Abstract
The study describes the whole-genome sequencing of two antibiotic-resistant representative Escherichia coli strains, isolated from poultry manure in 2020. The samples were obtained from a commercial chicken meat production facility in Poland. The antibiotic resistance profile was characterized by co-resistance to β-lactam antibiotics, aminoglycosides, and fluoroquinolones. The three identified resistance plasmids (R-plasmids), pECmdr13.2, pECmdr13.3, and pECmdr14.1, harbored various genes conferring resistance to tetracyclines (tetR[A]) for, aminoglycoside (aph, aac, and aad families), β-lactam (blaCMY-2, blaTEM-176), sulfonamide (sul1, sul2), fluoroquinolone (qnrS1), and phenicol (floR). These plasmids, which have not been previously reported in Poland, were found to carry IS26 insertion elements, the intI1-integrase gene, and conjugal transfer genes, facilitating horizontal gene transfer. Plasmids pECmdr13.2 and pECmdr14.1 also possessed a mercury resistance gene operon related to transposon Tn6196; this promotes plasmid persistence even without antibiotic selection pressure due to co-selection mechanisms such as co-resistance. The chicken manure–derived plasmids belonged to the IncX1 (narrow host range) and IncC (broad host range) incompatibility groups. Similar plasmids have been identified in various environments, clinical isolates, and farm animals, including cattle, swine, and poultry. This study holds significant importance for the One Health approach, as it highlights the potential for antibiotic-resistant bacteria from livestock and food sources, particularly E. coli, to transfer through the food chain to humans and vice versa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Years of misuse and overuse of antibiotics in the livestock and poultry industry have resulted in many irreversible changes, not only in the animal production sector but also in the everyday life of each human. Extensive, uncontrolled antibiotic use results in the presence of low, sub-inhibitory concentrations in the tissues and guts of treated animals (Lim et al. 2020) and the environment (Khan et al. 2013). The mechanisms of action of sublethal levels of antibiotics on bacteria are described in detail by Andersson and Hughes (2014). In food-producing animals, antibiotic use affects the functions of enteric bacteria and can temporarily increase antibiotic resistance in the fecal microbiome (Chee-Sanford et al. 2009; Broom 2017). The chronic application of such sub-therapeutic doses favors the selection of antibiotic-resistant bacteria (ARB) by promoting their growth or introducing de novo mutations. The resistance mechanism of a particular ARB depends on its harbored antibiotic resistance genes (ARGs). The majority of ARG transmission occurs via horizontal gene transfer (HGT), whereby mobile genetic elements (MGEs), such as plasmids or transposons coding for ARG, are exchanged between bacterial species, even those not closely related (Redondo-Salvo et al. 2020).
Escherichia coli is classified as a rod‐shaped, non-sporulating, facultatively anaerobic Gram‐negative bacteria belonging to the family Enterobacteriaceae. This species mainly lives in the lower intestinal tract of warm‐blooded animals, including livestock and poultry, and is often excreted into the environment with urine and feces. In addition to being a widespread gut commensal of vertebrates, E. coli is also a versatile pathogen, killing more than two million humans annually through intraintestinal and extraintestinal diseases (Tenaillon et al. 2010). The presence of E. coli in the natural environment has long been considered an indicator of recent fecal contamination (Anjum et al. 2021), but many studies show that many strains can survive and proliferate in such conditions and be incorporated into indigenous microbial communities (Jang et al. 2017; Marano et al. 2020).
The presence of E. coli and other commensal bacteria in the animals’ gut can be beneficial. Some help animals to digest complex molecules, which cannot otherwise be assimilated, thus producing vitamin K or certain B vitamins essential for the host; others prevent gut colonization by competing with harmful microorganisms, i.e., by consuming available nutrients, occupying physical space, and producing substances such as bacteriocins that inhibit the growth of pathogens. They can also play an essential role in the development and regulation of the immune system by stimulating it and helping it to differentiate between harmful and non-harmful organisms (Tenaillon et al. 2010; Abt and Pamer 2014; Hanning and Diaz-Sanchez 2015).
However, some strains of E. coli can be pathogenic, causing severe diseases in humans and animals. Infection with pathogenic E. coli strain can manifest in enteric or diarrheal disease, urinary tract infections, and meningitis or sepsis. The intestinal symptoms may be caused by EIEC (enteroinvasive E. coli), EHEC (enterohaemorrhagic E. coli), ETEC (enterotoxigenic E. coli), EPEC (enteropathogenic E. coli), DAEC (diffusely adherent E. coli), and EAEC (enteroaggregative E. coli) (Kaper et al. 2004). The most common extraintestinal E. coli infection is urinary tract infection, caused by UPEC (uropathogenic E. coli); however, the pathotype MNEC (meningitis-associated E. coli), responsible for meningitis and sepsis, is becoming increasingly prevalent. EPEC, EHEC, and ETEC can also cause animal disease using the same virulence factors present in human strains and colonization factors unique to animals. The avian pathogenic E. coli (APEC) is an additional animal pathotype, causing extraintestinal infections of poultry, primarily respiratory infections, pericarditis, and septicemia (Kaper et al. 2004).
The aim of the study was to examine the population of E. coli strains isolated from poultry (from intensive rearing facilities) feces. Based on the results of antibiotic susceptibility testing and plasmid profile characteristics, the most prevalent strains were subjected to sequencing with NovaSeq 6000 (Illumina) and MinIon (Oxford Nanopore) platforms for full plasmid structure recovery. Two representative multi-drug resistance (MDR) E. coli isolates were found to harbor three resistance plasmids (R-plasmids) that had not previously been reported in Poland: pECmdr13.3 (IncX1), pECmdr13.2, and pECmdr14.1 (IncC). These were found to carry genes coding for resistance to tetracyclines, aminoglycosides, β-lactams, sulfonamides, fluoroquinolones, and phenicol; the last two were accompanied by mercury resistance.
Materials and methods
Isolation of E. coli strains
Chicken waste was collected from a commercial chicken meat production facility located in the central part of Poland (Masovian district). The samples were taken from two points in the production chain: CM, chicken manure from laying hens, and CL, chicken litter from broilers. Sampling details were described previously by Błażejewska et al. (2022). Samples of chicken litter and manure (0.1 kg per sampling point) were collected from 10 locations for each waste type: the former in the chicken coop and the latter under the cages. The samples were then divided by type and pooled into one representative sample for each type for further analysis. All polled collections were taken in triplicate. The farm owners agreed to manure sampling.
The collected samples were transported to the laboratory in a refrigerator, stored at 4 °C, and processed within 24 h. To isolate antibiotic-resistant E. coli strains, the manure sample was first enriched in Luria Bertani broth (Biomaxima, Lublin, Poland) by adding 1 g of feces to 9 mL of liquid medium. The cultures were incubated at 30 °C and 37 °C for 24 h and 48 h, respectively. After incubation, the bacterial suspensions were diluted (10−1, 10−2, and 10−3), and 100 µL of undiluted sample and each dilution were plated on Eosin Methylene Blue agar (Biomaxima, Lublin, Poland) and MacConkey agar (Biomaxima, Lublin, Poland) supplemented with imipenem (16 mg/L) (Merck, Darmstadt, Germany) to search for carbapenem resistance phenotype; the same dilutions were also asdded to Eosin Methylene Blue agar and MacConkey agar both supplemented with cefotaxime (4 mg/L) (Merck, Darmstadt, Germany) for the extended-spectrum beta-lactamase phenotype (Tacconelli et al. 2018). Each isolation was performed as three biological replicates, followed by three technical ones.
The plates were incubated for 24 h at 37 °C to select animal pathogens and 30 °C for environmental strains. Next, 24 to 48 colonies showing the morphology of the intended bacteria were taken and subjected to three consecutive streaks to get pure colonies. The pure cultures were stored in PBS/glycerol stocks (20% v/v) for further analysis.
The susceptibility profiles of E. coli strains
The antibiotic susceptibility profiles of isolated strains were determined by The Kirby–Bauer test (Biemer 1973). For phenotype characterization, the following antibiotic disks were used: imipenem (β-lactam) (IMP, 10 µg), ciprofloxacin (fluoroquinolone) (CIP, 5 µg), cefotaxime (cephalosporin) (CTX, 5 µg), and gentamicin (aminoglycoside) (CN, 10 µg) (OXOID, USA). The inhibition zones were measured according to EUCAST recommendations, i.e., after incubation for 18 h at 37 °C. Strains with different antibiotic susceptibility profiles, indicated by the bacterial growth inhibition zone differing by approximately + / − 2 mm in diameter around at least one antibiotic disk, were considered non-repetitive and chosen for further research. The presence of E. coli was confirmed by MALDI-TOF MS/MS (matrix-assisted laser desorption/ionization system equipped with a time-of-flight mass spectrometer) (Bielen et al. 2024).
The analyses were performed in an external medical laboratory (ALAB Laboratoria Sp. z o. o., Warsaw, Poland), according to a standard diagnostic procedure. Identification was performed by aligning the peaks to the best-matching reference data. The resulting log score was classified as follows: ≥ 2.3, highly probable species; between 2.0 and 2.3, certain genus and probable species; between 1.7 and 2.0, probable genus; and < 1.7, non-reliable identification. Next, antibiotic susceptibility testing was performed with Vitek2 Compact equipment (BioMerieux, Marcy-l’Étoile, France) (Hogan et al. 2019). Based on the results, strains were classified as sensitive (S), resistant (R), or intermediate (I). The AST-N331 susceptibility card was used.
Strains with the same resistance profile and origin were considered clones. The plasmid profile of non-repetitive strains was determined in three ways: (1) Plasmid DNA was isolated with a commercially available Plasmid Mini kit (A&A Biotechnology, Gdynia, Poland), (2) rapid alkaline lysis for the isolation of plasmid DNA (Birnboim and Doly 1979), (3) plasmid visualization by Eckhardt electrophoresis (Eckhardt 1978). Plasmid DNA with similar profiles was additionally differentiated by digestion with the BamHI and HindIII restriction enzymes (NEB, Ipswich, MA, USA). The digestion was performed according to the manufacturer’s recommendation. Strains with different profiles were chosen for sequencing.
DNA extraction and sequencing
Genomic DNA from bacterial strains was isolated using a Genomic Mini kit (A&A Biotechnology, Gdynia, Poland). The DNA concentration was measured using a Qubit fluorometer and a dsDNA High Sensitivity Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA), and purity was determined by measuring the A260/A280 absorbance ratio with a NanoDrop spectrophotometer (Thermo Fisher, Waltham, MA, USA). Only samples with concentrations higher than 10 ng/µL and an A260/A280 ratio ranging from 1.8 to 2.0 were analyzed. DNA samples were stored at − 20 °C for further use. The DNA samples were isolated in triplicate.
Genomic DNA sequencing on Oxford Nanopore MinION
Libraries were prepared from 500 ng of DNA samples after mechanical fragmentation by the syringe-based method (0.4 × 20 mm needle, 1-mL glass syringe, 200 µL) and purification by solid-phase reversible immobilization with Kapa Pure Beads (Roche, cat. no. 07983298001, elution for 10 min at 37 °C). Native Barcoding method was applied (Oxford Nanopore, cat. no. SQK-NBD112.24, Version: NBE_9134_v112_revE_01Dec2021). The NanoPore MINIon MkB1 R10.4 flow cell and reagents (cat. no. FLO-MIN112) were used for sequencing with standard procedures. Guppy software (version 6.1.2, –flowcell FLO-MIN112 –kit SQK-NBD112-24 options) was applied for basecalling.
Genomic DNA sequencing on Illumina NovaSeq 6000
Briefly, 100 ng of genomic DNA was fragmented to 300 bp by ultrasonication (S220 Covaris, duty factor 10%, peak incident power 140, cycles per burst 200, treatment time 80 s) and subjected to library construction with the KAPA Hyper Prep Kit (Roche, cat. no. 07962363001), according to the manufacturer’s protocol, with five cycles of amplification; however, TruSeq DNA UD Index adapters were used (Illumina, cat. no. 20020590). Libraries were size-selected in a two-step SPRI method and with a sample-to-reagent volume ratio of (1) step—1:0.75 and (2) step—1:0.85 using Kapa HyperPure Beads (Roche, cat. no. 07983298001). Sequencing was performed using pair-end 2 × 100 cycle mode on the Illumina NovaSeq 6000 system (NovaSeq 6000 S1 Reagent Kit v1.5 200 cycles Illumina, cat. no. 20028317; 0.5% of the PhiX control library, Illumina, cat. no. FC-110–3001) and the standard clustering procedure.
Genome assembly and data analysis
Initial sequence quality metrics for Illumina data were obtained using FASTQC v.0.12.0 (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/) (Andrews 2010) and subjected to quality trimming using fastp v.0.23.2 (Chen et al. 2018). Nanopore reads were quality filtered with NanoFilt v.2.8.0, discarding reads below 1 kb and QV < 12 (De Coster et al. 2018), and residual adapters were removed using Porechop v.0.2.4 (https://github.com/rrwick/Porechop). The quality of the final long-read dataset was evaluated using NanoPlot v. 1.41.6 (De Coster et al. 2018).
Long-read assembly was performed using Trycycler v.0.5.3 pipeline (Wick et al. 2017). Nanopore reads were initially assembled using four long-read assemblers: Flye v2.9, Unicycler v0.4.8, Raven v1.8.1, and Miniasm v0.3-r179. The resulting assemblies were then reconciled and circularized, and a consensus sequence was generated, which underwent further polishing with medaka_consensus (https://github.com/nanoporetech/medaka). The long-read assembled contigs were further polished with short Illumina reads using polypolish (https://github.com/rrwick/Polypolish) v. 0.5.0 (Wick et al. 2017) and POLCA v. 4.0.5 (Zimin et al. 2017). Finally, all of the sequence errors and misassemblies were manually corrected using SeqMan software v. 9.1 (DNAStar) to obtain a complete nucleotide sequence for the bacterial genome.
The annotation of consensus sequences was performed using Bakta (Schwengers et al. 2021). Plasmid replicon sequences were searched against the PLSDB database (https://ccb-microbe.cs.uni-saarland.de/plsdb/). ARGs were identified and localized in the genomic sequences using abricate (https://github.com/tseemann/abricate). Mobile genetic element detection was performed using mobileelementfinder (Johansson et al. 2021), and the ARG-associated mobilome was characterized using VRprofile2 (Wang et al. 2022).
The plasmid sequences were visualized using SnapGene Viewer software (SnapGene® software from Dotmatics; available at snapgene.com).
Results
E. coli strain selection
Selective media were used to isolate 62 bacterial strains exhibiting growth characteristics of E. coli: 51 strains were identified on Eosin Methylene Blue (EMB) Agar and 11 on MacConkey agar. Of all isolated bacteria, 23 were identified as E. coli by MALDI-TOF MS/MS analysis: of these, 20 strains were isolated on EMB agar, three on MacConkey agar, 13 from chicken manure (CM), and 10 from chicken litter (CL). All bacteria strains were isolated on agar plates supplemented with cefotaxime. No strains resistant to imipenem were identified. All strains were subjected to antibiotic susceptibility profile determination by Vitek 2 Compact: the results are listed in Table S1. Isolates exhibiting similar antibiotic susceptibility profiles were grouped, and their plasmidic profiles were determined. After comparing the plasmidic profiles within the groups, the representative bacterial strains were chosen for plasmid structure determination by total DNA sequencing.
Genomes sequencing and assembly
High-quality genome sequence assemblies for the selected strains were obtained by combining scaffolds from Oxford Nanopore long-read technology (MINIon platform) with Illumina short-read technology (2 × 100 nt in pair-end mode, NovaSeq 6000, approximate 100 × coverage) for quality improvement. The chromosome was successfully assembled, resulting in close to 5 Mb contigs for each strain, and three additional contigs over 40 kb were reconstructed.
Plasmids
Six plasmids belonging to two bacterial isolates were identified (Table 1).
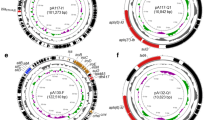
Three of the identified plasmids harbored different ARGs and MGEs (Table 2). The linear structure of the plasmid structures, highlighting the ARGs, MGEs, conjugal transfer genes, transposon transposase genes, heavy metal resistance genes, replication system, integron integrase, and partitioning system elements, is presented in Figs. 1, 2, and 3. Detailed analysis indicated close similarity between the identified plasmids and some bacterial plasmid sequences deposited in the PLSDB plasmid database (https://ccb-microbe.cs.uni-saarland.de/plsdb/); however, there were some differences between them. Plasmid pECmdr13.2 has 133 similar hits, isolated from varied sources located in different regions, plasmid pECmdr13.3 has 18 similar hits, and pECmdr14.1 has 155 similar hits (identity threshold 0.99). None of these plasmids has previously been reported in Poland, but they have been identified in many countries. Although plasmids similar to pECmdr13.2 and pECmdr14.1 have been found in the environment, clinical isolates, and farm animals (cattle, swine, and poultry/birds), plasmids similar to pECmdr13.3 have been identified in the same places but only from poultry. Plasmids that do not confer antibiotic resistance will be characterized further and deposited as a part of an additional project.
Linear and circular representation of pECmdr13.2 plasmid; pink-ARGs, red-conjugal transfer genes (TRA), blue-transposon transposase genes (TNP), brown-heavy metal resistance genes, yellow-replication system (REP), green-integron integrase, khaki-partitioning system PAR, gray-mob system (MOB), plum-pilus system (PIL); a name written in quotation marks indicates that the gene function has been assigned based on the homology of the protein it encodes
Linear and circular representation of pECmdr13.3 plasmid; pink-ARGs, red-conjugal transfer genes (TRA), blue-transposon transposase genes (TNP), brown-heavy metal resistance genes, yellow-replication system (REP), green-integron integrase, khaki-partitioning system (PAR), gray-mob system (MOB), plum-pilus system (PIL); a name given in quotation marks indicates that the gene function has been assigned based on the homology of the protein it encodes
Linear and circular representation of pECmdr14.1 plasmid; pink-ARGs, red-conjugal transfer genes (TRA), blue-transposon transposase genes (TNP), brown-heavy metal resistance genes, yellow-replication system (REP), green-integron integrase, khaki-partitioning system (PAR), gray-mob system (MOB), plum-pilus system (PIL); a name given in quotation marks indicates that the gene function has been assigned based on the homology of the protein it encodes
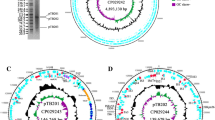
Alignment of ARG-coding sequences and MGE allowed the identification of ARGs located within defined and described MGE, such as IS26, ISEcp1, or “ISclustersTn,” which were in turn situated on the plasmids identified during the study (Fig. 4A–G).
ARGs located within MGE on the identified plasmids: A pECmdr13.2 IS26 (8965 bp) (pink-ARG, blue-transposon transposase genes TNP). B pECmdr13.2 ISclusterTn (18511 bp); pink-ARGs, blue-transposon transposase genes (TNP), green-integron integrase. C pECmdr13.2 ISEcp1 (2731 bp); pink-ARG. D pECmdr13.3 ISclusterTn (14527 bp); pink-ARGs, blue-transposon transposase genes (TNP). E pECmdr14.1 ISclusterTn (14527); pink-ARGs, blue-transposon transposase genes (TNP). F pECmdr14.1 ISEcp1 (2731 bp); pink-ARG. G pECmdr14.1 IS26 (13249 bp); pink-ARGs, blue-transposon transposase genes (TNP)
A detailed search of the PLSDB provides data on the isolation source, biosample origin, country, and first report of similar plasmids (Table 3), as well as previously reported plasmid host range (Table 4).
Discussion
Due to its presence in the veterinary, medical, and natural environment, E. coli is considered a specific vector for the transmission of ARGs, so-called acquired resistance (Zalewska et al. 2021; Męcik et al. 2023). The bacteria found in chicken manure or chicken litter are not limited to E. coli; they often co-isolate with other foodborne bacteria such as Salmonella and Campylobacter spp. (Merchant et al. 2012; Viegas et al. 2012; Błażejewska et al. 2022). This is an important issue since such material is utilized as fertilizer in agriculture globally (Pujiastuti et al. 2018; Błażejewska et al. 2022). These bacteria pose a serious risk of antibiotic resistance transmission between animals, humans, and the environment, i.e., the three links of the One Health concept, and demonstrate considerable survival in water, soil, and crops: the lifespan of pathogenic bacteria ranges from several days to 10 years in soil and from several days to a year on plants (Oliveira et al. 2012; Anokyewaa Appau and Ofori 2024).
The main process responsible for disseminating ARGs is horizontal gene transfer (HGT) (Aminov 2011). The principal route of HGT in bacterial communities is most likely conjugation, and conjugation elements, such as conjugative plasmids, often harbor multiple ARGs (Wozniak and Waldor 2010). In addition, ARGs are often encoded on non-conjugative plasmids, transposons, integrative, and bacteriophages (Abe et al. 2020), whose presence facilitates HGT. HGT is most likely to occur when bacterial density is high, particularly where high numbers of bacteria are present in a given environment, under stress conditions, and selective pressure (Zalewska et al. 2021). Acquired genes may confer adaptive advantages under certain growth or environmental conditions that may contain antimicrobials, xenobiotics, metals, sucrose, and other compounds. Also, abiotic factors may affect conjugal transfer, e.g., the rate of plasmid transfer in the soil varies depending on abiotic factors, such as soil moisture and temperature, pH, and soil type (Aminov 2011). HGT is also affected by genetic diversity in the environment: while high biodiversity may increase the pool of potentially exchangeable genes in the bacterial population, with HGT occurring even between distantly related species, high diversity is considered a barrier to HGT (Klümper et al. 2024).
Antibiotic resistance is undoubtedly associated with resistance plasmids, which spread rapidly through conjugation. Accordingly, plasmids play a significant role in the worldwide dissemination of resistance, including MDR (Partridge et al. 2018; Pinilla-Redondo et al. 2018; San Millan 2018). Therefore, identifying and characterizing R-plasmids and their association with different bacterial hosts is key to understanding the epidemiology and spreading of antibiotic resistance. So far, little information exists on the fully characterized plasmids identified in chicken manure isolates. A suitable example could be the aminoglycoside resistance plasmids (arr-3 and aacA) pRKZ3 (IncQ) and pKANJ7 (IncX), which were isolated from pig and chicken manure (Pu et al. 2019). The present study identified two IncC R-plasmids, pECmdr13.2 and pECmdr14.1, carrying genes for resistance to tetracyclines, aminoglycosides, β-lactams, sulfonamides, and fluoroquinolones and to mercury; in addition, pECmdr14.1 demonstrated resistance to phenicol. The study also identified one IncX1 pECmdr13.3 R-plasmid, carrying genes for resistance to aminoglycosides, β-lactams, tetracyclines, and fluoroquinolones. So far, such plasmids have not been reported in Poland.
Incompatibility Group C (IncC) plasmids have a broad host range; the group can be divided into several types based on differences in sequence arising from homologous recombination events between type 1 and type 2 IncC backbones (Harmer and Hall 2014; Zhang et al. 2020). This variability also results from insertion and deletion events or inversion mechanisms involving IS26 (Ambrose et al. 2018). IncC plasmids occur widely in Gram-negative bacteria and are responsible for transmitting resistance to several different antibiotics, such as aminoglycoside and fluoroquinolone, as well as cephalosporins (blaCMY cephalosporinase genes) and carbapenems (blaNDM carbapenemase genes) which have considerable clinical significance (Shoma et al. 2014; Harmer and Hall 2014; Wasyl et al. 2015; Partridge et al. 2018). In contrast, the IncX plasmids, and their subgroups (IncX1—IncX8), have a narrow spectrum of hosts, including E. coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Salmonella enterica (Dobiasova and Dolejska 2016). These plasmids have been associated with antibiotic resistance to β-lactams, including carbapenems, aminoglycosides, quinolones, tetracyclines, streptomycin, and amphenicols, which are associated with mobile elements, such as insertion sequences, integrons, and transposons (Fang et al. 2018). As shown in Table 2, the plasmids identified in this work have host ranges encompassing pathogenic and opportunistic bacteria capable of living in natural, veterinary, and clinical environments, which further emphasizes the importance of characterizing R-plasmids in these bacteria. Particularly noteworthy is the fact that most of these bacteria are categorized as critical and high-priority on the global priority pathogens list (Tacconelli et al. 2018).
Previous studies found a sulfonamide resistance gene (sul1), a tetracycline resistance gene (tetA), and three aminoglycoside resistance genes (aadA, strA, and strB) in plasmids isolated from chicken manure (Dobiasova and Dolejska 2016; Le Devendec et al. 2016). This is undoubtedly related to antibiotic therapy used in poultry farming: the most frequently administered pharmaceuticals are beta-lactams, macrolides, polymyxins, quinolones, sulfonamides, and tetracyclines (Chauvin et al. 2007). There has been a surge of reports on multidrug-resistant E. coli strains isolated from chicken manure, resistant to β-lactams with ESBL (extended-spectrum beta-lactamase) and AmpC (clinically important β-lactamases; cephalosporinases) phenotypes, which are often also resistant to gentamicin (Zalewska et al. 2021). However, few studies have reported about plasmids coding for both antibiotic and metal resistance genes occurring in bacterial strains isolated from chicken feces. However, one study recently described the presence of ESBL/AmpC and mcr-5-carrying MDR plasmids isolated from E. coli and K. pneumoniae strains in Paraguayan poultry farms (Nesporova et al. 2021). IncHI2 plasmids identified in E. coli isolates from food-producing animals have also been found to carry the antibiotic resistance genes blaCTX-M/oqxAB with aac (6′)-Ib-cr, floR, fosA3, and rmtB, as well as the heavy metal resistance genes pco and sil responsible for increasing the minimal inhibitory concentrations of CuSO4 and AgNO3 (Fang et al. 2016). Previously, similar IncHI2 plasmids carrying tetracycline, trimethoprim, and sulfonamide resistance genes and transposon Tn1696 related to mercury resistance were identified in two MDR S. enterica serovar Typhimurium isolates from Australian food-producing animals (Cain et al. 2010). Another study of E. coli strains from pig slaughterhouses in the UK showed various combinations of resistance to oxytetracycline, streptomycin, sulphonamide, ampicillin, chloramphenicol, trimethoprim–sulfamethoxazole, ceftiofur, amoxicillin–clavulanic acid, aztreonam, and nitrofurantoin, together with resistance to mercury, silver, or copper (merA, merC, and pcoE and silA, silB, and silE genes were detected) (Yang et al. 2020). A similar relationship was found in a 2024 study on the distribution and relationships of ARGs, heavy metal resistance genes, virulence factors, and their transmission mechanisms of an MDR E. coli strain isolated from livestock manure and fertilized soil (Tan et al. 2024).
Therefore, the presence of metal resistance genes and ARGs on the identified plasmids supports their transmission by HGT and subsequent maintenance in the bacterial cell, even in the absence of selection pressure caused by antibiotics. This co-occurrence of genes specifying resistant phenotypes on one MGE is referred to as co-resistance (Baker-Austin et al. 2006; Pal et al. 2017). Such genetic linkage between metal- and antibiotic-resistant traits has been reported on plasmids in Enterobacteriaceae and in other bacteria isolated from other animal feces, soil, or sewage (Summers et al. 1993; Ghosh et al. 2000; Hasman and Aarestrup 2002; Rozwandowicz et al. 2018). It has been shown that metals used in animal feed accumulate and persist in food animals and may impact the development of AMR in primary animal and food production environments (James et al. 2023). Many studies indicate that the spread of AMR introduced through the application of manure to agricultural fields leads to its further spread within the food chain and may pose a risk to human health (Do et al. 2022; Błażejewska et al. 2022; Zalewska et al. 2023, 2024). Such a risk is underlined by the presence of plasmids carrying shared antibiotic and metal resistance in MDR strains of E. coli, a vector known to transmit AMR between One Health sectors: E. coli strains have been found to potentially transfer resistance between humans and chickens (Norizuki et al. 2017).
AMR continues to evolve and spread, with the main mechanism being HGT through plasmids. Therefore, there is a pressing need to identify and characterize R-plasmids and their relationships with different bacterial hosts to understand their involvement in the transfer of AMR determinants. The molecular identification of plasmid genotypes, the transposons and integrons located within them, or other insertion sequences involved in this process would provide a clearer picture of the mechanism of AMR dissemination and its possible range. In turn, the characteristics of their hosts, i.e., the specific strains of bacteria, can improve the prediction of the risk to human and animal health.
Conclusion
The study characterizes three E. coli plasmids (pECmdr13.2, pECmdr13.3, pECmdr14.1) from chicken manure, each carrying ARGs, insertion elements (IS), transposons (Tn26, Tn6196), and a class 1 integron-integrase gene (intI1), common in resistance plasmids from key pathogens, and a mercury resistance operon promoting maintenance without antibiotic pressure. These plasmids confer an MDR phenotype, carry conjugal transfer genes, facilitating HGT, and belong to incompatibility groups IncX1 (narrow host range, Enterobacteriaceae and Pseudomonas spp.) and IncC (broad host range). No such plasmids have been previously reported in Poland, though similar ones have been identified in crucial human pathogens globally, and in natural settings like soil and water. This underscores the urgent need for vigilant monitoring of R-plasmids prevalence in the human, animal, and natural environments and their relationships with different bacterial hosts to understand their involvement in the transfer of AMR determinants.
Data availability
The genome sequences of E. coli strains generated during this study have been deposited in GenBank (NCBI) with the accession number PRJNA942482. Plasmid datasets generated during this study have been deposited in the University of Warsaw repository with the DOI number https://doi.org/10.58132/8ADBIC.
References
Abe K, Nomura N, Suzuki S (2020) Biofilms: hot spots of horizontal gene transfer (HGT) in aquatic environments, with a focus on a new HGT mechanism. FEMS Microbiol Ecol 96:fiaa031. https://doi.org/10.1093/femsec/fiaa031
Abt MC, Pamer EG (2014) Commensal bacteria mediated defenses against pathogens. Curr Opin Immunol 29:16–22. https://doi.org/10.1016/j.coi.2014.03.003
Ambrose SJ, Harmer CJ, Hall RM (2018) Evolution and typing of IncC plasmids contributing to antibiotic resistance in Gram-negative bacteria. Plasmid 99:40–55. https://doi.org/10.1016/j.plasmid.2018.08.001
Aminov R (2011) Horizontal gene exchange in environmental microbiota. Front Microbiol 2:158. https://doi.org/10.3389/fmicb.2011.0015
Andersson DI, Hughes D (2014) Microbiological effects of sublethal levels of antibiotics. Nat Rev Microbiol 12:465–478. https://doi.org/10.1038/nrmicro3270
Andrews S (2010) FastQC: a quality control tool for high throughput sequence data. https://www.bioinformatics.babraham.ac.uk/projects/fastqc/. Accessed 22 Dec 2023
Anjum MF, Schmitt H, Börjesson S et al (2021) The potential of using E. coli as an indicator for the surveillance of antimicrobial resistance (AMR) in the environment. Curr Opin Microbiol 64:152–158. https://doi.org/10.1016/j.mib.2021.09.011
Anokyewaa Appau AA, Ofori LA (2024) Antibiotic resistance profile of E. coli isolates from lettuce, poultry manure, irrigation water, and soil in Kumasi, Ghana. Int J Microbiol 2024:6681311. https://doi.org/10.1155/2024/6681311
Baker-Austin C, Wright MS, Stepanauskas R, McArthur JV (2006) Co-selection of antibiotic and metal resistance. Trends Microbiol 14:176–182. https://doi.org/10.1016/j.tim.2006.02.006
Bielen A, Babić I, Vuk Surjan M et al (2024) Comparison of MALDI-TOF mass spectrometry and 16S rDNA sequencing for identification of environmental bacteria: a case study of cave mussel-associated culturable microorganisms. Environ Sci Pollut Res 31:21752–21764. https://doi.org/10.1007/s11356-024-32537-1
Biemer JJ (1973) Antimicrobial susceptibility testing by the Kirby-Bauer disc diffusion method. Ann Clin Lab Sci 3:135–140
Birnboim HC, Doly J (1979) A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res 7:1513–1523
Błażejewska A, Zalewska M, Grudniak A, Popowska M (2022) A Comprehensive Study of the Microbiome, Resistome, and Physical and Chemical Characteristics of Chicken Waste from Intensive Farms. Biomolecules 12:1132. https://doi.org/10.3390/biom12081132
Broom LJ (2017) The sub-inhibitory theory for antibiotic growth promoters. Poult Sci 96:3104–3108. https://doi.org/10.3382/ps/pex114
Cain AK, Liu X, Djordjevic SP, Hall RM (2010) Transposons related to Tn1696 in IncHI2 plasmids in multiply antibiotic resistant Salmonella enterica serovar Typhimurium from Australian animals. Microb Drug Resist 16:197–202. https://doi.org/10.1089/mdr.2010.0042
Chauvin C, Clement C, Bruneau M, Pommeret D (2007) Time-patterns of antibiotic exposure in poultry production—A Markov chains exploratory study of nature and consequences. Prev Vet Med 80:230–240. https://doi.org/10.1016/j.prevetmed.2007.02.010
Chee-Sanford JC, Mackie RI, Koike S et al (2009) Fate and transport of antibiotic residues and antibiotic resistance genes following land application of manure waste. J Environ Qual 38:1086–1108. https://doi.org/10.2134/jeq2008.0128
Chen S, Zhou Y, Chen Y, Gu J (2018) fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34:i884–i890. https://doi.org/10.1093/bioinformatics/bty560
De Coster W, D’Hert S, Schultz DT et al (2018) NanoPack: visualizing and processing long-read sequencing data. Bioinformatics 34:2666–2669. https://doi.org/10.1093/bioinformatics/bty149
Do TT, Nolan S, Hayes N et al (2022) Metagenomic and HT-qPCR analysis reveal the microbiome and resistome in pig slurry under storage, composting, and anaerobic digestion. Environ Pollut 305:119271. https://doi.org/10.1016/j.envpol.2022.119271
Dobiasova H, Dolejska M (2016) Prevalence and diversity of IncX plasmids carrying fluoroquinolone and β-lactam resistance genes in Escherichia coli originating from diverse sources and geographical areas. J Antimicrob Chemother 71:2118–2124. https://doi.org/10.1093/jac/dkw144
Eckhardt T (1978) A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid 1:584–588. https://doi.org/10.1016/0147-619x(78)90016-1
Fang L, Li X, Li L et al (2016) Co-spread of metal and antibiotic resistance within ST3-IncHI2 plasmids from E. coli isolates of food-producing animals. Sci Rep 6:1–8. https://doi.org/10.1038/srep25312
Fang H, Feng J, Xu Y et al (2018) Sequencing of pT5282-CTXM, p13190-KPC and p30860-NR, and comparative genomics analysis of IncX8 plasmids. Int J Antimicrob Agents 52:210–217. https://doi.org/10.1016/j.ijantimicag.2018.04.012
Ghosh A, Singh A, Ramteke PW, Singh VP (2000) Characterization of large plasmids encoding resistance to toxic heavy metals in Salmonella abortus equi. Biochem Biophys Res Commun 272:6–11. https://doi.org/10.1006/bbrc.2000.2727
Hanning I, Diaz-Sanchez S (2015) The functionality of the gastrointestinal microbiome in non-human animals. Microbiome 3:51. https://doi.org/10.1186/s40168-015-0113-6
Harmer CJ, Hall RM (2014) pRMH760, a precursor of A/C2 plasmids carrying blaCMY and blaNDM genes. Microb Drug Resist 20:416–423. https://doi.org/10.1089/mdr.2014.0012
Hasman H, Aarestrup FM (2002) tcrB, a gene conferring transferable copper resistance in Enterococcus faecium: occurrence, transferability, and linkage to macrolide and glycopeptide resistance. Antimicrob Agents Chemother 46:1410–1416. https://doi.org/10.1128/AAC.46.5.1410-1416.2002
Hogan CA, Watz N, Budvytiene I, Banaei N (2019) Rapid antimicrobial susceptibility testing by VITEK®2 directly from blood cultures in patients with Gram-negative rod bacteremia. Diagn Microbiol Infect Dis 94:116–121. https://doi.org/10.1016/j.diagmicrobio.2019.01.001
James C, James SJ, Onarinde BA et al (2023) A critical review of AMR risks arising as a consequence of using biocides and certain metals in food animal production. Antibiotics 12:1569. https://doi.org/10.3390/antibiotics12111569
Jang J, Hur H-G, Sadowsky MJ et al (2017) Environmental Escherichia coli: ecology and public health implications—a review. J Appl Microbiol 123:570–581. https://doi.org/10.1111/jam.13468
Johansson MHK, Bortolaia V, Tansirichaiya S et al (2021) Detection of mobile genetic elements associated with antibiotic resistance in Salmonella enterica using a newly developed web tool: MobileElementFinder. J Antimicrob Chemother 76:101–109. https://doi.org/10.1093/jac/dkaa390
Kaper JB, Nataro JP, Mobley HLT (2004) Pathogenic Escherichia coli. Nat Rev Microbiol 2:123–140. https://doi.org/10.1038/nrmicro818
Khan GA, Berglund B, Khan KM et al (2013) Occurrence and abundance of antibiotics and resistance genes in rivers, canal and near drug formulation facilities – a study in Pakistan. PLoS One 8. https://doi.org/10.1371/journal.pone.0062712
Klümper U, Gionchetta G, Catão E et al (2024) Environmental microbiome diversity and stability is a barrier to antimicrobial resistance gene accumulation. Commun Biol 7:1–13. https://doi.org/10.1038/s42003-024-06338-8
Le Devendec L, Mourand G, Bougeard S et al (2016) Impact of colistin sulfate treatment of broilers on the presence of resistant bacteria and resistance genes in stored or composted manure. Vet Microbiol 194:98–106. https://doi.org/10.1016/j.vetmic.2015.11.012
Lim S-K, Kim D, Moon D-C et al (2020) Antibiotic resistomes discovered in the gut microbiomes of Korean swine and cattle. GigaScience 9. https://doi.org/10.1093/gigascience/giaa043
Marano RBM, Fernandes T, Manaia CM et al (2020) A global multinational survey of cefotaxime-resistant coliforms in urban wastewater treatment plants. Environ Int 144:106035. https://doi.org/10.1016/j.envint.2020.106035
Męcik M, Buta-Hubeny M, Paukszto Ł et al (2023) Poultry manure-derived microorganisms as a reservoir and source of antibiotic resistance genes transferred to soil autochthonous microorganisms. J Environ Manag 348:119303. https://doi.org/10.1016/j.jenvman.2023.119303
Merchant LE, Rempel H, Forge T et al (2012) Characterization of antibiotic-resistant and potentially pathogenic Escherichia coli from soil fertilized with litter of broiler chickens fed antimicrobial-supplemented diets. Can J Microbiol 58:1084–1098. https://doi.org/10.1139/w2012-082
Nesporova K, Valcek A, Papagiannitsis C et al (2021) Multi-drug resistant plasmids with ESBL/AmpC and mcr-5.1 in Paraguayan poultry farms: the linkage of antibiotic resistance and hatcheries. Microorganisms 9:866. https://doi.org/10.3390/microorganisms9040866
Norizuki C, Wachino J, Suzuki M et al (2017) Specific blaCTX-M-8/IncI1 plasmid transfer among genetically diverse Escherichia coli isolates between humans and chickens. Antimicrob Agents Chemother 61:e00663-e717. https://doi.org/10.1128/AAC.00663-17
Oliveira M, Viñas I, Usall J et al (2012) Presence and survival of Escherichia coli O157:H7 on lettuce leaves and in soil treated with contaminated compost and irrigation water. Int J Food Microbiol 156:133–140. https://doi.org/10.1016/j.ijfoodmicro.2012.03.014
Pal C, Asiani K, Arya S et al (2017) Metal resistance and its association with antibiotic resistance. Adv Microb Physiol 70:261–313. https://doi.org/10.1016/bs.ampbs.2017.02.001
Partridge SR, Kwong SM, Firth N, Jensen SO (2018) Mobile genetic elements associated with antimicrobial resistance. Clin Microbiol Rev 31:https://doi.org/10.1128/cmr.00088-17. https://doi.org/10.1128/cmr.00088-17
Pinilla-Redondo R, Cyriaque V, Jacquiod S et al (2018) Monitoring plasmid-mediated horizontal gene transfer in microbiomes: recent advances and future perspectives. Plasmid 99:56–67. https://doi.org/10.1016/j.plasmid.2018.08.002
Pu C, Gong X, Sun Y (2019) Characteristics of two transferable aminoglycoside resistance plasmids in Escherichia coli isolated from pig and chicken manure. Front Environ Sci Eng 13:35. https://doi.org/10.1007/s11783-019-1119-2
Pujiastuti ES, Tarigan JR, Sianturi E, Ginting BB (2018) The effect of chicken manure and beneficial microorganisms of EM-4 on growth and yield of kale (Brassica oleraceae acephala) grown on andisol. IOP Conf Ser: Earth Environ Sci 205:012020. https://doi.org/10.1088/1755-1315/205/1/012020
Redondo-Salvo S, Fernández-López R, Ruiz R et al (2020) Pathways for horizontal gene transfer in bacteria revealed by a global map of their plasmids. Nat Commun 11:3602. https://doi.org/10.1038/s41467-020-17278-2
Rozwandowicz M, Brouwer MSM, Fischer J et al (2018) Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J Antimicrob Chemother 73:1121–1137. https://doi.org/10.1093/jac/dkx488
San Millan A (2018) Evolution of plasmid-mediated antibiotic resistance in the clinical context. Trends Microbiol 26:978–985. https://doi.org/10.1016/j.tim.2018.06.007
Schwengers O, Jelonek L, Dieckmann MA et al (2021) Bakta: rapid and standardized annotation of bacterial genomes via alignment-free sequence identification. Microbial Genomics 7:000685. https://doi.org/10.1099/mgen.0.000685
Shoma S, Kamruzzaman M, Ginn AN et al (2014) Characterization of multidrug-resistant Klebsiella pneumoniae from Australia carrying blaNDM-1. Diagn Microbiol Infect Dis 78:93–97. https://doi.org/10.1016/j.diagmicrobio.2013.08.001
Summers AO, Wireman J, Vimy MJ et al (1993) Mercury released from dental “silver” fillings provokes an increase in mercury- and antibiotic-resistant bacteria in oral and intestinal floras of primates. Antimicrob Agents Chemother 37:825–834. https://doi.org/10.1128/aac.37.4.825
Tacconelli E, Carrara E, Savoldi A et al (2018) Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 18:318–327. https://doi.org/10.1016/S1473-3099(17)30753-3
Tan Y, Zhao K, Yang S et al (2024) Insights into antibiotic and heavy metal resistance interactions in Escherichia coli isolated from livestock manure and fertilized soil. J Environ Manag 351:119935. https://doi.org/10.1016/j.jenvman.2023.119935
Tenaillon O, Skurnik D, Picard B, Denamur E (2010) The population genetics of commensal Escherichia coli. Nat Rev Microbiol 8:207–217. https://doi.org/10.1038/nrmicro2298
Viegas C, Carolino E, Malta-Vacas J et al (2012) Fungal contamination of poultry litter: a public health problem. J Toxicol Environ Health A 75:1341–1350. https://doi.org/10.1080/15287394.2012.721165
Wang M, Goh Y-X, Tai C et al (2022) VRprofile2: detection of antibiotic resistance-associated mobilome in bacterial pathogens. Nucleic Acids Res 50:W768–W773. https://doi.org/10.1093/nar/gkac321
Wasyl D, Kern-Zdanowicz I, Domańska-Blicharz K et al (2015) High-level fluoroquinolone resistant Salmonella enterica serovar Kentucky ST198 epidemic clone with IncA/C conjugative plasmid carrying blaCTX-M-25 gene. Vet Microbiol 175:85–91. https://doi.org/10.1016/j.vetmic.2014.10.014
Wick RR, Judd LM, Gorrie CL, Holt KE (2017) Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol 13:e1005595. https://doi.org/10.1371/journal.pcbi.1005595
Wozniak RAF, Waldor MK (2010) Integrative and conjugative elements: mosaic mobile genetic elements enabling dynamic lateral gene flow. Nat Rev Microbiol 8:552–563. https://doi.org/10.1038/nrmicro2382
Yang H, Wei S-H, Hobman JL, Dodd CER (2020) Antibiotic and metal resistance in Escherichia coli isolated from pig slaughterhouses in the United Kingdom. Antibiotics (basel) 9:746. https://doi.org/10.3390/antibiotics9110746
Zalewska M, Błażejewska A, Czapko A, Popowska M (2023) Pig manure treatment strategies for mitigating the spread of antibiotic resistance. Sci Rep 13:11999. https://doi.org/10.1038/s41598-023-39204-4
Zalewska M, Błażejewska A, Czapko A, Popowska M (2021) Antibiotics and antibiotic resistance genes in animal manure – consequences of its application in agriculture. Front Microbiol, vol 12. https://doi.org/10.3389/fmicb.2021.610656
Zalewska M, Błażejewska A, Szadziul M et al (2024) Effect of composting and storage on the microbiome and resistome of cattle manure from a commercial dairy farm in Poland. Environ Sci Pollut Res 31:30819–30835. https://doi.org/10.1007/s11356-024-33276-z
Zhang Y, Lei C-W, Chen X et al (2020) Characterization of IncC plasmids in Enterobacterales of food-producing animals originating from China. Front Microbiol 11:580960. https://doi.org/10.3389/fmicb.2020.580960
Zimin AV, Puiu D, Luo M-C et al (2017) Hybrid assembly of the large and highly repetitive genome of Aegilops tauschii, a progenitor of bread wheat, with the MaSuRCA mega-reads algorithm. Genome Res 27:787–792. https://doi.org/10.1101/gr.213405.116
Acknowledgements
The research was conducted in the frame of the JPI-EC-AMR Joint Transnational Call (JPIAMR), JPI-EC-AMR JTC 2017, project INART—“Intervention of antibiotic resistance transfer into the food chain” granted to MP, grant no. 2017/25/Z/NZ7/03026, National Science Centre, Poland.
Funding
The research was funded by National Science Centre, Poland (2017/25/Z/NZ7/03026), grant under the European Horizon 2020, in the frame of the JPI-EC-AMR Joint Transnational Call (JPIAMR), JPI-EC-AMR JTC 2017, project INART—“Intervention of antibiotic resistance transfer into the food chain” to MP and partially in the frame of the “Excellence Initiative—Research University (2020–2026)” Program at the University of Warsaw. Sequencing was performed in GCF CeNT UW (RRID: SCR_022718), using NovaSeq 6000 platform financed by the Polish Ministry of Science and Higher Education (decision no. 6817/IA/SP/2018 of 2018–04-10).
Author information
Authors and Affiliations
Contributions
M.Z.—methodology, formal analysis, investigation, data curation, writing—original draft, visualization; A.B.—formal analysis, investigation; J.G.—formal analysis, data curation; A.D., S.M., K.G.—sequencing and primary data curation; P.K.—investigation, data curation; M.P.—conceptualization, writing—review and editing, project administration, funding acquisition.
Corresponding author
Ethics declarations
Ethics approval
Following the local legislation and institution requirements, collecting cow manure and conducting research using it does not require consent. The farm owner agreed to the manure being sampled and shared the history of the use of antibiotics on the farm.
Consent to participate
Not applicable.
Consent for publication
All authors have approved the final version of the manuscript to be published.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Diane Purchase
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11356_2024_34283_MOESM1_ESM.xlsx
Supplementary file1 Table S1. The antimicrobial susceptibility profile of bacterial isolates (E. coli), determined by Vitek 2 Compact (XLSX 12 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zalewska, M., Błażejewska, A., Gawor, J. et al. The IncC and IncX1 resistance plasmids present in multi-drug resistant Escherichia coli strains isolated from poultry manure in Poland. Environ Sci Pollut Res (2024). https://doi.org/10.1007/s11356-024-34283-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11356-024-34283-w