Abstract
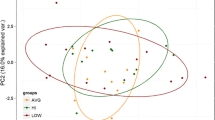
Microbiota composition has been linked to physical activity, health measures, and biological age, but a shared profile has yet to be shown. The aim of this study was to examine the associations between microbiota composition and measures of function, such as a composite measure of physical capacity, and biological age in midlife, prior to onset of age-related diseases. Seventy healthy midlife individuals (age 44.58 ± 0.18) were examined cross-sectionally, and their gut-microbiota profile was characterized from stool samples using 16SrRNA gene sequencing. Biological age was measured using the Klemera-Doubal method and a composition of blood and physiological biomarkers. Physical capacity was calculated based on sex-standardized functional tests. We demonstrate that the women had significantly richer microbiota, p = 0.025; however, microbiota diversity was not linked with chronological age, biological age, or physical capacity for either women or men. Men had slightly greater β-diversity; however, β-diversity was positively associated with biological age and with physical capacity for women only (p = 0.01 and p = 0.04; respectively). For women, an increase in abundance of Roseburia faecis and Collinsella aerofaciens, as well as genus Ruminococcus and Dorea, was significantly associated with higher biological age and lower physical capacity; an increase in abundance of Akkermansia muciniphila and genera Bacteroides and Alistipes was associated with younger biological age and increased physical capacity. Differentially abundant taxa were also associated with non-communicable diseases. These findings suggest that microbiota composition is a potential mechanism linking physical capacity and health status; personalized probiotics may serve as a new means to support health-promoting interventions in midlife. Investigating additional factors underlying this link may facilitate the development of a more accurate method to estimate the rate of aging.





Similar content being viewed by others
Data availability
The microbiota data used here is publicly available in the European Bioinformatics Institute (EBI) database, https://www.ebi.ac.uk/ena/browser/view/PRJEB63185.
References
Jazwinski SM, Kim S. Examination of the dimensions of biological age. Front Genet. 2019;10:263. https://doi.org/10.3389/fgene.2019.00263.
Oksuzyan A, Juel K, Vaupel JW, Christensen K. Men: good health and high mortality. Sex differences in health and aging. Aging Clin Exp Res. 2008;20(2):91–102. https://doi.org/10.1007/bf03324754.
Cummings SR, Kritchevsky SB. Endpoints for geroscience clinical trials: health outcomes, biomarkers, and biologic age. GeroScience. 2022;44(6):2925–31. https://doi.org/10.1007/s11357-022-00671-8.
Jylhävä J, Pedersen NL, Hägg S. Biological age predictors. EBioMedicine. 2017;21:29–36. https://doi.org/10.1016/j.ebiom.2017.03.046.
Bell CG, Lowe R, Adams PD, et al. DNA methylation aging clocks: challenges and recommendations. Genome Biol. 2019;20(1). https://link.springer.com/article/10.1186/s13059-019-1824-y.
Belsky DW, Moffitt TE, Cohen AA, et al. Eleven telomere, epigenetic clock, and biomarker-composite quantifications of biological aging: do they measure the same thing? Am J Epidemiol. 2018;187(6):1220–30. https://doi.org/10.1093/aje/kwx346.
Finkel D, Sternäng O, Jylhävä J, Bai G, Pedersen NL. Functional aging index complements frailty in prediction of entry into care and mortality. J Gerontol Ser A. 2019;74(12):1980–6. https://doi.org/10.1093/gerona/glz155.
Li X, Ploner A, Wang Y, et al. Longitudinal trajectories, correlations and mortality associations of nine biological ages across 20-years follow-up. eLife. 2020;9. https://elifesciences.org/articles/51507.
Badal VD, Vaccariello ED, Murray ER, et al. The gut microbiome, aging, and longevity: a systematic review. Nutrients. 2020;12(12):3759. https://doi.org/10.3390/nu12123759.
Galkin F, Mamoshina P, Aliper A, et al. Human gut microbiome aging clock based on taxonomic profiling and deep learning. iScience. 2020;23(6):101199. https://doi.org/10.1016/j.isci.2020.101199.
Tzemah Shahar R, Koren O, Matarasso S, Shochat T, Magzal F, Agmon M. Attributes of physical activity and gut microbiome in adults: a systematic review. Int J Sports Med. 2020;41(12):801–14. https://doi.org/10.1055/a-1157-9257.
Corbin CBP, Robert P, Franks, B. Don. Definitions: health, fitness, and physical activity. President's Council on Physical Fitness and Sports, Washington, DC. 2000;(Series 3 n9).
Tzemah-Shahar R, Hochner H, Iktilat K, Agmon M. What can we learn from physical capacity about biological age? A systematic review. Ageing Res Rev. 2022;77:101609. https://doi.org/10.1016/j.arr.2022.101609.
McGee SL, Hargreaves M. Exercise adaptations: molecular mechanisms and potential targets for therapeutic benefit. Nat Rev Endocrinol. 2020;16(9):495–505. https://doi.org/10.1038/s41574-020-0377-1.
Duggal NA, Niemiro G, Harridge SDR, Simpson RJ, Lord JM. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nat Rev Immunol. 2019;19(9):563–72. https://doi.org/10.1038/s41577-019-0177-9.
Tzemah-Shahar R, Shapiro I, Kodesh E, et al. Physical-capacity based evaluation battery for rate of aging at midlife: a preliminary study. Submitted to European Review of Aging and Physical Activity, 2023.
Ramos C, Gibson GR, Walton GE, Magistro D, Kinnear W, Hunter K. Systematic review of the effects of exercise and physical activity on the gut microbiome of older adults. Nutrients. 2022;14(3):674. https://doi.org/10.3390/nu14030674.
Baquero F, Nombela C. The microbiome as a human organ. Clin Microbiol Infect. 2012;18:2–4. https://doi.org/10.1111/j.1469-0691.2012.03916.x.
Bana B, Cabreiro F. The microbiome and aging. Annu Rev Genet. 2019;53:239–61. https://doi.org/10.1146/annurev-genet-112618-043650.
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: an expanding universe. Cell. 2023;186(2):243–78. https://doi.org/10.1016/j.cell.2022.11.001.
Nagpal R, Mainali R, Ahmadi S, et al. Gut microbiome and aging: physiological and mechanistic insights. Nutr Healthy Aging. 2018;4(4):267–85. https://doi.org/10.3233/NHA-170030.
Noce A, Marrone G, Di Daniele F, et al. Impact of gut microbiota composition on onset and progression of chronic non-communicable diseases. Nutrients. 2019;11(5):1073. https://doi.org/10.3390/nu11051073.
Bennett JE, Stevens GA, Mathers CD, et al. NCD countdown 2030: worldwide trends in non-communicable disease mortality and progress towards sustainable development goal target 3.4. The Lancet. 2018;392(10152):1072–88. https://doi.org/10.1016/s0140-6736(18)31992-5.
Belsky DW, Caspi A, Houts R, et al. Quantification of biological aging in young adults. Proc Natl Acad Sci. 2015;112(30):E4104–10. https://doi.org/10.1073/pnas.1506264112.
Huang R, Ju Z, Zhou PK. A gut dysbiotic microbiota-based hypothesis of human-to-human transmission of non-communicable diseases. Sci Total Environ. 2020;745:141030. https://doi.org/10.1016/j.scitotenv.2020.141030.
Zhong H, Ren H, Lu Y, et al. Distinct gut metagenomics and metaproteomics signatures in prediabetics and treatment-naïve type 2 diabetics. EBioMedicine. 2019;47:373–83. https://doi.org/10.1016/j.ebiom.2019.08.048.
Harlap S, Davies AM, Deutsch L, et al. The Jerusalem perinatal study cohort, 1964?2005: methods and a review of the main results. Paediatr Perinat Epidemiol. 2007;21(3):256–73. https://doi.org/10.1111/j.1365-3016.2007.00799.x.
Klemera P, Doubal S. A new approach to the concept and computation of biological age. Mech Ageing Dev. 2006;127(3):240–8. https://doi.org/10.1016/j.mad.2005.10.004.
Kwon D, Belsky DW. A toolkit for quantification of biological age from blood chemistry and organ function test data: BioAge. Geroscience. 2021;43(6):2795–808. https://doi.org/10.1007/s11357-021-00480-5.
Uzan-Yulzari A, Morr M, Tareef-Nabwani H, et al. The intestinal microbiome, weight, and metabolic changes in women treated by adjuvant chemotherapy for breast and gynecological malignancies. BMC Med. 2020;18(1):281. https://doi.org/10.1186/s12916-020-01751-2.
Bolyen E, Rideout JR, Dillon MR, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37(8):852–7. https://doi.org/10.1038/s41587-019-0209-9.
Kim BR, Shin J, Guevarra R, et al. Deciphering diversity indices for a better understanding of microbial communities. J Microbiol Biotechnol. 2017;27(12):2089–93. https://doi.org/10.4014/jmb.1709.09027.
Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R. UniFrac: an effective distance metric for microbial community comparison. ISME J. 2011;5(2):169–72. https://doi.org/10.1038/ismej.2010.133.
Santiago A, Pozuelo M, Poca M, et al. Alteration of the serum microbiome composition in cirrhotic patients with ascites. Sci Rep. 2016;6(1):25001. https://doi.org/10.1038/srep25001.
Breban M, Tap J, Leboime A, et al. Faecal microbiota study reveals specific dysbiosis in spondyloarthritis. Ann Rheum Dis. 2017;76(9):1614–22. https://doi.org/10.1136/annrheumdis-2016-211064.
Jolivet-Gougeon MB-MA. Roseburia spp.: a marker of health. Future Microbiol. 2017;12(2):157–70. https://doi.org/10.2217/fmb-2016-0130.
Zheng Y, Fang Z, Xue Y, et al. Specific gut microbiome signature predicts the early-stage lung cancer. Gut Microbes. 2020;11(4):1030–42. https://doi.org/10.1080/19490976.2020.1737487.
Boursier J, Mueller O, Barret M, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63(3):764–75. https://doi.org/10.1002/hep.28356.
Da Silva HE, Teterina A, Comelli EM, et al. Nonalcoholic fatty liver disease is associated with dysbiosis independent of body mass index and insulin resistance. Sci Rep. 2018;8(1). https://www.nature.com/articles/s41598-018-19753-9.
Kovács T, Mikó E, Ujlaki G, et al. The involvement of oncobiosis and bacterial metabolite signaling in metastasis formation in breast cancer. Cancer Metastasis Rev. 2021;40(4):1223–49. https://doi.org/10.1007/s10555-021-10013-3.
Emoto T, Yamashita T, Kobayashi T, et al. Characterization of gut microbiota profiles in coronary artery disease patients using data mining analysis of terminal restriction fragment length polymorphism: gut microbiota could be a diagnostic marker of coronary artery disease. Heart Vessels. 2017;32(1):39–46. https://doi.org/10.1007/s00380-016-0841-y.
Liu Z, Li J, Liu H, et al. The intestinal microbiota associated with cardiac valve calcification differs from that of coronary artery disease. Atherosclerosis. 2019;284:121–8. https://doi.org/10.1016/j.atherosclerosis.2018.11.038.
Jie Z, Xia H, Zhong S-L, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 2017;8(1). https://www.nature.com/articles/s41467-017-00900-1.
Nakatsu G, Li X, Zhou H, et al. Gut mucosal microbiome across stages of colorectal carcinogenesis. Nat Commun. 2015;6(1):8727. https://doi.org/10.1038/ncomms9727.
Kim S, Rigatto K, Gazzana MB, et al. Altered gut microbiome profile in patients with pulmonary arterial hypertension. Hypertension. 2020;75(4):1063–71. https://doi.org/10.1161/hypertensionaha.119.14294.
Ettehad Marvasti F, Moshiri A, Taghavi MS, et al. The first report of differences in gut microbiota composition between obese and normal weight Iranian subjects. Iran Biomed J. 2020;24(3):148–54. https://doi.org/10.29252/ibj.24.3.148.
Zhu L, Baker SS, Gill C, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57(2):601–9. https://doi.org/10.1002/hep.26093.
Jiang W, Wu N, Wang X, et al. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci Rep. 2015;5(1):8096. https://doi.org/10.1038/srep08096.
Loke MF, Chua EG, Gan HM, et al. Metabolomics and 16S rRNA sequencing of human colorectal cancers and adjacent mucosa. Plos One. 2018;13(12):e0208584. https://doi.org/10.1371/journal.pone.0208584.
Kim S, Goel R, Kumar A, et al. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin Sci. 2018;132(6):701–18. https://doi.org/10.1042/cs20180087.
Raman M, Ahmed I, Gillevet PM, et al. Fecal Microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2013;11(7):868-875.e3. https://doi.org/10.1016/j.cgh.2013.02.015.
Li Q, Chang Y, Zhang K, Chen H, Tao S, Zhang Z. Implication of the gut microbiome composition of type 2 diabetic patients from northern China. Sci Rep. 2020;10(1). https://www.nature.com/articles/s41598-020-62224-3.
Ortiz-Alvarez L, Xu H, Martinez-Tellez B. Influence of exercise on the human gut microbiota of healthy adults: a systematic review. Clin Transl Gastroenterol. 2020;11(2):e00126. https://doi.org/10.14309/ctg.0000000000000126.
Larsen OFA, Claassen E. The mechanistic link between health and gut microbiota diversity. Sci Rep. 2018;8(1). https://www.nature.com/articles/s41598-018-20141-6.
Dogra SK, Dore J, Damak S. Gut microbiota resilience: definition, link to health and strategies for intervention. Front Microbiol. 2020;11:572921. https://doi.org/10.3389/fmicb.2020.572921.
Aya V, Flórez A, Perez L, Ramírez JD. Association between physical activity and changes in intestinal microbiota composition: a systematic review. Plos One. 2021;16(2):e0247039. https://doi.org/10.1371/journal.pone.0247039.
Rinninella E, Raoul P, Cintoni M, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7(1):14. https://doi.org/10.3390/microorganisms7010014.
Hawley JA, Joyner MJ, Green DJ. Mimicking exercise: what matters most and where to next? J Physiol. 2021;599(3):791–802. https://doi.org/10.1113/jp278761.
Langsetmo L, Johnson A, Demmer RT, et al. The association between objectively measured physical activity and the gut microbiome among older community dwelling men. J Nutr Health Aging. 2019;23(6):538–46. https://doi.org/10.1007/s12603-019-1194-x.
Ma Z, Li L, Gotelli NJ. Diversity-disease relationships and shared species analyses for human microbiome-associated diseases. ISME J. 2019;13(8):1911–9. https://doi.org/10.1038/s41396-019-0395-y.
Dziewiecka H, Buttar HS, Kasperska A, et al. Physical activity induced alterations of gut microbiota in humans: a systematic review. BMC Sports Sci Med Rehabil. 2022;14(1). doi:https://doi.org/10.1186/s13102-022-00513-2.
Divella R, De Palma G, Tufaro A, et al. Diet, probiotics and physical activity: the right allies for a healthy microbiota. Anticancer Res. 2021;41(6):2759–72. https://doi.org/10.21873/anticanres.15057.
Lv WQ, Lin X, Shen H, et al. Human gut microbiome impacts skeletal muscle mass via gut microbial synthesis of the short-chain fatty acid butyrate among healthy menopausal women. J Cachexia Sarcopenia Muscle. 2021;12(6):1860–70. https://doi.org/10.1002/jcsm.12788.
Barton W, Penney NC, Cronin O, et al. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut. 2017:gutjnl-2016–313. https://gut.bmj.com/content/67/4/625.abstract?casa_token=uG5E_mzSPdsAAAAA:IpoRgMfcqa5L6qynNLApYQjQWbj166O_ICGZOi1lhIuDc6PZdZZtlDZRHkNWxVApc7eM0WVNz7U.
Allam-Ndoul B, Castonguay-Paradis S, Veilleux A. Gut microbiota and intestinal trans-epithelial permeability. Int J Mol Sci. 2020;21(17):6402. https://doi.org/10.3390/ijms21176402.
Park JH, Moon JH, Kim HJ, Kong MH, Oh YH. Sedentary lifestyle: overview of updated evidence of potential health risks. Korean J Fam Med. 2020;41(6):365–73. https://doi.org/10.4082/kjfm.20.0165.
Tett A, Pasolli E, Masetti G, Ercolini D, Segata N. Prevotella diversity, niches and interactions with the human host. Nat Rev Microbiol. 2021;19(9):585–99. https://doi.org/10.1038/s41579-021-00559-y.
OECD, Organization WH. Step up! Tackling the burden of insufficient physical activity in Europe. 2023.
Bhargava A, Arnold AP, Bangasser DA, et al. Considering sex as a biological variable in basic and clinical studies: an Endocrine Society scientific statement. Endocr Rev. 2021;42(3):219–58. https://doi.org/10.1210/endrev/bnaa034.
Valeri F, Endres K. How biological sex of the host shapes its gut microbiota. Front Neuroendocrinol. 2021;61:100912. https://doi.org/10.1016/j.yfrne.2021.100912.
Shin JH, Park YH, Sim M, Kim SA, Joung H, Shin DM. Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome. Res Microbiol Jun-Aug. 2019;170(4–5):192–201. https://doi.org/10.1016/j.resmic.2019.03.003.
Hall CV, Lord A, Betzel R, et al. Co-existence of network architectures supporting the human gut microbiome. iScience. 2019;22:380–91. https://doi.org/10.1016/j.isci.2019.11.032.
(CDC) CfDCaP. Data from: National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services.
Shapiro I, Belsky DW, Israel S, Youssim I, Friedlander Y, Hochner H. Familial aggregation of the aging process: biological age measured in young adult offspring as a predictor of parental mortality. GeroScience. 2023;45(2):901–13. https://doi.org/10.1007/s11357-022-00687-0.
Caporaso JG, Lauber CL, Walters WA, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6(8):1621–4. https://doi.org/10.1038/ismej.2012.8.
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13(7):581–3. https://doi.org/10.1038/nmeth.3869.
DeSantis TZ, Hugenholtz P, Larsen N, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72(7):5069–72. https://doi.org/10.1128/AEM.03006-05.
McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8(4):e61217. https://doi.org/10.1371/journal.pone.0061217.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12). https://genomebiology.biomedcentral.com/articles/10.1186/s13059-014-0550-8?ref=https://githubhelp.com.
Wickham H. Programming with ggplot2. Springer International Publishing; 2016:241–253. https://link.springer.com/chapter/10.1007/978-3-319-24277-4_12.
Douglas GM, Maffei VJ, Zaneveld JR, et al. PICRUSt2 for prediction of metagenome functions. Nat Biotechnol. 2020;38(6):685–8. https://doi.org/10.1038/s41587-020-0548-6.
Caspi R, Billington R, Keseler IM, et al. The MetaCyc database of metabolic pathways and enzymes - a 2019 update. Nucleic Acids Res. 2019;48(D1):D445–53. https://doi.org/10.1093/nar/gkz862.
Acknowledgements
We are thankful to all the participants for their willingness to contribute to this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Tzemah-Shahar, R., Turjeman, S., Sharon, E. et al. Signs of aging in midlife: physical function and sex differences in microbiota. GeroScience 46, 1477–1488 (2024). https://doi.org/10.1007/s11357-023-00905-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-023-00905-3