Abstract
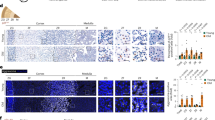
Debate exists on life-course adrenocortical zonal function trajectories. Rapid, phasic blood steroid concentration changes, such as circadian rhythms and acute stress responses, complicate quantification. To avoid pitfalls and account for life-stage changes in adrenocortical activity indices, we quantified zonae fasciculata (ZF) and reticularis (ZR) across the life-course, by immunohistochemistry of key regulatory and functional proteins. In 28 female baboon adrenals (7.5–22.1 years), we quantified 12 key proteins involved in cell metabolism, division, proliferation, steroidogenesis (including steroid acute regulatory protein, StAR), oxidative stress, and glucocorticoid and mitochondrial function. Life-course abundance of ten ZF proteins decreased with age. Cell cycle inhibitor and oxidative stress markers increased. Seven of the 12 proteins changed in the same direction for ZR and ZF. Importantly, ZF StAR decreased, while ZR StAR was unchanged. Findings indicate ZF function decreased, and less markedly ZR function, with age. Causes and aging consequences of these changes remain to be determined.



Similar content being viewed by others
Data Availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data are located in controlled access data storage at Texas Biomedical Research Institute.
References
Clegg A, Hassan-Smith Z. Frailty and the endocrine system. Lancet Diabetes Endocrinol. 2018;6(9):743–52. https://doi.org/10.1016/S2213-8587(18)30110-4.
Adekunbi DA, Li C, Nathanielsz PW, Salmon AB. Age and sex modify cellular proliferation responses to oxidative stress and glucocorticoid challenges in baboon cells. Geroscience. 2021. https://doi.org/10.1007/s11357-021-00395-1.
Zambrano E, Reyes-Castro LA, Nathanielsz PW. Aging, glucocorticoids and developmental programming. Age (Dordrecht, Netherlands). 2015;37(3):9774. https://doi.org/10.1007/s11357-015-9774-0.
Honnebier MB, Jenkins SL, Nathanielsz PW. Circadian timekeeping during pregnancy: endogenous phase relationships between maternal plasma hormones and the maternal body temperature rhythm in pregnant rhesus monkeys. Endocrinology. 1992;131(5):2051–8. https://doi.org/10.1210/endo.131.5.1330486.
Nathanielsz PW, Huber HF, Li C, Clarke GD, Kuo AH, Zambrano E. The nonhuman primate hypothalamo-pituitary-adrenal axis is an orchestrator of programming-aging interactions: role of nutrition. Nutr Rev. 2020;78(Supplement 2):48–61. https://doi.org/10.1093/nutrit/nuaa018.
Magyar DM, et al. Time-trend analysis of plasma cortisol concentrations in the fetal sheep in relation to parturition. Endocrinology. 1980;107(1):155–9. https://doi.org/10.1210/endo-107-1-155.
Downs JL, Mattison JA, Ingram DK, Urbanski HF. Effect of age and caloric restriction on circadian adrenal steroid rhythms in rhesus macaques. Neurobiol Aging. 2008;29(9):1412–22. https://doi.org/10.1016/j.neurobiolaging.2007.03.011.
Zambrano E, et al. Developmental programming-aging interactions have sex-specific and developmental stage of exposure outcomes on life course circulating corticosterone and dehydroepiandrosterone (DHEA) concentrations in rats exposed to maternal protein-restricted diets. Nutrients. 2023;15(5):1239. https://doi.org/10.3390/nu15051239.
Yang S, et al. A decline in female baboon hypothalamo-pituitary-adrenal axis activity anticipates aging. Aging (Albany NY). 2017;9(5):1375–85. https://doi.org/10.18632/aging.101235.
Willis EL, Wolf RF, White GL, McFarlane D. Age- and gender-associated changes in the concentrations of serum TGF-1β, DHEA-S and IGF-1 in healthy captive baboons (Papio hamadryas anubis). Gen Comp Endocrinol. 2014;195:21–7. https://doi.org/10.1016/j.ygcen.2013.10.004.
Willis EL, Eberle R, Wolf RF, White GL, McFarlane D. The effects of age and cytomegalovirus on markers of inflammation and lymphocyte populations in captive baboons. PLoS ONE. 2014;9(9):e107167. https://doi.org/10.1371/journal.pone.0107167.
Cox LA, et al. Baboons as a model to study genetics and epigenetics of human disease. ILAR J. 2013;54(2):106–21. https://doi.org/10.1093/ilar/ilt038.
Choi J, Li C, McDonald TJ, Comuzzie A, Mattern V, Nathanielsz PW. Emergence of insulin resistance in juvenile baboon offspring of mothers exposed to moderate maternal nutrient reduction. Am J Physiol Regul Integr Comp Physiol. 2011;301(3):R757–62. https://doi.org/10.1152/ajpregu.00051.2011.
Huber HF, Nathanielsz PW, Clarke GD. Summary and assessment of studies on cardiac aging in nonhuman primates. Comp Med. 2021;71(6):460–5. https://doi.org/10.30802/AALAS-CM-21-000038.
Kuo AH, Li C, Li J, Huber HF, Nathanielsz PW, Clarke GD. Cardiac remodelling in a baboon model of intrauterine growth restriction mimics accelerated ageing. J Physiol (Lond). 2017;595(4):1093–110. https://doi.org/10.1113/JP272908.
Bronikowski AM, Alberts SC, Altmann J, Packer C, Carey KD, Tatar M. The aging baboon: comparative demography in a non-human primate. PNAS. 2002;99(14):9591–5. https://doi.org/10.1073/pnas.142675599.
Grieves JL, et al. Barbiturate euthanasia solution-induced tissue artifact in nonhuman primates. J Med Primatol. 2008;37(3):154–61. https://doi.org/10.1111/j.1600-0684.2007.00271.x.
Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168(6):960–76. https://doi.org/10.1016/j.cell.2017.02.004.
Hu Q, Huang T. Regulation of the cell cycle by ncRNAs affects the efficiency of CDK4/6 inhibition. Int J Mol Sci. 2023;24(10):8939. https://doi.org/10.3390/ijms24108939.
Juríková M, Danihel Ľ, Polák Š, Varga I. Ki67, PCNA, and MCM proteins: markers of proliferation in the diagnosis of breast cancer. Acta Histochem. 2016;118(5):544–52. https://doi.org/10.1016/j.acthis.2016.05.002.
Galano M, Venugopal S, Papadopoulos V. Role of STAR and SCP2/SCPx in the transport of cholesterol and other lipids. Int J Mol Sci. 2022;23(20):12115. https://doi.org/10.3390/ijms232012115.
Auchus RJ. Overview of dehydroepiandrosterone biosynthesis. Semin Reprod Med. 2004;22(4):281–8. https://doi.org/10.1055/s-2004-861545.
Fadel L, et al. Modulating glucocorticoid receptor actions in physiology and pathology: insights from coregulators. Pharmacol Ther. 2023;251:108531. https://doi.org/10.1016/j.pharmthera.2023.108531.
Brand MD, Orr AL, Perevoshchikova IV, Quinlan CL. The role of mitochondrial function and cellular bioenergetics in ageing and disease. Br J Dermatol. 2013;169 Suppl 2(0 2):1–8. https://doi.org/10.1111/bjd.12208.
Zhou S, Yu Q, Zhang L, Jiang Z. Cyclophilin D-mediated mitochondrial permeability transition regulates mitochondrial function. Curr Pharm Des. 2023;29(8):620–9. https://doi.org/10.2174/1381612829666230313111314.
Shahab M, Jamesdaniel S. Nitrative stress and auditory dysfunction. Pharmaceuticals (Basel). 2022;15(6):649. https://doi.org/10.3390/ph15060649.
Li C, et al. Effects of maternal global nutrient restriction on fetal baboon hepatic insulin-like growth factor system genes and gene products. Endocrinology. 2009;150(10):4634–42. https://doi.org/10.1210/en.2008-1648.
Li C, et al. Up-regulation of the fetal baboon hypothalamo-pituitary-adrenal axis in intrauterine growth restriction: coincidence with hypothalamic glucocorticoid receptor insensitivity and leptin receptor down-regulation. Endocrinology. 2013;154(7):2365–73. https://doi.org/10.1210/en.2012-2111.
Dodds RM, et al. Grip strength across the life course: normative data from twelve British studies. PLoS ONE. 2014;9(12):e113637. https://doi.org/10.1371/journal.pone.0113637.
Coelho AM. Baboon dimorphism: growth in weight, length and adiposity from birth to 8 years of age. In: Watts ES, editor. Nonhuman primate models for human growth and development. New York: Alan R. Liss; 1985. p. 125–59.
Sharma M, et al. Circadian rhythms of melatonin and cortisol in aging. Biol Psychiatry. 1989;25(3):305–19. https://doi.org/10.1016/0006-3223(89)90178-9.
Masoro EJ. Glucocorticoids and aging. Aging (Milano). 1995;7(6):407–13. https://doi.org/10.1007/BF03324354.
Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9(3):153–66. https://doi.org/10.1038/nrc2602.
Shivji KK, Kenny MK, Wood RD. Proliferating cell nuclear antigen is required for DNA excision repair. Cell. 1992;69(2):367–74. https://doi.org/10.1016/0092-8674(92)90416-a.
Barr AR, et al. DNA damage during S-phase mediates the proliferation-quiescence decision in the subsequent G1 via p21 expression. Nat Commun. 2017;8:14728. https://doi.org/10.1038/ncomms14728.
Arakane F, Kallen CB, Watari H, Stayrook SE, Lewis M, Strauss JF. Steroidogenic acute regulatory protein (StAR) acts on the outside of mitochondria to stimulate steroidogenesis. Endocr Res. 1998;24(3–4):463–8. https://doi.org/10.3109/07435809809032634.
Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32(1):81–151. https://doi.org/10.1210/er.2010-0013.
Djikić D, et al. Ethanol and nitric oxide modulate expression of glucocorticoid receptor in the rat adrenal cortex. Pharmacol Rep. 2012;64(4):896–901. https://doi.org/10.1016/s1734-1140(12)70884-8.
Paust H-J, et al. Expression of the glucocorticoid receptor in the human adrenal cortex. Exp Clin Endocrinol Diabetes. 2006;114(1):6–10. https://doi.org/10.1055/s-2005-873007.
Myers DA, Robertshaw D, Nathanielsz PW. Effect of bilateral splanchnic nerve section on adrenal function in the ovine fetus. Endocrinology. 1990;127(5):2328–35. https://doi.org/10.1210/endo-127-5-2328.
de Picciotto NE, et al. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell. 2016;15(3):522–30. https://doi.org/10.1111/acel.12461.
Gillen JB, Martin BJ, MacInnis MJ, Skelly LE, Tarnopolsky MA, Gibala MJ. Twelve weeks of sprint interval training improves indices of cardiometabolic health similar to traditional endurance training despite a five-fold lower exercise volume and time commitment. PLoS ONE. 2016;11(4):e0154075. https://doi.org/10.1371/journal.pone.0154075.
Rooyackers OE, Adey DB, Ades PA, Nair KS. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci U S A. 1996;93(26):15364–9. https://doi.org/10.1073/pnas.93.26.15364.
Amato R, Gardin JF, Tooze JA, Cline JM (2022) Organ weights in relation to age and sex in cynomolgus monkeys (Macaca fascicularis). Toxicol Pathol:1926233221088283. https://doi.org/10.1177/01926233221088283.
Nonaka K, et al. Correlation between telomere attrition of zona fasciculata and adrenal weight reduction in older men. J Clin Endocrinol Metab. 2020;105(3):dgz214. https://doi.org/10.1210/clinem/dgz214.
Popplewell PY, Azhar S. Effects of aging on cholesterol content and cholesterol-metabolizing enzymes in the rat adrenal gland. Endocrinology. 1987;121(1):64–73. https://doi.org/10.1210/endo-121-1-64.
Popplewell PY, Tsubokawa M, Ramachandran J, Azhar S. Differential effects of aging on adrenocorticotropin receptors, adenosine 3’5’-monophosphate response, and corticosterone secretion in adrenocortical cells from Sprague-Dawley rats. Endocrinology. 1986;119(5):2206–13. https://doi.org/10.1210/endo-119-5-2206.
Azhar S, Cao L, Reaven E. Alteration of the adrenal antioxidant defense system during aging in rats. J Clin Invest. 1995;96(3):1414–24. https://doi.org/10.1172/JCI118177.
Calabrese V, et al. Analytical approaches to the diagnosis and treatment of aging and aging-related disease: redox status and proteomics. Free Radic Res. 2015;49(5):511–24. https://doi.org/10.3109/10715762.2015.1020799.
Franke K, et al. Premature brain aging in baboons resulting from moderate fetal undernutrition. Front Aging Neurosci. 2017;9:92. https://doi.org/10.3389/fnagi.2017.00092.
Kuo AH, Li C, Huber HF, Nathanielsz PW, Clarke GD. Ageing changes in biventricular cardiac function in male and female baboons (Papio spp.). J Physiol (Lond). 2018;596(21):5083–98. https://doi.org/10.1113/JP276338.
Kuo AH, Li C, Huber HF, Clarke GD, Nathanielsz PW. Intrauterine growth restriction results in persistent vascular mismatch in adulthood. J Physiol. 2018;596(23):5777–90. https://doi.org/10.1113/JP275139.
Kuo AH, Li J, Li C, Huber HF, Nathanielsz PW, Clarke GD. Poor perinatal growth impairs baboon aortic windkessel function. J Dev Orig Health Dis. 2018;9(2):137–42. https://doi.org/10.1017/S2040174417000770.
Lomas-Soria C, et al. Maternal obesity has sex-dependent effects on insulin, glucose and lipid metabolism and the liver transcriptome in young adult rat offspring. J Physiol (Lond). 2018;596(19):4611–28. https://doi.org/10.1113/JP276372.
Acknowledgements
We would like to thank Karen Moore for administrative support and to Dr. Edward Dick, Dr. Patrice Frost, Dr. Corinna Ross, and all of the staff of SNPRC for their support in animal care, veterinary procedures, and facility maintenance.
Funding
This study is supported by NIA U19AG057758, NIH P51OD011133.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted and ethical approval was obtained from the Texas Biomedical Research Institute Institutional Animal Care and Use Committee (IACUC), Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) Animal Welfare Assurance Number D16-00048.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Huber, H.F., Li, C., Xie, D. et al. Female baboon adrenal zona fasciculata and zona reticularis regulatory and functional proteins decrease across the life course. GeroScience 46, 3405–3417 (2024). https://doi.org/10.1007/s11357-024-01080-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-024-01080-9