Abstract
Based on the premise that physical activity/exercise impacts hippocampal structure and function, we investigated if hippocampal metabolites for neuronal viability and cell membrane density (i.e., N-acetyl aspartate (NAA), choline (Cho), creatine (Cr)) were higher in older adults performing supervised exercise compared to following national physical activity guidelines. Sixty-three participants (75.3 ± 1.9 years after 3 years of intervention) recruited from the Generation 100 study (NCT01666340_date:08.16.2012) were randomized into a supervised exercise group (SEG) performing twice weekly moderate- to high-intensity training, and a control group (CG) following national physical activity guidelines of ≥ 30-min moderate physical activity ≥ 5 days/week. Hippocampal body and head volumes and NAA, Cho, and Cr levels were acquired at 3T with magnetic resonance imaging and spectroscopic imaging. Sociodemographic data, peak oxygen uptake (VO2peak), exercise characteristics, psychological health, and cognition were recorded. General linear models were used to assess group differences and associations corrected for age, sex, education, and hippocampal volume. Both groups adhered to their training, where SEG trained at higher intensity. SEG had significantly lower NAA/Cr in hippocampal body than CG (p = 0.04). Across participants, higher training intensity was associated with lower Cho/Cr in hippocampal body (p < 0.001). Change in VO2peak, increasing VO2peak from baseline to 3 years, or VO2peak at 3 years were not associated with hippocampal neurochemicals. Lower NAA/Cr in hippocampal body was associated with poorer psychological health and slightly higher cognitive scores. Thus, following the national physical activity guidelines and not training at the highest intensity level were associated with the best neurochemical profile in the hippocampus at 3 years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Physical activity and/or exercising are suggested as avenues to preserve brain function and structure in older adults and even to stave off dementia [1,2,3,4]. The hippocampus is considered a brain region particularly adaptable to the positive effects of physical activity and/or exercising, while also being highly susceptible to pathology associated with Alzheimer’s disease and vascular dementia [5,6,7,8]. In animal studies, exercise is demonstrated to increase hippocampal neurogenesis and improve memory functions, supporting the notion that exercising is particularly relevant for preserving hippocampal structure and function [9,10,11,12,13]. In humans, structural magnetic resonance image (MRI) studies have demonstrated larger hippocampal volumes, perhaps located to the hippocampal head, in more physically active or exercising individuals in intervention and randomized control trials [7, 14,15,16,17]. However, either no effects or even negative effects of exercising on the hippocampus volume have also been described [18,19,20,21,22,23,24]. Likewise, inconsistent results have also been reported between physical activity or exercising and cognition in humans [25,26,27,28].
Investigations into the molecular characteristics of the hippocampus might provide new insight into mechanisms through which physical activity and/or exercise impact both brain structure and function. While structural MRI of the hippocampus is widespread and well known, the measurement of cerebral metabolite levels in vivo by magnetic resonance spectroscopic imaging (MRSI) is less frequently applied. Metabolites commonly identified include N-acetyl aspartate (NAA) and choline-containing compounds, often referred as choline (Cho) and creatine (Cr). NAA is used as a proxy for neuronal viability with diminished levels of NAA found in conditions such as Alzheimer’s disease [29], mild cognitive impairment [30], and normal aging [31], suggesting neuronal or synaptic losses in these conditions. Cho-containing molecules are components of membrane phospholipids, with decreased or increased levels suggesting changes in number of membranes related to for instance cellular density, turnover, breakdown, or inflammation, and associated with cognitive impairments in aging [32]. Cr provides a proxy measure of cellular energy and represents the sum of creatine and phosphocreatine which are present in both neurons and glial cells. This signal is often used as an internal standard for comparison of metabolites across different groups/conditions [33].
NAA/Cr or Cho/Cr levels are hence considered biomarkers of neuronal health and neural cell membrane density, respectively. Even though MRSI is available on clinical scanners, this technique is seldom employed compared to structural MRI. MRSI in the hippocampus, a core region of interest in the study of the effects of exercise and physical activity on the brain, has not been performed. So far, only one observational study has investigated the effect of exercising on brain metabolite levels in cortex using single-voxel magnetic resonance spectroscopy (MRS) [34]. They reported that middle-aged adults (45–60 years) who performed endurance training on a regular basis had higher NAA/Cr and Cho/Cr levels in frontal and occipitoparietal cortices, respectively, compared to their sedentary counterparts. Furthermore, maximal oxygen uptake (VO2max) was positively associated with frontal NAA/Cr and occipital Cho/Cr levels.
The aim of this study was to assess NAA/Cr and Cho/Cr in the hippocampal head and body in older adults taking part in the Generation 100 study, a randomized clinical trial (RCT) of supervised exercise versus following national physical activity guidelines [35]. Previous studies suggest a particularly beneficial effect of exercising on the hippocampal head [14] while atrophy of CA1 subfield of the hippocampal body is associated with risk of Alzheimer’s disease [36]. We therefore employed MRSI with a semi-localized adiabatic selective refocusing (sLASER) pulse sequence [37] to acquire metabolic profiles from voxels in the hippocampal head and body. Hippocampal head and body volumes from structural MRI were used to correct for volume differences. We predicted that the supervised exercise group (SEG) would have a higher NAA/Cr and Cho/Cr in the hippocampal head compared to the control group (CG) after 3 years of intervention. In line with the cardiorespiratory fitness hypothesis [38], we assumed that NAA/Cr and Cho/Cr levels in both hippocampal head and body would be positively associated with gains in peak oxygen uptake (VO2peak) as well as VO2peak at time of MRSI. We explored associations between measures related to exercising and physical activity, i.e., its intensity and duration, to evaluate possible association between these and hippocampal metabolites to extend the knowledge on how exercising may influence the brain. Finally, we investigated if NAA/Cr and Cho/Cr were associated with psychological distress and general cognition at 3 years to link our results to clinical outcomes.
Methods
Ethics
The RCT and the substudy were approved by the Regional Committee for Medical Research Ethics (REK 2012/381 B and REK 2012/849, respectively). Both studies adhered to the Helsinki Declaration and participants gave their written informed consent to both.
The RCT study
Participants were from the RCT Generation 100 study (NCT01666340, ClinicalTrials.gov registry) which originally set out to evaluate the effect of 3 years of supervised exercise training versus following the national physical activity guidelines on morbidity and mortality in older adults. The MRS acquisition was planned to be an outcome after end of the intervention at 3 years. At 3 years, the study period was extended to 5 years due to lower mortality in the cohort than anticipated based on national numbers [35]. MRS was, however, not repeated at the new endpoint.
All inhabitants of Trondheim, Norway, born between 1936 and 1942 registered in the National Population Registry (n = 6966) received an invitation letter in 2012. A total of 1790 declared interest while 1422 declined. Consequently, 1567 persons (790 women) were invited to baseline examination; 174 did not show up or withdrew and 49 were excluded. The inclusion criterion was having the physical ability to take part in an exercise intervention. Exclusion criteria were somatic diseases precluding vigorous physical activity or associated with a significantly shortened life expectancy, dementia, and any condition which limited ability to exercise as well as participating in another exercise study.
Participants were stratified by sex and cohabitation status before being randomized into a supervised exercise group performing either high-intensity interval training (HIIT) (n = 400) or moderate-intensity continuous training (MICT) (n = 387), and a control group (n = 780). Before randomization in the Generation 100 study, the participants were asked if they were interested in also taking part in a brain MRI study. Baseline data collection before the start of the intervention was performed between August 2012 and June 2013.
The Generation 100 MRI study
A total of 111 (55 men, 56 women) agreed to participate. Six were excluded due to MRI contraindications or previous neurosurgical disorders, leaving 105 participants (53 men, 52 women). The participants underwent a standardized structural MRI protocol [20], and at the 3-year follow-up, MRSI data was acquired. In total, 90 participants were still in the MRI study at the 3-year follow-up, of which 11 participants were not scanned with MRSI due to inability to stay in the scanner after the structural scans were acquired. MRSI data from another 16 participants could not be used due to insufficient quality because of movement (Fig. 1).
Interventions
Participants in the SEG were randomized to twice weekly MICT or HIIT sessions. The MICT sessions consisted of 50 min of continuous exercise at about 70% of peak heart rate corresponding to a rating of perceived exertion of about 13 on the Borg scale [39]. The HIIT sessions started with 10-min warm-up followed by 4 × 4 min intervals at about 90% peak heart rate corresponding to a rating of perceived exertion of about 16 [40]. Every 6th week, participants in the SEG attended mandatory spinning classes where they exercised with a heart rate monitor to ensure compliance with the prescribed training intensity. The participants in the active control group were asked to follow the Norwegian physical activity guideline for 2012, which states at least 30 min of moderate-level physical activity at least 5 days a week [35]. Yearly questionnaires were used to assess adherence to the allocated program, defined as performing at least 50% of the prescribed exercise sessions or physical activity recommendations (for details, see [40]).
Sociodemographic, health, fitness, and exercise variables
Date of birth, sex, and level of education (primary school, high school, and university) were obtained at baseline [35]. Data on cohabitation status and current smoking (yes/no) was obtained from annual questionnaires. Psychological health was assessed with a Norwegian validated version of the HADS questionnaire [41, 42]. Total HADS score was used [43, 44]. The Norwegian validated version of Montréal Cognitive Assessment (MoCA) scale was administrated [45] and total score reported.
Clinical measurements, including body mass index (BMI), blood pressure, and resting heart rate, were acquired at baseline and after 3 years of intervention using standard practices [35]. At the same timepoints, fasting blood samples were obtained, and high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides (TG), blood glucose, hemoglobin A1c (HbA1C), and high-sensitivity C-reactive protein (hsCRP) were assessed.
Cardiorespiratory fitness was assessed objectively as VO2peak using graded maximal exercise testing on a treadmill or exercise bike at baseline and 3-year follow-up [46]. Participants with previous heart diseases were tested under ECG monitoring, and participants with known cardiovascular disease were tested according to the American College of Cardiology/American Heart Association guidelines for exercise testing of patients with known cardiovascular disease [47]. Maximal oxygen uptake (VO2max) was reached when the respiratory exchange ratio was ≥ 1.05 and the participant did not increase oxygen uptake more than 2 mL during the last 30 s despite the increased workload. If these criteria were not met, participants were described as having reached VO2peak. Many participants (see Table 2) did not meet the criteria for VO2max which is common in aging populations [40], and instead reached VO2peak. Only VO2peak data from participants who stopped the test due to exhaustion were included while those who stopped due to factors such as pain or lack of motivation were excluded. VO2max and VO2peak data were combined depending on which was achieved by each participant, into a variable termed VO2peak.
A physical activity questionnaire including questions on frequency and duration of exercise was used to calculate weekly exercise duration (min/WK) at 3 years (for details, see [40]), while the Borg 6–20 rating of perceived exertion scale [39] was used to assess exercise intensity.
Brain MRI and MRSI
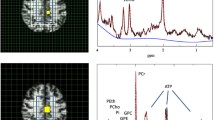
MRI/MRSI data were acquired on 3T Magnetom Skyra scanner (Siemens AG, Erlangen, Germany) equipped with a 32-channel head coil for homogenous signal-to-noise ratio (SNR) due to radio frequency (RF or B1) uniformity. Following advanced static magnetic field (B0) shimming, the MRI scan used in this study, a high-resolution 3D T1-weighted MPRAGE (TR = 1900; TE = 3.16; FOV = 256 × 256; slice thickness = 1 mm; gap = 0 mm), was acquired. From the T1-weighted MPRAGE scans, we obtained hippocampal long-axis volumes, i.e., hippocampal head, body, and tail volumes, in Freesurfer v. 6.0 (http://surfer.nmr.mgh.harvard.edu/) [48]. The MPRAGE scan was used for positioning of the MRSI grid which was angulated parallel to right hippocampus long axis (Fig. 2). A 2D MRSI method based on sLASER sequence with 4 preparation scans in a 16 × 6 × 1 grid with TR = 1700 ms, TE = 40 ms, flip angle = 90°, water suppression band width = 50 Hz, averages = 6, field of view = 120 × 120, volume = 60 × 60 mm, and slice thickness 10 mm was used.
Magnetic resonance spectroscopic imaging (MRSI) acquisition and representative spectra. A Positioning of the MRSI volume/grid in one Generation 100 study participant. The grid was angulated along the hippocampal long axis. The tip of the hippocampal head was always the third lower row voxel within the white outlined 16 × 16 grid. In the upper image, the turquoise square outlines the hippocampal head voxel, while the yellow square outlines the hippocampal head voxel. B Typical spectra from the hippocampal body. The upper spectrum is from a participant in CG and the lower spectrum from a participant in SEG. Metabolites shown in spectra (left) are N-acetyl aspartate (NAA), creatine (Cr), choline (Cho), and myoinositol (Ino). The MRSI maps (right) reflect the total NAA (tNAA) map for respective subjects
MRSI processing and metabolite quantification of NAA, Cho, and Cr
MRSI processing consisted of two main steps: preprocessing and fitting. Preprocessing included zero filling, water peak removal, phase correction, baseline correction, eddy current, and noise filtering. Although water suppression was used during MRSI acquisition, the spectra still contained a residual water peak. Usually, this peak width is in the frequency range < 45 Hz. We used Hankel singular value decomposition (HSVD) filter [49] where the water signal is first estimated using a subspace-based decomposition into a sum of complex damped exponentials and subsequently removed from the measured signal to suppress the water component in the specified frequency region, while the other frequency regions are minimally affected. For baseline correction, local minima of spectra were found and then iteratively generated envelopes were averaged and used to estimate baseline, which was subtracted from spectrum to generate baseline-corrected spectrum.
Metabolite quantification was achieved with LCModel version 6.3-1K (Stephen Provencher, Inc., Oakville, Ontario, Canada) [50]. Chemical shift and J-coupling values from Govindaraju et al. were used to simulate the metabolites, which included lipids and macromolecules to get the basis set [51]. Each metabolite was simulated with a Lorentzian lineshape with a full width at half maximum of 0.8 Hz. The accuracy of this basis set compared with experimentally derived metabolite spectra has been previously demonstrated [52]. Simulation of the collection of basis functions (basis set) is the preferred method in regions such as the hippocampus where MR spectra can be of lesser quality due to for instance partial volume effects [53,54,55]. However, the first few points of the FID contain very broad signals, not easily modeled with a basis set. Therefore, starting the fitting point was set to be 24th data point with endpoint set to 2048.
1H-MRSI techniques use rectangular or cubic voxels, which do not usually correspond with the curved shapes of the hippocampus. Hence, MRSI voxels can include a combination of cerebrospinal fluid (CSF), gray matter (GM), and white matter (WM). Because CSF has no measurable metabolites, the presence of a large portion of CSF within the voxel could underestimate the metabolite concentrations. Furthermore, metabolite concentrations are different in GM and WM portions [56, 57]. When a voxel contains a heterogeneous tissue composition, the spectroscopic signal acquired from this voxel will consist of the signal from different tissues making reliable measurements difficult. To avoid contribution of different tissues and CSF inside voxels, we did partial volume correction [58,59,60,61]. LCModel quantification accuracy was assessed through Cramer-Rao lower bounds (CRLB) [62]. Metabolites quantified with a CRLB above 50% were considered not detected. The average CRLB for NAA, Cho, and Cr were 6.9%, 7.0%, and 5.5%, respectively. The LCModel fitting was performed over the spectral range from 0 to 5 ppm. Total NAA, Cho, and Cr values were quantified and reported as NAA/Cr and Cho/Cr ratios.
Statistics
Mean, standard deviation (SD), 95% confidence intervals (CI), and % were used as appropriate to display the distribution of variables. Statistical comparisons of sociodemographic, health, fitness, and exercise variables were performed with t-tests, Mann–Whitney U, and Pearson chi-square as appropriate. Effect size calculated using Cohen’s d was provided for NAA/Cr and Cho/Cr in the hippocampal head and body. Across all subjects, a paired t-test was used to compare differences in the spatial distribution of NAA/Cr and Cho/Cr between voxels in the hippocampal head and body.
Group differences in NAA/Cr and Cho/Cr in the hippocampal head and body were assessed in general linear models including the covariates sex, age, education, and volume of the hippocampal head or body, respectively.
Since increasing VO2peak is suggested to mediate the positive effects of exercise on the brain, we investigated if degree of change in VO2peak (VO2peak 3-years - VO2 peak Baseline) was associated with NAA/Cr and Cho/Cr in the hippocampal head and body in general linear models. Subsequently, we stratified participants into groups with increasing (54.5%) versus decreasing (45.5%) VO2peak from baseline to 3 years and evaluated the presence of a group difference. Finally, we investigated the association between VO2peak at 3 years with NAA/Cr and Cho/Cr. These analyses were performed across all participants (SEG&CG) in general linear models including sex, age, education, and volume of the hippocampal head or body as covariates. Sensitivity analyses were performed limited to those reaching VO2max.
To investigate potential associations between variables related to exercising at time of MRS, we assessed the relationships between NAA/Cr and Cho/Cr and weekly exercise duration and intensity across all subjects in general linear models including sex, age, education, and volume of the hippocampal head or body as covariates.
Finally, we investigated if HADS and MoCa scores were associated with NAA/Cr or Cho/Cr in the body or head of the hippocampus across all subjects (SEG&CG) using one general linear model for NAA/Cr and one for Cho/Cr with sex, age, education, and hippocampal volume at 3 years, to evaluate the potential clinical impact of these MRSI biomarkers.
Based on the group sizes in the only previously published exercise and brain metabolite study, n = 28 in each group was considered a sufficient number of participants needed to detect a group difference [34].
SPSS (IBM Corp. Released 2010. IBM SPSS Statistics for Windows, Version 28.0. Armonk, NY: IBM Corp) was used for all analyses. The statistical significance threshold was set to p < 0.05.
Results
Usable spectral data were obtained in 63 (32 SEG/31 CG) of the 79 participants in whom MRSI scans were conducted. Motion, small hippocampal volumes, and technical processing/analysis issues were reasons for lack and exclusion of MRSI data. There were no significant differences in sociodemographic variables or VO2peak at 3 years between participants with and without MRSI data (Table 1).
Sociodemographic, health and fitness, and exercise variables
An overview of the sociodemographic and health characteristics of the included participants in the two groups at baseline and after 3 years is presented in Table 2. There were a similar number of women and men in both groups, and no significant differences between the groups in any of the variables at baseline or 3-year follow-up. VO2peak was not significantly different in the two groups at baseline or after 3 years.
Both CG and SEG adhered similarly well to their respective regimes, with an adherence of 87.5% and 84.4%, respectively, at 3 years (Table 3). There was no significant difference in average weekly exercise duration between the groups, but exercise intensity and the percentage of participants exercising per the HIIT protocol were significantly higher in SEG than CG (Table 3).
Hippocampal metabolite ratios, intervention groups, VO2peak, and exercise variables
The observed mean, SD, 95% CI, and Cohen’s d effect size of NAA/Cr and Cho/Cr ratios in the voxels in the hippocampal head and body for each group are presented in Table 4. For NAA/Cr ratios in the hippocampal body and head, moderate differences were present, while for Cho/Cr, small to moderate effect sizes were found between SEG and CG. The paired comparison of NAA/Cr and Cho/Cr levels, respectively, showed higher NAA/Cr levels in the hippocampal body than head within the subjects.
The general linear model revealed a significant effect of group on NAA/Cr ratio in the hippocampal body with lower levels in SEG compared to CG after controlling for age, sex, education, and hippocampal body volume. No significant group effect was uncovered for Cho/Cr in hippocampal body or for NAA/Cr or Cho/Cr in the hippocampal head (Table 5).
There were no associations between change in VO2peak from baseline to 3 years across all participants (SEG&CG) and hippocampal metabolites correcting for age, sex, education, and hippocampal head or body volume (Table 5). There was also no difference in hippocampal metabolite ratios between the participants in the increasing VO2peak versus the declining VO2peak group (Table 5). Furthermore, there were no significant associations between VO2peak at 3 years and hippocampal metabolites at 3 years. Sensitivity analyses with only participants reaching VO2max (n = 37) revealed the same results (results not shown).
Across all participants (SEG&CG), weekly exercise duration was not associated with NAA/Cr or Cho/Cr in neither the hippocampal head nor body, correcting for age, sex, education, and hippocampal head or body volume. Higher exercise intensity at 3 years was associated with lower Cho/Cr level in the hippocampal body correcting for age, sex, education, and hippocampal body volume. No other associations were present for exercise intensity (Table 5).
Hippocampal metabolite ratios and associations with HADS and MoCA scores
A significant negative association was present between HADS score and NAA/Cr in the hippocampal body but not hippocampal head, with lower NAA/Cr associated with poorer psychological health (Fig. 3, Table 6). There was no significant effect of age, sex, education, or hippocampal volume on HADS score. No relationships between HADS scores and Cho/Cr were uncovered (Table 6).
Performance on MoCA was associated with NAA/Cr in the hippocampal body but not in the head, with a lower level of NAA/Cr linked to slightly higher scores, correcting for age, sex, education, and hippocampal volume in the same model (Fig. 3, Table 6). There was no significant effect of age, sex, education, or hippocampal volume on MoCA score. No relationships between MoCA scores and Cho/Cr were uncovered (Table 6).
Discussion
This is the first exercise RCT in older adults examining metabolites in the hippocampus, a region considered highly modifiable by training and physical activity and a key structure in dementia. Opposed to our predictions, the CG had a higher NAA/Cr in the hippocampal body than the SEG after 3 years of intervention. Likewise, neither change in VO2peak, increasing VO2peak from baseline to 3-year intervention, nor VO2peak at 3 years were associated with more favorable hippocampal metabolites, as predicted based on the cardiorespiratory fitness hypothesis [38]. Furthermore, greater exercise intensity, which is connected to larger gains in cardiorespiratory fitness [63], was negatively associated with Cho/Cr in the hippocampal body.
The CG and SEG were well-matched at baseline, and both trained according to their specification with the SEG exercising at a higher intensity as intended. Still, the CG had a significantly higher level of NAA/Cr in the hippocampal body compared to the SEG. NAA/Cr is considered a marker of neuronal viability as it is located to neuronal mitochondria which are enriched in the synapses [64]. The lower NAA/Cr in the SEG suggests a reduction in synaptic health in the SEG even after controlling for hippocampal volume. We have previously shown that hippocampal atrophy rate from baseline was greater in the right hippocampus (where MRSI data were acquired) in the SEG compared to the CG after 3 and 5 years of intervention [20]. Our results were unexpected given the prevailing view of exercising being particularly beneficial for hippocampal structure and function. However, the literature is inconsistent with two recent meta-analyses reporting no effect of exercising on hippocampal volume [65, 66], while another finding reports a small positive effect on right and left hippocampus in older adults [67] suggesting that there are unanswered questions related to exercising and the hippocampus in humans. The lower level of NAA/Cr in the hippocampal body of SEG in our study is in line with the greater rate of atrophy located specifically to the hippocampal body in this group after 3 years [20]. Since we corrected for hippocampal body volume, the lower NAA/Cr level implies that the effect of SEG membership on hippocampal metabolites surpassed that explained by hippocampal volume reduction, suggesting that structural MRI and MRSI provide additive information. The group difference in NAA/Cr in the hippocampal body of CG and SEG was observed despite similar VO2peak levels in these groups. This concurs with our finding that none of the VO2peak measures were related to hippocampal metabolite levels. Interestingly, the previous observational exercise study implementing single-voxel MRS found a higher NAA/Cr ratio in the frontal cortex in the endurance-trained group than their sedentary counterparts [34]. The endurance-trained group had a similar physical activity level as our CG and age-average VO2max levels, like our participants [68]. This suggests that the levels of physical activity/exercise in our CG (and Gonzales et al.’s endurance training group) combined with an age-average VO2max/peak level provide brain health benefits. Contrary to the findings of Gonzales et al., however, we did not uncover a group difference in Cho/Cr, although a trend was evident (CG > SEG), but the effect sizes were small and larger samples are needed to determine group differences in Cho/Cr than NAA/Cr based on our data. Nevertheless, the negative relationship between Cho/Cr and exercise intensity across all participants (SEG&CG) implies that hard cardiorespiratory training had a negative effect on the density of cellular membranes across all neural cells, not just affecting neurons in the hippocampus. Overall, our findings, together with those of Gonzales et al., indicate that there might be a reversed J or inverted U-shaped relationship between training intensity and brain health outcomes. This concurs with previous descriptions of a reversed J-shaped relationship between exercise and premature mortality/cardiovascular disease, where a very high dose of strenuous activity is associated with higher risks compared to less extreme doses while being sedentary comes out worst [69]. Our findings imply that both neurons and glial cells in the hippocampus are sensitive to exercising and that following national physical activity guidelines, having an age-average VO2peak level [68], and not performing training at very high-intensity levels might be ideal for preserving hippocampal metabolites for older adults.
There was no relationship between change in VO2peak from baseline to 3 years, being in the group with an increasing versus declining VO2peak from baseline to 3 years of intervention, or VO2peak at 3 years and hippocampal metabolite levels. Thus, no support for the cardiorespiratory fitness hypothesis was found. This could be due the inclusion of participants from the general population with average VO2peak values [68]. Additionally, both the SEG and CG were physically active. Many studies include sedentary participants allocated to exercising versus remaining sedentary [14, 18, 34]. In our study, however, the CG diligently followed the national guidelines for physical activity, with 87.1% adherence rate. Although the CG were physically active, they exercised with a lower intensity level compared to SEG as shown by lower scores on the Borg scale and a lower percentage of participants following the HIIT protocol. The only previous MRS study on exercise effects reported a significant association between VO2max and NAA/Cr in the frontal cortex [34]. We did not find a similar association in the sensitivity analysis limited to participants who reached VO2max (n = 37). The discrepancy could arise from the broader distribution of VO2max in the Gonzales et al. study due to their inclusion of a sedentary control group. Their VO2max values spanned 34 units, while our VO2peak spanned 16 units based on the SDs. A greater range of VO2max/peak values is present when sedentary and exercising individuals are combined in the same study and a greater percentage gain in VO2max/peak is found in sedentary people who begin to exercise [70]; likewise, age may play a role, resulting in discrepant results. The relatively fit participants in the SEG and CG remained remarkably stable in measures considered to be positively affected by exercising such as BMI, blood glucose, and mood from baseline to 3 years [71]. Given the well-matched groups, stable clinical health in both the SEG and CG, lack of association between VO2peak and hippocampal metabolites, and the lower NAA/Cr level in the hippocampal body in the SEG most likely stemmed from the intervention. Indeed, NAA/Cr and Cho/Cr ratios were lower in both voxels in the SEG compared to the CG group, albeit they did not reach statistical significance, pointing to a general trend of reduced levels of neurochemicals.
All significant findings related to effects of exercising on hippocampal metabolites were uncovered in the hippocampal body only, but not its head. The hippocampal head volume has previously been suggested as linked to higher levels of cardiorespiratory fitness [14]. In this cohort though, the hippocampal body was the region with greater atrophy rate in the SEG compared to CG [20], providing an independent line of evidence for the hippocampal body as being susceptible to negative effects of certain types of training in older adults. High-intensity training has been shown to affect hippocampal perfusion and increase lactate levels [15, 72,73,74,75] and stress responses [76,77,78] in older adults and animals. Any or all these mechanisms can influence neuronal and glial metabolism and lead to the differences between SEG or CG, and the association related to exercise intensity.
Higher psychological distress, as measured with HADS, was associated with lower NAA/Cr in the hippocampal body across both groups. As can be seen in Fig. 3, there was a flooring effect in the HADS score as many participants did not report mental health problems. There were six participants, three in each group, with HADS scores > 11 which is considered a cutoff for a clinically relevant level of psychological distress [41, 79]. These participants had similarly high scores on HADS at earlier and later assessments, suggesting stable high mental health problems. Since the six individuals with high HADS scores were equally divided between SEG and CG, and mean HADS scores was slightly higher but not significantly, in the CG group, it is unlikely that HADS scores contributed to the observed CG versus SEG group difference in NAA/Cr sin the hippocampal body. A relationship between NAA/Cr and HADS scores were not present in the hippocampal head. This might be due to the observed differences in NAA/Cr levels in the MRSI voxel in the head and body of the hippocampus and their relationship to clinical measures. If/Whether neurochemicals measured in different hippocampal voxels with MRSI are differently associated with clinical measures, needs to be verified in other cohorts. A lower level of NAA/Cr has been reported with single-voxel MRS in the hippocampus of bipolar patients compared to healthy control earlier [80], providing some support for an effect of psychological distress on hippocampal NAA/Cr levels.
The lower NAA/Cr level uncovered in the SEG group could have cognitive implications [81, 82], but we did not detect evidence of this. Firstly, a lower NAA/Cr level in the hippocampal body was negatively associated with general cognitive ability (MoCA scores) across both groups, i.e., lower level and better MoCA scores, although the relationship was weak. Secondly, no difference were detected in MoCA scores between SEG versus CG in the MRS cohort (Table 2) or the entire RCT Generation 100 study SEG and CG groups after 5 years of intervention [83]. MoCA is a screening tool for mild cognitive impairment [45], and has a limited resolution with a notable ceiling effect, as can be seen in Fig. 3. This is a shortcoming of this measurement and could affect the results. We used the raw scores of the test, and corrected for age, sex and level of education in the statistical model, which are variables known to be associated with performance on the MoCA test [84, 85]. Only body NAA/Cr, not age, sex, education, right hippocampal volume, nor head NAA/Cr, was associated with MoCA performance in this sample. Even though significant, the variance in MoCA scores explained by NAA/Cr in the hippocampal body was much lower than HADS scores explained. To further complicate matters, the relationship between NAA/Cr in the hippocampal head and MoCA scores was not significant, but very weakly positively correlated as evidenced in both the statistical analysis and the scatter plot. A strong positive relationship between NAA/Cr in gray matter regions of interests using single-voxel MRS has been reported previously [82, 86]. One could speculate that the differences in the NAA/Cr in the hippocampal head and body demonstrated in the within-subject analysis could be related to the functional specialization along the hippocampal long axis [87]. This could potentially explain our findings, but it is highly speculative and needs validation.
Medial temporal lobe NAA/Cr has been suggested as a potential marker of dementia [88, 89]. Two early studies using single-voxel MRS reported an uncorrected group difference in NAA/Cr levels in the right hippocampus of 16.5% between controls and dementia patients [29], and the left hippocampus of 17.9% between controls and mild cognitive impairment (MCI) patients [30]. The uncorrected mean percentage difference between NAA/Cr in SEG and CG was 17.9% in our study. Still, we did not observe a negative effect of NAA/Cr level on general cognition, but rather a weak positive effect. Other MRS studies in the precuneus of patients with MCI or dementia consistently report that lower NAA/Cr is associated with or predicts lower general cognitive function, MCI, or dementia [29, 90,91,92]. This could imply that the observed difference in NAA/Cr in the hippocampal body is still within what is normal or supports normal functioning. The group difference in NAA/Cr might thus be within the range of typical aging but could render the SEG participants with less brain reserve as they age. Finally, none of the covariates (sex, age, education, and hippocampal volume) was associated with MoCA or HADS scores, implying that MRSI might be more sensitive to functional outcomes than the standard variables described as linked to mental health and cognitive outcomes.
Strengths and Limitations
The main strength was the study design with recruitment from the general population into an RCT, the detailed clinical assessment from baseline, objective VO2peak measurements, and long-term supervised exercising for three years. Participants adhered well to their assigned training regime, but since also the CG subjects were meticulous in their training, there were few differences between the SEG and CG, except for exercise intensity. In Norway, 32% of older adults (65 + years) follow the national physical activity guidelines [93], while in the present study, 87.1% in the CG did, demonstrating remarkable compliance. The excellent compliance to the national physical activity guidelines makes it difficult to generalize the results from CG to the general population.
All statistical models were corrected for variables known to be connected to hippocampal size such as age [94], sex [95], and education [96] as well as volume per se. Many statistical analyses were performed in this study following the main outcome analysis of the intervention group effect (SEG versus CG). We did not employ methods for correcting for multiple comparisons. Implementation of Bonferroni correction in the across all participant general linear model results related to exercise characteristics would give an adjusted p-values < 0.005 (p = 0.05 divided by 10 analyses for NAA/Cr and 10 analyses for Cho/Cr), and for the prediction of MoCA and HADS score, p-values < 0.025 (p = 0.05 divided by two analyses). Thus, the p-values uncovered would have been significant also after implementation of such correction.
A limitation of the study was the low number of participants in whom we were able to obtain acceptable MRSI data. Still, the final number was similar to the n in the only previous study investigating the effect of exercise on brain metabolites [34]. Nevertheless, in this age group, single-voxel MRS could be a good alternative to MRSI due to better SNR and shorter acquisition times. Still, using MRSI with sLASER for investigating hippocampal metabolites in distinct long-axis regions of the hippocampus is novel and provided a map of the spatial variation of neurochemicals not obtained with single-voxel MRS. The fact that we found lower NAA/Cr in the hippocampal body, the same region where we have previously shown greater atrophy rate in the SEG compared to the CG [20], and that there were significant differences in NAA/CR levels between the hippocampal head and body within subjects supports the notion that MRSI with sLASER reflects variability between anatomical regions even within a structure as small as the hippocampus. sLASER has the advantage to provide a sharper voxel localization and low sensitivity to B0 and B1 inhomogeneities. These features could be advantageous for MRSI of the hippocampus considering its small size and proximity to structures such as the petrous bone and sinuses which can cause broader linewidth and relatively low spectral resolution compared to other brain regions. Most studies that have applied MRS to study neurochemicals in the hippocampus have implemented large voxels compared to the size of the hippocampus and higher magnetic field than 3T [97,98,99,100,101]. However, sLASER suffers from longer minimum TE and higher RF energy deposition being directly limited by specific absorption rate restrictions which must be considered, especially at higher magnetic fields. The Cr resonance is widely used as internal reference as its level is high and comparable in different tissue types in the brain. Since Cr is present in neural and glial cells, the interpretation of Cr levels can be difficult, and any difference in a ratio can arise from differences in either the numerator (i.e., NAA or Cho) or denominator (i.e., Cr). Moreover, other metabolites of interest could have been examined (e.g., myoinositol/Cr levels) from the existing MRSI data, which have been shown to be related specifically to MCI and dementia [89, 91, 92, 102, 103]. In the future, more advanced MRS methods with J-coupling could be employed to investigate other neurochemicals of importance, such as excitatory (glutamate) and inhibitory (γ-aminobutyric acid) neurotransmitters, as well as energy metabolites like lactate or β-hydroxybutyrate [104]. Also, unlike the structural MRI protocol, MRSI was only collected after 3 years, the original RCT endpoint. The problems experienced during the MRSI acquisition at 3 years, leading to loss of 22% of the MRSI data due to motion, small hippocampal volumes, and technical processing/analysis issues, led us to decide not to repeat MRS acquisition at 5 years, the updated RCT endpoint. As a comparison, we did not lose any structural MRI data due to motion.
The lack of longitudinal MRSI data is a shortcoming of this study. It is possible differences existed in hippocampal metabolites between groups already at baseline, but given the lack of SEG and CG differences on other variables, this is likely not to be a serious problem. The results at the 3-year timepoint would have been even stronger if repeated at 5 years.
Conclusion
This study demonstrated that SEG had significantly lower NAA/Cr in the hippocampal body after 3 years of supervised exercise intervention compared to an active CG following the national physical activity guidelines. Moreover, higher exercise intensity, irrespective of group, was associated with lower Cho/Cr in the same part of the hippocampus. The MRSI results are in line with structural MRI results from the same cohort showing that the SEG group had higher rates of hippocampal atrophy [20] and white matter hyperintensity growth [105] than the CG. Additionally, our results demonstrate that sLASER allows for a detailed investigation of spatially specific metabolic profiles along the hippocampal long axis, and that the neurochemical profiles were more closely associated with functional outcome than for instance the hippocampal volume. In summary, this study demonstrated that diligently following the national physical activity guidelines and training at moderate intensity were linked to the healthiest neurochemical profile in the hippocampus and that metabolites from both neurons and glia cells were sensitive to exercise regimes.
Data Availability
Data used in this manuscript can be accessed by contacting the corresponding author. Access to data by qualified investigators will be subject to ethical and scientific review (to ensure the data is being requested for valid scientific research) and must comply with the European Union General Data Protection Regulations (GDPR), Norwegian laws and regulations, and NTNU regulations. The completion of a material transfer agreement (MTA) signed by an institutional official will be required.
References
Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819–28. https://doi.org/10.1016/S1474-4422(11)70072-2.
Laurin D, Verreault R, Lindsay J, et al. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58(3):498–504. https://doi.org/10.1001/archneur.58.3.498.
Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–46. https://doi.org/10.1016/S0140-6736(20)30367-6.
Norton S, Matthews FE, Barnes DE, et al. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788–94. https://doi.org/10.1016/S1474-4422(14)70136-X.
Arba F, Quinn T, Hankey G, et al. Cerebral small vessel disease, medial temporal lobe atrophy and cognitive status in patients with ischaemic stroke and transient ischaemic attack. Eur J Neurol. 2017;24(2):276–82. https://doi.org/10.1111/ene.13191.
Domingos C, Pêgo J, Santos N. Effects of physical activity on brain function and structure in older adults: a systematic review. Behav Brain Res. 2021;402:113061. https://doi.org/10.1016/j.bbr.2020.113061.
Niemann C, Godde B, Voelcker-Rehage C. Not only cardiovascular, but also coordinative exercise increases hippocampal volume in older adults. Front Aging Neurosci. 2014;6:170. https://doi.org/10.3389/fnagi.2014.00170.
Wu A, Sharrett AR, Gottesman RF, et al. Association of brain magnetic resonance imaging signs with cognitive outcomes in persons with nonimpaired cognition and mild cognitive impairment. JAMA Netw Open. 2019;2(5):e193359–e193359. https://doi.org/10.1001/jamanetworkopen.2019.3359.
Bhattacharya TK, Pence BD, Ossyra JM, et al. Exercise but not (–)-epigallocatechin-3-gallate or β-alanine enhances physical fitness, brain plasticity, and behavioral performance in mice. Physiol Behav. 2015;145:29–37. https://doi.org/10.1016/j.physbeh.2015.03.023.
Brockett AT, LaMarca EA, Gould E. Physical exercise enhances cognitive flexibility as well as astrocytic and synaptic markers in the medial prefrontal cortex. PLoS ONE. 2015;10(5):e0124859. https://doi.org/10.1371/journal.pone.0124859.
Kim T-W, Choi H-H, Chung Y-R. Treadmill exercise alleviates impairment of cognitive function by enhancing hippocampal neuroplasticity in the high-fat diet-induced obese mice. J Exerc Rehabil. 2016;12(3):156. https://doi.org/10.12965/jer.1632644.322.
Vilela TC, Muller AP, Damiani AP, et al. Strength and aerobic exercises improve spatial memory in aging rats through stimulating distinct neuroplasticity mechanisms. Mol Neurobiol. 2017;54(10):7928–37. https://doi.org/10.1007/s12035-016-0272-x.
Park H-S, Kim C-J, Kwak H-B, et al. Physical exercise prevents cognitive impairment by enhancing hippocampal neuroplasticity and mitochondrial function in doxorubicin-induced chemobrain. Neuropharmacology. 2018;133:451–61. https://doi.org/10.1016/j.neuropharm.2018.02.013.
Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017–22. https://doi.org/10.1073/pnas.1015950108.
Maass A, Düzel S, Goerke M, et al. Vascular hippocampal plasticity after aerobic exercise in older adults. Mol Psychiatry. 2015;20(5):585–93. https://doi.org/10.1038/mp.2014.114.
Rosano C, Guralnik J, Pahor M, et al. Hippocampal response to a 24-month physical activity intervention in sedentary older adults. Am J Geriatr Psychiatry. 2017;25(3):209–17. https://doi.org/10.1016/j.jagp.2016.11.007.
Wilckens KA, Stillman CM, Waiwood AM, et al. Exercise interventions preserve hippocampal volume: A meta-analysis. Hippocampus. 2021;31(3):335–47. https://doi.org/10.1002/hipo.23292.
Jonasson LS, Nyberg L, Kramer AF, et al. Aerobic exercise intervention, cognitive performance, and brain structure: results from the Physical Influences on Brain in Aging (PHIBRA) study. Front Aging Neurosci. 2016;8:336. https://doi.org/10.3389/fnagi.2016.00336.
Matura S, Fleckenstein J, Deichmann R, et al. Effects of aerobic exercise on brain metabolism and grey matter volume in older adults: results of the randomised controlled SMART trial. Transl Psychiatry. 2017;7(7):e1172–e1172. https://doi.org/10.1038/tp.2017.135.
Pani J, Reitlo LS, Evensmoen HR, et al. Effect of 5 years of exercise intervention at different intensities on brain structure in older adults from the general population: a generation 100 substudy. Clin Interv Aging. 2021;16:1485. https://doi.org/10.2147/CIA.S318679.
Scheewe TW, van Haren NE, Sarkisyan G, et al. Exercise therapy, cardiorespiratory fitness and their effect on brain volumes: a randomised controlled trial in patients with schizophrenia and healthy controls. Eur Neuropsychopharmacol. 2013;23(7):675–85. https://doi.org/10.1016/j.euroneuro.2012.08.008.
Stephen R, Liu Y, Ngandu T, et al. Brain volumes and cortical thickness on MRI in the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER). Alzheimers Res Ther. 2019;11(1):1–10. https://doi.org/10.1186/s13195-019-0506-z.
Venkatraman VK, Sanderson A, Cox KL, et al. Effect of a 24-month physical activity program on brain changes in older adults at risk of Alzheimer’s disease: the AIBL active trial. Neurobiol Aging. 2020;89:132–41. https://doi.org/10.1016/j.neurobiolaging.2019.02.030.
Wagner G, Herbsleb M, Fdl Cruz, et al. Hippocampal structure, metabolism, and inflammatory response after a 6-week intense aerobic exercise in healthy young adults: a controlled trial. J Cereb Blood Flow Metab. 2015;35(10):1570–8. https://doi.org/10.1038/jcbfm.2015.125.
Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14(2):125–30. https://doi.org/10.1111/1467-9280.t01-1-01430.
Smith PJ, Blumenthal JA, Hoffman BM, et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239. https://doi.org/10.1097/PSY.0b013e3181d14633.
Young J, Angevaren M, Rusted J, et al. Aerobic exercise to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev 2015;(4):CD005381. https://doi.org/10.1002/14651858.CD005381.pub4.
Sokołowski DR, Hansen TI, Rise HH, et al. 5 years of exercise intervention did not benefit cognition compared to the physical activity guidelines in older adults, but higher cardiorespiratory fitness did. A generation 100 substudy. Front. Aging Neurosci. 2021;13:742587. https://doi.org/10.3389/fnagi.2021.742587.
Block W, Jessen F, Träber F, et al. Regional N-acetylaspartate reduction in the hippocampus detected with fast proton magnetic resonance spectroscopic imaging in patients with Alzheimer disease. Arch Neurol. 2002;59(5):828–34. https://doi.org/10.1001/archneur.59.5.828.
Targosz-Gajniak MG, Siuda JS, Wicher MM, et al. Magnetic resonance spectroscopy as a predictor of conversion of mild cognitive impairment to dementia. J Neurol Sci. 2013;335(1–2):58–63. https://doi.org/10.1016/j.jns.2013.08.023.
Schuff N, Amend DL, Knowlton R, et al. Age-related metabolite changes and volume loss in the hippocampus by magnetic resonance spectroscopy and imaging☆. Neurobiol Aging. 1999;20(3):279–85. https://doi.org/10.1016/s0197-4580(99)00022-6.
Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278(5337):412–9. https://doi.org/10.1126/science.278.5337.412.
Ross AJ, Sachdev PS. Magnetic resonance spectroscopy in cognitive research. Brain Res Rev. 2004;44(2–3):83–102. https://doi.org/10.1016/j.brainresrev.2003.11.001.
Gonzales MM, Tarumi T, Kaur S, et al. Aerobic fitness and the brain: increased N-acetyl-aspartate and choline concentrations in endurance-trained middle-aged adults. Brain Topogr. 2013;26(1):126–34. https://doi.org/10.1007/s10548-012-0248-8.
Stensvold, Viken H, Rognmo O, et al. A randomised controlled study of the long-term effects of exercise training on mortality in elderly people: study protocol for the Generation 100 study. BMJ Open. 2015;5(2):e007519. https://doi.org/10.1136/bmjopen-2014-007519.
Greene SJ, Killiany RJ, Initiative AsDN. Hippocampal subregions are differentially affected in the progression to Alzheimer’s disease. Anat Rec: Adv Integr Anat Evol Biol. 2012;295(1):132–40. https://doi.org/10.1002/ar.21493.
Scheenen TW, Klomp DW, Wijnen JP, et al. Short echo time 1H-MRSI of the human brain at 3T with minimal chemical shift displacement errors using adiabatic refocusing pulses. Magn Reson Med. 2008;59(1):1–6. https://doi.org/10.1002/mrm.21302.
Voss M. The chronic exercise-cognition interaction: fMRI research. In: T. McMorris. Editor. Exercise-cognition interaction. London: Elsevier Academic Press. 2016;187–209. https://doi.org/10.1016/B978-0-12-800778-5.00009-8.
Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–81.
Stensvold, Viken H, Steinshamn SL, et al. Effect of exercise training for five years on all cause mortality in older adults—the Generation 100 study: randomised controlled trial. BMJ. 2020;371:m3485. https://doi.org/10.1136/bmj.m3485.
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
Mykletun A, Stordal E, Dahl AA. Hospital Anxiety and Depression (HAD) scale: factor structure, item analyses and internal consistency in a large population. Br J Psychiatry. 2001;179(6):540–4. https://doi.org/10.1192/bjp.179.6.540.
Haug TT, Mykletun A, Dahl AA. The association between anxiety, depression, and somatic symptoms in a large population: the HUNT-II study. Psychosom Med. 2004;66(6):845–51. https://doi.org/10.1097/01.psy.0000145823.85658.0c.
Bjerkeset O, Mykletun A, Dahl AA, et al. Mortality in relation to self-reported mixed anxiety and depression symptoms–The HUNT study. Nord J Psychiatry. 2007;61(1):6–11. https://doi.org/10.1080/08039480601121926.
Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9. https://doi.org/10.1111/j.1532-5415.2005.53221.x.
Stensvold D, Sandbakk SB, Viken H, et al. Cardiorespiratory Reference Data in Older Adults: The Generation 100 Study. Med Sci Sports Exerc. 2017;49(11):2206–15. https://doi.org/10.1249/mss.0000000000001343.
Gibbons RJ, Balady GJ, Beasley JW, et al. ACC/AHA guidelines for exercise testing. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing). J Am Coll Cardiol. 1997;30(1):260–311. https://doi.org/10.1016/s0735-1097(97)00150-2.
Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774–81.
Cabanes E, Confort-Gouny S, Le Fur Y, et al. Optimization of residual water signal removal by HLSVD on simulated short echo time proton MR spectra of the human brain. J Magn Reson. 2001;150(2):116–25. https://doi.org/10.1006/jmre.2001.2318.
Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–9. https://doi.org/10.1002/mrm.1910300604.
Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13(3):129–53.
Wilson M, Davies NP, Sun Y, et al. A comparison between simulated and experimental basis sets for assessing short-TE in vivo 1H MRS data at 1.5 T. NMR Biomed. 2010;23(10):1117–26. https://doi.org/10.1002/nbm.1538.
Helms G. The principles of quantification applied to in vivo proton MR spectroscopy. Eur J Radiol. 2008;67(2):218–29. https://doi.org/10.1016/j.ejrad.2008.02.034.
Landheer K, Swanberg KM, Juchem C. Magnetic resonance Spectrum simulator (MARSS), a novel software package for fast and computationally efficient basis set simulation. NMR Biomed. 2021;34(5):e4129. https://doi.org/10.1002/nbm.4129.
Near J, Harris AD, Juchem C, et al. Preprocessing, analysis and quantification in single-voxel magnetic resonance spectroscopy: experts’ consensus recommendations. NMR Biomed. 2021;34(5):e4257. https://doi.org/10.1002/nbm.4257.
Hetherington H, Spencer D, Vaughan J, et al. Quantitative 31P spectroscopic imaging of human brain at 4 Tesla: assessment of gray and white matter differences of phosphocreatine and ATP. Magn Reson Med. 2001;45(1):46–52.
McLean MA, Woermann FG, Barker GJ, et al. Quantitative analysis of short echo time 1H-MRSI of cerebral gray and white matter. Magn Reson Med. 2000;44(3):401–11.
Träber F, Block W, Lamerichs R, et al. 1H metabolite relaxation times at 3.0 tesla: measurements of T1 and T2 values in normal brain and determination of regional differences in transverse relaxation. J Magn Reson Imaging. 2004;19(5):537–45. https://doi.org/10.1002/jmri.20053.
Kirov II, Fleysher L, Fleysher R, et al. Age dependence of regional proton metabolites T2 relaxation times in the human brain at 3 T. Magn Reson Med. 2008;60(4):790–5. https://doi.org/10.1002/mrm.21715.
Posse S, Otazo R, Caprihan A, et al. Proton echo-planar spectroscopic imaging of J-coupled resonances in human brain at 3 and 4 Tesla. Magn Reson Med. 2007;58(2):236–44. https://doi.org/10.1002/mrm.21287.
Mlynárik V, Gruber S, Moser E. Proton T 1 and T 2 relaxation times of human brain metabolites at 3 Tesla. NMR Biomed. 2001;14(5):325–31. https://doi.org/10.1002/nbm.713.
Cavassila S, Deval S, Huegen C, et al. Cramer-Rao bound expressions for parametric estimation of overlapping peaks: influence of prior knowledge. J Magn Reson. 2000;143(2):311–20. https://doi.org/10.1006/jmre.1999.2002.
Weston KS, Wisløff U, Coombes JS. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: a systematic review and meta-analysis. Br J Sports Med. 2014;48(16):1227–34. https://doi.org/10.1136/bjsports-2013-092576.
Murray ME, Przybelski SA, Lesnick TG, et al. Early Alzheimer’s disease neuropathology detected by proton MR spectroscopy. J Neurosci. 2014;34(49):16247–55. https://doi.org/10.1523/JNEUROSCI.2027-14.2014.
Frederiksen KS, Gjerum L, Waldemar G, et al. Effects of physical exercise on Alzheimer’s disease biomarkers: a systematic review of intervention studies. J Alzheimers Dis. 2018;61(1):359–72. https://doi.org/10.3233/JAD-170567.
Gogniat MA, Robinson TL, Miller LS. Exercise interventions do not impact brain volume change in older adults: a systematic review and meta-analysis. Neurobiol Aging. 2021;101:230–46. https://doi.org/10.1016/j.neurobiolaging.2021.01.025.
Firth J, Stubbs B, Vancampfort D, et al. Effect of aerobic exercise on hippocampal volume in humans: a systematic review and meta-analysis. Neuroimage. 2018;166:230–8. https://doi.org/10.1016/j.neuroimage.2017.11.007.
Loe H, Steinshamn S, Wisløff U. Cardio-respiratory reference data in 4631 healthy men and women 20–90 years: the HUNT 3 fitness study. PLoS ONE. 2014;9(11):e113884. https://doi.org/10.1371/journal.pone.0113884.
O’Keefe EL, Torres-Acosta N, O’Keefe JH, et al. Training for longevity: the reverse J-curve for exercise. Mo Med. 2020;117(4):355.
Storen O, Helgerud J, Saebo M, et al. The effect of age on the V O2max response to high-intensity interval training. Med Sci Sports Exerc. 2017;49(1):78–85. https://doi.org/10.1249/mss.0000000000001070.
Fock KM, Khoo J. Diet and exercise in management of obesity and overweight. J Gastroenterol Hepatol. 2013;28:59–63. https://doi.org/10.1111/jgh.12407.
Calverley TA, Ogoh S, Marley CJ, et al. HIITing the brain with exercise: mechanisms, consequences and practical recommendations. J Physiol. 2020;598(13):2513–30. https://doi.org/10.1113/JP275021.
Lucas SJ, Cotter JD, Brassard P, et al. High-intensity interval exercise and cerebrovascular health: curiosity, cause, and consequence. J Cereb Blood Flow Metab. 2015;35(6):902–11. https://doi.org/10.1038/jcbfm.2015.49.
Quistorff B, Secher NH, Van Lieshout JJ. Lactate fuels the human brain during exercise. FASEB J. 2008;22(10):3443–9. https://doi.org/10.1096/fj.08-106104.
Vestergaard MB, Jensen ML, Arngrim N, et al. Higher physiological vulnerability to hypoxic exposure with advancing age in the human brain. J Cereb Blood Flow Metab. 2020;40(2):341–53. https://doi.org/10.1177/0271678X18818291.
Inoue K, Okamoto M, Shibato J, et al. Long-term mild, rather than intense, exercise enhances adult hippocampal neurogenesis and greatly changes the transcriptomic profile of the hippocampus. PLoS ONE. 2015;10(6):e0128720. https://doi.org/10.1371/journal.pone.0128720.
Shih P-C, Yang Y-R, Wang R-Y. Effects of exercise intensity on spatial memory performance and hippocampal synaptic plasticity in transient brain ischemic rats. PLoS ONE. 2013;8(10):e78163. https://doi.org/10.1371/journal.pone.0078163.
Soya H, Mukai A, Deocaris CC, et al. Threshold-like pattern of neuronal activation in the hypothalamus during treadmill running: establishment of a minimum running stress (MRS) rat model. Neurosci Res. 2007;58(4):341–8. https://doi.org/10.1016/j.neures.2007.04.004.
Bjelland I, Dahl AA, Haug TT, et al. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J Psychosom Res. 2002;52(2):69–77. https://doi.org/10.1016/s0022-3999(01)00296-3.
Scherk H, Backens M, Schneider-Axmann T, et al. Neurochemical pathology in hippocampus in euthymic patients with bipolar I disorder. Acta Psychiatr Scand. 2008;117(4):283–8. https://doi.org/10.1111/j.1600-0447.2007.01142.x.
Ackl N, Ising M, Schreiber YA, et al. Hippocampal metabolic abnormalities in mild cognitive impairment and Alzheimer’s disease. Neurosci Lett. 2005;384(1–2):23–8. https://doi.org/10.1016/j.neulet.2005.04.035.
Liu Y, Cai Z-L, Xue S, et al. Proxies of cognitive reserve and their effects on neuropsychological performance in patients with mild cognitive impairment. J Clin Neurosci. 2013;20(4):548–53. https://doi.org/10.1016/j.jocn.2012.04.020.
Zotcheva E, Håberg AK, Wisløff U, et al. Effects of 5 years aerobic exercise on cognition in older adults: the Generation 100 study: a randomized controlled trial. Sports Med. 2022;52(7):1689–99. https://doi.org/10.1007/s40279-021-01608-5.
Borland E, Nägga K, Nilsson PM, et al. The Montreal cognitive assessment: normative data from a large Swedish population-based cohort. J Alzheimers Dis. 2017;59(3):893–901. https://doi.org/10.3233/JAD-170203.
Engedal K, Gjøra L, Bredholt T, et al. Sex differences on Montreal cognitive assessment and mini-mental state examination scores and the value of self-report of memory problems among community dwelling people 70 years and above: The HUNT Study. Dement Geriatr Cogn Disord. 2021;50(1):74–84. https://doi.org/10.1159/000516341.
Sun D, Zhang J, Fan Y, et al. Abnormal levels of brain metabolites may mediate cognitive impairment in stroke-free patients with cerebrovascular risk factors. Age Ageing. 2014;43(5):681–6. https://doi.org/10.1093/ageing/afu027.
Poppenk J, Evensmoen HR, Moscovitch M, et al. Long-axis specialization of the human hippocampus. Trends Cogn Sci. 2013;17(5):230–40. https://doi.org/10.1016/j.tics.2013.03.005.
Schuff N, Meyerhoff DJ, Mueller S, et al. N-acetylaspartate as a marker of neuronal injury in neurodegenerative disease. Adv Exp Med Biol. 2006;576:241–262; discussion 361–3. https://doi.org/10.1007/0-387-30172-0_17
Wang Z, Zhao C, Yu L, et al. Regional metabolic changes in the hippocampus and posterior cingulate area detected with 3-Tesla magnetic resonance spectroscopy in patients with mild cognitive impairment and Alzheimer disease. Acta Radiol. 2009;50(3):312–9. https://doi.org/10.1080/02841850802709219.
Modrego PJ, Fayed N. Longitudinal magnetic resonance spectroscopy as marker of cognitive deterioration in mild cognitive impairment. Am J Alzheimers Dis Other Demen. 2011;26(8):631–6. https://doi.org/10.1177/1533317511433809.
Kantarci K, Smith GE, Ivnik RJ, et al. 1H magnetic resonance spectroscopy, cognitive function, and apolipoprotein E genotype in normal aging, mild cognitive impairment and Alzheimer’s disease. J Int Neuropsychol Soc. 2002;8(7):934–42. https://doi.org/10.1017/s1355617702870084.
Griffith HR, Okonkwo OC, den Hollander JA, et al. Brain metabolic correlates of decision making in amnestic mild cognitive impairment. Aging Neuropsychol Cogn. 2010;17(4):492–504. https://doi.org/10.1080/13825581003646135.
Hansen BH, Anderssen SA, Steene-Johannessen J, et al. Fysisk aktivitet og sedat tid blant voksne og eldre i Norge—Nasjonal kartlegging 2014–2015. Helsedirektoratet. 2015. IS-2367. https://www.helsedirektoratet.no/rapporter/fysisk-aktivitet-kartleggingsrapporter/Fysisk%20aktivitet%20og%20sedat%20tid%20blant%20voksne%20og%20eldre%20i%20Norge.pdf/_/attachment/inline/7d460cdf-051a-4ecd-99d6-7ff8ee07cf06:eff5c93b46b28a3b1a4d2b548fc53b9f51498748/Fysisk%20aktivitet%20og%20sedat%20tid%20blant%20voksne%20og%20eldre%20i%20Norge.pdf
Walhovd KB, Fjell AM, Reinvang I, et al. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005;26(9):1261–70. https://doi.org/10.1016/j.neurobiolaging.2005.05.020.
Pintzka CW, Hansen TI, Evensmoen HR, et al. Marked effects of intracranial volume correction methods on sex differences in neuroanatomical structures: a HUNT MRI study. Front Neurosci. 2015;9:238. https://doi.org/10.3389/fnins.2015.00238.
Noble KG, Grieve SM, Korgaonkar MS, et al. Hippocampal volume varies with educational attainment across the life-span. Front Hum Neurosci. 2012;6:307. https://doi.org/10.3389/fnhum.2012.00307.
Geurts JJ, Barkhof F, Castelijns JA, et al. Quantitative 1H-MRS of healthy human cortex, hippocampus, and thalamus: metabolite concentrations, quantification precision, and reproducibility. J Magn Reson Imaging. 2004;20(3):366–71. https://doi.org/10.1002/jmri.20138.
Hammen T, Stadlbauer A, Tomandl B, et al. Short TE single-voxel 1H-MR spectroscopy of hippocampal structures in healthy adults at 1.5 Tesla—how reproducible are the results? NMR in Biomed. 2005;18(3):195–201. https://doi.org/10.1002/nbm.958.
Venkatraman TN, Hamer RM, Perkins DO, et al. Single-voxel 1H PRESS at 4.0 T: precision and variability of measurements in anterior cingulate and hippocampus. NMR Biomed. 2006;19(4):484–91. https://doi.org/10.1002/nbm.1055.
Kassem MN, Bartha R. Quantitative proton short-echo-time LASER spectroscopy of normal human white matter and hippocampus at 4 Tesla incorporating macromolecule subtraction. Magn Reson Med. 2003;49(5):918–27. https://doi.org/10.1002/mrm.10443.
Hsu Y-Y, Chen M-C, Lim K-E, et al. Reproducibility of hippocampal single-voxel proton MR spectroscopy and chemical shift imaging. Am J Roentgenol. 2001;176(2):529–36. https://doi.org/10.2214/ajr.176.2.1760529.
Griffith HR, den Hollander JA, Okonkwo O, et al. Executive function is associated with brain proton magnetic resonance spectroscopy in amnestic mild cognitive impairment. J Clin Exp Neuropsychol. 2007;29(6):599–609. https://doi.org/10.1080/13803390600826595.
Kantarci K, Petersen RC, Przybelski SA, et al. Hippocampal volumes, proton magnetic resonance spectroscopy metabolites, and cerebrovascular disease in mild cognitive impairment subtypes. Arch Neurol. 2008;65(12):1621–8. https://doi.org/10.1001/archneur.65.12.1621.
Koush Y, Rothman DL, Behar KL, et al. Human brain functional MRS reveals interplay of metabolites implicated in neurotransmission and neuroenergetics. J Cereb Blood Flow Metab. 2022;(6):911–934. https://doi.org/10.1177/0271678X221076570.
Arild A, Vangberg T, Nikkels H, et al. Five years of exercise intervention at different intensities and development of white matter hyperintensities in community dwelling older adults, a Generation 100 sub-study. Aging (Albany NY). 2022;14(2):596. https://doi.org/10.18632/aging.203843.
Acknowledgements
The authors thank all the participants for taking part in this study, and Torill E. Sjøbakk for help with the recruitment. We thank Hanne Nikkels, Stine Bjøralt, and the radiographers at the 3T scanner for help with the MRI/MRSI data collection. We also thank those involved with the clinical testing, including VO2peak testing, performed at the NeXt Move Core Facility, Norwegian University of Science and Technology (NTNU), and the other clinical measurements performed at the Clinical Research Facility, St. Olavs Hospital.
Funding
Open access funding provided by NTNU Norwegian University of Science and Technology (incl St. Olavs Hospital - Trondheim University Hospital) The Generation 100 study was supported by the Research Council of Norway; The K.G. Jebsen Foundation for medical research, Norway; Norwegian University of Science and Technology (NTNU); Central Norway Regional Health Authority; St. Olavs Hospital, Trondheim, Norway; and the National Association for Public Health, Norway. The brain MR acquisition was supported by Norwegian Advisory Unit for fMRI, Department of Radiology and Nuclear Medicine, St. Olavs Hospital, Trondheim.
Author information
Authors and Affiliations
Contributions
Line Skarsem Reitlo contributed to the data quality control, statistical analysis, figures, drafting, and revision of the manuscript. Jelena Mihailovic performed the MRSI data analyses, wrote methods, and revised the manuscript. Dorthe Stensvold and Ulrik Wisløff supervised the RCT Generation 100 study; were responsible for exercise, health, and clinical data collection; and revised the manuscript. Fahmeed Hyder supervised MRSI analyses and revised the manuscript. Asta Håberg supervised the MRI substudy in Generation 100, organized the data collection, collected data, performed statistical analysis, and drafted and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reitlo Line S. and Mihailovic Jelena M. shared first authorship.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Reitlo, L.S., Mihailovic, J.M., Stensvold, D. et al. Hippocampal neurochemicals are associated with exercise group and intensity, psychological health, and general cognition in older adults. GeroScience 45, 1667–1685 (2023). https://doi.org/10.1007/s11357-022-00719-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-022-00719-9