Abstract
Age-related deterioration of condylar cartilage is an etiological factor in temporomandibular joint-osteoarthritis (TMJ-OA). However, its underlying mechanism remains unknown. Therefore, we examined age-related changes and the relationship between mTOR signaling and primary cilia in condylar cartilage to determine the intrinsic mechanisms of age-related TMJ-OA. Age-related morphological changes were analyzed using micro-computed tomography and safranin O-stained histological samples of the mandibular condyle of C57BL/6J mice (up to 78 weeks old). Immunohistochemistry was used to assess the activity of mTOR signaling, primary cilia frequency, and Golgi size of condylar chondrocytes. Four-week-old mice receiving an 11-week series of intraperitoneal injections of rapamycin, a potent mTOR signaling inhibitor, were used for the histological evaluation of the condylar cartilage. The condylar cartilage demonstrated an age-related reduction in cartilage area, including chondrocyte size, cell density, and cell size distribution. The Golgi size, primary cilia frequency, and mTOR signaling also decreased with age. Rapamycin injections resulted in both diminished cartilage area and cell size, resembling the phenotypes observed in aged mice. Rapamycin-injected mice also exhibited a smaller Golgi size and lower primary cilia frequency in condylar cartilage. We demonstrated that a loss of primary cilia due to a decline in mTOR signaling was correlated with age-related deteriorations in condylar cartilage. Our findings provide new insights into the tissue homeostasis of condylar cartilage, contributing to understanding the etiology of age-related TMJ-OA.






Similar content being viewed by others
Abbreviations
- TMJ:
-
Temporomandibular joint
- OA:
-
Osteoarthritis
- ECM:
-
Extracellular matrix
- IFT:
-
Intraflagellar transport
- mTOR:
-
Mechanistic/mammalian target of Rapamycin
- pS6:
-
Phosphorylated ribosomal protein S6 kinase
References
Glyn-Jones S, Palmer AJ, Agricola R, Price AJ, Vincent TL, Weinans H, Carr AJ. Osteoarthr Lancet. 2015;386:376–87. https://doi.org/10.1016/S0140-6736(14)60802-3.
Al-Ani Z. Temporomandibular joint osteoarthrosis: a review of clinical aspects and management. Prim Dent J. 2021;10:132–40. https://doi.org/10.1177/2050168420980977.
Kelemen K, Konig J, Czumbel M, Szabo B, Hegyi P, Gerber G, Borbely J, Mikulas K, Schmidt P, Hermann P. Additional splint therapy has no superiority in myogenic temporomandibular disorders: a systematic review and meta-analysis of randomized controlled trials. J Prosthodont Res. 2024;68:12–9. https://doi.org/10.2186/jpr.JPR_D_22_00264.
Zhou Y, Al-Naggar IMA, Chen PJ, Gasek NS, Wang K, Mehta S, Kuchel GA, Yadav S, Xu M. Senolytics alleviate the degenerative disorders of temporomandibular joint in old age. Aging Cell. 2021;20:e13394. https://doi.org/10.1111/acel.13394.
Pereira FJ Jr, Lundh H, Westesson PL. Morphologic changes in the temporomandibular joint in different age groups. An autopsy investigation. Oral Surg Oral Med Oral Pathol. 1994;78:279–87.
Chen PJ, Dutra EH, Mehta S, O’Brien MH, Yadav S. Age-related changes in the cartilage of the temporomandibular joint. Geroscience. 2020;42:995–1004. https://doi.org/10.1007/s11357-020-00160-w.
Delpachitra SN, Dimitroulis G. Osteoarthritis of the temporomandibular joint: a review of aetiology and pathogenesis. Br J Oral Maxillofac Surg. 2022;60:387–96. https://doi.org/10.1016/j.bjoms.2021.06.017.
Ootake T, Ishii T, Sueishi K, Watanabe A, Ishizuka Y, Amano K, Nagao M, Nishimura K, Nishii Y. Effects of mechanical stress and deficiency of dihydrotestosterone or 17β-estradiol on temporomandibular joint osteoarthritis in mice. Osteoarthritis Cartilage. 2021;29:1575–89. https://doi.org/10.1016/j.joca.2021.08.005.
Sangani D, Suzuki A, VonVille H, Hixson JE, Iwata J. Gene mutations associated with temporomandibular joint disorders: A systematic review. OAlib. 2015;2:e1583. https://doi.org/10.4236/oalib.1101583.
Lai Y, Zheng W, Qu M, Xiao CC, Chen S, Yao Q, Gong W, Tao C, Yan Q, Zhang P, Wu X, Xiao G. Kindlin-2 loss in condylar chondrocytes causes spontaneous osteoarthritic lesions in the temporomandibular joint in mice. Int J Oral Sci. 2022;14:33. https://doi.org/10.1038/s41368-022-00185-1.
Nakao Y, Konno-Nagasaka M, Toriya N, Arakawa T, Kashio H, Takuma T, Mizoguchi I. Proteoglycan expression is influenced by mechanical load in TMJ discs. J Dent Res. 2015;94:93–100. https://doi.org/10.1177/0022034514553816.
Knudson CB, Knudson W. Cartilage proteoglycans. Semin Cell Dev Biol. 2001;12:69–78. https://doi.org/10.1006/scdb.2000.0243.
Livne E. Matrix synthesis in mandibular condylar cartilage of aging mice. Osteoarthr Cartil. 1994;2:187–97. https://doi.org/10.1016/s1063-4584(05)80068-8.
Yang MC, Nakamura M, Kageyama Y, Igari Y, Sasano Y. Age-related gene and protein expression in mouse mandibular condyle analyzed by cap analysis of gene expression and immunohistochemistry. Gerontology. 2023;69:1295–306. https://doi.org/10.1159/000533921.
Elshawi A, Wakamatsu N, Iinuma M, Nagayama M, Tamura Y. TMJ degenerative changes in SAMP3 mice by occlusal disharmony and aging. J Hard Tissue Biol. 2012;2012(21):399–406. https://doi.org/10.2485/jhtb.21.399.
Ishizuka Y, Shibukawa Y, Nagayama M, Decker R, Kinumatsu T, Saito A, Pacifici M, Koyama E. TMJ degeneration in SAMP8 mice is accompanied by deranged Ihh signaling. J Dent Res. 2014;2014(93):281–7. https://doi.org/10.1177/0022034513519649.
Cardoneanu A, Macovei LA, Burlui AM, Mihai IR, Bratoiu I, Rezus II, Richter P, Tamba BI, Rezus E. Temporomandibular joint osteoarthritis: Pathogenic mechanisms involving the cartilage and subchondral bone, and potential therapeutic strategies for joint regeneration. Int J Mol Sci. 2022;24:171. https://doi.org/10.3390/ijms24010171.
Shi Y, Hu X, Cheng J, Zhang X, Zhao F, Shi W, Ren B, Yu H, Yang P, Li Z, Duan X, Fu X, Zhang J, Wang J, Ao Y. A small molecule promotes cartilage extracellular matrix generation and inhibits osteoarthritis development. Nat Commun. 2019;10:1914. https://doi.org/10.1038/s41467-019-09839-x.
Chen HY, Kelley RA, Li T, Swaroop A. Primary cilia biogenesis and associated retinal ciliopathies. Semin Cell Dev Biol. 2021;110:70–88. https://doi.org/10.1016/j.semcdb.2020.07.013.
Noda K, Kitami M, Kitami K, Kaku M, Komatsu Y. Canonical and noncanonical intraflagellar transport regulates craniofacial skeletal development. Proc Natl Acad Sci U S A. 2016;113:E2589–97. https://doi.org/10.1073/pnas.1519458113.
Berbari NF, O’Connor AK, Haycraft CJ, Yoder BK. The primary cilium as a complex signaling center. Curr Biol. 2009;19:R526–35. https://doi.org/10.1016/j.cub.2009.05.025.
Kaku M, Komatsu Y. Functional diversity of ciliary proteins in bone development and disease. Curr Osteoporos Rep. 2017;15:96–102. https://doi.org/10.1007/s11914-017-0351-6.
Chinipardaz Z, Liu M, Graves DT, Yang S. Role of primary cilia in bone and cartilage. J Dent Res. 2022;101:253–60. https://doi.org/10.1177/00220345211046606.
Arseni L, Lombardi A, Orioli D. From structure to phenotype: Impact of collagen alterations on human health. Int J Mol Sci. 2018;19:1407. https://doi.org/10.3390/ijms19051407.
Collins I, Wann AKT. Regulation of the extracellular matrix by ciliary machinery. Cells. 2020;9:278. https://doi.org/10.3390/cells9020278.
Yuan X, Yang S. Deletion of ift80 impairs epiphyseal and articular cartilage formation due to disruption of chondrocyte differentiation. PLoS ONE. 2015;10:e0130618.
McGlashan SR, Haycraft CJ, Jensen CG, Yoder BK, Poole CA. Articular cartilage and growth plate defects are associated with chondrocyte cytoskeletal abnormalities in Tg737orpk mice lacking the primary cilia protein polaris. Matrix Biol. 2007;26:234–46. https://doi.org/10.1016/j.matbio.2006.12.003.
Kitami M, Yamaguchi H, Ebina M, Kaku M, Chen D, Komatsu Y. IFT20 is required for the maintenance of cartilaginous matrix in condylar cartilage. Biochem Biophys Res Commun. 2019;509:222–6. https://doi.org/10.1016/j.bbrc.2018.12.107.
Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. https://doi.org/10.1016/j.cell.2006.01.016.
Lai Y, Jiang Y. Reciprocal regulation between primary cilia and mTORC1. Genes (Basel). 2020;11:711. https://doi.org/10.3390/genes11060711.
DiBella LM, Park A, Sun Z. Zebrafish TSC1 reveals functional interactions between the cilium and the TOR pathway. Hum Mol Genet. 2009;18:595–606. https://doi.org/10.1093/hmg/ddn384.
Rosengren T, Larsen LJ, Pedersen LB, Christensen ST, Moller LB. TSC21 and TSC2 regulate cilia length and canonical hedgehog signaling via different mechanisms. Cell Mol Life Sci. 2018;75:2663–80. https://doi.org/10.1007/s00018-018-2761-8.
Yuan S, Li J, Diener DR, Choma MA, Rosenbaum JL, Sun Z. Target-of-rapamycin complex 1 (Torc1) signaling modulates cilia size and function through protein synthesis regulation. Proc Natl Acad Sci U S A. 2012;109:2021–6. https://doi.org/10.1073/pnas.1112834109.
Hartman TR, Liu D, Zilfou JT, Robb V, Morrison T, Watnick T, Henske EP. The tuberous sclerosis proteins regulate formation of the primary cilium via a rapamycin-insensitive and polycystin 1-independent pathway. Hum Mol Genet. 2009;18:151–63. https://doi.org/10.1093/hmg/ddn325.
Yan B, Zhang Z, Jin D, Cai C, Jia C, Liu W, Wang T, Li S, Zhang H, Huang B, Lai P, Wang H, Liu A, Zeng C, Cai D, Jiang Y, Bai X. mTORC1 regulates PTHrP to coordinate chondrocyte growth, proliferation and differentiation. Nat Commun. 7:11151. https://doi.org/10.1038/ncomms11151.
Charan J, Kantharia ND. How to calculate sample size in animal studies? J Pharmacol Pharmacother. 2013;4:303–6. https://doi.org/10.4103/0976-500X.119726.
Zhao S, Chen C, Wang S, Ji F, Xie Y. Mhy1485 activates mTOR and protects osteoblasts from dexamethasone. Biochem Biophys Res Commun. 2016;481:212–8. https://doi.org/10.1016/j.bbrc.2016.10.104.
Yang J, Kitami M, Pan H, Nakamura MT, Zhang H, Liu F, Zhu L, Komatsu Y, Mishina Y. Augmented BMP signaling commits cranial neural crest cells to a chondrogenic fate by suppressing autophagic β-catenin degradation. Sci Signal. 2021;14:eaaz9368. https://doi.org/10.1126/scisignal.aaz9368
Ramos FJ, Chen SC, Garelick MG, Dai DF, Liao CY, Schreiber KH, MacKay VL, An EH, Strong R, Ladiges WC, Rabinovitch PS, Kaeberlein M, Kennedy BK. Rapamycin reverses elevated mTORC1 signaling in lamin A/C-deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci Transl Med. 2012;4:144ra103. https://doi.org/10.1126/scitranslmed.3003802
Bitto A, Ito TK, Pineda VV, LeTexier NJ, Huang HZ, Sutlief E, Tung H, Vizzini N, Chen B, Smith K, Meza D, Yajima M, Beyer RP, Kerr KF, Davis DJ, Gillespie CH, Snyder JM, Treuting PM, Kaeberlein M. Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. Elife. 2016;5:e16351. https://doi.org/10.7554/eLife.16351.
Cameron DA. The Golgi apparatus in bone and cartilage cells. Clin Orthop Relat Res. 1968;58:191–211.
Kinumatsu T, Shibukawa Y, Yasuda T, Nagayama M, Yamada S, Serra R, Pacifici M, Koyama E. TMJ development and growth require primary cilia function. J Dent Res. 2011;90:988–94. https://doi.org/10.1177/0022034511409407.
Sherpa RT, Atkinson KF, Ferreira VP, Nauli SM. Rapamycin increases length and mechanosensory function of primary cilia in renal epithelial and vascular endothelial cells. Int Educ Res J. 2016;2:91–7.
Jamal MH, Nunes ACF, Vaziri ND, Ramchandran R, Bacallao RL, Nauli AM, Nauli SM. Rapamycin treatment correlates changes in primary cilia expression with cell cycle regulation in epithelial cells. Biochem Pharmacol. 2020;178:114056. https://doi.org/10.1016/j.bcp.2020.114056.
Mukhopadhyay S, Frias MA, Chatterjee A, Yellen P, Foster DA. The enigma of rapamycin dosage. Mol Cancer Ther. 2016;15:347–53. https://doi.org/10.1158/1535-7163.MCT-15-0720.
Blagosklonny MV. Cell senescence, rapamycin and hyperfunction theory of aging. Cell Cycle. 2022;21:1456–67. https://doi.org/10.1080/15384101.2022.2054636.
Sasaki N, Itakura Y, Toyoda M. Rapamycin promotes endothelial-mesenchymal transition during stress-induced premature senescence through the activation of autophagy. Cell Commun Signal. 2020;18:43. https://doi.org/10.1186/s12964-020-00533-w.
Caramés B, Taniguchi N, Seino D, Blanco FJ, D’Lima D, Lotz M. Mechanical injury suppresses autophagy regulators and pharmacologic activation of autophagy results in chondroprotection. Arthritis Rheum. 2012;64:1182–92. https://doi.org/10.1002/art.33444.
Caramés B, Hasegawa A, Taniguchi N, Miyaki S, Blanco FJ, Lotz M. Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Ann Rheum Dis. 2012;71:575–81. https://doi.org/10.1136/annrheumdis-2011-200557.
Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–37. https://doi.org/10.1038/nri2546.
Mondino A, Mueller DL. mTOR at the crossroads of T cell proliferation and tolerance. Semin Immunol. 2007;19:162–72. https://doi.org/10.1016/j.smim.2007.02.008.
Kasahara K, Inagaki M. Primary ciliary signaling: Links with the cell cycle. Trends Cell Biol. 2021;31:954–64. https://doi.org/10.1016/j.tcb.2021.07.009.
Funding
This study was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JP19K19088 and JP21K17035 to MKi).
Author information
Authors and Affiliations
Contributions
Conception and design: M.Ki; Conducting the experiments: M.Ki. L.T.; Analysis and interpretation of the data: M.Ki; Drafting of the article: M.Ki.; Review and editing of the article: M.Ka and T.M.
Corresponding authors
Ethics declarations
Ethical statement
All animal experimental protocols were approved by the Animal Care and Experimentation Committee of Niigata University (SA00959).
Competing interests
No potential conflict of interest was reported by the authors.
(A) Micro-CT analysis of mouse mandibular condyle at 5, 15, 26, 52, and 78 weeks. Bar, 0.5 mm. (B) Bone mineral density (BMD) of the mandibular condyle. (C) The mandibular condyle was stained with safranin O at five, 15, 26, 52, and 78 weeks. Bar, 50 μm. (D–G) Cartilage area, safranin O-positive area, cell density, and cell size distribution were quantified in the condylar cartilage with age. Data in (B), (D), (E), and (F) are represented as mean ± SD; n = 5 in each group. aP < 0.05, vs. 5 w; AP < 0.01, vs. 5 w; bP < 0.05, vs. 15 w; BP < 0.01, vs. 15 w; cP < 0.05, vs. 26 w; CP < 0.01, vs. 26 w; DP < 0.01, vs. 52 w.
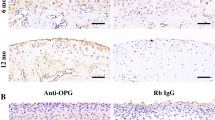
(A) Immunohistochemistry of cis-Golgi marker GM130 in condylar cartilage. Bars, 10 μm. (B) The Golgi apparatus size of chondrocytes of all layers in the condylar cartilage was quantified. (C) Age-related changes in primary cilia were examined via immunohistochemistry of the condylar cartilage using anti-acetylated tubulin and anti-gamma-tubulin antibodies. Bars, 10 μm. (D) Primary cilia frequency in chondrocytes. (E) Immunohistochemistry analysis of pS6. Bars, 10 μm. (F) The ratio of pS6-positive chondrocytes. The white square indicates the region of interest in the high-magnification image shown in (A) (C) and (E). Data in (B) (D) and (F) are represented as mean ± SD; n = 5 in each group. aP < 0.05, vs. 5 w; AP < 0.01, vs. 5 w; bP < 0.05, vs. 15 w; BP < 0.01, vs. 15 w; cP < 0.05, vs. 26 w.
(A-D) ATDC5 cells, a chondroprogenitor cell line, were treated with DMSO as control (Cont) or MHY1485. (A) Immunocytochemistry analysis using anti-acetylated tubulin, anti-gamma-tubulin, and anti-pS6. Cells were counterstained with DAPI. Bars, 10 μm. (B) Cell number. (C) The ratio of pS6-positive cells. (D) Primary cilia frequency. (E–H) ATDC5 cells were treated with ethanol as control (Cont) or Rapamycin (RAP). (E) Immunocytochemistry analysis using anti-acetylated tubulin, anti-gamma-tubulin, and anti-pS6. Cells were counterstained with DAPI. Bars, 10 μm. (F) Cell number. (G) The ratio of pS6-positive cells. (H) Primary cilia frequency. The white square indicates the region of interest in the high-magnification image shown in (A) and (E). Arrowheads indicate the primary cilia. Data in (B), (C), (D), (F), (G), and (H) are represented as mean ± SD; n = 6 in each group. *: P < 0.05, as compared to vehicle.
(A-C) Mice were injected with the vehicle as control (Cont) or rapamycin (RAP) for one week. (A) Immunohistochemistry analysis using anti-acetylated tubulin, anti-gamma-tubulin, and anti-pS6 antibodies in the condylar cartilage. Bars, 10 μm. (B) The ratio of pS6-positive cells. (C) Primary cilia frequency. (D-F) Mice were injected with a vehicle as control (Cont) or rapamycin (RAP) for 11 weeks. (D) Immunohistochemistry analysis in the condylar cartilage. Bars, 10 μm. (E) The ratio of pS6-positive cells. (F) Primary cilia frequency. The white square indicates the region of interest in the high-magnification image shown in (A) and (D). Arrowheads indicate the primary cilia. Data in (B), (C), (E), and (F) are represented as mean ± SD; n = 6 in each group. *: P < 0.05, as compared to control.
Mice were injected with a vehicle as control (Cont) or rapamycin (RAP) for 11 weeks. (A) The mandibular condylar cartilage was stained with safranin O Bars, 100 μm. (B–E) Cartilage area, safranin O-positive area, cell density, and cell size distribution of the condylar cartilage in rapamycin/vehicle-injected mice. (F) cis-Golgi marker GM130 in condylar cartilage in rapamycin/vehicle-injected mice. Bars, 10 μm. (G) Golgi apparatus size. The white square indicates the region of interest in the high-magnification image shown in (F). Data in (B), (C), (D), and (G) are represented as mean ± SD; n = 6 in each group. *: P < 0.05, as compared to control.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kitami, M., Kaku, M., Thant, L. et al. A loss of primary cilia by a reduction in mTOR signaling correlates with age-related deteriorations in condylar cartilage. GeroScience (2024). https://doi.org/10.1007/s11357-024-01143-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11357-024-01143-x