Abstract
In this work, the efficacy of two metal–organic frameworks (MIL-101(Fe) and NH2-MIL-101(Fe)) in eliminating acetamiprid (ATP) insecticide and eosin Y (EY) dye from aqueous solution is tested. An analysis was conducted on the developed nanocomposite’s optical, morphological, and structural characteristics. The adsorption isotherm, kinetics, thermodynamics, reusability, and mechanisms for ATP and EY dye removal were assessed. NH2-MIL-101(Fe) adsorbed 76% and 90% of ATP pesticide and EY dye, respectively after 10 to 15 min in optimum conditions. For both adsorbents, with regard to explaining the isotherm data, the Langmuir model offered the most accurate description. Moreover, the adsorption of ATP and EY dye is described by the pseudo-second-order kinetic model. The maximum adsorption capacities of ATP and EY dye on MIL-101(Fe) were 57.6 and 48.9 mg/g compared to 70.5 and 97.8 mg/g using NH2-MIL-101(Fe). The greatest amount of ATP and EY dye clearance was obtained at a neutral medium for both adsorbents. The results of this investigation demonstrate the effectiveness of MIL-101(Fe) and NH2-MIL-101(Fe) as effective substances in the adsorption process for removing pesticides and dyes from aqueous solution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water pollution is still a problem, and the security of water resources has a significant impact on human life (Zhang et al. 2022). A rise in emerging pollutants in the environment is being caused by anthropogenic activities and industrial outputs (Marican & Duran-Lara 2018). Due to the risks it poses to the environment and public health, water contamination by organic and inorganic substances is a pressing issue that has attracted attention worldwide (Doan et al. 2021). Because of the pollutants’ toxicity, persistence, and concentration, there are negative effects on the environment, public health, and the economy (Fua et al. 2014). Aside from other demands, there has been a significant growth in the water demand, with the agricultural, industrial, and home sectors absorbing, respectively, 70%, 22%, and 8% of the freshwater supply. Approximately 500 million tonnes of these microcontaminants are produced annually on a worldwide scale (Saleh et al. 2020). Large volumes of wastewater containing a variety of contaminants have been produced as a result of this. Wastewater must thus be treated prior to release into the environment (Zainorizuan et al. 2017). Due to their bio-accumulative property, which has a harmful influence on the environment and human health, pesticides of various types are a threat to the cosmos. Generally, hazardous chemical identification is a time-consuming and expensive operation on the other hand. Pesticide treatment from water faces many difficulties, including the impactful composition, the variety of pesticide physical structures, and the pH of the pesticide-tainted water. The variety of amounts of pesticides in various water sources is 0.1 to 107 mg. L−1. However, the effluent from the manufacture of pesticides needs to be thoroughly treated before it is mixed with domestic wastewater. Consequently, given their high concentrations and recalcitrance in wastewater and water sources treated with pesticides is a crucial scientific study (Anand et al. 2020; Saleh et al. 2020). Nowadays, neonicotinoid insecticides have been demonstrated to be a novel group of active components. Acetamiprid, clothianidin, thiamethoxam, and imidacloprid are the most widely used neonicotinoid insecticides (Ghiasi et al. 2022). Acetamiprid is a relatively new and effective neonicotinoid pesticide and traces of it have a critical impact on water (2.50 ng. L−1, 0.2–7.7 μg. L−1) (Zoumenou et al. 2019). In rats, oral administration of 217 mg.kg−1 is the average fatal dosage. The buildup of this pesticide in different tissues is tightly linked to both direct and indirect exposure to it (Pisa et al. 2021). Research has shown that mice’s liver, kidney, and other tissues contain notable amounts of acetamiprid and its metabolites (Ford & Casida 2006). According to several research on acetamiprid exposure, oxidative stress production might be the overall mechanism behind the toxicity of pesticides to several organs in animal studies. Mammals exposed to acetamiprid have shown signs of oxidative stress, ptosis, and DNA damage (Annabi et al. 2019, Ayyavoo & Tamilselvan 2019).
The global manufacturing of synthetic dyes is estimated by tons of the total pigments produced annually, and about 10–60% of the effluent containing the dye which emitted into the environment without proper treatment. Dye metabolites that have been subjected to oxidation, heat, and light are very hazardous and have cancer-causing properties (Assefi et al. 2014, Borzelleca et al. 1987). When trying to remove it, we may face many difficulties including dye decomposition since these dyes are not biodegradable and have a high resilience against oxidation and light (Mahanna & El-Bendary 2022).
2-(2,4,5,6- Tetrabromo-6-oxido-3-oxido-3H xanthenes-9-yl) benzoate disodium salt is the IUPAC name for eosin yellow, an anionic dye (Ansari et al. 2016). It belongs to the fluorescein dye family and is very water-soluble. Eosin Yellow is frequently used in gram staining to distinguish between various bacterial species because of its remarkable capacity to absorb red blood cells and red color (Oladipo et al. 2021). EY dye is a unique acid dye often used for printing, tanning, and coloring leather, cotton, printing, and other materials (Jabbar et al. 2022) (Singh et al. 2017). According to Eosin Yellow’s toxicological data, the EY dye could seriously irritate skin and eyes. When the dye physically touches the skin, it irritates it, resulting in discomfort, redness, and swelling. When consumed, it has several negative consequences, especially on the body's essential organs including the liver and kidney. Direct dye contact with the eye can permanently damage the cornea. The DNA in living things’ gastrointestinal organs is also damaged, which leads to an assortment of ailments within a human being. The ability of the lungs to exchange gases is decreased by dye inhalation. Additionally, dyes can be poisonous and carcenogenic, metabolites (Sharma et al. 2019; Silva et al. 2018, Thabet & Ismaiel 2016).
Coordination polymers/networks or metal–organic frameworks (MOFs) are a novel category of hybrid porous crystalline materials that emerge from the interaction of multidentate organic linkers with inorganic vertices, such as metal ions or clusters, acting as nodes (Trung et al. 2011). MOFs are characterized by microporous structures that hold larger pore sizes called MIL (Material of Institute Lavoisier) (Rasheed et al. 2018). This type of material attracts more attention in many potential applications like heterogeneous catalysis (Wen et al. 2018), adsorption (Jiang et al. 2019), gas storage (Qasem et al. 2018), gas sensing (Wang et al. 2018a), drug delivery (Wu et al. 2017b), and photocatalysis (Xiao et al. 2019) because of their amazing characteristics, such as high porosity and large surface area, tunable functionality, and thermal and mechanical stability (Long & Yaghi 2009). Due to the low crystal density of MOFs, which have no sufficient dispersion forces to hold small molecules, an effective composite prepared of MOFs with another substrate solves this drawback (Wu et al. 2017a). Two subfamilies of MOFs are presented like zeolite imidazolate frameworks (ZIFs) and metal carboxylate frameworks (MOFs) (Orribas et al. 2016). In the metal carboxylate frameworks, the metal ions are iron (III), and terephthalic acid acts as a carboxylate linker. MIL-101(Fe) is processed in different catalytic reactions like Friedel–Crafts benzylation as it exhibits high stability and catalytic activity (Rasheed et al. 2018). One of the most effective MOFs is MIL-101 based on their unique properties such as high thermodynamically stable material with high surface area. These properties make MIL-101 good for the adsorption and separation of a wide variety of pollutants and CO2 gases from humid atmospheres (Mei et al. 2017). Besides, it is considered an eco-friendly material due to the central metal ion (Fe), low cost, and non-toxicity compared with other metal ions (Li et al. 2019a).
Adsorption is a physical phenomenon that occurs on surfaces and is heavily influenced by factors that include surface area, pore size, pH, solution temperature, and the kind of adsorbent and its substituent groups (Ahmad et al. 2010). Studying adsorption kinetics is crucial for determining the best adsorption system because it allows scientists to forecast the pace at which pollutants will be removed from the environment, determine how much residual adsorbate will remain in solution over time, and identify the mechanisms at play throughout the adsorption process given how straightforward and inexpensive it is compared to other methods, it is regarded as a well-developed procedure for removing dyes, pesticides, and heavy metals from wastewater (Joseph et al. 2021; Wang et al. 2020).
In this study, iron as a metal node, and 2-amino terephthalic acid was used as an organic linker in MIL-101(Fe) development. The designed adsorbent NH2-MIL-101(Fe) displayed a highly chelating affinity to adsorbent for pesticides and dyes attributed to the amine group that reinforced the adsorption capacity against hazardous waste from the solution. In this study, an efficient adsorbent was used to demonstrate the removal of Eosin Y dye and low and high concentrations of acetamiprid from polluted water. By using the techniques of FT-IR, XRD, XPS, SEM, EDX, TEM, and BET, samples were characterized and investigated. Other parameters were also studied, like initial concentrations of adsorbate, contact time, the initial pH of solutions, adsorbent dose, and temperature.
Experimental
Materials
All compounds’ substances and solvents which are applied in the adsorption studies were of the analytical grade, and none of them required further purification before usage. Ferric chloride hexahydrate (FeCl3.6H2O), 2-amino terephthalic acid (NH2-H2BDC), and Eosin yellow were purchased from Alfa Aesar. N, N-dimethyl formamide (DMF), and absolute ethanol were purchased from EDWIC, while terephthalic acid was acquired from ADVENT business. Pure acetamiprid pesticide (assay 96%) was obtained from Jiangsu Fengshan Group Co., LTD – China. Stock solutions of ATP pesticide and EY dye solutions were prepared via dist. purified water by a Milli-Q system.
Synthesis of MIL-101(Fe) and NH2-MIL-101(Fe)
MIL-101(Fe) was synthesized using a solvothermal method explained elsewhere (Skobelev et al. 2013) as shown in Fig. 1. Briefly, 15 mL of DMF was used to dissolve 0.675 g (2.45 mmol) FeCl36H2O and 0.206 g (1.24 mmol) BDC. Then the solution was moved to a Teflon autoclave made of stainless steel and sealed, for 20 h at 110 °C in a traditional oven. After centrifuging the powder, it was three times cleaned in DMF and hot ethanol before being left to dry overnight at 80 °C (Zorainy et al. 2021). The same precedence is applied in this instance synthesis of NH2-MIL-101 (Fe) but the linker changed to NH2-BDC 0.217 g)1.2 mmol).
Batch equilibrium studies
The 50 mg of adsorbent in the 50 mL of pesticide/dye solutions was used in batch adsorption experiments. In the cases of the pesticide and dye, the solutions were churned for 120 and 140 min, respectively, after being suspended using ultrasonic technology for a few minutes. Using diluted NaOH and HCl solutions, the ideal pH of solutions was adjusted. Applying Eqs. (1) and (2), the removal % and adsorption capabilities were assessed.
where V(L) represents the solution's volume, \({\mathrm{C}}_{\mathrm{i}}\) is the adsorbate concentration initially (mg. L−1), \({\mathrm{C}}_{\mathrm{e}}\) in balanced with the adsorbate concentration (mg.L−1), and \({\mathrm{m}}_{(\mathrm{g})}\) represents the dosage of adsorbent.
Kinetic studies
To ensure that investigate of the impact of the contact time, 50 mL of ATP and EY dye with different concentrations was added to 100 mL alongside 0.05 g of an absorbent at 298 K while stirring. At time intervals, after 5, 10, 15, 30, 45, 60, 75, 90, 105, 120, 180, and 240 min, the aliquots were collected. To separate the solid from the supernatant, the aqueous solutions were diluted and centrifuged in Falcon tubes. The optimum pH of ATP and EY dye adsorption was adjusted by making adjustments with solutions of HCl and NaOH. Also, the impact of the adsorbent dosage was carried out with various masses of NH2-MIL-101(Fe) (0.2, 0.6, 1.0, 1.4, 1.8, and 2 g.L−1). The ATP pesticide and EY dye concentrations in supernatants for all experiments were measured by HPLC–DAD, and UV–visible spectrophotometer, respectively, using Eq. (1).
Characterization
A record of the synthesized catalysts' crystal structure and phase purity was made at room temperature in the 2θ range from 4 to 70o via X-ray diffraction (XRD) using a Bruker axs Co D8 Discover, beside Cu-K radiation (40 kV, 40 mA); Germany. The surface morphology and crystal size spectroscopy (FTIR) were noted on a spectrometer (Thermo Scientific Nicolet iS10 Spectrometer) applying the typical KBr disc. In order to determine compositions of elemental and chemical valance referred to the fabricated frameworks, X-ray photoelectron spectroscopy (XPS, Thermo Fisher Scientific™ K-Alpha™, USA) beside monochromatic X-ray Al K-alpha radiation -10 to 1350 eV, Al-Kα micro-focused monochromator performed at an energy range up to 4 keV, during pressure 10–9 m bar the spot size was 400 µm along with full spectrum pass energy 200 eV and at narrow spectrum 50 eV was employed. A scanning electron microscope (SEM, JEOL JSM-6510LV, Japan) analyzed the surface morphology and crystal size of the prepared MOFs. Energy-dispersive X-ray spectroscopy (EDS) X-MaxN-20 (Oxford Instruments) was used to conduct an elemental analysis with high spatial resolution. A transmission electron microscope (TEM) (L120C—TEM—ThermoFisher—Europe) operating at 120 kV accelerating voltage was used to examine the microstructural properties of the samples. Brunauer–Emmett–Teller (BET) method was used to determine the specific surface area analysis was determined. The N2 physical absorption–desorption isotherms measurements were performed at 273 K applying Belsorb III equipment, Japan. The samples were evacuated at 150 °C before measurement. The remaining concentration in supernatants was evaluated using HPLC–DAD (high-pressure liquid chromatography-diode array detector Agilent Technologies 1200 series). A YP5 B C18 (125 mm × 4 mm) analytical column was utilized. 1.0 mL.min−1 is the rate of flow and A 30 L injection volume was used. The isocratic elution circumstances were acetonitrile/water (50:50, v/v); wavelength, 255 nm. A UV–visible spectrometer (UV-2000, UNICO, USA) was used to measure the light absorption qualities. The results for the UV–vis absorption spectra were acquired within the 200–800 nm test range.
Results and discussion
Design and characterization of MIL-101(Fe) and NH2-MIL-101(Fe)
NH2-MIL-101(Fe) and MIL-101(Fe) infrared spectra are displayed in Fig. 2a. As a result, the benzene-carboxylates were primarily visible in the distinctive IR spectra of MIL-101(Fe). MIL-101(Fe) spectrum displays a stretched band that is symmetrical and assigned to 3213 cm−1 attributed to \(-\) COOH vibration (Gecgel et al. 2019a). A band at 1659.38 cm−1 shows the presence of DMF molecules (Wu et al. 2013). The bands at 1593 and 1388 cm−1 were allocated to the C \(=\) O and C \(-\) C vibrational modes (Xie et al. 2017). The band at 749 cm−1 was associated with the benzene ring in the frameworks (Wang et al. 2018b). Furthermore, the observable band at 578 cm−1 was allocated to the Fe–O vibration (El-Bendary et al. 2021). NH2-MIL-101(Fe) exhibited the same characteristic bands of MIL-101(Fe) not including the bands at 3463 and 3367 cm−1, which enhances the symmetric and asymmetric amine of amino terephthalic acid (Gecgel et al. 2019a). Additionally, the band assigned to 1256 cm−1 is attributed to the extending approach to aromatic carbon C \(-\) N bonding (Xie et al. 2017).
Crystalline structures of the fabricated frameworks were distinguished by XRD as illustrated in Fig. 2b. Both fabricated MILs illustrated the main characteristic peak placed at 2θ of \(\approx\) 9.2°. XRD pattern of the blank MIL-101(Fe) displayed precisely defined diffraction peaks situated in 2θ of 12.6°, 16.7°, 18.7°, and 21.9° (Zhou et al. 2020). MIL-101(Fe) and NH2-MIL-101(Fe) showed comparable peals in their crystal structures (Xie et al. 2017). As a consequence, both frameworks have the same crystal structure, and the successful preparation is confirmed to match the previously published diffraction pattern (Wang & Li 2015).
Validating the surface composition and chemical makeup of the as-synthesized substances was done by an investigation of the XPS analysis. A comprehensive survey scan of NH2-MIL-101(Fe) reveals the presence of C 1 s, N 1 s, and O 1 s with Fe 2p and Cr 2p metals, respectively, confirming the accomplishment of MOF formation which shown in Fig. 2 e, f, g, h, and i. Four peaks at ranges of 284.6, 285.9, 288.2, and 291.1 eV may be seen in the HR-XPS of C 1 s, which are attributed to C–C (sp2 and sp3), C-O, C = O, and O-C = O, correspondingly (Li et al. 2019b). At around 530.0, 531.4, and 533.0 eV, respectively, the O 1 s deconvoluted into three peaks, each of which had a MIL attributed to H2O, metal-O, and -OH (Zhang et al. 2021). Concurrently, the presence of an amine group in the organic linker structure resulted in the division of the N 1 s peak into two contributions denoting which represent the amino group (C \(-\) NH2) and the protonated N species (C \(-{\mathrm{NH}}_{3}^{+}\)) (Solís et al. 2021). At 712.0 and 725.0 eV, respectively, the HR-XPS of the Fe 2p spectrum revealed the major peaks of Fe 2P1/2 and Fe 2p3/2. Fe+3 is responsible for the peaks at 714.0, 718.4, and 729.8 eV, while Fe+2 is responsible for the peaks at 711.1 and 724.7 (Quan et al. 2019).
The SEM and TEM images of the prepared MIL-101(Fe) and NH2-MIL-101(Fe) are shown in Fig. 3. SEM analysis was employed to determine differences in the surface morphology of the manufactured frameworks. Figure 3 a and b shows the as-prepared framework particles with unique microstructures and morphologies of the metal-dielectric structure. It is obvious that both MIL-101(Fe) and NH2-MIL-101(Fe) materials have a loose and uniform polyhedron structure with an average diameter of 0.3–1 μm and 1–2 μm (Fig. S10.a and b), which matches previous reports in the literature (Boontongto & Burakham 2019, Chen et al. 2021). The crystallite size (D) was found to be 0.065648–0.000159 μm as shown in Table S1, and the particles are regular octahedrons as shown in Fig. S10. In addition, Fig. S11 illustrates the elemental analysis of the MIL-101(Fe) and NH2-MIL-101 (Fe) taken from EDX. According to results confirmed The presence of C, O, Fe, and N as the only elementary components of frameworks that match with data obtained from XPS (Gecgel et al. 2019b). The unique porous morphologies of the products are characterized by TEM as illustrated in Fig. 3 c and d. The as-prepared NH2-MIL101(Fe) is shown to have maintained the MIL-101(Fe) original morphology. The sample surface exhibits a porous frame with a hierarchical structure, which is derived from the MOFs. The morphologies of MIL-101(Fe) and NH2-MIL-101(Fe) show an octahedral structure, which is similar to the Zr-based materials (Xie et al. 2017).
The surface area of NH2-MIL-101(Fe) and MIL-101(Fe) measured by Brunauer–Emmett–Teller (BET) is determined by the surface area analyzer (Belsorb III equipment). Figure 2 c and d displays a plot of adsorbed volume vs. relative pressure. The surface area of both frameworks is acquired through nitrogen adsorption/desorption at –77 K, respectively. The BET surface area for MIL-101(Fe) is 125 m2.g−1, which is less than the amino-MIL-101(Fe) whose surface area is 953 m2.g−1 which is in line with other reports in the literature (Dong et al. 2020; Mahdipoor et al. 2021; Shan et al. 2020). As can be seen, the surface morphology of the amino-MIL-101(Fe) (Fig. S11) is much less porous compared to MIL-101(Fe), which reduces the surface area of the MOF in the absence of the amine group. In other words, functionalization of MIL-101(Fe) with the amine group increases the surface area of MOF by about seven times, which means increasing the active sites for the absorption of environmental pollutants (Rahmani et al. 2022), In addition, the results showed that the mean pore diameter and total pore volume for MIL-101(Fe) and NH2-MIL-101(Fe) were 3.37 nm, 0.1075 cm3.g−1, 1.88 nm nm, and 0.2456 cm3.g−1, respectively.
Batch adsorption studies
The removal efficiency was studied in the presence of two Fe-based MOFs for removing ATP and EY from an aqueous solution. For ATP, both MOFs show high equilibrium capacities of 57.6 and 70.1 g.L−1 in 10 min of contact time for MIL-101(Fe) and NH2-MIL-101(Fe), respectively. in the instance of EY dye, the adsorption capacity increased from 48.9 g.L−1 in the instance of MIL-101(Fe) to 97.8 g.L−1 in 15 min contact time by utilizing amine functionalized MIL as illustrated in Fig. 4 a and b The rate of adsorption raise significantly in the instance of NH2-MIL-101(Fe) compared to MIL-101(Fe), it might be explained by the amino groups that it contains, which provide the adsorbate molecules more adsorption sites during the adsorption process. (Dong et al. 2020). Based on the adsorption capacity data, we can observe that the adsorption capacity of NH2-MIL-101(Fe) is higher than MIL-101(Fe). Furthermore, the surface area of NH2-MIL-101(Fe) is 953 m2g −1 which is greater than that MIL-101(Fe) (Dong et al. 2020; Rahmani et al. 2022; Shan et al. 2020; Xu et al. 2020).
Comparison study between MIL-101(Fe) and NH2-MIL-101(Fe) for a ATP [conditions: initial concentration = 100 g.L-1, dose = 0.05 g, contact time = 2 h, pH 6 and temperature = 298 K]. b EY dye [conditions: initial concentration = 100 g.L-1, dose = 0.05 g, contact time = 4 h, pH 7 and temperature = 298 K]
For the adsorption experiments, different initial concentrations were performed between 5 and 100 mg.L−1 under the same conditions of temperature, dose, and pH. Increased dye and pesticide initial concentration leads to an increase in equilibrium adsorption capability. Nevertheless, for NH2-MIL-101(Fe), it was discovered that at a low concentration (between 5 and 30 mg. = L−1), as the initial concentration of ATP rises, the adsorption capacity reached equilibrium rapidly, and the speed slowed when the concentration level was raised Fig. 5a. This is due to the fact that a high adsorbate concentration can supply the required driving force to get over the adsorbate mass transfer barrier between the aqueous and solid phases. However, EY’s adsorption was investigated at various initial concentrations of 5–150 g.L−1 under room temperature. Figure 5b illustrated that NH2-MIL-101(Fe) exhibited excellent removal of EY dye with an equilibrium concentration of 136.8 g.L−1. More than 90% of EY dye was eliminated from the aqueous solution for the first 15 min in the case of all initial concentrations. After that, it faded off with progressively more time of contact. This behavior is because all of the active sites on the adsorbent were unoccupied at first and was high concentration of dye. Because of the possession of the active sites by dye molecules, the number of active sites was reduced, and the rate of sorption decreased as time passed (Nazir et al. 2022). After the equilibrium was attained, the adsorption process was not feasible due to the possible monolayer of dye formation onto the adsorbent surface.
Effect of initial concentration for adsorption of a ATP [conditions: initial concentration = 5–100 g.L-1, dose = 0.05 g, contact time = 2 h, pH 6 and temperature = 298 K]. b EY dye [conditions: initial concentration = 5–150 g.L-1, dose = 0.05 g, contact time = 4 h, pH 7 and temperature = 298 K]. Effect of solution’s pH on adsorption of c ATP [conditions: initial concentration = 100 g.L-1, dose = 0.05 g, contact time = 2 h and temperature = 298 K]. d EY dye [conditions: initial concentration = 150 g.L-1, dose = 0.05 g, contact time = 2 h and temperature = 298 K]
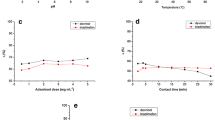
The concomitant effect of solution pH within the scope of 2–10 on the removal capacity of ATP was evaluated and the obtained contour graphs are displayed in Fig. 5c. The efficiency of removal of pesticides reached the maximum value at a neutral medium and reduced at lower and higher pH values. Because ATP has a low pKa of 0.7, it is found in aqueous solutions in its anionic form and its charge is unaffected by changes in solution pH (Dolatabadi et al. 2021). Therefore, at a lower pH, there is a positive charge on the adsorbent surface, and at a higher pH, negatively charged (Dolatabadi et al. 2021). The removal efficacy reduces at higher pH levels due to the electrostatic repulsion force among adsorbent and ATP molecules. At very low pH (pH = 2), the removal efficiency reduces, which is due to the as-synthesized frameworks that may undergo hydrolysis in a highly acidic medium. Besides, although NH2-MIL-101(Fe) was stable in both acids and water, it degraded quickly, especially under strong basic conditions. The weak acidic to neutral conditions of pH values should thus be maintained (pH ~ 6) to achieve a high adsorption capacity of ATP (Li et al. 2020b; Ma et al. 2022).
For EY dye removal, the pH range was between 5 and 10 and the optimum pH was between 5 and 7 as depicted in Fig. 5d. This resulted from the protonated amine group of NH2-MIL-101(Fe) interacting beside the dye anionic group during the adsorption process, which led to the creation of more cationic amines (Du et al. 2008). Furthermore, due to the higher electrostatic interactions, cause the dye molecules \((\mathrm{D}-\mathrm{COONa})\) to be absorbed by the frameworks \((\mathrm{R}-{\mathrm{NH}}_{2})\) to a greater extent as shown in Eq. (3–5). Therefore, the protonation of the amine group in the framework structure decreased at higher pH, and the removal capacity of EY dye decreased. The lower pH of the aqueous solution was not favored because most of the dye molecules were noticed to be precipitated and the color was changed during the adsorption of the dye.
The influence of adsorbent dosage on the removal of ATP utilizing the Fe-based frameworks was examined in the presence of different masses as shown in Fig. 6a. The rate of adsorption ATP enhanced through increasing the mass of adsorbent (0.2–1.8 g.L−1). Additionally, the same behavior was illustrated for the adsorption of EY dye on raising the dosage (0.2–2.0 g.L−1). The total number of adsorption sites rises dramatically at the high adsorbent dose, which leads to a larger removal of ATP and EY dye from the aqueous solution because the adsorbent active sites are more easily accessible and can interact with the analyte (Ghiasi et al. 2022; Mohammad et al. 2020). For ATP, the removal percentage reached 80.2% in the case of 1.0 g.L−1 compared to 94.3% when the dosage nearly doubled to 1.8 g.L−1. Therefore, the removal efficiency reached the highest of 316.9 mg.g−1 utilizing 2.0 g.L−1 in the case of EY dye Fig. 6b. Therefore, considering the economic cost, the optimal adsorbent dose is 1.0 g.L−1 that could be applied in the ensuing study.
Effect of adsorbent dose for adsorption of a ATP [conditions: initial concentration = 100 g.L-1, pH 6, contact time = 2 h and temperature = 298 K]. b EY dye [Conditions: initial concentration = 500 g L-1, pH 7, contact time = 2 h and temperature = 298 K]. Effect of reusability for adsorption of c ATP [conditions: initial concentration = 50 g.L-1, pH 6, contact time = 2 h and temperature = 298 K]. d EY dye [conditions: initial concentration = 50 g.L-1, pH 7, contact time = 4 h and temperature = 298 K]
The capacity to regenerate and reuse used adsorbents is crucial for cost-effective industrial applications. The reusability studies were carried out for ATP and EY dye for four cycles to confirm the industrial use of NH2-MIL-101(Fe). Upon completion of each cycle, the used adsorbent was extracted from the solution using a centrifuge, and it was thereafter repeatedly cleaned using distilled water and heated ethanol. Next, the repurposed adsorbent was dried at 80 °C and reused for the next cycles. For the initial cycle, the adsorption capacities were 96.5% and 93.4% for ATP and EY dye, as shown in Fig. 6c and d. After the fourth cycle, slight decreases in the removal percentages were noticed after four cycles. Moreover, Fig. 7a demonstrates that the FT-IR spectrum of the materials has no significant change. This means that there is no significant difference between the chemical bond and crystal structure of the sample before and after the reaction. Therefore, the NH2-Fe-MILs material has a strong reusability performance. Table S2 clearly shows that NH2-Fe-MILs material is an effective heterogeneous catalyst for the removal of ATP and EY dye from industrial wastewater which means it has the potential ability to remove other organic pollutants in industrial wastewater, and the XRD characterization of the material after four cycles showed that the peak intensity of the material was decreased. However, their crystalline structure was found to remain moderately intact after the repeated cycles (Fig. S13.). The use of the TEM technique revealed that after four cycles consecutive, there were no changes observed in the structure or crystallinity of the nanocomposite following the adsorption process. This observation suggests that the composite maintained high stability, as indicated in Fig. S12.
Figure 7a displayed the FTIR spectrum of the recycled frameworks after four cycles in the case of ATP and EY dye. The spectrum illustrates no considerable changes in the functional groups or their positions. This confirms that the prepared NH2-MIL-101(Fe) is an effective adsorbent in the removal of organic pollutants from aqueous solutions after various cycles.
The XRD patterns for MIL-101(Fe) and NH2-MIL-101(Fe) feature very similar peaks, as depicted in Fig. S13. This is because terephthalic acid, which is used to synthesis MIL-101(Fe), and 2-amino terephthalic acid, which is used to synthesis NH2-MIL-101(Fe), have similar structures. As a result, the products maintain similar crystal structures (Liu et al. 2022). Furthermore, the XRD pattern showed no discernible shift before or after adsorption, suggesting that the adsorbents’ structures were stable.
Utilizing transmission electron microscopy (TEM), an advanced imaging technique capable of high-resolution analysis provided crucial insights into the structural integrity of the nanocomposite. Through TEM analysis, it was established that there were no discernible alterations observed in the structural composition or crystalline characteristics of the nanocomposite subsequent to the adsorption process. This observation underscores the remarkable stability exhibited by the composite material. The findings, depicted graphically in Fig. S12, serve to highlight the robustness of the nanocomposite following its interaction with the adsorbate.
As listed in Table S3 and Table S4, a comparison of the ATP insecticide and EY dye adsorption in previous studies using different adsorbents with our work is illustrated. Remarkably, a high adsorption capacity was attained at a moderately brief time using MIL-101(Fe) and NH2-MIL-101(Fe) throughout this study.
Adsorption isotherm
In order to assess an adsorbent's sorption capacity and comprehend how the adsorbent binds and interacts with the adsorbate, it is crucial to use the adsorption isotherm method. This article aims to demonstrate the equilibrium nature of adsorption by examining many adsorption isotherm models. The constant parameters for each model were determined by fitting the experimental data with the Freundlich, Langmuir, D–R, Redlich-Peterson, and Temkin isotherm equations.
Freundlich and langmuir model
Adsorbent utilization can be improved by using the isotherm of adsorption; this is a general equilibrium relation that describes how an adsorbate and the binding sites scattered over a solid surface interact (Adly et al. 2021). Adsorption isotherm equations for the experimental findings were obtained by applying Langmuir and Freundlich (Xie et al. 2017).
Because of the adsorbent heterogeneous surface that exhibits multilayer adsorption, the Freundlich pattern is appropriate for application. The maximal adsorption capacity in this model is not known with certainty, since there are different adsorption sites with varying energies. The model of Freundlich is expressed as Eq. 6:
where, Kf (\(\mathrm{L}\).mg−1) and the constants of Freundlich that correspond to the adsorbent capacity for adsorption are represented via \(\mathrm{n}\), and adsorption intensity is represented as 1/n. The Freundlich coefficient, also known as the heterogeneity coefficient, is 1/n and it describes the deflection from linear adsorption. The sort of isotherm is either favorable (1/n < 1) or unfavorable (1/n > 2) based on the value of 1/n.
Because the surface is energetically homogenous, all adsorption sites are assumed to be equally active, and a monolayer surface coverage forms without any interaction among the adsorbed molecules, the Langmuir model is developed (Araújo et al. 2018). Equation 7 represents the Langmuir model:
where \({\mathrm{C}}_{\mathrm{e}}\) is the concentration of solute at equilibrium (mg.L−1), \({\mathrm{q}}_{\mathrm{e}}\) is solute adsorbed quantity per mass of adsorbent (mg.g−1), \({\mathrm{K}}_{\mathrm{L}}\) (L.mg−1) is the adsorption bonding energy-related Langmuir adsorption constant, and \({\mathrm{q}}_{\mathrm{m}}\) is the capability of Single-layer Langmuir adsorption (mg.g−1). Furthermore, this model posits a one-to-one relationship, wherein a single adsorbate can be drawn to each adsorptive site.
Table 1 provides the linear fitting process findings for Freundlich and Langmuir constants. The correlation coefficients found using an equation for Langmuir were greater than those using the equation for Freundlich. Therefore, as shown in Fig. 8, the model of Langmuir model performed better than the model Freundlich, showing that the adsorption of ATP insecticide and EY dye on the surface of NH2-MIL-101(Fe) was restricted to the creation of monolayers and that there were no interactions between them; the interaction between adsorbent and adsorbate was physical adsorption processes (Liu et al. 2021).
Figure 8 displays the adsorption isotherm of ATP pesticide and EY dye adsorbed by MIL-101(Fe) and NH2-MIL-101(Fe). Table 1 displays the isotherm parameters together with the matching correlation coefficients (R2) of isotherm models. The adsorption capability of MIL-101(Fe) and NH2-MIL-101(Fe) adsorbents to ATP insecticide and EY dye was very high. As seen in Fig. 8 and Table 1, the adsorption of ATP pesticide and EY dye is described very well by both Langmuir and Freundlich isotherms. On MIL-101(Fe) and NH2-MIL-101(Fe), the Langmuir maximum adsorption capacities of ATP insecticide and EY dye on MIL-101(Fe) were 57.6 and 48.9 mg/g while on NH2-MIL-101(Fe) was 70.5 and 97.8 mg/g, respectively. The isotherm of ATP pesticide and EY dye matched better with the Langmuir model (R2 = 0.9812, 0.93622, 0.9795 and 0.9999) than the Freundlich model (R2 = 0.8701,0.8916, 0.9795 and 0.9956) for both MIL-101(Fe) and NH2-MIL-101(Fe). This suggests that the ATP pesticide and EY dye adsorption on MIL-101(Fe) and NH2-MIL-101(Fe) may be controlled by the physical adsorption. Additionally, the favorable adsorption of ATP insecticide and EY dye onto MIL-101(Fe) and NH2-MIL-101(Fe) was verified by the values of 1/n (0.4968, 0.4944, 0.7192, and 0.8318).
Dubinin-radushkevich model
(D-R model) was put up as an empirical isotherm to demonstrate the vapor's adsorption onto solid materials (Dubinin 1960). The D-R model is developed on the basis of Polanyi theory and the premise that the distribution of pores in an adsorbent follows the Gaussian energy distribution. It is based on the theory that the process of adsorption is pore volume filling as opposed to a layer-by-layer build-up of a film on the pore walls (Wang & Guo 2020a) (Fig. 9a and b). The D-R model that is linear can represented according to the following equation:
where \({\mathrm{Q}}_{\mathrm{max}}\) (mg.g−1) represents the maximum amount of adsorbed substances, the constant that is associated with the computed average sorption energy E is KD (mol2.kJ−2), ε (kJ.mol−1) is Polanyi’s potential theory-based adsorption potential calculated by Eq. (10), R is gas’ constant, and the absolute temperature is T (Mozaffari Majd et al. 2022).
Using Eq. 11 will calculate the mean free energy (E, kJ.mol−1).
The index of energy consumed is denoted by the symbol E, which indicates the free energy per mole of metal ions going from infinity in solution to the sorbent surface. E is frequently used to assess whether a physical process (where E is less than 8 kJ.mol−1) or chemical process (8 < E < 16 kJ.mol−1) is dominant in the adsorption (Chabani et al. 2006).
The linear plot of ε2 vs ln (qe) is displayed in Fig. 9a and b. Table 1 displays the computed D-R constants and mean free energy for adsorption. The range of 1.570, 3.881, 0.382, and 2.953 kJ.mol–1 for the mean adsorption energy suggested that the adsorption process is physical. Depending on the correlation coefficient values as indicated in Table 1, the D-R isotherm model provides a good match to the experimental data.
Redlich-peterson isotherm model
Since it is flexible enough to be applied in both homogeneous and heterogeneous systems, the Redlich-Peterson isotherm model may be used to represent the adsorption equilibrium across a wide range of concentrations (Ayawei et al. 2017). According to (Foo & Hameed 2010, Redlich & Peterson 1959), this isotherm may be represented by the nonlinear equation Eq. (12) and the linear equation Eq. (13) and Fig. 9c and d shows this in addition to Table 1.
where Ce denotes the equilibrium concentration, \(\upbeta\) denotes the Redlich–Peterson isotherm exponent, B denotes the Redlich–Peterson isotherm constant (L.g–1), A denotes the Redlich–Peterson isotherm constant (L.g–1), and qe is the quantity of adsorbate in the adsorbent at equilibrium (mg. g–1).
Redlich-Peterson constants may be calculated by plotting ln (Ce) against ln (Ce/ qe), where β represents the slope and A the intercept (Brouers & Al-Musawi 2015).
This isotherm model is versatile enough to be used in both homogeneous and heterogeneous systems, with a linear dependence on concentration in the numerator and an exponential function in the denomination. Together, these features represent adsorption equilibrium over a wide range of adsorbate concentrations (Gimbert et al. 2008).
The R–P have also been applied to evaluate the fitting for the adsorption of ATP insecticide and EY dye (Fig. 9c and d). The calculated isotherm parameters and their corresponding coefficient of determination, R2, values are shown in Table 1.
Temkin isotherm model
Additionally, based on this model of isotherm, the impact of an indirect adsorbent and interactions of adsorbate on adsorption will cause the adsorption heat of all layers to drop linearly beside coverage. the model of Temkin isotherm is utilized to calculate the sorbent sorption capability for organic compounds (ATP pesticide and EY dye) and assumes that the adsorption heat drop is linear as opposed to logarithmic, as indicated by the Freundlich equation (Aharoni & Ungarish 1977, Ashouri et al. 2021). Typically, this paradigm is applied according to Eqs. (14) and (15) (Ashouri et al. 2021; Benjelloun et al. 2021):
where,
KR,aR g (0 < g < 1) are constants of isotherms, \({\mathrm{A}}_{\mathrm{t}}\) Temkin isotherm is the constant of binding for equilibrium that reflects the highest possible binding energy value (L.g−1), the positive value of constant B is 11.5160 and 12.4924 J.mol−1 MIL-101(Fe) (ATP and EY) 19.1147 and 33.3748 J.mol−1 NH2-MIL-101(Fe) (ATP and EY) reflects a process of endothermic calculated by Eq. (15). \({\mathrm{b}}_{\mathrm{t}}\) is the constant of Temkin isotherm that represents the heat of adsorption (J.mol−1), Ce: equilibrium concentration (mg.L−1),\({\mathrm{q}}_{\mathrm{e}}\): the adsorbate content of the adsorbent at equilibrium (mg.g−1); R is the constant of universal gas (8.314 J mol−1.K−1) and the absolute temperature is T (K).
The graph of ln (Ce) vs. qe (Fig. 10a and b) was used to calculate the Temkin isotherm constants, bt and At. Table 1 shows the correlation coefficient and Temkin constant values. The Langmuir model fits the experimental data better than the Temkin model, as shown by the derived R2 values of 0.9812, and 0.93622 for the Langmuir model of ATP insecticide and EY dye on MIL-101(Fe) while on NH2-MIL-101(Fe) was 0.9795 and 0.9999, respectively. The R2 of Temkin model for ATP and EY adsorption on MIL-101(Fe) was 0.89136 and 0.91393 while on NH2-MIL-101(Fe) was 0.88805, and 0.87822 respectively.
Adsorption kinetics on the MIL-101(Fe) and NH2-MIL-101(Fe) surface
Various kinetic models, including the pseudo-first-order, pseudo-second-order, Elvoich, Boyd kinetic, and intraparticle diffusion models, were employed to match the experimental data to examine the adsorption mechanism of ATP insecticide and EY dye onto MIL-101(Fe) and NH2-MIL-101(Fe).
Pseudo-first-order and second-order model
Lagergren model was applied to analyze the adsorption kinetics of MIL-101(Fe) and NH2-MIL-101(Fe) to better understand the link between the two compounds adsorption processes for the removal of ATP pesticide and EY dye (Liu et al. 2021). The adsorption rate constant may be determined by applying the 1st-order reaction kinetic model and pseudo-2nd-order kinetic model. The Lagergren first-order model linear form is illustrated as follows (Adly et al. 2021; Younes et al. 2021).
Pseudo-first order equation:
where \({\mathrm{k}}_{1}\) (min−1) is the constant of rate for the 1st-order model, \({\mathrm{q}}_{\mathrm{e}}\) and \({\mathrm{q}}_{\mathrm{t}}\) (mg.g−1) are the quantities adsorbed at equilibrium and time \(\mathrm{t}\) (min), correspondingly, when \({\mathrm{k}}_{1}\) is calculated from the t against \(\mathrm{log}\left({\mathrm{q}}_{\mathrm{e}}-{\mathrm{q}}_{\mathrm{t}}\right)\) plots for every sample at various concentrations and temperatures, a line that is straight and a slope of ( \({\mathrm{k}}_{1}\)/2.303) and an intercept of \({\mathrm{logq}}_{\mathrm{e}}\) is produced. The received information was assessed to see whether it was valid for either the Lagergren equation or pseudo-first order. The experimental findings \({\mathrm{q}}_{\mathrm{e}}\) as displayed in Table 2 and Fig. 11 are much greater than the estimated values. Because the model pseudo-1st order R2 values are lower than those of the model of the 2nd order, the adsorption of the ATP pesticide and EY dye does not follow the kinetics of pseudo-1st order when two various factors of concentration and temperatures.
Equation of pseudo-second order:
where \({\mathrm{k}}_{2}\) (g mg−1·min−1) is the model equilibrium rate constant for pseudo-2nd-order. The ATP pesticide and EY dye adsorbed amounts are \({\mathrm{q}}_{\mathrm{t}}\) and \({\mathrm{q}}_{\mathrm{e}}\), respectively, at equilibrium and at time t. Values of \({\mathrm{k}}_{2}\) and \({\mathrm{q}}_{\mathrm{e}}\) may be acquired by using the slope and intercept of plots t versus\(\mathrm{t}/{\mathrm{q}}_{\mathrm{t}}\). The linear relationship between \(\mathrm{t}\) and \(\mathrm{t}/{\mathrm{q}}_{\mathrm{t}}\) demonstrates that chemisorption is the primary controlling stage in the adsorption process. The values obtained for R2 and the computed values of maximal adsorption capacity \({\mathrm{q}}_{\mathrm{e}}\) for the model of pseudo-2nd order are consistent with the experimental data and showed the use of the kinetic model of pseudo-2nd order (Fig. 11c and d). Correlation coefficients (R2) were 0.999 for the two Fe-based MOFs for the adsorption of ATP and EY dye from aqueous solutions. Furthermore, a capability for equilibrium adsorption predicted from appropriate findings was compatible with the experimental data of MIL-101(Fe) and NH2-MIL-101(Fe), which are given in Table 2. These results suggest that the model of pseudo-2nd-order, which was created depending on the hypothesis that a chemisorption process encompassing valency forces through the sharing (or exchange) of electrons among the adsorbate and the adsorbent might be the rate-limiting phase, is followed by the adsorption of pesticides and organic dyes on Fe-based MIL-101 (Li et al. 2020a; Liu et al. 2021; Xie et al. 2017).
The adsorption behavior of ATP pesticide and EY dye removal by MIL-101(Fe) and NH2-MIL-101(Fe) was described using pseudo-1st and 2nd-order kinetics. Table 2 and Fig. 11 display the main findings of the kinetics parameters. The main findings of the kinetics parameters are displayed in Fig. 11 and Table 2. The pseudo-2nd -order model (R2 = 0.99947, 0.99997, 0.99953, and 0.99962) describes the experimental data more accurately than the pseudo-1st-order model (R2 = 0.72917, 0.27798, 0.093 and 0.1260), according to the kinetics findings of the ATP insecticide and EY dye adsorption onto both MIL-101(Fe) and NH2-MIL-101(Fe). Additionally, the chi-square values and the good agreement between the computed and experimental qe values support this. The pseudo-second model well fit of the ATP pesticide and EY dye adsorption on MIL-101(Fe) and NH2-MIL-101(Fe) demonstrating that chemisorption is the rate-limiting mechanism of the adsorption process and that the adsorption capacity is proportional to the active sites numbers onto adsorbents. Therefore, it was implied that ATP pesticide and EY dye were adsorbed onto the surface of MIL-101(Fe) and NH2-MIL-101(Fe) by chemical interactions, such as hydrogen bonding and the π–π EDA interaction (Isaeva et al. 2021; Li et al. 2023; Mahmoud et al. 2022).
Elovich model
The basic assumptions of the Elovich model are postulated as follows: (i) the activation energy grew as the adsorption period went on and (ii) the adsorbent surface was heterogeneous (Wang & Guo 2020b). In the presumption that the sorbent surface is energetically heterogeneous, this model is applied to clarify the pseudo-2nd-order kinetics (Yakub et al. 2020). As time goes on, the adsorption sites rise and multilayer. The Elovich model has also been used for the chemisorption of gases onto heterogeneous surfaces of adsorbent (Hoskins & Bray 1926). Elovich model describes the adsorption process as shown in Table 2 and Fig. 12 a and b by Eq. 18 as follows:
where \({\mathrm{q}}_{\mathrm{t}}\) adsorbate concentration in the adsorbent at equilibrium (mg.g−1) and at a given contact time t ( min), α is the initial sorption rate (mg.g−1 min−1), and the parameter β (mg.g−1 min−1/2). It is related to the extent of surface coverage and activation energy for chemisorption. It can be calculated from the slopes and the intercepts of the linear plot between ln(t) versus qt giving a straight line with the correlation coefficient 0.89638, 0.54127, 0.33117 and 0.0658 and β 1.8889, 1.9333, 0.64789, and 0.48158 (mg.g−1 min−1/2) (Benjelloun et al. 2021, Thabet & Ismaiel 2016).
Boyd kinetic model
It is a model that enables the differentiation of intraparticle diffusion from extra particle diffusion: The sorption is regulated by intraparticle diffusion if the plot of Bt against time is a straight line that passes through the origin; if not, the diffusion in the film (which is constrained by extra particular transport) controls the sorption (Okewale et al. 2013). This model is described by Eqs. 19 and 20. The results are shown in Fig. 12 c and d in addition to Table 2.
where qe is the amount of adsorbate on the adsorbent at equilibrium (mg.g−1), Bt is the Boyd constant calculated by Eq. (20)., and qt is the amount of adsorbate in the adsorbent at time t (mg.g−1). Graphing Bt as a function of contact time t and noting the coefficient of determination R2 makes it easy to evaluate this isotherm.
Figure 12 c and d shows the linear plot between t0.5 vs. Bt. The constants and R2 values are shown in Table 2. However, the obtained value of R2 for the Boyd kinetic model (0.62521, 0.48904, 0.24308 and 0.02631) was found to be less than that obtained for the other models.
Intraparticle diffusion model
It is possible to regulate the adsorption of ACT insecticide and EY dye onto NH2-MIL-101(Fe) adsorbent by either intraparticle diffusion, external film diffusion, or both (Mahanna & Azab 2020). The intraparticle diffusion model given in Eq. (21) is utilized to determine the diffusion process, and Fig. 13 shows this in addition to Table 2.
where kd (mg.g–1 min–0.5) is the intraparticle diffusion rate constant, C (mg.g–1) is the intercept that indicates the boundary layer thickness and qt (mg.g–1) is the adsorption capacity at time t (min).
Table 2 displays the constants and R2 values. These results verify that the adsorption of ATP insecticide and EY dye onto MIL-101(Fe) and NH2-MIL-101(Fe) adsorbent is significantly influenced by the intraparticle diffusion process. However, it was discovered that the intraparticle diffusion model's R2 value (0.82597, 0.3684, 0.2312, and 0.06303) was lower than the values obtained for the other models.
Results indicate that the pseudo-second-order model was able to match the experimental data more accurately than other models, according to the findings of the kinetic models of the adsorption of ATP pesticide and EY dye onto MIL-101(Fe) and NH2 MIL-101(Fe) adsorbent. The pseudo-2nd-order kinetics regression coefficient (R2) values are near unity (0.99947, 0.99997, 0.99953 and 0.99962). Different adsorption models have a lower correlation coefficient than other relevant models; the order is as follows: pseudo-2nd-order (0.99947, 0.99997, 0.99953, and 0.99962) > Elvoich model (0.89638, 0.54127, 0.33117 and 0.0658) > intraparticle diffusion (0.82597, 0.3684, 0.2312, and 0.06303) > pseudo-1st order (0.72917, 0.27798, 0.093 and 0.1260) > Boyd kinetic (0.62521, 0.48904, 0.24308, and 0.02631).
Thermodynamic parameters
Van’t hoff equation
Whether the sorption process follows physisorption or chemisorption is determined by the organic pollutants’ thermodynamic behavior as they sorb from an aqueous solution onto the NH2-MIL-101(Fe) adsorbent (Sahmoune 2018). Various adsorption parameters were studied at various temperatures.
The formulas equations listed below were applied to calculate the free energy of Gibbs (∆G°), enthalpy (ΔH°), and entropy (ΔS°) using Van't Hoff equation:
where ρ (g.L−1) is the density of water, R is a constant of an ideal gas (8.3145 J.mol−1 K−1), and the temperature is T (K). KC is the constant of equilibrium and was estimated as qe/Ce, while ΔH and ΔS were determined from the linear plot of 1/T against ln \((\uprho {\mathrm{K}}_{\mathrm{c}})\), including its slope and intercept Eq. 24. ΔG° is a change of Gibbs free energy, ΔH° is the enthalpy change, and ΔS° is the entropy change (Mohammad & El-Sayed 2020). Values of the equilibrium constant and qe/Ce are enumerated in Table 3. The negative ∆G° values reveal that the adsorption of the organic pollutants on nanoparticles is a spontaneous process for ACT pesticide and EY dye. Using the slope and intercept of the 1/T vs In (pKc). plot provided in Eqs. 22 and 23, we get the ΔH° and ΔS° values, in that order. If ∆H° is less than 20 kJ.mol−1, the adsorption is physisorption in nature and includes weak forces of attraction or Van der Waals forces, which are frequently thought of as outer-sphere complexation between the surface of the adsorbent and the dye the ∆S° negative value points that throughout the adsorption process, the state of orderliness at the solid/solution interface increased, and the adsorbate and adsorbents underwent some structural modifications.
Arrhenius equation
When the equation of Arrhenius is applied to determine the energy of activation, the linear form is expressed as follows: it is possible to identify the kind of adsorption process since the rate of reaction rises with increased temperature as shown in Fig. 15a and b by Eqs. 27 and 28 as follows:
Activation energy:
where A is a factor of pre-exponential and Ea is the energy of activation is calculated by Eg (28), and R is the constant of gas (8.31 kJ−1.mol−1). The value of Ea may be computed using the slope of the curve among 1/T and In (pKc) (Table 3, Fig. 14). From an equation of Arrhenius, the activation energy value is 5.428 kJ.mol−1 (ATP) and 2.012 kJ.mol−1 (EY) verified that the process of chemisorption based on the results which taken from the equation of Van’t Hoff. The intercept values were 10.74552 and 6.87577 dm3.mol−1 s−1 for ATP and EY dye, respectively.
Modified arrhenius equation (probability sticking)
As stated by the Arrhenius type equation modified, the activation energy (\({\mathrm{E}}_{\mathrm{a}}\)) from Table 3 and Fig. 15 c and d and the likelihood of sticking (\({\mathrm{S}}^{*}\)) from the surface covering (θ) may be calculated (31).
The \({\mathrm{S}}^{*}\) is considered a function of the adsorbate/adsorbent system that is the subject of the inquiry; it has a value that ranges from 0 < \({\mathrm{S}}^{*}\) < 1 and is influenced via the system's temperature. The value of θ may be obtained using the equation that follows.
The slope of the 1/T vs. ln (1 − θ) plot was used to determine the value of \({\mathrm{E}}_{\mathrm{a}}\). The size of the activation energy provides insight into the primary physical or chemical nature of the adsorption. Chemisorption is implied via higher activation energies (40–800 kJ.mol–1), whereas activation energies for physisorption processes usually lie between 5 and 40 kJ mol−1. The positive value of \({\mathrm{E}}_{\mathrm{a}}\) 4.553 (ATP) and 1.624 (EY) kJ.mol−1 showed that adsorption is a physisorption process that is exothermic in nature and prefers lower temperatures; the value of \({\mathrm{S}}^{*}\) is < 1, (0.025) (ATP) and (0.29) (EY) thus the sticking probability of acetamiprid and eosin Y uptake on the NH2-MIL-101(Fe) is very high.
Mechanism of MIL-101(Fe) and NH2-MIL-101(Fe) adsorbent
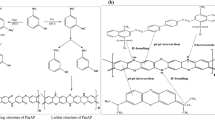
Possible adsorption mechanisms for dye and pesticide are depicted in Fig. 16 which shows that it might be portrayed to π-π stacking, hydrogen bonding, and inner sphere coordination (Isaeva et al. 2021; Li et al. 2023; Mahmoud et al. 2022, Mondol & Jhung 2021).
Because of their versatile complexing activity towards a wide range of metal ions, carboxylates are a family of ligands for the synthesis of coordination compounds that have received much research. Because it is feasible to add extra groups to the ring to provide beneficial characteristics and interactions for desired applications, benzene carboxylic acids have been a particularly wise option. Hydrogen bonding interactions can be conferred by functionalities including thio, hydroxyl, nitro, amino, and halogens by serving as hydrogen donors or acceptors (Aris et al. 2020; Lunardi et al. 2022). The aromaticity of the benzene ring can also result in π-π interactions along with other aromatics and be helpful for adsorptive separation. In contrast to MIL-101(Fe), NH2—MIL-101(Fe) exhibits structural variety because the amine group is added together with carboxylate ligands (Bagheri et al. 2021; Xu et al. 2021). The dye molecule’s aromatic structure is responsible for powerful interactions with the frameworks including electrostatic interactions, π-π interaction, and physical adsorption (Mondol & Jhung 2021).
Conclusion
Within this research, MIL-101(Fe) and NH2-MIL-101(Fe) nanocomposites were made up, and their capacities to extract ATP insecticide and EY dye from an aqueous solution were assessed. High adsorption capacities 57.6 and 48.9 mg/g of MIL-101(Fe) and 70.5 and 97.8 mg/g of NH2-MIL-101(Fe) for ATP and EY dye, respectively. To ensure that determine the removal effectiveness, the influences of contact duration, pH, the concentration of pollutants, and adsorbent dose were studied. The optimum contact duration by both adsorbents was discovered to be 10 min and 15 min for ATP pesticide and EY dye, correspondingly. The isotherm of both adsorbents matched well with the Langmuir model. The pseudo-2nd order model describes the adsorption kinetics rather well. The thermodynamic results show exothermic and non-spontaneous, and there is an increase in the state of orderness in the adsorption processes. According to a local energy decomposition study, electrostatic interactions are the main adsorption mechanism by which MIL-101(Fe) and NH2-MIL-101(Fe) remove pesticides and dyes. In summary, this work shows that MIL-101(Fe) and NH2-MIL-101(Fe) are efficient in eliminating dyes and pesticides from aqueous solutions.
Data availability
The experimental data of this study is available upon request
References
Adly MS, El-Dafrawy SM, Ibrahim AA, El-Hakam SA, El-Shall MS (2021) Efficient removal of heavy metals from polluted water with high selectivity for Hg(II) and Pb(II)by a 2-imino-4-thiobiuret chemically modified MIL125 metal–organic framework†. RSC Adv. https://doi.org/10.1039/D1RA00927C
Aharoni C, Ungarish M (1977) Kinetics of activated chemisorption. Part 2.—Theoretical models. J Chem Soc Faraday Trans 1. https://doi.org/10.1039/F19777300456
Ahmad T, Rafatullah M, Ghazali A, Sulaiman O, Hashim R, Ahmad A (2010) Removal of pesticides from water and wastewater by different adsorbents: a review. J Environ Sci Health c: Toxicol Carcinog. https://doi.org/10.1080/10590501.2010.525782
Anand KV, Subala AS, Sumathi KS, Merin SAL (2020) A review on the removal of dye, pesticide and pathogens from waste water using quantum dots. Chem Eur J 1:5. https://doi.org/10.24018/ejchem.2020.1.5.14
Annabi E, Ben Salem I, Abid-Essefi S (2019) Acetamiprid, a neonicotinoid insecticide, induced cytotoxicity and genotoxicity in PC12 cells. Toxicol Mech Methods. https://doi.org/10.1080/15376516.2019.1624907
Ansari F, Ghaedi M, Taghdiri M, Asfaram A (2016) Application of ZnO nanorods loaded on activated carbon for ultrasonic assisted dyes removal: experimental design and derivative spectrophotometry method. Ultrason Sonochem. https://doi.org/10.1016/j.ultsonch.2016.05.004
Araújo CST, Almeida ILS, Rezende HC, Marcionilio SMLO, Léon JJL, de Matos TN (2018) Elucidation of mechanism involved in adsorption of Pb(II) onto lobeira fruit (Solanum lycocarpum) using Langmuir, Freundlich and Temkin isotherms. Microchem J. https://doi.org/10.1016/j.microc.2017.11.009
Aris AZ, Mohd Hir ZA, Razak MR (2020) Metal-organic frameworks (MOFs) for the adsorptive removal of selected endocrine disrupting compounds (EDCs) from aqueous solution: a review. Appl Mater Today. https://doi.org/10.1016/j.apmt.2020.100796
Ashouri V, Adib K, Rahimi Nasrabadi M (2021) A new strategy for the adsorption and removal of fenitrothion from real samples by active-extruded MOF (AE-MOF UiO-66) as an adsorbent. New J Chem. https://doi.org/10.1039/D0NJ05693F
Assefi P, Ghaedi M, Ansari A, Habibi MH, Momeni MS (2014) Artificial neural network optimization for removal of hazardous dye Eosin Y from aqueous solution using Co2O3-NP-AC: Isotherm and kinetics study. J Ind Eng Chem. https://doi.org/10.1016/j.jiec.2013.11.027
Ayawei N, Ebelegi AN, Wankasi D (2017) Modelling and interpretation of adsorption isotherms. J Chem. https://doi.org/10.1155/2017/3039817
Ayyavoo DK, Tamilselvan DC (2019) Analytical method validation of acetamiprid+ bifenthrin formulation by Reverse Phase High Performance Liquid Chromatography (R-HPLC). Int. j. adv. res. idea, innov. Tech. http://www.ijariit.com/
Bagheri AR, Aramesh N, Bilal M (2021) New frontiers and prospects of metal-organic frameworks for removal, determination, and sensing of pesticides. Environ Res. https://doi.org/10.1016/j.envres.2020.110654
Benjelloun M, Miyah Y, Akdemir Evrendilek G, Zerrouq F, Lairini S (2021) Recent advances in adsorption kinetic models: their application to dye types. Arab J Chem. https://doi.org/10.1016/j.arabjc.2021.103031
Boontongto T, Burakham R (2019) Evaluation of metal-organic framework NH(2)-MIL-101(Fe) as an efficient sorbent for dispersive micro-solid phase extraction of phenolic pollutants in environmental water samples. Heliyon. https://doi.org/10.1016/j.heliyon.2019.e02848
Borzelleca JF, Capen CC, Hallagan JB (1987) Lifetime toxicity/carcinogenicity study of FD & C RED No. 3 (erythrosine) in rats. Food Chem Toxicol 25(10):723–733. https://doi.org/10.1016/0278-6915(87)90226-2
Brouers F, Al-Musawi TJ (2015) On the optimal use of isotherm models for the characterization of biosorption of lead onto algae. J Mol Liq. https://doi.org/10.1016/j.molliq.2015.08.054
Chabani M, Amrane A, Bensmaili A (2006) Kinetic modelling of the adsorption of nitrates by ion exchange resin. Chem Eng J. https://doi.org/10.1016/j.cej.2006.08.014
Chen M-L, Lu T-H, Long L-L, Xu Z, Ding L, Cheng Y-H (2021) NH2-Fe-MILs for effective adsorption and Fenton-like degradation of imidacloprid: removal performance and mechanism investigation. Environ Eng Res. https://doi.org/10.4491/eer.2020.702
Doan VD, Huynh BA, Pham HAL, Vasseghian Y, Le VT (2021) Cu2O/Fe3O4/MIL-101(Fe) nanocomposite as a highly efficient and recyclable visible-light-driven catalyst for degradation of ciprofloxacin. Environ Res. https://doi.org/10.1016/j.envres.2021.111593
Dolatabadi M, Naidu H, Ahmadzadeh S (2021) A green approach to remove acetamiprid insecticide using pistachio shell-based modified activated carbon; economical groundwater treatment. J Clean Prod. https://doi.org/10.1016/j.jclepro.2021.128226
Dong Y, Hu T, Pudukudy M, Su H, Jiang L, Shan S, Jia Q (2020) Influence of microwave-assisted synthesis on the structural and textural properties of mesoporous MIL-101(Fe) and NH2-MIL-101(Fe) for enhanced tetracycline adsorption. Mater Chem Phys. https://doi.org/10.1016/j.matchemphys.2020.123060
Du WL, Xu ZR, Han XY, Xu YL, Miao ZG (2008) Preparation, characterization and adsorption properties of chitosan nanoparticles for eosin Y as a model anionic dye. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2007.08.040
Dubinin MM (1960) The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem Rev. https://doi.org/10.1021/cr60204a006
El-Bendary N, El-Etriby HK, Mahanna H (2021) Reuse of adsorption residuals for enhancing removal of ciprofloxacin from wastewater. Environ Technol. https://doi.org/10.1080/09593330.2021.1952310
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J. https://doi.org/10.1016/j.cej.2009.09.013
Ford KA, Casida JE (2006) Unique and common metabolites of thiamethoxam, clothianidin, and dinotefuran in mice. J Am Chem Soc. https://doi.org/10.1021/tx0601859
Fua F, Dionysioub DD, Liuc H (2014) The use of zero-valent iron for groundwater remediation and wastewater treatment: a review. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2013.12.062
Gecgel C, Simsek UB, Gozmen B, Turabik M (2019a) Comparison of MIL-101(Fe) and amine-functionalized MIL-101(Fe) as photocatalysts for the removal of imidacloprid in aqueous solution. J Iran Chem Soc. https://doi.org/10.1007/s13738-019-01647-w
Gecgel C, Simsek UB, Turabik M, Ozdemir S (2019b) Synthesis of titanium doped iron based metal–organic frameworks and investigation of their biological activities. J Inorg Organomet Polym Mater. https://doi.org/10.1007/s10904-019-01329-3
Ghiasi A, Malekpour A, Mahpishanian S (2022) Aptamer functionalized magnetic metal-organic framework MIL-101(Cr)-NH2 for specific extraction of acetamiprid from fruit juice and water samples. Food Chem. https://doi.org/10.1016/j.foodchem.2022.132218
Gimbert F, Morin-Crini N, Renault F, Badot PM, Crini G (2008) Adsorption isotherm models for dye removal by cationized starch-based material in a single component system: error analysis. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2007.12.072
Hoskins WM, Bray WC (1926) The catalytic oxidation of carbon monoxide. 11. The adsorption of carbon dioxide, carbon monoxide, and oxygen by the catalysts, manganese dioxide, cupric oxide, and mixtures of these oxides. J Am Chem Soc 48(6):1454–1474. https://doi.org/10.1021/ja01417a002
Isaeva VI, Vedenyapina MD, Kurmysheva AY, Weichgrebe D, Nair RR, Nguyen NPT, Kustov LM (2021) Modern carbon-based materials for adsorptive removal of organic and inorganic pollutants from water and wastewater. Molecules. https://doi.org/10.3390/molecules26216628
Jabbar NM, Salman SD, Rashid IM, Mahdi YS (2022) Removal of an anionic Eosin dye from aqueous solution using modified activated carbon prepared from date palm fronds. Chemical Data Collections. https://doi.org/10.1016/j.cdc.2022.100965
Jiang D, Chen M, Wang H, Zeng G, Huang D, Cheng M, Wang Z (2019) The application of different typological and structural MOFs-based materials for the dyes adsorption. Coord Chem Rev. https://doi.org/10.1016/j.ccr.2018.11.002
Joseph L, Saha M, Kim S, Jun BM, Heo J, Park CM, Jang M, Flora JRV, Park CM, Yoon Y (2021) Removal of Cu2+, Cd2+, and Pb2+ from aqueous solution by fabricated MIL-100(Fe) and MIL-101(Cr): Experimental and molecular modeling study. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2021.106663
Li H, Liu F, Ma X, Wu Z, Li Y, Zhang L, Helian Y (2019) Catalytic performance of strontium oxide supported by MIL–100(Fe) derivate as transesterification catalyst for biodiesel production. Energy Convers Manag 180:401–410. https://doi.org/10.1016/j.enconman.2018.11.012
Li Z, Liu X, Jin W, Hu Q, Zhao Y (2019b) Adsorption behavior of arsenicals on MIL-101(Fe): the role of arsenic chemical structures. J Colloid Interface Sci. https://doi.org/10.1016/j.jcis.2019.07.046
Li J, Wang L, Liu Y, Zeng P, Wang Y, Zhang Y (2020a) Removal of Berberine from wastewater by MIL-101(Fe): performance and mechanism. ACS Omega. https://doi.org/10.1021/acsomega.0c03422
Li T, Lu M, Gao Y, Huang X, Liu G, Xu D (2020b) Double layer MOFs M-ZIF-8@ZIF-67: The adsorption capacity and removal mechanism of fipronil and its metabolites from environmental water and cucumber samples. J Adv Res. https://doi.org/10.1016/j.jare.2020.03.013
Li J, Lv Q, Bi L, Fang F, Hou J, Di G, Wei J, Wu X, Li X (2023) Metal-organic frameworks as superior adsorbents for pesticide removal from water: The cutting-edge in characterization, tailoring, and application potentials. Coord Chem Rev. https://doi.org/10.1016/j.ccr.2023.215303
Liu Z, He W, Zhang Q, Shapour H, Bakhtari MF (2021) Preparation of a GO/MIL-101(Fe) composite for the removal of methyl orange from aqueous solution. ACS Omega. https://doi.org/10.1021/acsomega.0c05091
Liu R, Xie Y, Cui K, Xie J, Zhang Y, Huang Y (2022) Adsorption behavior and adsorption mechanism of glyphosate in water by amino-MIL-101(Fe). J Phys Chem Solids. https://doi.org/10.1016/j.jpcs.2021.110403
Long JR, Yaghi OM (2009) The pervasive chemistry of metal–organic frameworks†. Chem Soc Rev. https://doi.org/10.1039/B903811F
Lunardi VB, Soetaredjo FE, Foe K, Putro JN, Wenten IG, Irawaty W, Ismadji S (2022) Pesticide elimination through adsorption by metal-organic framework and their modified forms. Environ Nanotechnol Monit Manag. https://doi.org/10.1016/j.enmm.2021.100638
Ma W, Yang B, Li J, Li X (2022) Amino-functional metal–organic framework as a general applicable adsorbent for simultaneous enrichment of nine neonicotinoids. Chem Eng J. https://doi.org/10.1016/j.cej.2022.134629
Mahanna H, Azab M (2020) Adsorption of Reactive Red 195 dye from industrial wastewater by dried soybean leaves modified with acetic acid. Desalin Water Treat. https://doi.org/10.5004/dwt.2020.24960
Mahanna H, El-Bendary N (2022) Enhanced catalytic oxidation of reactive dyes by reuse of adsorption residuals as a heterogeneous catalyst with persulfate/UV process. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-021-03856-4
Mahdipoor HR, Halladj R, Ganji Babakhani E, Amjad-Iranagh S, Sadeghzadeh Ahari J (2021) Synthesis, characterization, and CO(2) adsorption properties of metal organic framework Fe-BDC. RSC Adv. https://doi.org/10.1039/D0RA09292D
Mahmoud LAM, Dos Reis RA, Chen X, Ting VP, Nayak S (2022) Metal-organic frameworks as potential agents for extraction and delivery of pesticides and agrochemicals. ACS Omega. https://doi.org/10.1021/acsomega.2c05978
Marican A, Duran-Lara EF (2018) A review on pesticide removal through different processes. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-017-0796-2
Mei L, Jiang T, Zhou X, Li Y, Wang H, Li Z (2017) A novel DOBDC-functionalized MIL-100(Fe) and its enhanced CO2 capacity and selectivity. Chem Eng J. https://doi.org/10.1016/j.cej.2017.03.131
Mohammad SG, El-Sayed MMH (2020) Removal of imidacloprid pesticide using nanoporous activated carbons produced via pyrolysis of peach stone agricultural wastes. Chem Eng Commun. https://doi.org/10.1080/00986445.2020.1743695
Mohammad SG, Ahmed SM, Amr AEE, Kamel AH (2020) Porous activated carbon from lignocellulosic agricultural waste for the removal of acetampirid pesticide from aqueous solutions. Molecules. https://doi.org/10.3390/molecules25102339
Mondol MMH, Jhung SH (2021) Adsorptive removal of pesticides from water with metal–organic framework-based materials. Chem Eng J. https://doi.org/10.1016/j.cej.2021.129688
Mozaffari Majd M, Kordzadeh-Kermani V, Ghalandari V, Askari A, Sillanpaa M (2022) Adsorption isotherm models: a comprehensive and systematic review (2010–2020). Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2021.151334
Nazir MA, Najam T, Bashir MS, Javed MS, Bashir MA, Imran M, Azhar U, Shah SSA, Rehman AU (2022) Kinetics, isothermal and mechanistic insight into the adsorption of eosin yellow and malachite green from water via tri-metallic layered double hydroxide nanosheets. Korean J Chem Eng. https://doi.org/10.1007/s11814-021-0892-3
Okewale AO, Babayemi KA, Olalekan AP (2013) Adsorption isotherms and kinetics models of starchy adsorbents on uptake of water from ethanol – water systems. International Journal of Applied Science and Technology. https://www.ijastnet.com/journals/Vol_3_No_1_January_2013/5.pdf
Oladipo AC, Tella AC, Olayemi VT, Adimula VO, Dembaremba TO, Ogunlaja AS, Clayton HS, Clarkson GJ, Walton RI (2021) Synthesis, structural and DFT investigation of Zn(nba)2 (meim)2 for adsorptive removal of eosin yellow dye from aqueous solution. Z Fur Anorg Allg Chem. https://doi.org/10.1002/zaac.202000425
Orribas S, Zornoza B, Serra-Crespo P, Gascon J, Kapteijn F, Téllez C, Coronas J (2016) Synthesis and gas adsorption properties of mesoporous silica-NH2-MIL-53(Al) core-shell spheres. Microporous Mesoporous Mater. https://doi.org/10.1016/j.micromeso.2015.12.004
Pisa L, Goulson D, Yang EC, Gibbons D, Sanchez-Bayo F, Mitchell E, Aebi A, van der Sluijs J, MacQuarrie CJK, Giorio C, Long EY, McField M, Bijleveld van Lexmond M, Bonmatin JM (2021) An update of the Worldwide Integrated Assessment (WIA) on systemic insecticides. Part 2: impacts on organisms and ecosystems. Environ Sci Pollut Res 28(10):11749–11797. https://doi.org/10.1007/s11356-017-0341-3
Qasem NA, Ben-Mansour R, Habib MA (2018) An efficient CO2 adsorptive storage using MOF-5 and MOF-177. Appl Energy. https://doi.org/10.1016/j.apenergy.2017.11.011
Quan X, Sun Z, Meng H, Han Y, Wu J, Xu J, Xu Y, Zhang X (2019) Surface functionalization of MIL-101(Cr) by aminated mesoporous silica and improved adsorption selectivity toward special metal ions. Dalton Trans. https://doi.org/10.1039/C9DT00501C
Rahmani A, Shabanloo A, Zabihollahi S, Salari M, Leili M, Khazaei M, Alizadeh S, Nematollahi D (2022) Facile fabrication of amino-functionalized MIL-68(Al) metal-organic framework for effective adsorption of arsenate (As(V)). Sci Rep. https://doi.org/10.1038/s41598-022-16038-0
Rasheed HU, Lv X, Zhang S, Wei W, Ullah N, Xie J (2018) Ternary MIL-100(Fe)@Fe3O4/CA magnetic nanophotocatalysts (MNPCs): magnetically separable and Fenton-like degradation of tetracycline hydrochloride. Adv Powder Technol. https://doi.org/10.1016/j.apt.2018.09.011
Redlich O, Peterson DL (1959) A useful adsorption isotherm. J Phys Chem. https://doi.org/10.1021/j150576a611
Sahmoune MN (2018) Evaluation of thermodynamic parameters for adsorption of heavy metals by green adsorbents. Environ Chem Lett. https://doi.org/10.1007/s10311-018-00819-z
Saleh IA, Zouari N, Al-Ghouti MA (2020) Removal of pesticides from water and wastewater: chemical, physical and biological treatment approaches. Environ Technol Innov. https://doi.org/10.1016/j.eti.2020.101026
Shan Y, Xu C, Zhang H, Chen H, Bilal M, Niu S, Cao L, Huang Q (2020) Polydopamine-modified metal-organic frameworks, NH(2)-Fe-MIL-101, as pH-sensitive nanocarriers for controlled pesticide release. Nanomater (Basel). https://doi.org/10.3390/nano10102000
Sharma AK, Priya KBS, Panchal S, Bhatia JK, Bajaj S, Tanwar V, Sharma N (2019) Response surface methodology directed synthesis of luminescent nanocomposite hydrogel for trapping anionic dyes. J Environ Manage. https://doi.org/10.1016/j.jenvman.2018.10.038
Silva LS, Ferreira FJL, Silva MS, Cito A, Meneguin AB, Sabio RM, Barud HS, Bezerra RDS, Osajima JA, Silva Filho EC (2018) Potential of amino-functionalized cellulose as an alternative sorbent intended to remove anionic dyes from aqueous solutions. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2018.05.034
Singh NH, Kezo K, Debnath A, Saha B (2017) Enhanced adsorption performance of a novel Fe-Mn-Zr metal oxide nanocomposite adsorbent for anionic dyes from binary dye mix: response surface optimization and neural network modeling. Appl Organomet Chem. https://doi.org/10.1002/aoc.4165
Skobelev IY, Sorokin AB, Kovalenko KA, Fedin VP, Kholdeeva OA (2013) Solvent-free allylic oxidation of alkenes with O2 mediated by Fe- and Cr-MIL-101. J Catal. https://doi.org/10.1016/j.jcat.2012.11.003
Solís RR, Gómez-Avilés A, Belver C, Rodriguez JJ, Bedia J (2021) Microwave-assisted synthesis of NH2-MIL-125(Ti) for the solar photocatalytic degradation of aqueous emerging pollutants in batch and continuous tests. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2021.106230
Thabet MS, Ismaiel AM (2016) Sol-Gel γ-Al2O3 Nanoparticles assessment of the removal of eosin yellow using: adsorption, kinetic and thermodynamic parameters. J Encapsulation Adsorpt Sci. https://doi.org/10.4236/jeas.2016.63007
Trung TK, Déroche I, Rivera A, Yang Q, Yot P, Ramsahye N, Vinot SD, Devic T, Horcajada P, Serre C, Maurin G, Trens P (2011) Hydrocarbon adsorption in the isostructural metal organic frameworks MIL-53(Cr) and MIL-47(V). Microporous Mesoporous Mater. https://doi.org/10.1016/j.micromeso.2010.09.003
Wang J, Guo X (2020a) Adsorption isotherm models: classification, physical meaning, application and solving method. Chemosphere. https://doi.org/10.1016/j.chemosphere.2020.127279
Wang J, Guo X (2020b) Adsorption kinetic models: physical meanings, applications, and solving methods. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2020.122156
Wang X-F, Song X-Z, Sun K-M, Cheng L, Ma W (2018a) MOFs-derived porous nanomaterials for gas sensing. Polyhedron. https://doi.org/10.1016/j.poly.2018.06.037
Wang Y, Guo W, Li X (2018b) Activation of persulfates by ferrocene-MIL-101(Fe) heterogeneous catalyst for degradation of bisphenol A. RSC Adv. https://doi.org/10.1039/C8RA07007E
Wang Y, Wang K, Lin J, Xiao L, Wang X (2020) The preparation of nano-MIL-101(Fe)@chitosan hybrid sponge and its rapid and efficient adsorption to anionic dyes. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2020.10.073
Wang D, Li Z (2015) Bi-functional NH2-MIL-101(Fe) for one-pot tandem photo-oxidation/Knoevenagel condensation between aromatic alcohols and active methylene compounds. Catal Sci Technol. https://doi.org/10.1039/C4CY01464B
Wen Y, Zhang J, Xu Q, Wu X-T, Zhu Q-L (2018) Pore surface engineering of metal–organic frameworks for heterogeneous catalysis. Coord Chem Rev. https://doi.org/10.1016/j.ccr.2018.08.012
Wu B, Lin X, Ge L, Wu L, Xu T (2013) A novel route for preparing highly proton conductive membrane materials with metal-organic frameworks. Chem Commun. https://doi.org/10.1039/C2CC37045J
Wu SC, Yu LL, Xiao FF, You X, Yang C, , Cheng JH, (2017a) Synthesis of aluminum-based MOF/graphite oxide composite and enhanced removal of methyl orange. J Alloys Compd. https://doi.org/10.1016/j.jallcom.2017.07.095
Wu Z, Yuan X, Zhong H, Wang H, Jiang L, Zeng G, , Li Y, (2017b) Highly efficient adsorption of Congo red in single and binary water with cationic dyes by reduced graphene oxide decorated NH2 -MIL-68(Al). J Mol Liq. https://doi.org/10.1016/j.molliq.2017.09.112
Xiao H, Zhang W, Yao Q, Huang L, Chen L, Boury B, Chen Z (2019) Zn-free MOFs like MIL-53(Al) and MIL-125(Ti) for the preparation of defect-rich, ultrafine ZnO nanosheets with high photocatalytic performance. Appl Catal B. https://doi.org/10.1016/j.apcatb.2018.11.026
Xie Q, Li Y, Lv Z, Zhou H, Yang X, Chen J, Guo H (2017) Effective adsorption and removal of phosphate from aqueous solutions and eutrophic water by Fe-based MOFs of MIL-101. Sci Rep. https://doi.org/10.1038/s41598-017-03526-x
Xu Q, Fang L, Fu Y, Xiao Q, Zhang F, Zhu W (2020) Synthesis, characterization, and CO2 adsorption properties of metal–organic framework NH2–MIL–101(V). Mater Lett. https://doi.org/10.1016/j.matlet.2020.127402
Xu Y, Wang H, Li X, Zeng X, Du Z, Cao J, Jiang W (2021) Metal-organic framework for the extraction and detection of pesticides from food commodities. Compr Rev Food Sci Food Saf. https://doi.org/10.1111/1541-4337.12675
Yakub E, Agarry SE, Omoruwou F, Owabor CN (2020) Comparative study of the batch adsorption kinetics and mass transfer in phenol-sand and phenol-clay adsorption systems. Part Sci Technol. https://doi.org/10.1080/02726351.2019.1616862
Younes H, El-Etriby HK, Mahanna H (2021) High removal efficiency of reactive yellow 160 dye from textile wastewater using natural and modified glauconite. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-021-03528-3
Zainorizuan MJ, Amirza MAR, Adib MMR, Hamdan R, Yee Yong L, Alvin John Meng Siang L, Mohamad Hanifi O, SitiNazahiyah R, MohdShalahuddin A (2017) Application of agricultural wastes activated carbon for dye removal – an overview. MATEC Web Conf. https://doi.org/10.1051/matecconf/201710306013
Zhang F, Niu T, Wu F, Wu L, Wang G, Li J (2021) Highly oriented MIL-101(Cr) continuous films grown on carbon cloth as efficient polysulfide barrier for lithium-sulfur batteries. Electrochim Acta. https://doi.org/10.1016/j.electacta.2021.139028
Zhang S, Ding J, Tian D (2022) Incorporation of MIL-101 (Fe or Al) into chitosan hydrogel adsorbent for phosphate removal: performance and mechanism. J Solid State Chem. https://doi.org/10.1016/j.jssc.2021.122709
Zhou RY, Yu JX, Chi RA (2020) Selective removal of phosphate from aqueous solution by MIL-101(Fe)/bagasse composite prepared through bagasse size control. Environ Res. https://doi.org/10.1016/j.envres.2020.109817
Zorainy MY, Gar Alalm M, Kaliaguine S, Boffito DC (2021) Revisiting the MIL-101 metal–organic framework: design, synthesis, modifications, advances, and recent applications. J Mater Chem a. https://doi.org/10.1039/D1TA06238G
Zoumenou B, Aina MP, Imorou Toko I, Igout A, Douny C, Brose F, Schiffers B, Gouda I, Chabi Sika K, Kestemont P, Scippo ML (2019) Occurrence of acetamiprid residues in water reservoirs in the Cotton Basin of Northern Benin. Bull Environ Contam Toxicol. https://doi.org/10.1007/s00128-018-2476-4
Acknowledgements
The third author is supported by a research project by the Research Support Fund of Mansoura University No (MU-ENG-22-13) and by an internal research support of the Faculty of Engineering at Mansoura University.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The third author is supported by a research project by the Research Support Fund of Mansoura University No (MU-ENG-22–13) and by an internal research support of the Faculty of Engineering at Mansoura University.
Author information
Authors and Affiliations
Contributions
Conceptualization: Mohamed Sakr, Mina Shawky Adly; Methodology: Mohamed Sakr, Mohamed Gar Alalm; Formal analysis and investigation: Mohamed Gar Alalalm, Hani Mahanna, Mina Shawky Adly; Writing—original draft preparation: Mohamed Sakr, Mina Shawky Adly; Writing—review and editing:: Mohamed Gar Alalalm, Hani Mahanna; Funding acquisition:: Mohamed Gar Alalalm; Resources: Mina Shawky Adly, Mohamed Sakr; Supervision: Mina Shawky Adly, Mohamed Gar Alalalm, Hani Mahanna.
Corresponding author
Ethics declarations
Ethical approval
There are no human participants or biological materials involved in this research.
Consent to publish
All authors have checked the manuscript and have agreed to the publication of this work in Environmental Science and Pollution Research.
Consent to participate
There are no human participants or biological materials involved in this research.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sakr, M., Adly, M.S., Gar Alalm, M. et al. Effective removal of acetamiprid and eosin Y by adsorption on pristine and modified MIL-101(Fe). Environ Sci Pollut Res 31, 41221–41245 (2024). https://doi.org/10.1007/s11356-024-33821-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-33821-w