Abstract
Frailty is a complex clinical syndrome associated with aging and comorbidities, which correlates with unfavorable outcomes. However, in heart failure patients, frailty is very common, data is scarce about those, who are eligible for Cardiac Resynchronization Therapy (CRT) implantation. We investigated the incidence of frailty and the association of Frailty Index (FI) with the outcome. Thirty baseline clinical parameters were used by the Rockwood cumulative deficit method to determine patients' FI in our single-center cohort. Based on previous studies, patients with FI ≤ 0.210 were considered as non-frail, those with FI 0.10–0.210 were classified in Frail-1, with FI > 0.10 in Frail-2 groups, respectively. Echocardiographic response after 12 months and all-cause mortality were investigated by frailty groups. Among 1004 included patients, 75 (7%) were considered Non-frail, 271 (27%) grouped in Frail-1, and 658 (66%) in Frail-2 with a median FI of 0.36 (0.28–0.43). Patients in Frail-2 group were older, with more comorbidities compared with non-frail patients or those in Group Frail-1. During the median follow-up time of 4.8 years, 29 (39%) patients died in the Non-frail, 140 (52%) in Frail-1, and 471 (72%) in the Frail-2 groups (log-rank p < 0.001). Group Frail-2 showed an unfavorable outcome compared to the non-frail (HR 2.49, 95%CI 1.92–3.22; p < 0.001) and the Frail-1 group (1.83, 95%CI 1.55–2.16; p < 0.001). In our HFrEF patients eligible for CRT implantation, patients were exceedingly vulnerable with a high prevalence of frailty. The calculated frailty index was associated with outcome and proved to be prevalent in individual risk stratification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Frailty is a complex clinical condition that results from an aggregation of insults across multiple organ systems [1]. Frailty can be quantified by counting the number of ‘health deficits’ across a range of domains. Frailty is related to, but distinct from, both aging and comorbidities [2, 3]. The relationship between frailty and heart failure (HF) is of particular interest because these conditions often coexist, and each increases the likelihood of the other. Thus, patients with HF are up to six times more likely to be frail than the general population, and, due to shared pathophysiological mechanisms, HF may accelerate the development of frailty, and frail persons may be at higher risk for developing HF [4,5,6,7]. Frail patients with HF also have a substantially higher risk for death, hospitalizations, and functional decline than non-frail patients with HF. Reducing the risk for developing frailty, slowing its progression, and even reversing frailty are now recognized goals in the management of HF [8,9,10,11,12,13,14].
The subgroup of heart failure patients with reduced ejection fraction (HFrEF) and wide QRS is a selected patient population, that can show mortality and morbidity benefit after cardiac resynchronization therapy (CRT) implantation [15]. For those patients, who are proved to be responders, frailty can be modified and patients can be shifted to a better frailty class with an improved clinical state and organ functions [16]. However, the assessment and risk stratification by the patients’ clinical condition would be essential before CRT implantation, data about the long-term outcome by frailty is scarce in this cohort.
The aim of this study was to assess the prevalence of frailty in HFrEF patients eligible for CRT implantation from a real-world cohort, to classify them by severity using the Rockwood frailty method and to investigate its correlation with their long-term outcome. Additionally, to identify the most vulnerable patients whose management should be optimized.
Methods
Patient population
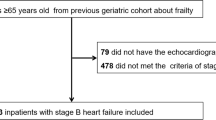
We retrospectively collected 2923 patients, who underwent successful CRT implantation at the Heart and Vascular Center of Semmelweis University between June 2000 and December 2020. As per the current guidelines those HFrEF patients with wide QRS (> 130 ms), symptoms (NYHA II-IVa), and lower left ventricular ejection fraction (LVEF) < 35% were implanted.
Frailty index
By using the original Rockwood method [17] we constructed a 30-item frailty index to identify frail patients. The 30 items were derived from medical history, other patient characteristics, and laboratory results (Table 1). Binary variables were scored 0/1 (absent/present); ordinal variables were scored from 0–1, 1 indicating the greatest severity. Continuous variables were dichotomized and scored as 0/1 (normal/non-normal). Patients with ≥ 20% missing variables were excluded from the analysis. FI score was calculated using the recommended approach, i.e., the sum of the deficits divided by the total number of non-missing deficits assessed. In keeping with previous studies, patients with FI ≤ 0.210 were classified as non-frail based on conventional cutoffs used by most authors; those with higher scores were further divided into two categories using increments in a score of 0.1.
Optimal medical and device therapy and response to CRT
We assessed the proportion of nonresponders and patients on optimal medical therapy in each frailty group. We also evaluated the ratio of CRT-pacemaker (CRT-P) or CRT with a defibrillator (CRT-D) by frailty groups. We defined responders when a relative 15% improvement in left ventricular ejection fraction (LVEF) could be observed in 12 months after CRT implantation assessed by echocardiography, which was performed by an experienced certified echocardiography specialist. Optimal medical therapy was considered complete if the patient was on a beta-blocker, ACE-inhibitor, Angiotensin II receptor blocker or ARNI, and mineralocorticoid receptor antagonist at the same time.
The type of the CRT devices (CRT-P vs. CRT-D) was assessed individually by the implanting physician as per the current guidelines, preferably using CRT-D for ischemic patients, males, younger patients or those who were expected to be non-responders.
Outcome data
The primary endpoint of the study was all-cause mortality. Patients’ status (dead or alive), and date of death were obtained for all patients by querying the National Health Insurance Database of Hungary in April 2022. The time to death was measured from the date of the CRT implantation.
Response to CRT was assessed 12 months after the implantation by echocardiography. Those more than 15% reative LVEF increase were considered as responders.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation (SD) or median and interquartile range (25th-75th). Statistical tests employed were unpaired Student’s t-test or Mann–Whitney U test or Spearman correlation (for continuous variables) and Chi-squared or Fisher’s exact test (for categorical variables), respectively. The survival of subgroups was visualized using Kaplan–Meier curves, and Log-rank tests were performed for comparison. Univariable and multivariable Cox proportional hazards models were used to compute hazard ratios (HR) with 95% confidence intervals (95% CI). A two-sided p-value of < 0.05 was considered statistically significant. All statistical analysis was performed in SPSS (version 25.0, IBM, Armonk, NY, USA), GraphPad Prism (version 8, Inc., GraphPad Software, SanDiego, CA, USA), and RStudio (version 1.8, RStudio PBC, Boston, MA, USA).
Results
Out of 2923 patients in our database, altogether 1004 were eligible to calculate the frailty index by their parameters. Their median age was 69 (61–76,) years, their median FI was 0.36 (0.28–0.43). Overall, 929 (92.5%) patients were frail, 75 (7.5%) patients were in the non-frail group (≤ 0.210-), 271 (27%) patients in Frail-1 Group (0.211–0.310), and 658 (65.5%) in Frail-2 Group (> 0.311) (Table 2).
Baseline clinical characteristics
In the different subgroups, the mean of age was associated with FI (r = 0.219396; p < 0.001) Compared to the non-frail group, frail patients were older [Median age was 63 (57–71) years in the Non frail and 70 (64–76) years in the Frail-2 group, p < 0.0001], more often men (67% vs. 76%; p = 0.159), and more likely to have cardiovascular and non-cardiovascular comorbidities: they had a higher uric acid (364 (296–417) vs. 432 (336–521) µmol/l, p < 0.001), creatinine (77 (68–87) vs. 113 (89–147) µmol/l, p < 0.001), and BUN (5.7 (4.6–6.7) vs. 9.5 (7.3–12.9) mmol/l, p < 0.001), levels, but lower hemoglobin (14 (13.2–14.7) vs. 13.6 (12.2.-14.7) g/dl, < 0.001), thrombocyte, (229 (192–267) vs. 194 (152–244) G/l, p < 0.001), and lymphocyte levels (24 (19–29) % vs 20 (15–26) %, p = 0.004). Patients with higher FI were more likely to have an ischemic etiology (12% vs. 52%, p < 0.001), worse NYHA functional class (NYHA III/IV: 34% vs. 60%, p < 0.001), and worse right ventricular function (TAPSE 22 (19–25) mm vs 18 (15–22) mm, p < 0.0001). They suffered more often from COPD (3% vs. 19%, p < 0.001), peripheral arterial disease (0% vs. 13%, p < 0.001), diabetes mellitus (13% vs. 45%, p < 0.001), and atrial fibrillation (17% vs. 48%, p < 0.001), than those with lower FI (Table 2).
The use of optimal medical treatment and device therapy at baseline
In the proportion of those patients received optimal heart failure medical therapy was similar between the three groups. (OMT vs. Non-OMT p = 0.517). The proportion of CRT-D and CRT-P device proved to be equal in the different frail groups (Non-frail: 55 vs 44%, Frail 1: 58 vs 42%, Frail 2: 27% vs 20%, p = 0.907).
Outcome data
Response to CRT by groups
When CRT response was investigated, FI was associated with a beneficial response, those in the non-frail group had a higher proportion of responders compared to Frail-2 group (79% vs. 58%, p = 0.044).
Primary endpoint
During the median follow-up time of 4.8 (2.3–7.2) years, a total of 640 (64%) patients reached the primary endpoint, 29 (39%) in the Non-frail group, 140 (52%) in Frail group 1, and 471 (72%) in the Frail group 2 (p < 0.001).
The less favorable outcome could be observed in Group Frail-2 compared to the non-frail (HR 2.49, 95%CI 1.92–3.22; p < 0.001) (Figs. 1 and 2) and the Frail-1 group (1.83, 95%CI 1.55–2.16; p < 0.001) (Fig. 3).
Discussion
In the current study of our large-scale, single-center, retrospective database, we demonstrated that in HFrEF patients who are eligible for CRT implantation, frailty is more frequent compared with previous HFrEF trials’ populations, affecting more than two third of our cohort. The calculated frailty index from clinically relevant covariates using the Rockwood cumulative deficit approach was correlated with the number of comorbidities and associated with an unfavorable outcome. Moreover, in those with greater frailty score, a less favorable treatment effect of CRT could be observed with a low rate of responders in both CRT-D and CRT-P patients. The proportion of CRT-D implantations was comparable in each group regardless of the frailty score, and in patients with higher frailty, the administration of optimal pharmacological treatment could be equally introduced.
Frailty and pre-frailty are very common in the heart failure population along the entire spectrum of the ejection fraction [18]. Due to the impaired heart function that leads to pathomechanisms affecting several organs, patients with heart failure have multiple times increased odds of being frail. However, age is linearly correlated with frailty, which is more frequent in the elderly, the impaired and systemic damage and the severity of heart failure also determine its prevalence.
Therefore, in the last decades, the terminology to characterize this condition has changed. As previously frailty phenotype was described as a biological syndrome with specific phenotypic presentations, nowadays frailty index incorporates the conditions of all deficits, most frequently used and calculated by Rockwood cumulative deficits approach.
Using this method, previous RCTs proved the ratio of frail patients and non-frail patients with lower than 0.21 FI lies between 38–50%, which is exceedingly high compared to our recent cohort. Moreover, patients with the highest FI higher than 0.311 ranged between 14–24%, which was 65.5% in our patient population, although the RCTs investigated a well-selected patient population, and precluded the enrollment of very high-risk patients [19].
In HFrEF cohorts those patients with higher FI are showing similar clinical characteristics; FI is correlated with older age and more severe HF symptoms [2]. Additionally, in our HFrEF cohort who were selected for a CRT device implantation, impaired renal function, anemia, atrial fibrillation, ischemic etiology, type II diabetes, and COPD were more frequent compared to less frail or non-frail patients. Moreover, when echocardiographic baseline parameters were assessed, those with the highest frailty had impaired right ventricular function as well showing a systemic, multiorgan impairment.
In line with these findings, the frailty score was described as a strong predictor of mortality. In the DELIVER trial, those with higher than 0.311 FI showed a 13.4-fold risk for mortality, which was a higher proportion than in our cohort, where patients with a FI higher than 0.311 showed a 2.5-fold risk for mortality [20]. In the analysis of PARADIGM and ATMOSPHERE studies, all-cause mortality and hospitalization from any cause occurred to have the strongest correlation with FI, showing that the assessment of FI incorporates the comorbidities and related risk of hospitalizations and mortality. Besides, patients with higher frailty complained of the worst quality of life, although after adding an effective treatment such as dapagliflozin, the improvement of QoL was the largest in the highest frailty group [8].
Sriwattanakomen et al. investigated an older CRT population with 469 HFrEF patients with age of > 75 years. During the mean follow-up period of 4.6 years, 82% of their patient died. In contrast with our results, they found that the choice of using a CRT-P was associated with higher FI and older age [21]. In our cohort, the rate of CRT-D was comparable in each FI group.
As a consequence of multiorgan failures and lower tolerance for pharmacological treatments, frail patients are less likely to receive new pharmacologic therapies and the occurrence of discontinuation of the optimal medical treatment is higher, and patients’ adherence could be lower [22]. However, in our recent study, those with the highest frailty received OMT in a comparable proportion regardless of the frailty. Despite such a balanced use of optimal pharmacological and device therapy, patients’ outcome already at a very early stage differed significantly, those with higher frailty failed to develop reverse remodeling, which was reflected in a less beneficial long-term outcome and high all-cause mortality rate.
Based on these results, early selection of frail patients in clinical practice is essential. Detecting those modifiable laboratory parameters (e.g. anemia, hyperuricemia, hyponatremia) and conditions (such as weight or BMI) that are described to correlate with unfavorable outcomes and, combined with a supervised exercise-based cardiac rehabilitation program might lead to a risk reduction in hospitalization or mortality in HFrEF patients [13]. Adding remote monitoring to the guideline directed medical therapy is associated with significantly improved clinical outcomes in patients with advanced HF [23] and could prevent frailty shift towards a worse status by the early detection of limited mobility [24]. Frailty is also proven to be a key factor in appropriate device selection. According to a recent study of Segar et al. baseline frailty modified the efficacy of ICD therapy with a significant mortality benefit observed among participants with HFrEF and a low frailty burden but not among those with a high frailty burden [25]. Additionally, those heart failure treatments, which showed a beneficial effect in decreasing cardiovascular mortality and heart failure events, should be used, their early administration is crucial in groups with higher frailty index.
Limitations
Our study has a few limitations. First, this is a single-center, retrospective, observational study, may resulting in some imbalances in the study groups and data were missing for a small proportion of patients. As the enrollment period is 20 years, further imbalances in the treatment may influence our results. The single-center, retrospective design of the study may also limit the generalizability of findings to a broader population and could introduce selection bias.
Conclusions
Based on our results, patients with heart failure and reduced ejection fraction, who are eligible for CRT implantation are exceedingly vulnerable with a high prevalence of frailty. The calculated frailty index was associated with higher rates of death from any cause and proved to be prevalent in individual risk stratification and can predict the outcome. Patients with high frailty index have a higher risk to fail to develop reverse remodeling despite having the same rate of optimal treatment both in terms of devices (such as CRT-D) and pharmacological therapy.
References
Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Sci World J. 2001;1:323–36. https://doi.org/10.1100/tsw.2001.58.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–57. https://doi.org/10.1093/gerona/56.3.m146.
Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:M255–63. https://doi.org/10.1093/gerona/59.3.m255.
Khan H, Kalogeropoulos AP, Georgiopoulou VV, et al. Frailty and risk for heart failure in older adults: the health, aging, and body composition study. Am Heart J. 2013;166:887–94. https://doi.org/10.1016/j.ahj.2013.07.032.
Woods NF, LaCroix AZ, Gray SL, et al. Women’s health initiative. Frailty: emergence and consequences in women aged 65 and older in the women’s health initiative observational study. J Am Geriatr Soc. 2005;53:1321–30. https://doi.org/10.1111/j.1532-5415.2005.53405.x.
Denfeld QE, Winters-Stone K, Mudd JO, et al. The prevalence of frailty in heart failure: A systematic review and meta-analysis. Int J Cardiol. 2017;236:283–9. https://doi.org/10.1016/j.ijcard.2017.01.153.
Bielecka-Dabrowa A, Ebner N, Dos Santos MR, et al. Cachexia, muscle wasting, and frailty in cardiovascular disease. Eur J Heart Fail. 2020;22:2314–26. https://doi.org/10.1002/ejhf.2011.
Dewan P, Jackson A, Jhund PS, et al. The prevalence and importance of frailty in heart failure with reduced ejection fraction - an analysis of paradigm-HF and atmosphere. Eur J Heart Fail. 2020;22:2123–33. https://doi.org/10.1002/ejhf.1832.
Sanders NA, Supiano MA, Lewis EF, et al. The frailty syndrome and outcomes in the TOPCAT trial. Eur J Heart Fail. 2018;20:1570–7. https://doi.org/10.1002/ejhf.1308.
Zhang Y, Yuan M, Gong M, et al. Frailty and clinical outcomes in heart failure: A systematic review and meta-analysis. J Am Med Dir Assoc. 2018;19:1003-1008.e1. https://doi.org/10.1016/j.jamda.2018.06.009.
Bottle A, Kim D, Hayhoe B, et al. Frailty and co-morbidity predict first hospitalisation after heart failure diagnosis in primary care: population-based observational study in England. Age Ageing. 2019;48:347–54. https://doi.org/10.1093/ageing/afy194.
Vidán MT, Blaya-Novakova V, Sánchez E, et al. Prevalence and prognostic impact of frailty and its components in non-dependent elderly patients with heart failure. Eur J Heart Fail. 2016;18:869–75. https://doi.org/10.1002/ejhf.518.
McDonagh TA, Metra M, Adamo M, et al. ESC scientific document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–726. https://doi.org/10.1093/eurheartj/ehab368.
Hoogendijk EO, Afilalo J, Ensrud KE, et al. Frailty: implications for clinical practice and public health. Lancet. 2019;394:1365–75. https://doi.org/10.1016/S0140-6736(19)31786-6.
Cleland JGF, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. https://doi.org/10.1056/NEJMoa050496.
Champ-Rigot L, Cornille AL, Ollitrault R, et al. Predictors of clinical outcomes after cardiac resynchronization therapy in patients ≥75 years of age: a retrospective cohort study. BMC Geriatr. 2019;19:325. https://doi.org/10.1186/s12877-019-1351-4.
Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatrics. 2008;8:24. https://doi.org/10.1186/1471-2318-8-24.
Denfeld QE, Winters-Stone K, Mudd JO, et al. The prevalence of frailty in heart failure: a systematic review and meta-analysis. Int J Cardiol. 2017;236:283–9. https://doi.org/10.1016/j.ijcard.2017.01.153.
Butt JH, Dewan P, Merkely B, et al. Efficacy and safety of dapagliflozin according to frailty in heart failure with reduced ejection fraction: a post hoc analysis of the dapa-hf trial. Ann Intern Med. 2022;175(6):820–30. https://doi.org/10.7326/M21-4776.
Butt JH, Jhund PS, Belohlávek J, et al. Efficacy and safety of dapagliflozin according to frailty in patients with heart failure: a prespecified analysis of the deliver trial. Circulation. 2022;146:1210–24. https://doi.org/10.1161/CIRCULATIONAHA.122.061754.
Sriwattanakomen R, Ahmad S, Mathew D et al. Frailty as a Predictor of Device Choice and Mortality in Older Cardiac Resynchronization Therapy Recipients. Circulation: Arrhythmia and Electrophysiology. 2021;14 https://doi.org/10.1161/CIRCEP.121.010185
Jankowska-Polańska B, Dudek K, Szymanska-Chabowska A, Uchmanowicz I. The influence of frailty syndrome on medication adherence among elderly patients with hypertension. Clin Interv Aging. 2016;11:1781–90. https://doi.org/10.2147/CIA.S113994.
Wintrich J, Pavlicek V, Brachmann J, et al. Remote monitoring with appropriate reaction to alerts was associated with improved outcomes in chronic heart failure: results from the optiLink HF study. Circ Arrhythm Electrophysiol. 2021;14(1):e008693. https://doi.org/10.1161/CIRCEP.120.008693.
Kramer DB, Tsai T, Natarajan P, et al. Frailty, physical activity, and mobility in patients with cardiac implantable electrical devices. J Am Heart Assoc. 2017;6(2):e004659. https://doi.org/10.1161/JAHA.116.004659.
Segar MW, Keshvani N, Singh S, et al. Frailty Status modifies the efficacy of ICD therapy for primary prevention among patients with HF. JACC Heart Fail. 2023;26:S2213-1779(23)00311-6. https://doi.org/10.1016/j.jchf.2023.06.009.
Funding
Open access funding provided by Semmelweis University. Project no. RRF-2.3.1–21-2022–00003 has been implemented with the support provided by the European Union. This study was supported by the National Research, Development and Innovation Office of Hungary (NKFIA; NVKP_16-1–2016-0017 National Heart Program).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
AKos receives consulting fees from Medtronic and Biotronik, and receives payment or honoraria for lectures from Novartis, Bayer, Boehringer Ingelheim, Astra Zeneca, Medtronic Biotronik, and Boston Scientific. BM receives grants or has contracts with Abbott, Astra Zeneca, Argint International, Biotronik, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, CSL Behring, Daiichi Sankyo, Cerenis Therapeutics SA DUKE Clinical Institut, Eli Lilly, Medtronic, Novartis, Terumo, St. Jude, Zoll and VIFOR Pharma, and receives lecture fees from Abbott, Astra Zeneca, Biotronik, Boehringer Ingelheim, and Novartis. LG receives lecture fees from Biotronik, Medtronic, Johnson & Johnson Medical, and Abbott outside the submitted work. EZ reports lecture and advisory fees from Biotronik, Medtronic, Boston Scientific, and Zoll Medical, outside the submitted work. I.O. received advisory fees from Biotronik, Medtronic, Abbott, Boston Scientific, Orchesra Biomed. AKov serves as Chief Medical Officer of Argus Cognitive, Inc. and Chief Executive Officer of CardioSight Hungary Ltd. and receives financial compensation for his work, outside the submitted paper. L.K.K. receives lecture fees from Boehringer, Novartis and Richter outside the submitted work. LM reports lecture fees from Biotronik, Medtronic, and Abbott outside the submitted work. EDM reports grants from Novartis and Boehringer Ingelheim. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Luca Katalin Kuthi and Walter Richard Schwertner contributed equally to the analysis and the drafting of the present manuscript.
Annamária Kosztin and Béla Merkely contributed equally to the supervising of the present manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kuthi, L.K., Schwertner, W.R., Veres, B. et al. The prevalence of frailty and its effect on the outcome in cardiac resynchronization therapy patients. GeroScience 46, 2671–2679 (2024). https://doi.org/10.1007/s11357-023-01023-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-023-01023-w