304. https://doi.org/10.1161/CIRCULATIONAHA.122.060315.
Blanc C, Blanc G, Boveda S, Calviere L, Combes N, Viguier A, Mondoly P, Albucher JF, Gollion C, Fabry V, et al. Left atrial appendage closure in patients with atrial fibrillation and coexisting cerebral amyloid angiopathy. Stroke. 2021;52:e792–3. https://doi.org/10.1161/STROKEAHA.121.037248.
Schrag M, Mac Grory B, Nackenoff A, Eaton J, Mistry E, Kirshner H, Yaghi S, Ellis CR. Left atrial appendage closure for patients with cerebral amyloid angiopathy and atrial fibrillation: the LAA-CAA cohort. Transl Stroke Res. 2021;12:259–65. https://doi.org/10.1007/s12975-020-00838-5.
Kelly J. New horizons: managing antithrombotic dilemmas in patients with cerebral amyloid angiopathy. Age Ageing. 2021;50:347–55. https://doi.org/10.1093/ageing/afaa275.
Boyle PA, Yu L, Nag S, Leurgans S, Wilson RS, Bennett DA, Schneider JA. Cerebral amyloid angiopathy and cognitive outcomes in community-based older persons. Neurology. 2015;85:1930–6. https://doi.org/10.1212/WNL.0000000000002175.
Koemans EA, Chhatwal JP, van Veluw SJ, van Etten ES, van Osch MJP, van Walderveen MAA, Sohrabi HR, Kozberg MG, Shirzadi Z, Terwindt GM, et al. Progression of cerebral amyloid angiopathy: a pathophysiological framework. Lancet Neurol. 2023;22:632–42. https://doi.org/10.1016/S1474-4422(23)00114-X.
Lee SR, Choi EK, Lee SW, Han KD, Oh S, Lip GY. Early rhythm control and incident dementia in patients with atrial fibrillation and prior stroke. JACC: Clin Electrophysiol. 2024.https://doi.org/10.1016/j.jacep.2024.03.007.
Guo J, Liu Y, Jia J, Lu J, Wang D, Zhang J, Ding J, Zhao X. Effects of rhythm-control and rate-control strategies on cognitive function and dementia in atrial fibrillation: a systematic review and meta-analysis. Age Ageing. 2024;53(2):afae009. https://doi.org/10.1093/ageing/afae009.
Saka E, Topcuoglu MA. Rhythm control and dementia in patients with atrial fibrillation: a role for glymphatic system? Can J Cardiol. 2023;39:198. https://doi.org/10.1016/j.cjca.2022.11.016.
Cosedis Nielsen J, Johannessen A, Raatikainen P, Hindricks G, Walfridsson H, Kongstad O, Pehrson S, Englund A, Hartikainen J, Mortensen LS, Hansen PS. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med. 2012;367:1587–95. https://doi.org/10.1056/NEJMoa1113566.
De Becker B, Van de Borne P. Treatment of hypertension to prevent atrial fibrillation. Curr Pharm Des. 2018;24:4397–403. https://doi.org/10.2174/1381612825666181127100437.
Gumprecht J, Lip GYH, Sokal A, Sredniawa B, Stokwiszewski J, Zdrojewski T, Rutkowski M, Grodzicki T, Kazmierczak J, Opolski G, Kalarus Z. Impact of diabetes mellitus severity, treatment regimen and glycaemic control on atrial fibrillation prevalence in the Polish population aged >/= 65. Sci Rep. 2023;13:17252. https://doi.org/10.1038/s41598-023-43939-5.
Alwafi H, Wong ICK, Banerjee A, Mongkhon P, Whittlesea C, Naser AY, Lau WCY, Wei L. Epidemiology and treatment of atrial fibrillation in patients with type 2 diabetes in the UK, 2001–2016. Sci Rep. 2020;10:12468. https://doi.org/10.1038/s41598-020-69492-z.
Valls-Pedret C, Lamuela-Raventos RM, Medina-Remon A, Quintana M, Corella D, Pinto X, Martinez-Gonzalez MA, Estruch R, Ros E. Polyphenol-rich foods in the Mediterranean diet are associated with better cognitive function in elderly subjects at high cardiovascular risk. J Alzheimers Dis. 2012;29:773–82. https://doi.org/10.3233/JAD-2012-111799W012188621153H61[pii].
Maggi S, Ticinesi A, Limongi F, Noale M, Ecarnot F. The role of nutrition and the Mediterranean diet on the trajectories of cognitive decline. Exp Gerontol. 2023;173:112110. https://doi.org/10.1016/j.exger.2023.112110.
Ungvari Z, Fazekas-Pongor V, Csiszar A, Kunutsor SK. The multifaceted benefits of walking for healthy aging: from Blue Zones to molecular mechanisms. Geroscience. 2023;45(6):3211–39. https://doi.org/10.1007/s11357-023-00873-8.
Molnar T, Szabo Z, Bartha E, Illes Z. “Cerebrovascular stressing”: dipyridamole-induced S100B elevation predicts ischemic cerebrovascular events. Clin Chem Lab Med. 2013;51:e69-72. https://doi.org/10.1515/cclm-2012-0337.
Molnar T, Horvath A, Szabo Z, Vamos Z, Doczi T, Illes Z. Detection of silent cerebral microcirculatory abnormalities in patients with manifest ischemic coronary disease: a perfusion brain MRI study combined with dipyridamole stress. Scand Cardiovasc J. 2021;55:97–101. https://doi.org/10.1080/14017431.2020.1821911.
Prodan CI, Batra A, Ungvari Z, Liotta EM. Stringent public health measures during COVID-19 across ischemic stroke care systems: the potential impact of patient perceptions on health care-seeking behaviors. Geroscience. 2022;44:1255–62. https://doi.org/10.1007/s11357-022-00566-8.
Van Dusen RA, Abernethy K, Chaudhary N, Paudyal V, Kurmi O. Association of the COVID-19 pandemic on stroke admissions and treatment globally: a systematic review. BMJ Open. 2023;13:e062734. https://doi.org/10.1136/bmjopen-2022-062734.
de Oliveira LC, Ponciano A, Kashani N, Guarda SNF, Hill MD, Smith EE, Stang JM, Viswanathan A, Turner AC, Ganesh A. Stroke metrics during the first year of the COVID-19 pandemic, a tale of two comprehensive stroke centers. Sci Rep. 2023;13:17171. https://doi.org/10.1038/s41598-023-44277-2.
Bojti PP, Szilagyi G, Dobi B, Stang R, Szikora I, Kis B, Kornfeld A, Ovary C, Eross L, Banczerowski P, et al. Impact of COVID-19 on ischemic stroke care in Hungary. Geroscience. 2021;43:2231–48. https://doi.org/10.1007/s11357-021-00424-z.
Zheng F, Liang J, Li C, Gao D, Xie W. Cognitive decline among older adults with depressive symptoms before and during the COVID-19 pandemic. J Affect Disord. 2024;344:407–13. https://doi.org/10.1016/j.jad.2023.10.051.
Matsui T, Mitsuma S, Nagata A, Matsushita S, Asahi T. Accelerated cognitive decline after the COVID-19 pandemic in a community population of older persons with cognitive impairment: a 4-year time series analysis in the Tokyo Metropolis area. Geriatr Gerontol Int. 2023;23:200–4. https://doi.org/10.1111/ggi.14543.
Jung J, Kim S, Kim B, Kim M, Yang J, Chung D, Won C. Accelerated cognitive function decline in community-dwelling older adults during COVID-19 pandemic: the Korean frailty and aging cohort study (KFACS). Int J Environ Res Public Health. 2022;19(17):10666. https://doi.org/10.3390/ijerph191710666.
Corbett A, Williams G, Creese B, Hampshire A, Hayman V, Palmer A, Filakovzsky A, Mills K, Cummings J, Aarsland D, et al. Cognitive decline in older adults in the UK during and after the COVID-19 pandemic: a longitudinal analysis of PROTECT study data. Lancet Healthy Longev. 2023;4:e591–9. https://doi.org/10.1016/S2666-7568(23)00187-3.
Zaheer K, Goncalves B, Ramalingam A, Rabbani NUA, Sayyed R, Nawab A, Puri R, Williams CJ, Mansoor K. Association of new-onset atrial fibrillation with all-cause mortality in COVID-19 patients. Cureus. 2023;15:e49785. https://doi.org/10.7759/cureus.49785.
Talaei F, Banga A, Pursell A, Gage A, Pallipamu N, Seri AR, Adhikari R, Kashyap R, Surani S. New-onset atrial fibrillation among COVID-19 patients: a narrative review. World J Crit Care Med. 2023;12:236–47. https://doi.org/10.5492/wjccm.v12.i5.236.
Molnar T, Lehoczki A, Fekete M, Varnai R, Zavori L, Erdo-Bonyar S, Simon D, Berki T, Csecsei P, Ezer E. Mitochondrial dysfunction in long COVID: mechanisms, consequences, and potential therapeutic approaches. Geroscience. 2024. https://doi.org/10.1007/s11357-024-01165-5.
Russell SJ, Parker K, Lehoczki A, Lieberman D, Partha IS, Scott SJ, Phillips LR, Fain MJ, Nikolich JZ. Post-acute sequelae of SARS-CoV-2 infection (Long COVID) in older adults. Geroscience. 2024. https://doi.org/10.1007/s11357-024-01227-8.
Achleitner M, Steenblock C, Danhardt J, Jarzebska N, Kardashi R, Kanczkowski W, Straube R, Rodionov RN, Bornstein N, Tselmin S, et al. Clinical improvement of Long-COVID is associated with reduction in autoantibodies, lipids, and inflammation following therapeutic apheresis. Mol Psychiatry. 2023;28:2872–7. https://doi.org/10.1038/s41380-023-02084-1.
Greene C, Connolly R, Brennan D, Laffan A, O’Keeffe E, Zaporojan L, O’Callaghan J, Thomson B, Connolly E, Argue R, et al. Blood-brain barrier disruption and sustained systemic inflammation in individuals with long COVID-associated cognitive impairment. Nat Neurosci. 2024. https://doi.org/10.1038/s41593-024-01576-9.
Woodruff MC, Bonham KS, Anam FA, Walker TA, Faliti CE, Ishii Y, Kaminski CY, Ruunstrom MC, Cooper KR, Truong AD, et al. Chronic inflammation, neutrophil activity, and autoreactivity splits long COVID. Nat Commun. 2023;14:4201. https://doi.org/10.1038/s41467-023-40012-7.
Fagyas M, Nagy B Jr, Ráduly AP, Mányiné IS, Mártha L, Erdősi G, Sipka S Jr, Enyedi E, Szabó AÁ, Pólik Z, Kappelmayer J. The majority of severe COVID-19 patients develop anti-cardiac autoantibodies. Geroscience. 2022;44(5):2347–60. https://doi.org/10.1007/s11357-022-00649-6.
Chang X, Ismail NI, Rahman A, Xu D, Chan RWY, Ong SG, Ong SB. Long COVID-19 and the heart: is cardiac mitochondria the missing link? Antioxid Redox Signal. 2023;38:599–618. https://doi.org/10.1089/ars.2022.0126.
Liu J, Deswal A, Khalid U. COVID-19 myocarditis and long-term heart failure sequelae. Curr Opin Cardiol. 2021;36:234–40. https://doi.org/10.1097/HCO.0000000000000832.
Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583–90. https://doi.org/10.1038/s41591-022-01689-3.
Prasad M, Leon M, Lerman LO, Lerman A. Viral endothelial dysfunction: a unifying mechanism for COVID-19. Mayo Clin Proc. 2021;96:3099–108. https://doi.org/10.1016/j.mayocp.2021.06.027.
Greenberg A, Pemmasani G, Yandrapalli S, Frishman WH. Cardiovascular and cerebrovascular complications with COVID-19. Cardiol Rev. 2021;29:143–9. https://doi.org/10.1097/CRD.0000000000000385.
McMaster MW, Dey S, Fishkin T, Wang A, Frishman WH, Aronow WS. The impact of long COVID-19 on the cardiovascular system. Cardiol Rev. 2024. https://doi.org/10.1097/CRD.0000000000000654.
McLaughlin M, Sanal-Hayes NEM, Hayes LD, Berry EC, Sculthorpe NF. People with long COVID and myalgic encephalomyelitis/chronic fatigue syndrome exhibit similarly impaired vascular function. Am J Med. 2023. https://doi.org/10.1016/j.amjmed.2023.09.013.
Osiaevi I, Schulze A, Evers G, Harmening K, Vink H, Kumpers P, Mohr M, Rovas A. Persistent capillary rarefication in long COVID syndrome. Angiogenesis. 2023;26:53–61. https://doi.org/10.1007/s10456-022-09850-9.
Vassiliou AG, Vrettou CS, Keskinidou C, Dimopoulou I, Kotanidou A, Orfanos SE. Endotheliopathy in acute COVID-19 and long COVID. Int J Mol Sci. 2023;24(9):8237. https://doi.org/10.3390/ijms24098237.
Fogarty H, Townsend L, Morrin H, Ahmad A, Comerford C, Karampini E, Englert H, Byrne M, Bergin C, O’Sullivan JM, et al. Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J Thromb Haemost. 2021;19:2546–53. https://doi.org/10.1111/jth.15490.
Charfeddine S, Ibn Hadj Amor H, Jdidi J, Torjmen S, Kraiem S, Hammami R, Bahloul A, Kallel N, Moussa N, Touil I, et al. Long COVID 19 syndrome: is it related to microcirculation and endothelial dysfunction? Insights from TUN-EndCOV study. Front Cardiovasc Med. 2021;8:745758. https://doi.org/10.3389/fcvm.2021.745758.
Kumar A, Shariff M, Bhat V, DeSimone C, Deshmukh A. Atrial fibrillation after vaccination for COVID-19: analysis of the vaccine adverse event reporting system. J Interv Card Electrophysiol. 2022;65:1–2. https://doi.org/10.1007/s10840-022-01263-4.
Pari B, Babbili A, Kattubadi A, Thakre A, Thotamgari S, Gopinathannair R, Olshansky B, Dominic P. COVID-19 vaccination and cardiac arrhythmias: a review. Curr Cardiol Rep. 2023;25:925–40. https://doi.org/10.1007/s11886-023-01921-7.
Acknowledgements
Project no. TKP2021-NKTA-47, implemented with the support provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-NKTA funding scheme; by funding through the National Cardiovascular Laboratory Program (RRF-2.3.1-21-2022-00003) provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund; Project no. 135784 implemented with the support provided from the National Research, Development and Innovation Fund of Hungary, financed under the K_20 funding scheme and the European University for Well-Being (EUniWell) program (grant agreement number: 101004093/ EUniWell/EAC-A02-2019 / EAC-A02-2019-1). The funding sources had no role in the writing of the manuscript; and in the decision to submit the article for publication. The 4.0 version of ChatGPT, developed by OpenAI, was used as a tool to refine our writing and enhancing the clarity of our work.
Funding
Open access funding provided by Semmelweis University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr. Eric Liotta serves as Associate Editor for GeroScience. All other authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Fekete, M., Liotta, E.M., Molnar, T. et al. The role of atrial fibrillation in vascular cognitive impairment and dementia: epidemiology, pathophysiology, and preventive strategies. GeroScience (2024). https://doi.org/10.1007/s11357-024-01290-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11357-024-01290-1