Abstract
Danube sturgeon (Acipenser gueldenstaedtii) which is identified as endangered species can be exposed to pollutants such as bisphenol A (BPA) that have a disruptive effect on the endocrine system at any time. Starting from this motivation, the current study focused on BPA toxicity in A. gueldenstaedtii juvenile individuals and its adverse effects in sub-lethal concentration. The median lethal concentration (LC50) of BPA was 5.03 mg/L in 96th hour. In the chronic period, 0.625 mg/L and 1.25 mg/L BPA concentrations were evaluated based on the result of acute study. Accordingly, growth performance was significantly decreased in BPA groups (1.25 mg/L BPA group was significantly lowest) compared to control (p < 0.05). In the acute period, behavioral disorders were standing at the bottom/corner of tank, slowing and stopping of gill movement, decreased response to stimuli, and death, respectively. While vacuolization was severe in the liver tissue of the fish in the acute period, intense necrosis and melanomacrophage centers were observed in the chronic period. In terms of genotoxicity, longer DNA migration was observed in all groups exposed to BPA than in the control group. In addition, lower erythrocyte and hemoglobin were observed in the BPA groups compared to control. As a result, the current study revealed toxic effect of BPA on A. gueldenstaedtii juvenile individuals and its negative results on fish physiology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bisphenol A (BPA) is a synthetic monomer used in the plastics industry that has a negative effect on the endocrine system (Vandenberg et al. 2010; Rubin 2011; Rebai et al. 2021). Especially with the increase in plastic production year by year, BPA accumulation in aquatic environments becomes hazardous. It has been reported that around 1 million pounds of BPA is released into the environment annually (McCracken et al. 2017). The presence of BPA in natural waters has been reported in various studies with both lower and higher concentrations ranging from 4.4 to 8000 ng/L (Belfroid et al. 2002; Jonkers et al. 2010; Lee et al. 2013). Although even the current highest concentration seems low, future projections support that BPA tends to increase rapidly in natural waters depending on plastic consumption (Flint et al. 2012). In addition, residual BPA concentrations have been observed in aquatic organisms, which are indispensable for human consumption as protein food (Belfroid et al. 2002; Basheer et al. 2004; Mita et al. 2011). Therefore, indirect exposure of BPA to humans in the food chain is inevitable. However, BPA production tends to increase continuously due to heavy plastic consumption (Vandenberg et al. 2009; Onay et al. 2023).
BPA has been reported as a toxic/moderately toxic chemical for vertebrates and invertebrates (Minaz et al. 2022b, 2023). In fish exposed to BPA, growth slows (Mccormick et al. 2010), reproduction is negatively affected (Bhandari et al. 2015), and mortality increases (Hanson et al. 2014). BPA disrupts the endocrine system, including acting as an estrogen agonist (Le Fol et al. 2017). BPA is more effective on organisms in the larval period than in the adult period (Brown et al. 2019). Fish are exposed to BPA during the larval period, which is the most vulnerable stage to external stressors (Minaz et al. 2024). However, in order to express this clearly, it needs to be investigated in individual fish species. Exposure of aquatic organisms to toxic substances such as BPA is an important mortality factor. The lower the trophic level of the organism exposed to BPA, the higher the effect of the exposure. For example, while the median lethal concentration (LC50) value of BPA is between 0.96 and 2.70 mg/L for aquatic invertebrate organisms, it varies between 6.8 and 17.9 mg/L for fish (Mathieu-Denoncourt et al. 2016). Organisms with low trophic levels have a short life cycle and respond quickly to environmental changes (Ventura et al. 2010).
Exposure to BPA in the aquatic ecosystem can be considered as a potential risk to all organisms (Diler et al. 2022). Especially for critically endangered species such as Danube sturgeon (Acipenser gueldenstaedtii) (Gessner et al. 2022; IUCN 2024), it is very important to investigate BPA and similar endocrine-disrupting toxic substances. Because sturgeon has delicious meat and high-value caviar, it is a commercial species for aquaculture and fishing (Song et al. 2022). This calls into question the potential of sturgeon exposed to BPA to be carriers of an endocrine-disrupting chemical onto the dinner table. In addition, the fact that they reach reproductive maturity in a long time and are in danger of extinction makes local governments even more sensitive for sturgeon. Anadromous migratory Danube sturgeon migrates between the Black Sea and the Danube River. They migrate more than 1200 km upriver, especially to spawn (Strat and Gheorghe 2023). It was previously stated that the Danube River, where A. gueldenstaedtii is most frequently reported, contains BPA because it is a very long river passing through the borders (city centers) of many countries (Chiriac et al. 2021). Therefore, the physiological response of Danube sturgeon exposed to BPA has become an interesting ecotoxicological issue. In light of all these reasons, a series of studies are needed to monitor the sturgeon, to determine its resistance to toxic substances and to take it under protection. For instance, it has been reported that BPA concentrations found in nature can degrade the antioxidant system in starlet (Acipenser ruthenus), thus causing a decrease in sperm quality and DNA damage (Hulak et al. 2013). On the other hand, in the BPA toxicity study conducted on Danube sturgeon larvae, 24th, 48th, and 72nd hour LC50 values were 803.4 μg/L, 63.1 μg/L, and 39.6 μg/L, respectively (Minaz et al. 2024). According to our null hypothesis, we consider that the BPA has the potential to have a toxic effect on Danube sturgeon juvenile. Current study reveals the LC50 of BPA on A. gueldenstaedtii juvenile. Afterwards, adverse effects of sub-lethal BPA concentration prepared based on LC50 were examined in the chronic period. The lack of previous studies on the potential effect of BPA, especially on sturgeon juvenile, is an important novelty that will fill this gap in the literature.
Material and method
System design and water quality parameters
A. gueldenstaedtii juvenile (~ 1 year) were provided from Aquaculture Application and Research Center in Recep Tayyip Erdoğan University. Because the fish have not reached sexual maturity, their gender is uncertain in this stage. Fish were placed in the tanks 1 week before the acute and chronic trial for adaptation. Trials were established in the 50 L aquariums with rested tap water under the condition of manipulated photoperiod (12 h light and 12 h dark). Air stones were used for aeration for each tank. Water exchange was not applied in the acute period since fish were not fed, whereas 100% water was daily changed in the chronic period. All experimental studies were checked and approved by the Ethical Local Committee of the Recep Tayyip Erdogan University (Decision No: 2023/05).
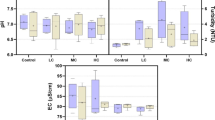
A portable multi-parameter (Hach, HQ40D 58258–00) was daily used for measurement of pH, dissolved oxygen (DO; mg/L), temperature (°C), and electrical conductivity (EC; µS/cm) as water quality parameters. Accordingly, pH (7.18 ± 0.3), DO (7.48 ± 0.4 mg/L), temperature (23.2 ± 0.8 °C), and EC (98.45 ± 12.87 µS/cm) were measured within the limit values that would not adversely affect the fish during experiments. Finally, water samples from each aquarium were analyzed in terms of BPA to prove the presence of BPA-free water.
Acute and chronic toxicity testing
All experiments in the acute and chronic period were processed in the Aquaculture Application and Research Center and toxicology laboratory at the Faculty of Fisheries, Recep Tayyip Erdoğan University, Rize, Türkiye. BPA (97% purity, Sigma Aldrich) used in chronic and acute trials was purchased commercially. The lethal concentration (LC50) that killed 50% of the target population was calculated in 24, 48, 72, and 96 h. Ten fish in triplicate (thirty fish in total) were used for each concentration group (six groups including control) in the experimental design. Five different BPA concentrations (3.0 mg/L, 4.5 mg/L, 6.0 mg/L, 7.5 mg/L, and 9.0 mg/L) and one control (no BPA exposure) were applied under static conditions in the acute period. The LC50 values in the 24th, 48th, 72nd, and 96th hour were calculated as 6.84, 6.14, 5.49, and 5.03 mg/L, respectively (Table 1). LC50-96 h was used for the measurement of sub-lethal concentration. Accordingly, two different sub-lethal (0.625 mg/L and 1.25 mg/L) BPA concentrations were determined based on 1/8 and 1/4 of the lethal concentration (Abdel-Tawwab and Hamed 2018). Ten fish for each group (thirty fish in total) were placed in triplicate. The chronic trial was established for 28 days under daily renewed water condition (%100 change per day).
To monitor BPA concentrations, 20 mL water samples were taken from each tank at the end of the periods in the acute study and weekly in the chronic study. Before analysis, the samples were conditioned with ammonium hydroxide and filtered through 0.2-micron filter papers. It was subsequently analyzed in a negative ionization LC–MS/MS system with a detection limit of 0.2–0.3 µg/L.
Monitoring of growth performance and behavioral changes
Fish were weighted by precision scales before trial to determine initial weight. Fish were daily fed (2% of the fish biomass) with commercial pelleted diet (~ 35% protein, Skretting). At the end of chronic experiment, fish were collected from each aquarium and specific growth rate (SGR); weight gain ratio (WGR), feed conversion ratio (FCR), and feed efficiency ratio (FER) were calculated as per the following equations:
where FW and IW are the weights of fish in final and initial of trial, respectively. In addition, FI, WG, and t represents feed intake, weight gain of fish, and experiment period (28 days), respectively.
Fish behaviors were monitored by the same researcher to avoid observational errors during acute period. Behavioral alterations were noted considering the moment when majority of the population (> 50%) exhibit the same behavior. Accordingly, eight different behaviors were taken into account from normal behavior to first death. It was inspired by another study for determining the behavioral endpoints over time (Kane et al. 2004).
Histological evaluation
Individuals in the chronic and acute periods were randomly taken for histological analyses. Fish were euthanized by clove oil before sampling. The liver and gill tissues were considered for histological evaluation. The neutral buffer solution (10%) was used for fixation of tissues. In the following day, tissues were placed in ethanol (50%) and stored at room temperature. All tissues were exposed to flowing water to remove ethanol. Then, tissues were treated by alcohol (80%, 90%, and 99.9% ethanol series, respectively “15 min per stage”) and xylene (99.9% throughout 15 min × 2 times) series and exposed to paraffin overnight at + 65 °C before the staining stage. Tissues were submerged and blocked into the paraffin in the last stage of the first step. Afterward, paraffin-blocked tissues were cut with a thickness of 5 µm with a microtome and placed on the slide. Slides were kept at + 65 °C overnight to remove paraffin. In the next stage, the tissues were again treated by alcohol (96% and 99.9% during 1 min) and stained with hematoxylin (5 min) and eosin (5 min) series then completed by xylene (1.5 h × 2 times). In the final, the slides were covered with an Entellan™ and cover-slip. Finally, preparates were examined under a light microscope.
A semi-quantitative model developed by Bernet et al. (1999) was used to evaluate quantitatively histological alterations (Table 2). Based on this model, histological alterations were divided into five reaction patterns (circulatory disturbances, regressive changes, progressive changes, inflammation, and tumor). In the current study, only some of these reaction patterns were observed. One importance factor (from 1 “minimal” to 3 “marked) for each reaction pattern was determined in this model. In addition, a Likert scale (from 0 “no change” to 6 “very severe”) was used to score severity of symptoms. In the final, the evaluation of each symptom was calculated by multiplying the importance factor and the score value. The final score for each pattern is determined by summing the assessment scores of the lesions.
Genotoxicity analysis
Comet assay was performed blood samples of fish in the end of both acute and chronic period (Singh et al. 1988). Alkali conditions (pH > 13) were provided to determine erythrocyte DNA damage. Approximately 10 µL blood sampled from caudal vein of fish was treated by phosphate buffer saline (PBS-without Ca++ and Mg++). Therefore, the cell suspension was formed. Then, 75 µL low melting agarose (LMP-0.5%) was mixed 10 µL cell suspension for final mixed suspension. In the first step, slides were frosted and cover by normal melting point agarose (NMP-1%). Then, slides were covered by this mixed suspension in the second layer. Then, 100 µL LMP agarose was used in the last layer for solidification. Slides were exposed to lysing solution (2.5 M NaCl, 100 mM EDTA, 10 mM Tris, pH 10–10.5, 1% Triton X-100, and 10% dimethyl sulfoxide) overnight at + 4 °C after solidification. After lysing treatment, slides were placed in a horizontal gel electrophoresis and immersed in the fresh alkaline electrophoresis buffer for 30 min at + 4 °C. Thus, the DNA unraveled and converted into single-stranded breaks of alkali-labile zones. Electrophoresis was run at 25 V and 300 mA for 30 min. Subsequently, the slides were washed with neutralized buffer and cold distilled water for 10 min, respectively. In the staining stage, 0.5 µg/mL ethidium bromide was used. Then, slides were washed with distilled water for the prevention of excessive staining. Blood samples in the positive control were treated with 100 µm H2O2 for 10 min at 4 °C. In the end, slides were examined under the fluorescent microscope (Leica DMR HC, Germany). In addition, cells were analyzed using Comet Score™ 2.0 Software (Tritek Crop., Sumemeruck, VA, USA).
Hematological indicators
Fish were randomly chosen from aquariums for hematological analyses at the end of acute and chronic periods. After that, fish were anesthetized by clove oil according to Ak et al.’s (2022) procedure. Blood samples were taken from the caudal vein of fish with a 2.5 mL syringe. Then, samples were transferred to EDTA K3 tubes. The erythrocyte (RBC), leukocyte (WBC), hematocrit (HCT), and hemoglobin (HGB) in each blood sample were analyzed by an automatic hematological assay (Prokan6800VET). The automatic hematological assay has been previously calibrated based on the fish and then checked (Minaz et al. 2022b).
Statistical analysis
All data were presented in mean ± standard deviation. As pre-test, normality test was performed with Kolmogorov–Smirnov test. Based on normality analyses, one-way ANOVA and Kruskal–Wallis H test were chosen to determine significant differences between groups for parametric and non-parametric groups, respectively. The significant p value was statistically preferred maximum of 0.05. All data sets were analyzed by SPSS 25 software package for Windows (Version 25, IBM Corp., Armonk, New York, USA).
Results
Lethal toxicity of BPA to A. gueldenstaedtii
Figure 1 shows the mortality rate in fish exposed BPA during acute period depending on increased logarithmic BPA concentration. Based on 95% confidence interval, LC50 of A. gueldenstaedtii was 5.04 mg/L in 96th hour. In addition, mortality rate model [\(y=A1+(A2-A1)/(1+{10}^{{{\text{log}}}_{x}0-x}\times p)\)] was designed for increased BPA concentration. Variables were assumed according to each exposure period. A1 and A2 are the bottom and top asymptotes, respectively. Moreover, y, x, and p represent mortality rate, BPA concentration, and hill slope, respectively.
Growth performance and behavioral assessment
The growth performance of fish in control and BPA-exposed groups was observed at the end of the chronic period (Table 3). Accordingly, final weight, specific growth rate, and weight gain rate were significantly higher in 1.25 mg/L BPA exposed group and lower in control group (p < 0.01). FCR was significantly higher in control group; 1.25 mg/L BPA-exposed group was significantly higher for FER expressing 1/FCR. Also, no mortality was observed in the groups.
Fish exposed to different BPA concentrations showed various behavior disorders in the acute period (Table 4). In the highest concentration (9 mg/L), fish showed all behavior disorders into the 12 h, and first death was noted in the end 12nd hour. While no death was observed in the 3 and 4.5 mg/L BPA concentration, first deaths were observed in the 36th and 16th hour for 6 and 7 mg/L BPA concentrations, respectively. There was no weakness in the gill movement and decreased response to stimuli in the minimum BPA concentration (3 mg/L) till end of acute period. The behavioral responses in the current study were designed entirely descriptively, and therefore, there are some limitations in their interpretation and analysis.
Histological alterations
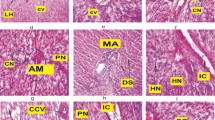
Histological analyses were performed in liver and gill tissues (Fig. 2). In the liver tissues of the acute period, very severe vacuolization was observed, while mild necrosis and melanomacrophage centers were formed (Table 5). In addition, the formation of melanomacrophage centers was severe in the chronic period groups. Hyperplasia was dominant symptom in the gill tissues of BPA groups. Moreover, fusion of secondary lamella and necrosis was also severe in the 1.25 mg/L BPA-exposed group. A model was used to determine quantitatively histological alterations (Table 6). The results showed that liver tissues were significantly affected in terms of regressive changes and inflammation in all BPA groups and chronic BPA groups, respectively (p < 0.01). On the other hand, reaction indices such as regressive changes, progressive changes, and circulatory disturbances were significantly lower in the control group compared to BPA groups (p < 0.01).
Outputs of DNA damage
As a result of alkaline comet assay analysis, DNA migration was observed in the experimental groups exposed to BPA (Fig. 3). DNA damage in the chronic period was affected more than the acute period and control group according to the quantitative index of DNA migration (Table 7). In the control group, head %DNA was significantly highest, whereas tail %DNA, tail olive moment, and tail length were significantly lowest compared to other groups (p < 0.01). All parameters were significantly higher in chronic period groups than even positive control and acute period (p < 0.01). On the other hand, there were no significant differences between positive control and acute period for tail olive moment and tail length (p > 0.05).
Hematological results
Figure 4 shows WBC, RBC, HGB, and HCT in different groups as blood indicators. Accordingly, there were no statistically significant differences between groups in WBC and HCT (p > 0.05). However, RBC in the control group was significantly higher than 0.625 mg/L BPA-exposed group (p < 0.05). On the other hand, HGB in the control group was significantly higher than all BPA-exposed groups, while BPA groups in the chronic period were significantly lowest (p < 0.01).
Discussion
While various pollutants continue to be released into the environment around the world, endangered organisms try to survive and continue their future generations. In this context, countries have many conservation strategies to ensure that endangered animals such as sturgeon can reproduce for future generations (Wang et al. 2011). Even though there are currently strict rules regarding illegal fishing and dams on Danube sturgeon, both negligence in complying with these rules and exposure to toxic substances such as BPA make this fish critically endangered (Gessner et al. 2022). The negative effect of BPA, which is frequently used especially in plastic production, on the endocrine system of aquatic organisms constitutes the motivation of the present study. Although A. gueldenstaedtii has a high resistance to conditions such as poor water quality, the fact that this species is in danger of extinction in recent years has brought the possible consequences of possible BPA exposure into the focus of attention. Previous studies have revealed that water quality is adversely affected by acute BPA exposure (Minaz et al. 2022b). In this study, water quality parameters affected the survival standard of A. gueldenstaedtii as minimally during the acute and chronic trials. Daily water changes were provided to prevent/minimize possible poor water quality. Thus, it is aimed that all physiological and behavioral changes in fish are due to BPA concentration.
The general physiological response of fish to threatening situations is called stress, and a stress response is initiated immediately upon perception of a stressor (Schreck and Tort 2016). Teleost fish are often used in toxicity studies as they are highly sensitive to toxic substances and present stress responses similar to mammals (Sancho et al. 2000). BPA is classified as “moderately toxic” and “toxic” to the aquatic environment with an LC50 value of 1–10 mg/L (Alexander et al. 1988). In various studies of BPA toxicity on fish, the LC50 ranged between these values (Faheem and Lone 2013; Asifa and Chitra 2015; Krishnapriya et al. 2017). Based on this issue, five different BPA concentrations were tested in the present study. Especially, 9 mg/L BPA concentration caused the death of all individuals even at the 24th hour. According to the “general adaptation syndrome,” the concept of stress consists of three stages: the “alarm stage,” which activates the body’s defense mechanism, the “resistance stage,” in which tissue damage occurs in the subsequent process, and the “exhaustion stage,” which potentially causes death when the adaptive energy is exhausted (Galhardo and Oliveira 2009). While the alarm reaction and resistance phase of acute stress ended within the first 24 h, high mortality rate was observed with the exhaustion phase in the following process. In addition, the current study will shed light to researchers and commercial fish farming facilities with the dose response model in terms of BPA toxicity. According to this model [\(y=A1+(A2-A1)/(1+{10}^{{{\text{log}}}_{x}0-x}\times p)\)], the mortality rate in a population exposed known BPA concentration can be estimated. As a result, it is possible for large and small scale facilities to take measures.
Exposure to the toxic material BPA caused an adverse effect on growth performance of A. gueldenstaedtii in the current study. Accordingly, final weight, specific growth rate, weight gain rate, and feed efficiency ratio decreased with increasing BPA concentration, while feed conversion ratio increased. Similar negative growth performance in BPA-exposed fish has been observed in previous studies (Chitra and Maiby 2014; Minaz et al. 2022a). Stress and growth axes in fish are intricately interconnected and all stressors, biotic or abiotic, suppress growth performance (McCormick et al. 1998). Against current stress conditions, fish begin to waste/consume energy in a complex manner to restore homeostasis and maintain functional integrity (Guderley and Pörtner 2010). Because the energy available for growth is limited, fish may divert energy substrate away from growth in coping with stress. This negatively affects fish growth performance (Sadoul et al. 2017).
In toxicological studies, the swimming behavior of fish provides an estimate of how the toxic material affects fish welfare. On the other hand, the determination of the lethal concentration is quite effective as a quantitative result. In general, exposure to BPA has previously been reported to produce effects on fish such as loss of mobility and anorexia (Akram et al. 2021a). In the current acute study, the first 24 h can be considered as the critical period. Because it can be characterized as the hour when the inactive waiting (no mobility) starts at a concentration close to LC50. This low mobility and poor swimming were observed in our previous study (Minaz et al. 2022b). Moreover, other studies have proven that exposure to BPA causes rapid operculum movement (Naveira et al. 2021; Sharma and Chadha 2021). Different behavioral movements such as jerky movement and erratic swimming have also been observed (Faheem et al. 2017; Akram et al. 2021a). In general, it should not be ignored that the behavioral differences observed in fish exposed to toxic substances may be due to possible neurotoxic effects (Sharma and Chadha 2021). Toxic substances degrade nerve cells, axons, and myelin sheath, altering the synthesis of neurotransmitters involved in behavior (Sharma and Borade 2019).
Histopathological indicators are important biomarkers that reveal population health in an ecosystem (Faria et al. 2014). Histological alterations in the different types of tissues serve as a guide in revealing the biological effect of the toxic substance on fish health and welfare (Rautenberg et al. 2015). In fish, the liver is the main organ for detoxification of xenobiotic pollutants such as BPA (Faheem et al. 2016). Therefore, the liver of fish is an important indicator for estimating the water quality of the aquatic environment (Colin et al. 2016). In this study, severe vacuolization was observed in the acute period, while necrosis and melanomacrophage centers were moderate in the chronic period. Although the presence of melanomacrophage centers in tissues is an expected situation, similar to our study, severe melanomacrophage center is an indicator of a response to toxic substance. Because macrophages are indicator cells of innate immunity in fish and other vertebrates and have the ability to phagocytosis, they have functions such as post-inflammatory repair, tissue regeneration, and elimination of old cells (Sayed and Younes 2017). Vacuolization of hepatocytes is an indication of the disappearance of the cell nucleus and the emergence of the intracellular lipid layer. Therefore, vacuolization can be considered as the primary degradation caused by malathion-like necrosis. Similar necrosis and vacuolization have been previously reported in fish exposed to pesticides (Magar and Shaikh 2013).
Since the gills are the first organs to come into contact with water in fish, the effect of pollutants in the water is primarily evaluated with gill histology (Barišić et al. 2015). The toxic effect of a pollutant is highly correlated with the severity of the damage (Faheem et al. 2016). In the current study, chronic lesions in the gills exposed to 1.25 mg/L BPA concentration were noted. Especially the fusion of secondary lamella has been the dioristic symptom in the chronic period. On the other hand, regressive changes, progressive changes, and circulatory disturbances were significantly detected in the BPA groups compared to the control group. In particular, the main reason of the regressive changes was caused by the intense necrosis symptom in the gill tissues. In our previous study, the necrosis symptom in the gill tissues between the BPA and the control group overlaps with the current study (Minaz et al. 2022a). It has been previously proven that the symptom of necrosis in the gills increases due to the increased BPA concentration (Akram et al. 2021b).
Although fish is an example of model organism to reveal the toxic, carcinogenic, and mutagenic effects of various pollutants in the aquatic ambient (Guerrera et al. 2021), there are limited studies showing that BPA toxicity is genotoxic or mutagenic (Akram et al. 2021a; Frenzilli et al. 2021; Afzal et al. 2022; Ďurovcová et al. 2022). These substances are a source of degradation in the DNA sequence. In this purpose, comet assay technique has been developed to detect DNA damage of organisms exposed to various contaminants (Garaj-Vrhovac and Zeljezic 2000). In contrast to the control group, DNA migration was seen in the groups chronically and acutely exposed to BPA, including the positive control group. Exposure to BPA throughout the chronic period caused significant damage to DNA. Even higher DNA damage was observed than the positive control induced with H2O2. In a study that revealed the effect of BPA on freshwater with aquatic insect as a bio-indicator species, it proved the disruptive effect of BPA on DNA tail parameters even in the acute process (Martínez-Paz et al. 2013). In a study conducted on rats, it has been proven that BPA has genotoxicity even if it does not provide a mutagenic effect (Tiwari et al. 2012). BPA-induced DNA strand breakage in HepG2 cells in the comet assay (Quinn-Hosey et al. 2012). BPA has been reported to cause DNA strand breaks on aquatic organisms such as Daphnia magna and Chironomus tentans (Park and Choi 2007).
Hematological alterations are quite rapid tests to quantify stress on fish. Therefore, it can be considered among the primary stress indicators of fish exposed to toxic materials such as BPA. RBC and HGB decreased in the current study depending on BPA exposure. Exposure to sub-lethal BPA concentration during the chronic period resulted in decreased hemoglobin concentration. This reduction in hemoglobin may put a strain on the hematopoietic system of the larvae (Ahmad et al. 2021). The erythropoietic site in fish is the head kidney, and the erythropoiesis process is similar to that in other vertebrates. Fishes show a weak barrier between hematopoietic tissue and circulating blood in which numerous immature cells are present, often comprising over 10% of all erythrocytes. As in other vertebrates, fish erythrocytes contain tetrameric hemoglobin of different oxygen affinity—lower in species living in well-oxygenated water than in those that experience hypoxia (Witeska 2013). In our previous study, the fact that RBC and HGB were lower in BPA-exposed groups was attributed to apoptosis of blood cells by BPA (Minaz et al. 2022a). In relation to histological results, the symptom of hyperplasia and inflammation, especially in the gill tissues, prevented the diffusion of oxygen from the gill surface to the erythrocyte cell membrane (Elahee and Bhagwant 2007).
Conclusion
The current study focused on the acute and chronic toxicity of bisphenol A in Danube sturgeon (Acipenser gueldenstaedtii) juvenile individuals. The lethal concentration of BPA in A. gueldenstaedtii was detected after the acute period. The sub-lethal BPA concentration determined according to the acute period affected the fish adversely. The 24th hour was determined as critical point in terms of fish behavior. According to the control group, the growth performance was adversely affected in the experimental group exposed to BPA. In addition, different types of histological symptoms, DNA migrations in erythrocyte cells, and negative results in hematological indicators (red blood cell and hemoglobin) were observed in the BPA groups compared to control. As a result, exposure of endangered sturgeon to BPA in both their natural habitats and aquaculture environments will cause some physiological effects. The current study provides some justification as to why there is a need for more regulation to limit plastic use and waste.
Data availability
All datasets used during the current study are available from the corresponding author on reasonable request.
References
Abdel-Tawwab M, Hamed HS (2018) Effect of bisphenol A toxicity on growth performance, biochemical variables, and oxidative stress biomarkers of Nile tilapia, Oreochromis niloticus (L.). J Appl Ichthyol 34:1117–1125. https://doi.org/10.1111/JAI.13763
Afzal G, Ahmad HI, Hussain R et al (2022) Bisphenol A induces histopathological, hematobiochemical alterations, oxidative stress, and genotoxicity in common carp (Cyprinus carpio L.). Oxid Med Cell Longev 2022. https://doi.org/10.1155/2022/5450421
Ahmad Z, Alkahem Al-Balawi HF, Al-Ghanim KA et al (2021) Risk assessment of malathion on health indicators of catfish: food and water security prospective research. J King Saud Univ - Sci 33:101294. https://doi.org/10.1016/J.JKSUS.2020.101294
Ak K, Minaz M, Er A, Aslankoç R (2022) The using potential of a new natural anesthetic agent on rainbow trout (Oncorhynchus mykiss): chamomile oil (Matricaria chamomilla). Aquaculture 561:738742. https://doi.org/10.1016/J.AQUACULTURE.2022.738742
Akram R, Iqbal R, Hussain R et al (2021a) Evaluation of oxidative stress, antioxidant enzymes and genotoxic potential of bisphenol A in fresh water bighead carp (Aristichthys nobils) fish at low concentrations. Environ Pollut 268:115896. https://doi.org/10.1016/J.ENVPOL.2020.115896
Akram R, Iqbal R, Hussain R (2021b) Ali M (2021b) Effects of bisphenol a on hematological, serum biochemical, and histopathological biomarkers in bighead carp (Aristichthys nobilis) under long-term exposure. Environ Sci Pollut Res 1:1–16. https://doi.org/10.1007/S11356-021-17329-1
Alexander HC, Dill DC, Smith LW et al (1988) Bisphenol a: acute aquatic toxicity. Environ Toxicol Chem 7:19–26. https://doi.org/10.1002/ETC.5620070104
Asifa KP, Chitra KC (2015) Evaluation of lc50 and behavioural responses of bisphenol a in the cichlid fish, Etroplus maculatus. Int J Curr Res 7:16725–16729
Barišić J, Dragun Z, Ramani S et al (2015) Evaluation of histopathological alterations in the gills of Vardar chub (Squalius vardarensis Karaman) as an indicator of river pollution. Ecotoxicol Environ Saf 118:158–166. https://doi.org/10.1016/J.ECOENV.2015.04.027
Basheer C, Lee HK, Tan KS (2004) Endocrine disrupting alkylphenols and bisphenol-A in coastal waters and supermarket seafood from Singapore. Mar Pollut Bull 48:1161–1167. https://doi.org/10.1016/J.MARPOLBUL.2004.04.009
Belfroid A, Van Velzen M, Van der Horst B, Vethaak D (2002) Occurrence of bisphenol A in surface water and uptake in fish: evaluation of field measurements. Chemosphere 49:97–103. https://doi.org/10.1016/S0045-6535(02)00157-1
Bernet D, Schmidt H, Meier W et al (1999) Histopathology in fish: proposal for a protocol to assess aquatic pollution. J Fish Dis 22:25–34. https://doi.org/10.1046/J.1365-2761.1999.00134.X
Bhandari RK, Deem SL, Holliday DK et al (2015) Effects of the environmental estrogenic contaminants bisphenol A and 17α-ethinyl estradiol on sexual development and adult behaviors in aquatic wildlife species. Gen Comp Endocrinol 214:195–219. https://doi.org/10.1016/J.YGCEN.2014.09.014
Brown AR, Green JM, Moreman J et al (2019) Cardiovascular effects and molecular mechanisms of bisphenol A and its metabolite MBP in zebrafish. Environ Sci Technol 53:463–474. https://doi.org/10.1021/ACS.EST.8B04281/SUPPL_FILE/ES8B04281_SI_002.XLSX
Chiriac FL, Paun I, Pirvu F, et al (2021) Occurrence and fate of bisphenol A and its congeners in two wastewater treatment plants and receiving surface waters in Romania. Environ Toxicol Chem 40:435–446. https://doi.org/10.1002/ETC.4929
Chitra KC, Maiby S (2014) Oxidative stress of bisphenol-A and its adverse effect on the liver of fresh water fish, Oreochromis mossambicus. Int J Sci Res 3:221–224
Colin N, Porte C, Fernandes D et al (2016) Ecological relevance of biomarkers in monitoring studies of macro-invertebrates and fish in Mediterranean rivers. Sci Total Environ 540:307–323. https://doi.org/10.1016/J.SCITOTENV.2015.06.099
Diler Ö, Özil Ö, Nane İD et al (2022) The effects of bisphenol A on oxidative stress, antioxidant defence, histopathological alterations and lysozyme activity in narrow-clawed crayfish (Pontastacus leptodactylus). Turkish J Fish Aquat Sci 22. https://doi.org/10.4194/TRJFAS19877
Ďurovcová I, Kyzek S, Fabová J et al (2022) Genotoxic potential of bisphenol A: a review. Environ Pollut 306:119346. https://doi.org/10.1016/J.ENVPOL.2022.119346
Elahee KB, Bhagwant S (2007) Hematological and gill histopathological parameters of three tropical fish species from a polluted lagoon on the west coast of Mauritius. Ecotoxicol Environ Saf 68:361–371. https://doi.org/10.1016/J.ECOENV.2006.06.003
Faheem M, Lone K (2013) Acute toxicity and behavioural response of Cirrhinus mrigala fingerlings to Bisphenol-A. Int J Open Sci Res 1:28–37
Faheem M, Jahan N, Lone K (2016) Histopathological effects of bisphenol-A on liver, kidneys and gills of Indian major carp, Catla catla (Hamilton, 1822). J Anim Plant Sci 26:514–522
Faheem M, Khaliq S, Ahmad HU, Lone KP (2017) Bisphenol-A (BPA) alters plasma thyroid hormones and sex steroids in female Pakistani major carp (Catla catla; Cyprinidae). Pak Vet J 37:326–330
Faria M, Ochoa V, Blázquez M et al (2014) Separating natural from anthropogenic causes of impairment in zebra mussel (Dreissena polymorpha) populations living across a pollution gradient. Aquat Toxicol 152:82–95. https://doi.org/10.1016/J.AQUATOX.2014.03.020
Flint S, Markle T, Thompson S, Wallace E (2012) Bisphenol A exposure, effects, and policy: a wildlife perspective. J Environ Manage 104:19–34. https://doi.org/10.1016/J.JENVMAN.2012.03.021
Frenzilli G, Martorell-Ribera J, Bernardeschi M et al (2021) Bisphenol A and bisphenol S induce endocrine and chromosomal alterations in brown trout. Front Endocrinol (lausanne) 12:645519. https://doi.org/10.3389/FENDO.2021.645519/BIBTEX
Galhardo L, Oliveira RF (2009) Psychological stress and welfare in fish. Annu Rev Biomed Sci 11:1–20
Garaj-Vrhovac V, Zeljezic D (2000) Evaluation of DNA damage in workers occupationally exposed to pesticides using single-cell gel electrophoresis (SCGE) assay. Pesticide genotoxicity revealed by comet assay. Mutat Res - Genet Toxicol Environ Mutagen 469:279–285. https://doi.org/10.1016/S1383-5718(00)00092-9
Gessner J, Freyhof J, Kottelat M (2022) Acipenser gueldenstaedtii. In: IUCN Red List Threat. Species 2022 e.T232A135063140. https://www.iucnredlist.org/species/232/135063140. Accessed 18 Mar 2024
Guderley H, Pörtner HO (2010) Metabolic power budgeting and adaptive strategies in zoology: examples from scallops and fish. Can J Zool 88:753–763. https://doi.org/10.1139/Z10-039
Guerrera MC, Aragona M, Porcino C et al (2021) Micro and nano plastics distribution in fish as model organisms: histopathology, blood response and bioaccumulation in different organs. Appl Sci 11:5768. https://doi.org/10.3390/APP11135768
Hanson AM, Kittilson JD, Martin LE, Sheridan MA (2014) Environmental estrogens inhibit growth of rainbow trout (Oncorhynchus mykiss) by modulating the growth hormone-insulin-like growth factor system. Gen Comp Endocrinol 196:130–138. https://doi.org/10.1016/J.YGCEN.2013.11.013
Hulak M, Gazo I, Shaliutina A, Linhartova P (2013) In vitro effects of bisphenol A on the quality parameters, oxidative stress, DNA integrity and adenosine triphosphate content in sterlet (Acipenser ruthenus) spermatozoa. Comp Biochem Physiol Part C Toxicol Pharmacol 158:64–71. https://doi.org/10.1016/J.CBPC.2013.05.002
IUCN (2024) Acipenser gueldenstaedtii (Russian Sturgeon). In: IUCN Red List Threat. Species 2022 e.T232A135063140. https://doi.org/10.2305/IUCN.UK.2022-1.RLTS.T232A135063140.en. Accessed 18 Mar 2024
Jonkers N, Sousa A, Galante-Oliveira S et al (2010) Occurrence and sources of selected phenolic endocrine disruptors in Ria de Aveiro, Portugal. Environ Sci Pollut Res 17:834–843. https://doi.org/10.1007/S11356-009-0275-5/TABLES/2
Kane AS, Salierno JD, Gipson GT et al (2004) A video-based movement analysis system to quantify behavioral stress responses of fish. Water Res 38:3993–4001. https://doi.org/10.1016/J.WATRES.2004.06.028
Krishnapriya K, Shobana G, Narmadha S et al (2017) Sublethal concentration of bisphenol A induces hematological and biochemical responses in an Indian major carp Labeo rohita. Ecohydrol Hydrobiol 17:306–313. https://doi.org/10.1016/J.ECOHYD.2017.06.003
Le Fol V, Aït-Aïssa S, Sonavane M et al (2017) In vitro and in vivo estrogenic activity of BPA, BPF and BPS in zebrafish-specific assays. Ecotoxicol Environ Saf 142:150–156. https://doi.org/10.1016/J.ECOENV.2017.04.009
Lee CC, Jiang LY, Kuo YL et al (2013) The potential role of water quality parameters on occurrence of nonylphenol and bisphenol A and identification of their discharge sources in the river ecosystems. Chemosphere 91:904–911. https://doi.org/10.1016/J.CHEMOSPHERE.2013.02.006
Magar RS, Shaikh A (2013) Effect of malathion toxicity on detoxifying organ of fresh water fish Channa punctatus. Int J Pharm Chem Biol Sci 3:723–728
Martínez-Paz P, Morales M, Martínez-Guitarte JL, Morcillo G (2013) Genotoxic effects of environmental endocrine disruptors on the aquatic insect Chironomus riparius evaluated using the comet assay. Mutat Res Toxicol Environ Mutagen 758:41–47. https://doi.org/10.1016/J.MRGENTOX.2013.09.005
Mathieu-Denoncourt J, Wallace SJ, de Solla SR, Langlois VS (2016) Influence of lipophilicity on the toxicity of bisphenol A and phthalates to aquatic organisms. Bull Environ Contam Toxicol 97:4–10. https://doi.org/10.1007/S00128-016-1812-9
McCormick SD, Shrimpton JM, Carey JB et al (1998) Repeated acute stress reduces growth rate of Atlantic salmon parr and alters plasma levels of growth hormone, insulin-like growth factor I and cortisol. Aquaculture 168:221–235. https://doi.org/10.1016/S0044-8486(98)00351-2
Mccormick JM, Paiva MS, Häggblom MM et al (2010) Embryonic exposure to tetrabromobisphenol A and its metabolites, bisphenol A and tetrabromobisphenol A dimethyl ether disrupts normal zebrafish (Danio rerio) development and matrix metalloproteinase expression. Aquat Toxicol 100:255–262. https://doi.org/10.1016/J.AQUATOX.2010.07.019
McCracken KE, Tat T, Paz V, Yoon JY (2017) Smartphone-based fluorescence detection of bisphenol A from water samples. RSC Adv 7:9237–9243. https://doi.org/10.1039/c6ra27726h
Minaz M, Er A, Ak K et al (2022a) Investigation of long-term bisphenol A exposure on rainbow trout (Oncorhynchus mykiss): hematological parameters, biochemical indicator, antioxidant activity, and histopathological examination. Chemosphere 303:135136. https://doi.org/10.1016/J.CHEMOSPHERE.2022.135136
Minaz M, Er A, Ak K et al (2022b) Short-term exposure to bisphenol A (BPA) as a plastic precursor: hematological and behavioral effects on Oncorhynchus mykiss and Vimba vimba. Water, Air, Soil Pollut 233:1–12. https://doi.org/10.1007/S11270-022-05585-X
Minaz M, Er A, Ak K et al (2023) Bisphenol A used in plastic industry negatively affects wild vimba bream (Vimba vimba). Turkish J Fish Aquat Sci 23. https://doi.org/10.4194/TRJFAS22598
Minaz M, Er A, Ak K et al (2024) Acute toxicity and histopathological assessment of bisphenol A in Danube sturgeon (Acipenser gueldenstaedtii) larvae. Pol J Environ Stud 33:1–6. https://doi.org/10.15244/PJOES/171760
Mita L, Bianco M, Viggiano E et al (2011) Bisphenol A content in fish caught in two different sites of the Tyrrhenian Sea (Italy). Chemosphere 82:405–410. https://doi.org/10.1016/J.CHEMOSPHERE.2010.09.071
Naveira C, Rodrigues N, Santos FS et al (2021) Acute toxicity of bisphenol A (BPA) to tropical marine and estuarine species from different trophic groups. Environ Pollut 268:115911. https://doi.org/10.1016/J.ENVPOL.2020.115911
Onay H, Minaz M, Ak K et al (2023) Decade of microplastic alteration in the southeastern black sea: an example of seahorse gastrointestinal tracts. Environ Res 218:115001. https://doi.org/10.1016/J.ENVRES.2022.115001
Park SY, Choi J (2007) Cytotoxicity, genotoxicity and ecotoxicity assay using human cell and environmental species for the screening of the risk from pollutant exposure. Environ Int 33:817–822. https://doi.org/10.1016/J.ENVINT.2007.03.014
Quinn-Hosey KM, Roche JJ, Fogarty AM et al (2012) Screening for genotoxicity and oestrogenicity of endocrine disrupting chemicals in vitro. J Environ Prot (irvine, Calif) 3:902–914. https://doi.org/10.4236/JEP.2012.328105
Rautenberg GE, Amé MV, Monferrán MV et al (2015) A multi-level approach using Gambusia affinis as a bioindicator of environmental pollution in the middle-lower basin of Suquía River. Ecol Indic 48:706–720. https://doi.org/10.1016/J.ECOLIND.2014.09.025
Rebai I, Fernandes JO, Azzouz M et al (2021) Urinary bisphenol levels in plastic industry workers. Environ Res 202:111666. https://doi.org/10.1016/J.ENVRES.2021.111666
Rubin BS (2011) Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol 127:27–34. https://doi.org/10.1016/J.JSBMB.2011.05.002
Sadoul B, Birceanu O, Aluru N et al (2017) (2017) Bisphenol A in eggs causes development-specific liver molecular reprogramming in two generations of rainbow trout. Sci Rep 71(7):1–11. https://doi.org/10.1038/s41598-017-13301-7
Sancho E, Cerón JJ, Ferrando MD (2000) Cholinesterase activity and hematological parameters as biomarkers of sublethal molinate exposure in Anguilla anguilla. Ecotoxicol Environ Saf 46:81–86. https://doi.org/10.1006/EESA.1999.1888
Sayed A, Younes HM (2017) Melanomacrophage centers in Clarias gariepinus as an immunological biomarker for toxicity of silver nanoparticles. J Microsc Ultrastruct 5:97–104. https://doi.org/10.1016/J.JMAU.2016.07.003
Schreck CB, Tort L (2016) The concept of stress in fish. Fish Physiol 35:1–34. https://doi.org/10.1016/B978-0-12-802728-8.00001-1
Sharma S, Borade JS (2019) Clinical profile of PCOS patients in a rural tertiary care hospital. Int J Clin Obstet Gynaecol 3:01–05. https://doi.org/10.33545/GYNAE.2019.V3.I4A.281
Sharma P, Chadha P (2021) Bisphenol A induced toxicity in blood cells of freshwater fish Channa punctatus after acute exposure. Saudi J Biol Sci 28:4738–4750. https://doi.org/10.1016/J.SJBS.2021.04.088
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191. https://doi.org/10.1016/0014-4827(88)90265-0
Song H, Dong T, Hu M et al (2022) First single-step genomic prediction and genome-wide association for body weight in Russian sturgeon (Acipenser gueldenstaedtii). Aquaculture 561:738713. https://doi.org/10.1016/J.AQUACULTURE.2022.738713
Strat D, Gheorghe IF (2023) Conservation status and effectiveness of the national and international policies for the protection and conservation of sturgeons in the Danube River and Black Sea Basin. Divers 15:568. https://doi.org/10.3390/D15040568
Tiwari D, Kamble J, Chilgunde S et al (2012) Clastogenic and mutagenic effects of bisphenol A: an endocrine disruptor. Mutat Res Toxicol Environ Mutagen 743:83–90. https://doi.org/10.1016/J.MRGENTOX.2011.12.023
Vandenberg LN, Maffini MV, Sonnenschein C et al (2009) Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev 30:75–95. https://doi.org/10.1210/ER.2008-0021
Vandenberg LN, Chahoud I, Heindel JJ et al (2010) Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect 118:1055–1070. https://doi.org/10.1289/EHP.0901716
Ventura SPM, Gonçalves AMM, Gonçalves F, Coutinho JAP (2010) Assessing the toxicity on [C3mim][Tf2N] to aquatic organisms of different trophic levels. Aquat Toxicol 96:290–297. https://doi.org/10.1016/J.AQUATOX.2009.11.008
Wang JH, Wei QW, Zou YC (2011) Conservation strategies for the Chinese sturgeon, Acipenser sinensis: an overview on 30 years of practices and future needs. J Appl Ichthyol 27:176–180. https://doi.org/10.1111/J.1439-0426.2011.01716.X
Witeska M (2013) Erythrocytes in teleost fishes: a review. Zool Ecol 23:275–281. https://doi.org/10.1080/21658005.2013.846963
Acknowledgements
We thank the scientific and administrative staff at Recep Tayyip Erdogan University, Rize, Turkey Fisheries Research Center for their valuable assistance.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This work was supported by the Research Fund of the Recep Tayyip Erdogan University [grant number: FDK-2023–1480].
Author information
Authors and Affiliations
Contributions
Mert Minaz: visualization, methodology, writing—original draft, and data curation. İlker Zeki Kurtoğlu: resources and supervision.
Corresponding author
Ethics declarations
Ethics approval
The current study was checked and approved by the Ethical Local Committee of the Recep Tayyip Erdogan University.
Consent to participate
The authors have consent to participate.
Consent for publication
The authors have consent to publish.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Minaz, M., Kurtoğlu, İ.Z. Long-term exposure of endangered Danube sturgeon (Acipenser gueldenstaedtii) to bisphenol A (BPA): growth, behavioral, histological, genotoxic, and hematological evaluation. Environ Sci Pollut Res 31, 30836–30848 (2024). https://doi.org/10.1007/s11356-024-33168-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-33168-2