Abstract
Globally, per- and polyfluoroalkyl substances (PFAS)–related research on paper products has focused on food packaging with less consideration on the presence of PFAS at different stages of the paper recycling chain. This study analysed the prevalence of PFAS in paper grades used for the manufacture of recycled paperboard. The presence of PFAS was attributed to the use of PFAS-containing additives, consumer usage, exposure to packed goods as well as contamination during mingling, sorting, collection, and recovery of paper recycling material. Q Orbitrap mass spectrometry was used to analyse the paper samples after accelerated solvent extraction and solid phase extraction. The distribution and possible propagation of 22 PFAS were determined in pre-consumer, retail and post-consumer paper products. Post-consumer samples had the highest combined average concentration (ΣPFAS) at 213 ng/g, while the ΣPFAS in retail (159 ng/g) and pre-consumer samples (121 ng/g) was detected at lower concentrations. This study showed that waste collection and recycling protocols may influence PFAS propagation and that measures must be developed to minimise and possibly eliminate exposure opportunities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past 5 years, more than 50 million tons of paper products were recycled annually, with almost half of these being used to manufacture corrugated boxes (World Economic Forum 2022). In the same period, the global paper recycling rate was 68% (Van Ewijk et al. 2021), with South Africa having an average five-year paper recovery rate of 67% (Paper Manufacturers Association of South Africa 2022). The growing emphasis on circular economy and thereby paper recycling (Semple et al. 2022) means that it is imperative to investigate how potentially toxic substances such as per- and polyfluoroalkyl substances (PFAS) propagate across the paper recycling value chain. PFAS may be introduced during the initial manufacture of packaging if they are applied as moisture and grease repellents (Schultes et al. 2019). In addition to the manufacturing stage, PFAS exposure may occur during transportation, storage, use, and disposal of paper products as well as from the mingling of recovered paper with other waste materials during collection, sorting, and repulping (Reinhart et al. 2023). In addition, PFAS present in the soil, air, and water (Solan et al. 2023) may interact with paper destined for recycling. PFAS have been shown to be present at waste disposal sites and landfills (Helmer et al. 2022) and can thereby be present at recycling facilities where recycling includes paper recovered from solid waste disposal sites and landfills. The true contribution of PFAS from paper recycling processes is currently not fully understood in developed and developing countries (Geueke et al. 2018).
PFAS, often referred to as “forever chemicals”, are classified as very persistent, bioaccumulative, and toxic (Cousins et al. 2020; Chambers et al. 2021; Liu and Sun 2021; Brunn et al. 2023). Long-chain PFAS, which typically contain fluoroalkyl chains of six or more carbons (Chambers et al. 2021; Baldwin et al. 2023), possess extremely stable carbon–fluorine bonds, making them more resistant to degradation (Chambers et al. 2021). These longer-chain PFAS have increased hydrophobicity which promotes their retention in paper’s organic, cellulose lattice (Liu and Sun 2021). Initial research into PFAS suggested that PFAS compounds with alkyl chain lengths of five carbons or less were significantly less bioaccumulative and less toxic (Quinete et al. 2010). This led manufacturers to a shift towards shorter-chain PFAS which have now also been shown to be very stable (Brendel et al. 2018), toxic (Lu et al. 2017; Solan et al. 2023) and persistent in the environment (Vierke et al. 2014; Brendel et al. 2018). These short-chain PFAS often have a higher mobility in aqueous environments owing to their higher solubility (Vierke et al. 2014). Since paper manufacturing is associated with high water use (Blanco et al. 2013), PFAS may easily circulate during the manufacture of recycled paper.
The analysis of PFAS in different paper grades used in the manufacture of recycled paper is important to further understand the global occurrence of PFAS. Globally, PFAS-related research on paper products has been focused on food packaging (Trier et al. 2011; Rosenmai et al. 2016; Curtzwiler et al. 2021; Timshina et al. 2021) with less focus on other grades of paper such as newspapers, magazines and packaging used for non-food applications (Glüge et al. 2020). This study aimed to fill this gap in understanding the possible presence of PFAS in different paper recycling grades used to manufacture recycled paperboard. This investigation is crucial as it addresses the challenges of paper recycling processes by pinpointing the possibility of PFAS circulation through the mechanisms employed in solid waste management. The findings of the present study are important for showcasing a need to develop measures that could assist in minimising and eliminating PFAS exposure opportunities.
Materials and methods
Chemicals and buffers
The chemicals and buffers used were LC–MS grade methanol (Anatech, Cape Town, South Africa), water (Merck, Johannesburg, South Africa), ammonium formate (Merck, Johannesburg, South Africa), HPLC grade toluene (Merck, Johannesburg) and analytical grade ammonium hydroxide (Merck, Johannesburg, South Africa). Certified reference standards made up in methanol were obtained from AccuStandard (Stargate, Johannesburg, South Africa), the details of which are shown in Table S1. The mixture standard contained both short- and long-chain per- and polyfluorocarboxylic acids (PFCAs) and sulfonic acids (PFSAs). Perfluorohexanesulfonic acid (PFHxS) and perfluorooctanesulfonic acid (PFOS) were present as linear and branched isomers with both isomers considered in the calibration standards and quantification. The calibration standards were made up in methanol in two concentration ranges, from 0.2 to 120 µg/L and from 30 to 450 µg/L.
Sampling
Thirty-nine paper samples were collected from Cape Town, South Africa, and consisted of various grades of paper used for recycling (Fig. 1; Table S2). The pre-consumer and retail samples consisted only of boards, while the post-consumer samples consisted of paper grades typically used in the manufacture of recycled boards. These included cartonboards, newspapers, corrugated boards (boxes), office paper and magazines. The pre-consumer samples were sourced directly from paper mills and paper conversion sites, whereas retail samples were collected from South African grocery stores. Post-consumer samples were collected at recycling facilities, solid waste disposal sites, household waste and informal waste pickers.
Sample preparation
Paper samples were prepared in triplicate (1 g each), followed by accelerated solvent extraction (ASE) using a Dionex 350 ASE system (Anatech, Cape Town, South Africa). The extraction method was optimised for ethanol:methanol solvent combination (Table S3), with the final optimised conditions being a 3:2 ethanol:methanol combination at 70 °C oven temperature for three cycles at 12 min each (MacLennan et al. 2019). After extraction, the sample was evaporated to 1 mL under nitrogen at 40 °C using a Biotage TurboVap (Anatech, Cape Town, South Africa) in preparation for SPE clean-up. The SPE process was based on weak ionic exchange (WAX) mechanism. The SPE steps were guided by the United States (US) Environmental Protection Agency (EPA) Draft Method 1633, EPA Method 533 and Barola et al. (2020) (Wendelken and EPA 2019; Barola et al. 2020; EPA 2021). For this study, 500 mg sorbent bed/6 mL Enviro-Clean WAX SPE cartridges (Stargate Scientific, Johannesburg, South Africa) were used. The cartridges were mounted on a SPE manifold (Stargate Scientific, Johannesburg, South Africa) and operated under vacuum using a DryVac 400 vacuum pump (Air & Vacuum Technologies, Johannesburg, South Africa). The SPE was initiated by conditioning the cartridges with 3 mL of methanol followed with 5 mL of 0.1 M ammonium formate buffer. This was followed by loading 500 µL of sample and elution of analytes using 6 mL of 1% methanolic ammonium hydroxide. The eluted compounds were concentrated to 1 mL under 40 °C nitrogen and filtered into a polypropylene vial with 0.22-µm filter (Stargate Scientific, Johannesburg, South Africa) before analysis by UHPLC-HRMS.
Instrumentation
High-resolution LC–MS analysis was achieved using a Thermo Scientific Vanquish UHPLC+ Q Exactive Focus Orbitrap (Anatech, Cape Town, South Africa). A Hypersil Gold aQ 100 mm × 2.1 mm × 1.9 µm column and Hypersil Gold 50 mm × 3 mm × 1.9 µm delay column (Anatech, Cape Town, South Africa) were used at 35 °C. The autosampler temperature was set to 7 °C. The instrument operating method was adapted from Zhu and Walker (2020), Silva et al. (2021) and Curtzwiler et al. (2021) with the final optimised conditions consisting of solvent A as 0.1% formic acid in water and 10 mM ammonium formate in methanol as solvent B (Zhu and Walker 2020; Silva et al. 2021; Curtzwiler et al. 2021). The initial flowrate was 0.400 mL/min in 30% solvent B which increased to 100% solvent B at 13 min (at 1 mL/min), held until 17 min, before returning to 30% solvent B at 21.1 min (Table S4). The acquisition mode was full scan with data-dependent acquisition (ddMS2) in negative mode. The PFAS transitions and retention times are tabled in Table S5.
Quality assurance
PFAS are ubiquitous compounds (Evich et al. 2022) and are used extensively in analytical chemistry laboratory items such as PTFE-lined reagent bottle caps, O-rings, septa linings and syringe filters. It was therefore important to investigate latent PFAS contamination in the PFAS analysis workflow. The necessary steps were included to measure the possible contamination from sample collection (Table S6) and the UHPLC-HRMS system (Table S7). Preparation blanks were analysed to subtract the analyte concentrations found in the blanks from the detected concentrations in the samples (Table S8). Additionally, a stringent washing and cleaning protocol was established using toluene, 1% methanolic ammonium hydroxide and methanol. The target compounds absent in the preparation blanks were quantified using external calibration quantification, whereas those found to be present were semi-quantitatively determined by subtracting the preparation blank from the detected concentration. PFBA, PFPeA, PFDoA, PFTeDA, PFBS, PFOS, PFNS, PFDS and 4:2FTS were identified as system contaminants and were semi-quantitatively determined. To evaluate the external calibration curves, the coefficient of determination (R2) was used as an indicator of linearity. The limit of detection (LOD) and limit of quantification (LOQ), which were used as sensitivity indicators, were measured at signal-to-noise ratios of 3 and 10, respectively. Relative recovery and %RSD were used to evaluate the accuracy and precision of the method, respectively. Virgin, unconverted, unprinted paper and paperboard samples were used to evaluate the method performance by spiking the samples at three different concentrations in triplicate. The data was corrected for the relative recoveries obtained by considering the unspiked paper and paperboard samples. Due to the use of external standards and expected variability, the quantified concentrations were reported with their associated uncertainty using Eq. (1):
where s2 is the variance of the sample, umatrix is the uncertainty of the sample repeatability due to matrix effects, urecovery is the uncertainty of the recovery of the target compound and uprep. blank is the uncertainty related to the preparation blanks (Rasul et al. 2017; Ellison and Williams 2012). The equations related to each term are shown in equations (S1) to (S4).
Data analysis
Instrument data analysis was performed using Thermo Scientific Xcalibur™. Further data analysis was performed using Microsoft Excel, MetaboAnalyst 5.0 and TIBCO Statistica 14.0. The statistical analysis aimed to identify possible correlations between the target analytes. TIBCO Statistica 14.0. was used to determine if the data followed normal or non-normal distributions. For normally distributed data, the Pearson correlation coefficient would be employed, while the Spearman rank correlation, a non-parametric correlation, would be used for non-normal data that contains outliers (Leon 1998). Once established, the Spearman correlation was plotted using MetaboAnalyst 5.0. The Spearman rank equation is defined as
where the Spearman rank coefficient rs is calculated by determining the sum of the squared differences in distance (d2) for n observations. A value of rs = 1 indicates a linear positive relationship, while a value of rs < 0 indicates a negative relationship between two variables, where an increase in the value of one variable would be associated with a decrease in the second variable (Leon 1998). Hierarchical clustering analysis was performed in conjunction with Spearman correlation. Hierarchical clustering allows for similarity analysis of one sample to the entire dataset and can provide insight into any heterogeneity or homogeneity present in different groups (Kumar et al. 2014).
Results and discussion
Method validation
The R2 of the calibration curves ranged from 0.98 for PFHpA to 0.99 for PFDS, indicating acceptable overall linearity of the target compounds (Table S9). The relative recoveries of paper and board were determined by spiking samples at 10, 20 and 100 ng/g; 20, 40 and 200 ng/g; and 40, 80 and 400 ng/g, depending on the concentration present in the standard mixture. Relative recoveries ranged from 55% for PFHxA to 139% for PFOA (except for 6:2 FTS) (Table S9). For the target PFAS investigated, 19 of the 22 fell within the generally accepted range of 70–130% (Srivastava et al. 2022). PFHxA (10 ng/g paperboard spike), PFOA (10 ng/g paperboard spike) and 6:2 FTS showed poor recovery. This may have been due to the lack of isotopically labelled internal standards that would have likely corrected any matrix effects that would have resulted in changes in ionisation. According to Harris et al. (2022) and Rawn et al. (2022), the use of isotopically labelled internal standards is critical in ensuring absolute quantification and correcting for matrix effects (Rawn et al. 2022; Harris et al. 2022). Other factors, such as SPE interaction, the influence of the buffer solvent system, interaction with other PFAS compounds present and salts, ions and organic matter in the paper samples, may have further influenced the recovery of these target PFAS (Zabaleta et al. 2016; Kaiser et al. 2020; Harris et al. 2022). The %RSD for the relative recoveries ranged from 1.0 to 9.8%, indicating acceptable precision (Srivastava et al. 2022). The method LODs and LOQs were found to be generally lower for the compounds present in the lower concentration in the calibration standard mix with LODs ranging from 1.47 pg/g for PFDA to 10.1 pg/g for PFUnA for compounds present as 2 µg/mL in methanol (Table S9). For the compounds present in larger concentrations, the LODs ranged from 11.2 pg/g for PFPeA to 53.1 pg/g for PFBA. In addition to differences in carbon chain lengths and chemical structures, the stability and possible interactions of the different PFAS compounds in methanol may also have contributed to the differences found in the LOQs and LODs. To date, there have been limited studies on the quantification of paper-based samples using UHPLC Orbitrap HRMS. However, most studies have focused on the use of LC–MS/MS or LC-QTOF-MS (Zabaleta et al. 2016, 2020; Blanco-Zubiaguirre et al. 2021; Boisacq et al. 2023; Vera et al. 2024). The LODs in this study were thus compared with the analysis of PFAS in food matrices and landfill leachate, where the instrument of analysis was an HRMS Q Orbitrap. This study achieved more sensitive detection limits for common PFAS (Rawn et al. 2022; Rehnstam et al. 2023). Rawn et al. (2022) reported LOQs ranging from 18 g/g food for L-PFDS to 12.4 ng/g PFBA food (Rawn et al. 2022). In this study, the limits of quantification ranged from 0.0049 ng/g paper for PFDA to 0.18 ng/g paper for PFBA, further highlighting the sensitivity achieved in this study.
Occurrence of PFAS
The combined sum of average concentrations (ΣPFAS) of the 22 analysed PFAS compounds was highest for post-consumer samples (213 ng/g), followed by retail samples (159 ng/g) and pre-consumer samples (121 ng/g), as shown in Fig. 2. Overall, PFAS contamination increased from pre-consumer to post-consumer, with the highest concentration associated with post-consumer samples where recovered material had likely interacted with the environment and possibly mingled with other waste materials. The pre-consumer samples analysed consisted of samples made with a recycled fibre component, whereas the retail and post-consumer samples were expected to contain virgin, mixed and/or recycled fibre. The occurrence of PFAS in these samples was strongly related to PFAS intentionally and/or unintentionally used in manufacturing, retention of PFAS from recycling and, in retail samples, exposure from transport, distribution and packed goods. When looking at the categorical box and whiskers chart plotted using Statistica ® (Table S10), it was further evident that more PFAS were detected in post-consumer samples, with the categorical data being found to statistically follow normal distributions (Fig. S1 to Fig. S22). In terms of the PFAS compounds, 6:2 FTS was the most prevalent target analyte with the highest average concentration of 55.2 ng/g for post-consumer samples, 34.3 ng/g for retail and 22.1 ng/g for pre-consumer samples. This 6-fluoroalkyl chain telomer has become a predominant polymer processing alternative to PFOS and PFOA (Lu et al. 2017) (Schwartz-Narbonne et al. 2023; Solan et al. 2023). In a study by Dueñas-Mas et al. (2023) on food packaging in fast-food restaurants in France, 6:2 FTS and PFHxA were detected in all 47 samples analysed (Dueñas-Mas et al. 2023). It has also been reported in fast-food packaging in the USA (Schaider et al. 2017). This corroborated the prevalence of 6:2 FTS in this study. It is important to note that matrix enhancement effects may have given results that were biased high due to the high 6:2 FTS recovery rate obtained. Along with 6:2 FTS, PFBA, PFHxA, PFDoA and PFTeDA were also frequently detected in the samples. This highlighted the possible circulation of short-chain PFAS. According to Palma et al. (2022) and Rehnstam et al. (2023), shorter-chain PFAS such as PFPeA, PFHxA and PFBA are the main degradation products of both PFOA and PFOS in the environment (Palma et al. 2022). PFPeA and PFHxA are common breakdown products of stain- and grease-proof coatings in food packaging and household products (Liu and Liu 2016).
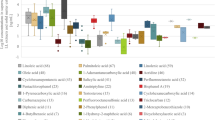
The PFAS range was plotted to compare the ranges of the detected PFAS (Fig. 3). The smallest range was for PFNS from (0–0.04 ng/g), and the largest was for 6:2 FTS (0–354.5 ng/g). The majority of the PFAS compounds detected were below 50 ng/g, with PFDoA (58.3 ± 0.5 ng/g for a recycling facility coffee box), PFPeA (70.4 ± 3.1 ng/g for a waste picker corrugated box), PFHxA (156.9 ± 9.6 ng/g for a retail chocolate box) and 4:2FTS (164.79 ± 7.1 ng/g for the same waste picker corrugated box) all being higher than 50 ng/g. In comparison, the PFAS concentrations reported in Dueñas-Mas et al. (2023) were considerably lower in food packaging with concentrations detected found to be less than 10 ng/g (Dueñas-Mas et al. 2023).
PFAS profile of samples
The PFAS profiles of the samples are plotted in Fig. 4 for the detected concentrations (Tables S11 and S12). When examining the individual PFAS in specific samples, the three highest ΣPFAS were found in post-consumer samples, namely a recycling facility tequila carton (ΣPFAS of 647.9 ng/g), a recycling facility newspaper (ΣPFAS of 400.2 ng/g) and a waste picker corrugated food box (ΣPFAS of 548.0 ng/g). The corrugated food box had the highest number of different PFAS detected with 21 of the 22 quantified PFAS detected. An egg carton collected from household waste was found to contain 18 of the 22 quantified PFAS detected, albeit in smaller combined concentrations (ΣPFAS of 127.6 ng/g) than the waste picker corrugated food box. The lowest concentration of combined PFAS concentration samples was found in a newsletter collected at a solid waste disposal site (ΣPFAS of 2.6 ng/g), a recycling facility cereal carton (ΣPFAS = 14.5 ng/g), a colouring book cover (ΣPFAS = 17.2 ng/g) and a recycling facility corrugated fruit box (ΣPFAS 31.9 ng/g). Among the retail samples, a chocolate carton had the highest combined PFAS concentration (ΣPFAS = 235.1 ng/g) with PFHxA being the dominant PFAS. Across the paper recycling chain, the average ΣPFAS in the corrugated boxes increased from pre-consumer (99 ng/g) to retail (102 ng/g) to post-consumer (145 ng/g). All samples analysed in this study were found to contain PFAS, further demonstrating the prevalence of PFAS compounds. This differed Zabaleta et al. (2020) and other findings related to targeted quantification (Zafeiraki et al. 2014; Zabaleta et al. 2016), where the majority of the food packaging material was found to contain PFAS at very low concentrations or not detected at all. However, it correlated with Dueñas-Mas et al. (2023) and Sapozhnikova et al. (2023). In the study by Sapozhnikova et al. (2023), PFHpA, PFDA, 6:2 fluorotelomer phosphate diester and PFHxS were the most frequently detected PFAS(Sapozhnikova et al. 2023). These comparisons showed that the detection of PFAS may be dependent on the region, circulating PFAS, exposure pathways, analysis protocols and analytical method sensitivity.
The uncategorised data followed non-normal distributions with outliers (Fig. S23). Spearman rank correlation (Eq. (1)) was used as the distance measure for the correlation heat map which was constructed with its associated dendrogram using MetaboAnalyst 5.0. In Fig. 5, a correlation of 1 (shown in dark red) represented a directly proportional relationship and − 0.4 indicated the most negative correlation, shown in dark blue. The correlation between the different PFAS under investigation showed that the strongest correlation was between PFPeA and PFTrDA (0.69) and between 8:2 FTS and PFDoS (0.30). This indicated that the presence of PFPeA was accompanied by the detection of PFTrDA in the same sample. Furthermore, the detection of PFTeDA was associated with the presence of PFDA and PFUnA. The PFAS found to likely co-occur to some extent were PFNA, PFHpS, PFDS, PFBS, 8:2 FTS and PFDoS. Conversely, PFTeDA and PFHpS had a strong negative correlation at − 0.261. A hierarchical clustering dendrogram as shown in Fig. 6 was based on the principle that variables with similar numerical values would also be close to each other in space (Kumar et al. 2014). The dendrogram indicated distinct clustering in the presence of several target PFAS in various papers. For instance, it could be seen that PFTeDA, PFDA, PFUnA, PFOA and PFDoA clustered together when compared to the remaining target analytes. In addition, further clustering was evident between PFTeDA and PFDA with PFUnA compared to PFOA and PFDoA. The investigation of such clustering was beneficial for understanding the detection of numerous types of poly- and perfluorocarboxylic and sulfonic acids in different paper samples and environmental media (Trier et al. 2011; Zabaleta et al. 2020; Bai and Son 2021; Kurwadkar et al. 2022; Bugsel et al. 2022). This further suggested that it would be difficult to isolate a single PFAS compound for targeted removal, for instance, without determining all possible co-occurrences.
Stockholm-listed PFAS
In 2009, PFOS and its salts were the first PFAS to be listed in the United Nations Stockholm Convention list of persistent organic pollutants. The listing of PFOA followed in 2019, with PFHxS formally listed most recently in 2022 (UNEP 2019a). When looking specifically at the Stockholm Convention–listed PFAS, the highest detection was at 19.9 ± 2.1 ng/g for PFHxS in a pre-consumer corrugated board. PFHxS was the most prevalent PFAS in the Stockholm Convention–listed PFAS, followed by PFOS and PFOA (as shown in Fig. 6). Sapozhnikova et al. (2023) reported that PFHxS was one of the most frequently detected PFAS in their study of globally sourced food packaging (Sapozhnikova et al. 2023). PFHxS is used as a surfactant in various applications, including firefighting foams, metal plating and textiles (Boucher et al. 2019). PFHxS and its related compounds have also been associated with paper production (UNEP 2019b; Langberg et al. 2021), which could explain their detection in this study. In addition, PFHxS has been found to form from perfluoroalkyl sulfonamide derivatives during chlorination and chloramination during water disinfection (Li et al. 2023) and bioaccumulate in the environment (Zhong et al. 2021), which may be another exposure pathway in the paper recycling chain. When examining the other listed PFAS in this study, PFOS was more prominent in the household waste samples with the highest concentration detected in a fast-food container (44.4 ± 1.6 ng/g). PFOA, on the other hand, was less prominent in household waste and pre-consumer samples but was more prevalent in post-consumer solid waste disposal and recycling facility samples, in the colouring sheet sample (14.3 ± 0.7 ng/g) and magazine sample (7.0 ± 1.4 ng/g), respectively. This shows that these longer-chain PFAS of concern may be circulating in the environment and consumer-good value chains. Many developing countries are guided by the Stockholm Convention and the ratification thereof. The co-occurrences shown in this study are therefore important for the Global South as they open avenues for further research that can be used to motivate for legislation that is driven towards the restriction of all PFAS.
Concluding remarks
The analysis of PFAS using UHPLC-HRMS highlighted the difficulty of PFAS analysis given the ubiquitous nature of PFAS in the samples as well as in laboratory ware, solvents and instrumentation. Blank samples were largely able to account for contamination issues; however, the lack of internal standards meant that the matrix effects could not be accounted for. When comparing the results obtained, it was shown that the combined average concentration of PFAS was lowest in the pre-consumer samples and highest in the post-consumer samples. This study further showed that the co-mingling, sorting and collection protocols may influence PFAS propagation, as the highest PFAS concentrations were detected at post-consumer sites. When considering paper recycling and the circular economy, this study illustrated the need to further understand and investigate activities in the entire paper recycling chain. The initial manufacturing, usage, sorting, collection, disposal, recovery and remanufacturing design protocols for recycled paper are key to ensuring minimal PFAS circulation in the paper recycling chain. Research on different exposure pathways is still required to further unpack PFAS contamination, exposure and propagation to fully evaluate the safety and impact of PFAS in paper recycling.
Data Availability
All data generated in this study is available in the article supplementary material.
References
Bai X, Son Y (2021) Perfluoroalkyl substances (PFAS) in surface water and sediments from two urban watersheds in Nevada, USA. Sci Total Environ 751:141622. https://doi.org/10.1016/j.scitotenv.2020.141622
Baldwin WS, Davis TT, Eccles JA (2023) Per- and polyfluoroalkyl substances (PFAS) and their toxicology as evidenced through disease and biomarkers. In: Patel, V.B., Preedy, V.R., Rajendram, R. (eds) Biomarkers in toxicology. Biomarkers in disease: methods, discoveries and applications. Springer, Cham. https://doi.org/10.1007/978-3-031-07392-2_67
Barola C, Moretti S, Giusepponi D, Paoletti F, Saluti G, Cruciani G, Brambilla G, Galarini R (2020) A liquid chromatography-high resolution mass spectrometry method for the determination of thirty-three per- and polyfluoroalkyl substances in animal liver. J Chromatogr A 1628:461442. https://doi.org/10.1016/j.chroma.2020.461442
Blanco A, Miranda R, Monte MC (2013) Extending the limits of paper recycling: improvements along the paper value chain. For Syst 22(3):471–483. https://doi.org/10.5424/fs/2013223-03677
Blanco-Zubiaguirre L, Zabaleta I, Prieto A, Olivares M, Zuloaga O, Elizalde MP (2021) Migration of photoinitiators, phthalates and plasticizers from paper and cardboard materials into different simulants and foodstuffs. Food Chem 344:128597. https://doi.org/10.1016/j.foodchem.2020.128597
Boisacq P, De Keuster M, Prinsen E, Jeong Y, Bervoets L, Eens M, Covaci A, Willems T, Groffen T (2023) Assessment of poly- and perfluoroalkyl substances (PFAS) in commercially available drinking straws using targeted and suspect screening approaches. Food Additiv Contam - Part A Chem Anal Control Expo Risk Assess 40(9):1230–1241. https://doi.org/10.1080/19440049.2023.2240908
Boucher JM, Cousins IT, Scheringer M, Hungerbühler K, Wang Z (2019) Toward a comprehensive global emission inventory of C4–C10 perfluoroalkanesulfonic acids (PFSAs) and related precursors: focus on the life cycle of C6- and C10-based products. Environ Sci Technol Lett 6:1–7. https://doi.org/10.1021/acs.estlett.8b00531
Brendel S, Fetter E, Staude C, Vierke L, Biegel-Engler A (2018) Short-chain perfluoroalkyl acids: environmental concerns and a regulatory strategy under REACH. Environ Sci Eur 30:9. https://doi.org/10.1186/s12302-018-0134-4
Brunn H, Arnold G, Körner W, Rippen G, Steinhäuser KG, Valentin I (2023) PFAS: forever chemicals—persistent, bioaccumulative and mobile. Reviewing the status and the need for their phase out and remediation of contaminated sites. Environ Sci Eur 35:20. https://doi.org/10.1186/s12302-023-00721-8
Bugsel B, Bauer R, Herrmann F, Maier ME, Zwiener C (2022) LC-HRMS screening of per- and polyfluorinated alkyl substances (PFAS) in impregnated paper samples and contaminated soils. Anal Bioanal Chem 414:1217–1225. https://doi.org/10.1007/s00216-021-03463-9/Published
Chambers WS, Hopkins JG, Richards SM (2021) A review of per- and polyfluorinated alkyl substance impairment of reproduction. Front Toxicol 3:732436. https://doi.org/10.3389/ftox.2021.732436
Cousins IT, Dewitt JC, Glüge J, Goldenman G, Herzke D, Lohmann R, Ng CA, Scheringer M, Wang Z (2020) The high persistence of PFAS is sufficient for their management as a chemical class. Environ Sci Process Impacts 22:2307–2312
Curtzwiler GW, Silva P, Hall A, Ivey A, Vorst K (2021) Significance of perfluoroalkyl substances (PFAS) in food packaging. Integr Environ Assess Manag 17:7–12. https://doi.org/10.1002/ieam.4346
Dueñas-Mas MJ, Ballesteros-Gómez A, de Boer J (2023) Determination of several PFAS groups in food packaging material from fast-food restaurants in France. Chemosphere 339:139734. https://doi.org/10.1016/j.chemosphere.2023.139734
Ellison SLR, Williams A (Eds) (2012) EUROCHEM/CITAC guide quantifying uncertainty in analytical measurement Third Ed. QUAM:2012.P1 ISBN 978–0–948926–30–3. www.eurachem.org . Accessed 21 February 2024
Evich MG, Davis MJB, McCord JP, Acrey B, Awkerman JA, Knappe DRU, Lindstrom AB, Speth TF, Tebes-Stevens C, Strynar MJ, Wang Z, Weber EJ, Henderson WM, Washington JW (2022) Per- and polyfluoroalkyl substances in the environment. Science 375 (6580). https://doi.org/10.1126/science.abg9065
Geueke B, Groh K, Muncke J (2018) Food packaging in the circular economy: overview of chemical safety aspects for commonly used materials. J Clean Prod 193:491–505
Glüge J, Scheringer M, Cousins IT, Dewitt JC, Goldenman G, Herzke D, Lohmann R, Ng CA, Trier X, Wang Z (2020) An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ Sci Process Impacts 22:2345–2373. https://doi.org/10.1039/d0em00291g
Harris KJ, Munoz G, Woo V, Sauvé S, Rand AA (2022) Targeted and suspect screening of per- and polyfluoroalkyl substances in cosmetics and personal care products. Environ Sci Technol 56:14594–14604. https://doi.org/10.1021/acs.est.2c02660
Helmer RW, Reeves DM, Cassidy DP (2022) Per- and polyfluorinated alkyl substances (PFAS) cycling within Michigan: contaminated sites, landfills and wastewater treatment plants. Water Res 210:117983. https://doi.org/10.1016/j.watres.2021.117983
Kaiser A-M, Aro R, Kärrman A, Weiss S, Hartmann C, Uhl M, Forsthuber M, Gundacker C, Yeung LWY (2020) Comparison of extraction methods for per- and polyfluoroalkyl substances (PFAS) in human serum and placenta samples—insights into extractable organic fluorine (EOF). Anal Bioanal Chem 413(3):856–876. https://doi.org/10.1007/s00216-020-03041-5/Published
Kumar N, Bansal A, Sarma GS, Rawal RK (2014) Chemometrics tools used in analytical chemistry: An overview. Talanta 123:186–199
Kurwadkar S, Dane J, Kanel SR, Nadagouda MN, Cawdrey RW, Ambade B, Struckhoff GC, Wilkin R (2022) Per- and polyfluoroalkyl substances in water and wastewater: a critical review of their global occurrence and distribution. Sci Total Environ 809:151003. https://doi.org/10.1016/j.scitotenv.2021.151003
Langberg HA, Arp HPH, Breedveld GD, Slinde GA, Høiseter Å, Grønning HM, Jartun M, Rundberget T, Jenssen BM, Hale SE (2021) Paper product production identified as the main source of per- and polyfluoroalkyl substances (PFAS) in a Norwegian lake: source and historic emission tracking. Environ Pollut 273:116259. https://doi.org/10.1016/j.envpol.2020.116259
Leon A (1998) Descriptive and inferential statistics. In: Comprehensive clinical psychology. Elsevier Science Ltd., pp 243–285. https://doi.org/10.1016/B0080-4270(73)00264-9
Li Z, Lu Y, Chen T, He A, Huang Y, Li L, Pan W, Li J, Zhu N, Wang Y, Jiang G (2023) Generation mechanism of perfluorohexanesulfonic acid from polyfluoroalkyl sulfonamide derivatives during chloramination in drinking water. Environ Sci Technol 57(41):18462–18472. https://doi.org/10.1021/acs.est.2c07881
Liu C, Liu J (2016) Aerobic biotransformation of polyfluoroalkyl phosphate esters (PAPs) in soil. Environ Pollut 212:230–237. https://doi.org/10.1016/j.envpol.2016.01.069
Liu YL, Sun M (2021) Ion exchange removal and resin regeneration to treat per- and polyfluoroalkyl ether acids and other emerging PFAS in drinking water. Water Res 207:117781. https://doi.org/10.1016/j.watres.2021.117781
Lu M, Cagnetta G, Zhang K, Huang J, Yu G (2017) Mechanochemical mineralization of “very persistent” fluorocarbon surfactants - 6:2 fluorotelomer sulfonate (6:2FTS) as an example. Sci Rep 7(1):17180. https://doi.org/10.1038/s41598-017-17515-7
MacLennan M, Ng D, Hope D (2019) Extraction of poly- and perfluorinated alkyl substances (PFAS) from solid matrices. In: society of Ecotoxicology and Chemistry (SETAC) North America, 40th Annual Meeting, Toronto, Poster. https://www.pacificrimlabs.com/uploads/content/PFASatSETACTORONTO2019.pdf. Accessed 5 Nov 2021
Palma D, Richard C, Minella M (2022) State of the art and perspectives about non-thermal plasma applications for the removal of PFAS in water. Chem Eng J Adv 10:100253
Paper Manufacturers Association of South Africa (2022) Paper Production, Collection and Recycling Statistics South Africa. https://thepaperstory.co.za/wp-content/uploads/2023/07/PAMSA-Recycling-Stats-2022.pdf. Accessed 17 Jan 2023
Quinete N, Orata F, Maes A, Gehron M, Bauer KH, Moreira I, Wilken RD (2010) Degradation studies of new substitutes for perfluorinated surfactants. Arch Environ Contam Toxicol 59:20–30. https://doi.org/10.1007/s00244-009-9451-3
Rasul SB, Monsur Kajal A, Khan AH (2017) Quantifying uncertainty in analytical measurements. J Bangladesh Acad Sci 41(2):145–163. https://doi.org/10.3329/jbas.v41i2.35494
Rawn DFK, Ménard C, Feng SY (2022) Method development and evaluation for the determination of perfluoroalkyl and polyfluoroalkyl substances in multiple food matrices. Food Addit Contam - Part A Chem Anal Control Expo Risk Assess 39(4):752–776. https://doi.org/10.1080/19440049.2021.2020913
Rehnstam S, Czeschka MB, Ahrens L (2023) Suspect screening and total oxidizable precursor (TOP) assay as tools for characterization of per- and polyfluoroalkyl substance (PFAS)-contaminated groundwater and treated landfill leachate. Chemosphere 334:138925. https://doi.org/10.1016/j.chemosphere.2023.138925
Reinhart DR, Bolyard SC, Chen J (2023) Fate of per- and polyfluoroalkyl substances in postconsumer products during waste management. J Environ Eng 149(4):03123002. https://doi.org/10.1061/JOEEDU.EEENG-7060
Rosenmai AK, Taxvig C, Svingen T, Trier X, van Vugt-Lussenburg BMA, Pedersen M, Lesné L, Jégou B, Vinggaard AM (2016) Fluorinated alkyl substances and technical mixtures used in food paper-packaging exhibit endocrine-related activity in vitro. Andrology 4(4):662–672. https://doi.org/10.1111/andr.12190
Sapozhnikova Y, Taylor RB, Bedi M, Ng C (2023) Assessing per- and polyfluoroalkyl substances in globally sourced food packaging. Chemosphere 337:139. https://doi.org/10.1016/j.chemosphere.2023.139381
Schaider LA, Balan SA, Blum A, Andrews DQ, Strynar MJ, Dickinson ME, Lunderberg DM, Lang JR, Peaslee GF (2017) Fluorinated compounds in U.S. fast food packaging. Environ Sci Technol Lett 4(3):105–111. https://doi.org/10.1021/acs.estlett.6b00435
Schultes L, Peaslee GF, Brockman JD, Majumdar A, McGuinness SR, Wilkinson JT, Sandblom O, Ngwenyama RA, Benskin JP (2019) Total fluorine measurements in food packaging: how do current methods perform? Environ Sci Technol Lett 6(2):73–78. https://doi.org/10.1021/acs.estlett.8b00700
Schwartz-Narbonne H, Xia C, Shalin A, Whitehead HD, Yang D, Peaslee GF, Wang Z, Wu Y, Peng H, Blum A, Venier M, Diamond ML (2023) Per- and polyfluoroalkyl substances in Canadian fast food packaging. Environ Sci Technol Lett 10:343–349. https://doi.org/10.1021/acs.estlett.2c00926
Semple KE, Zhou C, Rojas OJ, Nkeuwa WN, Dai C (2022) Moulded pulp fibers for disposable food packaging: a state-of-the-art review. Food Packag Shelf Life 33:100908. https://doi.org/10.1016/j.fpsl.2022.100908
Silva P, Vorst K, Curtzwiler W (2021) The effect of particle size reduction techniques on extraction and recovery of 16 PFAS in food-contact paper packaging matrices. Thermo Fisher Scientific AN65960-en 0421M. https://assets.thermofisher.com/TFS-Assets/CMD/Application-Notes/an-65960-lc-ms-pfas-food-packaging-matrices-an65960-en.pdf. Accessed 5 Nov 2021
Solan ME, Koperski CP, Senthilkumar S, Lavado R (2023) Short-chain per- and polyfluoralkyl substances (PFAS) effects on oxidative stress biomarkers in human liver, kidney, muscle, and microglia cell lines. Environ Res 223:115424. https://doi.org/10.1016/j.envres.2023.115424
Srivastava P, Williams M, Du J, Navarro D, Kookana R, Douglas G, Bastow T, Davis G, Kirby JK (2022) Method for extraction and analysis of per- and poly-fluoroalkyl substances in contaminated asphalt. Anal Methods 14(17):1678–1689. https://doi.org/10.1039/d2ay00221c
Timshana A, Aristizabal-Henao JJ, Da Silva BF, Bowden JA (2021) The last straw: Characterization of per- and polyfluoroalkyl substances in commercially-available plant-based drinking straws. Chemosphere 277:130238. https://doi.org/10.1016/j.chemosphere2021.130238
Trier X, Granby K, Christensen JH (2011) Polyfluorinated surfactants (PFS) in paper and board coatings for food packaging. Environ Sci Pollut Res 18:1108–1120. https://doi.org/10.1007/s11356-010-0439-3
UNEP (2019a) Stockholm Convention. In: United Nations Environment Programme. https://chm.popd.int/default.aspx. Accessed 20 Aug 2023
UNEP (2019b) Big Year for chemicals & waste continues as UN experts take steps to recommend eliminating UV-328 (a toxic plastic additive). https://chm.pops.int/tabid/243/. Accessed 11 October 2023
US Environmental Protection Agency (2021) Draft Method 1633 Analysis of Per- and Polyfluoroalkyl Substances (PFAS) in Aqueous, Solid, Biosolids, and Tissue Samples by LC-MS/MS. EPA 821-D-21-001. https://www.epa.gov/system/files/documents/2021-09/method_1633_draft_aug-2021.pdf. Accessed 27 Sept 2021
Van Ewijk S, Stegemann JA, Ekins P (2021) Limited climate benefits of global recycling of pulp and paper. Nat Sustain 4:180–187. https://doi.org/10.1038/s41893-020-00624-z
Vera P, Canellas E, Dreolin N, Goshawk J, Nerín C (2024) The analysis of the migration of per and poly fluoroalkyl substances (PFAS) from food contact materials using ultrahigh performance liquid chromatography coupled to ion-mobility quadrupole time-of-flight mass spectrometry (UPLC- IMS-QTOF). Talanta 266:124999. https://doi.org/10.1016/j.talanta.2023.124999
Vierke L, Möller A, Klitzke S (2014) Transport of perfluoroalkyl acids in a water-saturated sediment column investigated under near-natural conditions. Environ Pollut 186:7–13. https://doi.org/10.1016/j.envpol.2013.11.011
Wendelken SC, U.S. EPA (2019) Method 533: Determination of Per- and Polyfluoroalkyl Substances in Drinking Water by Isotope Dilution Anion Exchange Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry. EPA 815-V-19-020. https://www.epa.gov/sites/default/files/2019-12/documents/method-533-815b19020.pdf. Accessed 27 Sept 2021
World Economic Forum (2022) World recycling facts for 2022_ Plastic, paper and more _ World Economic Forum. In: World Economic Forum. https://www.weforum.org/agenda/2022/06/recycling-global-statistics-facts-plastic-paper. Accessed 31 Aug 2023
Zabaleta I, Bizkarguenaga E, Bilbao D, Etxebarria N, Prieto A, Zuloaga O (2016) Fast and simple determination of perfluorinated compounds and their potential precursors in different packaging materials. Talanta 152:353–363. https://doi.org/10.1016/j.talanta.2016.02.022
Zabaleta I, Blanco-Zubiaguirre L, Baharli N, Olivares M, Prieto A, Zuloaga O, Elizalde MP (2020) Occurrence of per- and polyfluorinated compounds in paper and board packaging materials and migration to food simulants and foodstuffs. Food Chem 321:126746. https://doi.org/10.1016/j.foodchem.2020.126746
Zafeiraki E, Costopoulou D, Vassiliadou I, Bakeas E, Leondiadis L (2014) Determination of perfluorinated compounds (PFCs) in various foodstuff packaging materials used in the Greek market. Chemosphere 94:169–176. https://doi.org/10.1016/j.chemosphere.2013.09.092
Zhong H, Zheng M, Liang Y, Wang Y, Gao W, Wang Y, Jiang G (2021) Legacy and emerging per- and polyfluoroalkyl substances (PFAS) in sediments from the East China Sea and the Yellow Sea: occurrence, source apportionment and environmental risk assessment. Chemosphere 282:131042. https://doi.org/10.1016/j.chemosphere.2021.131042
Zhu M, Walker E (2020) Analysis of perfluoroalkyl substances (PFAS) using high-resolution accurate mass data https://assets.thermofisher.cn/TFS-Assets/CMD/posters/pn-73525-lcms-pfas-water-pn73525-en.pdf. Accessed 15 October 2022
Acknowledgements
Anatech Instruments Pty (Ltd) is thanked for the opportunity to utilise their Thermo Scientific Vanquish UHPLC+ Q Exactive Focus Orbitrap and for providing excellent training, expertise and support.
Funding
Open access funding provided by University of the Witwatersrand. This study was supported financially through the Innovation, Research and Development Division of Mpact Operations Pty (Ltd), a paper and plastics manufacturing business operating in southern Africa.
Author information
Authors and Affiliations
Contributions
Nondumiso Nomonde Mofokeng, Lawrence Mzukisi Madikizela, Ineke Tiggelman, Edmond Sanganyado and Luke Chimuka contributed to the study’s conception and design. Material preparation, data collection and analysis were performed by Nondumiso Nomonde Mofokeng. The first draft of the manuscript was written by Nondumiso Nomonde Mofokeng. Lawrence Mzukisi Madikizela, Ineke Tiggelman, Edmond Sanganyado and Luke Chimuka reviewed, edited and commented on previous versions of the manuscript. Nondumiso Nomonde Mofokeng, Lawrence Mzukisi Madikizela, Ineke Tiggelman, Edmond Sanganyado and Luke Chimuka read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors give consent to publish.
Competing interests
Nondumiso Nomonde Mofokeng and Ineke Tiggelman declare employment at Mpact Operations Pty (Ltd). Lawrence Mzukisi Madikizela, Edmond Sanganyado and Luke Chimuka declare they have no competing interests.
Additional information
Responsible Editor: Roland Peter Kallenborn
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mofokeng, N.N., Madikizela, L.M., Tiggelman, I. et al. Determination of per- and polyfluoroalkyl compounds in paper recycling grades using ultra-high-performance liquid chromatography–high-resolution mass spectrometry. Environ Sci Pollut Res 31, 30126–30136 (2024). https://doi.org/10.1007/s11356-024-33250-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-33250-9