Abstract
Recently, one of the main purposes of wastewater treatment plants is to achieve a neutral or positive energy balance while meeting the discharge criteria. Aerobic granular sludge (AGS) technology is a promising technology that has low energy and footprint requirements as well as high treatment performance. The effect of co-treatment of municipal wastewater and food waste (FW) on the treatment performance, granule morphology, and settling behavior of the granules was investigated in the study. A biochemical methane potential (BMP) test was also performed to assess the methane potential of mono- and co-digestion of the excess sludge from the AGS process. The addition of FW into wastewater enhanced the nutrient treatment efficiency in the AGS process. BMP of the excess sludge from the AGS process fed with the mixture of wastewater and FW (195 ± 17 mL CH4/g VS) was slightly higher than BMP of excess sludge from the AGS process fed with solely wastewater (173 ± 16 mL CH4/g VS). The highest methane yield was observed for co-digestion of excess sludge from the AGS process and FW, which was 312 ± 8 mL CH4/g VS. Integration of FW as a co-substrate in the AGS process would potentially enhance energy recovery and the quality of effluent in municipal wastewater treatment.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With increasing urbanization and industrialization, the assimilation capacity of the environment has been decreasing. Therefore, the treatment of wastewater has become a critical task for public and environmental sanitation. Biological treatment is the most frequent method in wastewater treatment to remove pollutants discharged into the aquatic environment. Biological nutrient removal (BNR) systems are widely used for nitrogen and phosphorus removal from wastewater by appropriate microorganisms under different operational conditions. Among conventional BNR processes, the A2O process has a wide range of uses in wastewater treatment. The A2O process has anaerobic-anoxic-aerobic compartments operating at various redox potentials and a settling tank for the separation of liquid–solid. The main drawbacks of the A2O process are the high footprint and energy requirement (Nancharaiah et al. 2019). Thus, the need for novel treatment technologies is increasing to eliminate these bottlenecks for efficient wastewater treatment. Research on biological wastewater treatment is currently directed toward resource recovery (Franca et al. 2018; Guven et al. 2023). Aerobic granular sludge (AGS) technology, an innovative technology that permits simultaneous carbon and nutrient removal, has been increasingly used for municipal wastewater treatment (de Bruin et al. 2004; de Kreuk et al. 2007; Winkler et al. 2015; Kosar et al. 2022a; Cicekalan et al. 2023). Compared to conventional activated sludge processes using flocculent sludge, the AGS process provides better settling ability and higher biomass retention having the ability to withstand high-strength wastewater and shock load (Zheng et al. 2005).
Aerobic granulation has been fundamentally acquired in sequencing batch reactors (SBR) and is strongly associated with operation conditions, which should be favorable for microorganisms to form aggregates and/or granular particles (Liu and Tay 2004). SBRs are operated in a cyclic mode that includes a series of phases such as anaerobic feeding, aeration, settling, idling, and discharge. Microorganisms are exposed to high substrate concentration in the feeding phase by means of discontinuous operation in SBRs, and hence, it allows the substrate to penetrate the inner layers of the granules. It was indicated that substrate cannot permeate into deeper layers in reactors that are continuously fed (Beun et al. 2002). To ensure nitrogen removal, the concentration of dissolved oxygen (DO) in the aeration phase is controlled so that the inner layers of granules are not sustained to a high level of DO. Such an air feeding regime promotes the growth of nitrifiers and denitrifiers in the outer and inner layers of granules, respectively (de Kreuk et al. 2005). Polyphosphate-accumulating organisms (PAOs) in the core of granules have become prominent in the AGS reactor fed under anaerobic conditions. Hence, the AGS process can provide enhanced biological phosphorus removal (EBPR) (Coma et al. 2012).
The most preferred approach for enhancing the energy self-sufficiency of wastewater treatment plants (WWTPs) is the anaerobic digestion of excess sludge (Ersahin 2018). There are several applications in order to enhance methane production from excess sludge (Silvestre et al. 2015; Guven et al. 2019; Abdelrahman et al. 2021). For example, anaerobic co-digestion can be described as an instantaneous digestion of two or more substrates. Anaerobic co-digestion provides benefits such as enhanced nutrient balance, the growth of microbial synergy, increasing the load of biodegradable organic matter, and improvement of methane production with high digestion rates (de Castro et al. 2020). The organic fraction of municipal solid waste (OFMSW) that involves a high amount of biodegradable matter is one of the most frequently applied co-substrate for co-digestion applications (Bolzonella et al. 2006). Food waste (FW) is an easily biodegradable substrate for anaerobic digestion with its high content of carbohydrates, lipids, protein, and moderate moisture. Co-digestion of FW and sewage sludge is an attractive approach for increasing methane yield (Mehariya et al. 2018). FW from households may be diverted to WWTPs via typical solid waste collection vehicles. Kitchen grinders, installed in households, can be considered an option for conveying organic solid waste into WWTPs (Mattsson et al. 2014). Hence, co-treatment of wastewater and FW was proposed as an alternative waste management concept to lessen the negative impacts of waste transportation on the environment as well as providing benefits at WWTPs through anaerobic digestion (Guven et al. 2019).
Several studies have been focused on the impact of co-treatment of municipal wastewater and FW on treatment performance and energy recovery in the last decades (Sankai et al. 1997; Bolzonella et al. 2003; Monino et al. 2016, 2017; Barrios-Hernández et al. 2020). A pilot-scale anaerobic membrane bioreactor (AnMBR) was operated for the co-treatment of municipal wastewater and FW (Monino et al. 2017). They found that increasing the influent COD caused a considerable enhancement in methane production. The effects of the co-treatment of synthetic fecal sludge and wastewater in an AGS system were investigated by Barrios-Hernández et al. (2020). It was indicated that the addition of fecal sludge in the AGS reactor decreased the settleability of granular sludge. OFMSW and FW are potential co-substrates for anaerobic digestion. Cabbai et al. (2013) found an improvement of 16% and 48% in methane yield when co-digesting sewage sludge with OFMSW and FW, respectively. In the study of Iqbal et al. (2022), the effect of FW integration with biological wastewater treatment was investigated by the life cycle assessment analysis method in terms of wastewater treatment performance and energy balance. Two scenarios were studied in that study: co-treatment of FW with wastewater + anaerobic digestion and anaerobic co-digestion of FW with sewage sludge. The FW addition enhanced the total methane production by ~ 73% and ~ 130% in the co-treatment of FW with wastewater + anaerobic digestion scenario and co-digestion of FW with sewage sludge scenario, respectively. The addition of FW did not have a negative impact on the operation of WWTPs in terms of treatment capacity and meeting the discharge criteria (Iqbal et al. 2022).
There are several studies in which FW was used as a co-substrate (Cabbai et al. 2013; Guven et al. 2019; Montecchio et al. 2019; Zan and Hao 2020). Although there are few studies available on the effect of co-treatment with several substrates in the AGS process in the literature, there is no study conducted on the investigation of FW as a co-substrate in the AGS process. The purpose of this study was to comprehensively explore the viability of the co-management of FW with municipal wastewater and excess sludge from the AGS process. The impact of co-treatment of municipal wastewater with FW on the morphology of granules and treatment performance of the AGS process was investigated. Besides, the biochemical methane potential (BMP) test was performed to determine the methane yields of the digestion of the excess sludge from the AGS system and co-digestion of the excess sludge from the AGS system with FW. The mass balance based on COD was performed to investigate the effect of the FW addition on methane production.
Material and methods
Experimental procedure
To investigate the effect of co-treatment of wastewater and FW, two stages were used in this study. At stage 1, raw wastewater from a full-scale municipal WWTP was fed to a laboratory-scale AGS reactor, whereas a mixture of wastewater and FW was fed to the AGS reactor at stage 2. Afterward, to have a better understanding of the digestibility of the excess sludge from the AGS process, BMP tests were conducted with three different scenarios (Fig. 1):
-
Scenario 1: The excess sludge (taken at stage 1) from the AGS process fed with solely wastewater was used for the BMP test.
-
Scenario 2: The excess sludge (taken at stage 2) from the AGS process fed with the mixture of wastewater and FW was used for the BMP test.
-
Scenario 3: Mixture of the excess sludge from the AGS process (taken at stage 1) and FW was used for the BMP test.
Seed sludge characteristics
The laboratory-scale AGS system was seeded with the inoculum obtained from another laboratory-scale AGS system. The inoculum characteristics was indicated in Table 1. The ratio between volatile suspended solids (VSS) and total suspended solids (TSS) was 0.73 ± 0.09 with an average TSS concentration of 7200 ± 680 mg/L in the inoculum. The median particle size (d50) of the inoculum, and average granule diameter were 74.4 ± 0.6 µm, and 0.68 ± 0.25 mm, respectively.
Substrate characteristics
The laboratory-scale AGS system was operated under two stages in the study. At stage 1, the AGS reactor was fed with raw wastewater, whereas the AGS reactor was fed with the mixture of wastewater and FW at stage 2. A commercial kitchen FW grinder (InSinkErator Model 45, UK) was used to obtain pulped FW. Pulped FW, composed of vegetable and fruit leftovers as well as bakery products on a roughly equal weight basis, was introduced to the AGS reactor. The FW was collected from a nearby cafeteria, grocery store, and bakery. It should be noted that the pulped FW did not include any fat oil grease. The wastewater and FW were sieved through a 2-mm mesh screen for removal of the coarse particles, and they were kept at 4 °C till use. Characterizations of the substrates are given in Table 2. At each stage, the AGS reactor was operated at the similar organic loading rate (OLR), 0.97 ± 0.03 kg COD/m3∙day and 0.96 ± 0.02 kg COD/m3∙day at stage 1 and stage 2, respectively. At stage 2, the relative contribution (as a percentage) of the wastewater and FW to OLR was 60% and 40%, respectively. The addition of FW led to an increase in total suspended solids (TSS) and organic matter in the influent. Calcium and magnesium concentrations at stage 1 were 116 ± 11 mg/L and 126 ± 36 mg/L, respectively, while they were 130 ± 8 mg/L and 140 ± 24 mg/L at Stage 2, respectively.
Laboratory-scale AGS system
The laboratory-scale AGS system consisted of a plexiglass reactor with a diameter of 4.5 cm and a water depth of 70 cm. In order to feed wastewater into the reactor from the bottom, a peristaltic feed pump (Watson Marlow SCIQ 323, Belgium) was used (Fig. 2). The discharge was accomplished using a vacuum pump (LongerPump YZ1515x, China). The air was supplied with a fine bubble air diffuser (Atman HP-8000, China) with its header at the bottom of the reactor. The AGS system was kept in a temperature-controlled room between 20 and 25 °C.
Operational conditions
The AGS reactor was operated with a cycle time of 4 h, consisting of 3 min of feeding, 30 min of settling, 120 min of aeration, 65 min of anaerobic phase, 2 min of idle phase, and 20 min of decanting. The height-to-diameter ratio of the AGS reactor was 16. The AGS system reached steady-state conditions on the 52nd day and then, stable biomass concentration in the reactor and treatment performance were achieved. Afterward, the system was operated for 82 days at stage 1. At stage 2, the FW was mixed with wastewater before feeding to the AGS system. At stage 2, the AGS system was operated for 82 days. COD/TN ratio was monitored during each stage and obtained as 6.98 ± 0.22 at stage 1 and 9.65 ± 0.57 at stage 2. Nitrogen loading rates at stage 1 and stage 2 were 0.14 ± 0.003 kg N/m3 day and 0.10 ± 0.002 kg N/m3 day, respectively. At stage 1, the phosphorus loading rate was 0.014 ± 0.0004 kg P/m3 day, while it was 0.010 ± 0.0003 kg P/m3.day at stage 2.
Experimental analysis
Analysis techniques
TSS, VSS, COD, TN, NH4-N, and TP parameters were measured based on Standard Methods (APHA 2017). The samples were filtered through 0.45-μm syringe filters before the soluble COD analysis. Concentration of particulate COD (pCOD) was calculated by subtracting soluble COD (sCOD) from total COD concentration. A pH meter (XS pH 50 + DHS, Carpi MO, Italy) was used to determine the pH value. Turbidity was measured by using a turbidimeter (Hach 2100P, USA) having a detection range of 0–1000 NTU. CST analysis was performed using a CST analyzer with an 18 mm funnel (Triton Electronics, Type 304 M, UK). To reduce the impact of suspended solids concentration on CST, the normalized CST value was computed by dividing CST by the TSS concentration in the AGS reactor (Khan et al. 2008; Ersahin et al. 2014). Particle size distribution (PSD) of the AGS was determined with a Mastersizer 2000 (Malvern Instruments, Hydro 2000 MU, UK) with a detection range of 0.6–6000 μm. SVI30 measurement was performed based on the Standard Methods (APHA 2017). The values of SVI5 and SVI0 were measured by reading the height of the settled sludge after 5 and 10 min, respectively (van Loosdrecht et al. 2016). Ion chromatography was used for conducting the measurement of nitrate, nitrite, sulfate, potassium, magnesium, and calcium concentrations (Dionex ICS-300, USA).
The high-temperature sodium carbonate (Na2CO3) extraction method was used to extract extracellular polymeric substances (EPS) (Felz et al. 2016). Protein (PN) and carbohydrate (PS) fractions of the structural EPS were determined following the methods of Dubois et al. (1956) and the modified Lowry method (Frolund et al. 1995), respectively. The method described by Ghangrekar et al. (1996) was used to determine the integrity coefficient (IC) of the granules. Specific granule density was determined by a pycnometer (van Loosdrecht et al. 2016). Granular sludge was sieved through three different mesh-sized sieves (from top to bottom: 2 mm, 1 mm, and 0.5 mm) (van Loosdrecht et al. 2016). Based on their diameters, granules were categorized into three size classes including 0.5–1 mm, 1–2 mm, and > 2 mm. Granules were randomly chosen from each class and analyzed using light microscopy in order to determine the real diameter (Leica S8APO, Leica Microsystems, Germany).
Morphological analyses
The morphology of granules was visualized using an environmental scanning electron microscopy (ESEM) (Thermo Fisher Scientific, FEI Quanta FEG 250, USA). ESEM was combined with an energy-dispersive X-ray (EDX) spectroscopy to identify the major elements on the surface of the granules. Organic materials on the surface of the granules were detected by using Fourier transform infrared spectroscopy (FTIR) (Perkin-Elmer, Spectrum 100, USA).
BMP test
BMP tests were carried out in triplicate with the Automatic Methane Potential Test System II (AMPTS II) (Bioprocess Control, Sweden). The inoculum was taken from a full-scale anaerobic digester treating municipal wastewater. The ratio of inoculum to the substrate was chosen to be 2:1 on a VS basis. The working volume of the mixture of inoculum and substrates was 400 mL. All bottles were placed in a water bath with a temperature set to 37 °C. Each bottle was sparged with nitrogen gas for 3 min to remove oxygen from the headspace. Phosphate buffer solution, macronutrients, and trace elements were made by following the steps in the study of Zhang et al. (2014). Cellulose microcrystalline (Sigma Aldrich, USA) was used as a model substrate for the positive control (PC) of the BMP tests. The digestion process was simulated using the modified Gompertz model (Filer et al. 2019), as shown in the following equation.
where B(t) is the simulated cumulative methane yield (mL CH4/g VS), B0 is the simulated highest cumulative methane yield (mL CH4/g VS), Rm is the maximum methane production rate (mL CH4/g VS d), λ is the lag phase (d), and t is the digestion time (d).
Mass balance
Mass balance was constructed for each configuration given in Fig. 1 to understand the fate of COD. The mass balance was conducted based on the following equation.
where \({{\text{COD}}}_{{\text{influent}}}\) is the influent COD load (g/day), \({{\text{COD}}}_{{\text{effluent}}}\) is the effluent COD load (g/day), \({{\text{COD}}}_{{\text{sludge}}}\) is the COD load in the sludge (g/day), and \({{\text{COD}}}_{{\text{min}}}\) is mineralized COD during oxidation (g/day).
For anaerobic digestion, the COD load in the sludge (\({{\text{COD}}}_{{\text{sludge}}}\)) (g/day) was considered as the sum of COD which is transformed into methane gas (\({{\text{COD}}}_{{\text{methane}}}\)) (g/day) and the COD load that remained in the digestate (\({{\text{COD}}}_{{\text{digestate}}}\)) (g/day). \({{\text{COD}}}_{{\text{methane}}}\) was determined according to the following equation:
where \({Q}_{{\text{methane}}}\) (L/g VS) is the amount of methane produced per g VS of the sludge, 0.35 is the theoretical methane generation per g COD at a standard temperature of 273 K and an atmospheric pressure of 1 atm (L methane/g COD), and \({({\text{VS}}/{\text{COD}})}_{{\text{sludge}}}\) is the ratio of VS to COD in the sludge.
Results and discussions
Treatment performance
Treatment performances achieved at each stage are given in Fig. 3. Addition of FW to wastewater increased the pollutant concentrations in the influent of the AGS system. FW addition increased the average COD concentration in influent from 361 to 556 mg/L. The increase in pCOD concentration would be more than the increase in sCOD concentration after FW addition due to the higher pCOD/COD ratio in pulped FW (Guven et al. 2019). pCOD and TSS concentrations in the influent increased by 61% and 42% at stage 2, respectively. The increase in pCOD concentration was significantly influenced by the amount and composition of FW (Table 2). Marashlian and El-Fadel (2005) and Monino et al. (2017) found that the TSS concentration of the FW ranges between 1537 and 39,480 mg/L, which was 19,570 ± 625 mg/L in this study (Table 2). COD removal efficiencies were over 80% at each stage under steady-state conditions (Fig. 3a). The removal efficiency of COD was 85 ± 1% at stage 2, despite the contribution of FW in the organic load. A long anaerobic phase is necessary in the AGS process especially for the treatment of wastewaters including particulate matter in order to ensure enough time for the hydrolysis of particulates to soluble forms, which is available for anaerobic microorganisms inside the granules (Wagner et al. 2015). By minimizing the attached particulate matter on the granule surface during the aerobic phase, it is possible to prevent the growth of ordinary heterotrophic microorganisms promoting degranulation and growth of filamentous. The anaerobic period (65 min) in this study was enough to hydrolyze particulate matter at stage 2.
Average TN removal efficiency was obtained to be 63 ± 2% and 75 ± 2% at stage 1 and stage 2, respectively (Fig. 3b). It was enough slowly growing nitrifiers for completing nitrification at each stage because the NH4-N removal was nearly complete (> 99%). Ni et al. (2009) reported that the AGS process treating domestic wastewater had ~ 95% of NH4-N removal efficiency, which is consistent with the results obtained in this study. Nitrite concentrations were less than 0.05 mg NO2-N/L at each stage in the influent and effluent. Nitrite concentration in the effluent of an AGS process fed with synthetic municipal wastewater was found less than 0.06 mg NO2-N/L (Kosar et al. 2022b). Nitrate concentration in the effluent was 15.4 ± 1.1 mg NO3-N/L at Stage 1, whereas it was 9.9 ± 0.8 mg NO3-N/L at Stage 2. The reason for the lower removal efficiency of TN at stage 1 was associated with inadequate denitrification, which might be confirmed by higher nitrate concentrations in the effluent at stage 1. It was observed that the majority of the TN corresponded to NO2-N and NO3-N, indicating incomplete denitrification (Barrios-Hernández et al. 2020). Pulped FW was not rich in nitrogen and phosphorus. Thus, FW addition to wastewater had little impact on TN and TP concentrations in the influent. Influent TN and TP concentrations at stage 2 were similar with those at stage 1; therefore, the COD/TN ratio of the influent at stage 2 increased from 6.98 ± 0.22 to 9.65 ± 0.57. An increase in the COD/TN ratio provided a better denitrification efficiency and lower NO3-N concentration in effluent at stage 2 compared to stage 1. The higher TP and TN removal efficiencies obtained at stage 2 might be due to sufficient carbon availability for PAOs and anoxic denitrifiers. The increase in the COD/TN ratio led to an enhancement in the removal efficiency of TN from 63 ± 2% to 75 ± 2% at stage 2. Kim et al. (2021) reported that TN removal efficiency increased from 57.5 to 79.1% with an increase in the COD/TN ratio from 5 to 20. TP concentrations were 2.07 ± 0.11 mg/L and 1.65 ± 0.09 mg/L in the effluent and TP removal efficiencies were 60 ± 2% and 71 ± 2% at stage 1 and stage 2, respectively (Fig. 3c). Effluent TP concentrations were reported in the range of 0.9–3.2 mg/L in full-scale AGS processes treating domestic wastewater (Giesen and Thompson 2013; Pronk et al. 2017).
After steady-state conditions, the average TSS concentration in the effluent was 17 ± 2 mg/L at Stage 1, while it was 33 ± 4 mg/L at stage 2. The addition of FW to wastewater might be the reason behind the higher effluent TSS concentration obtained at stage 2. Similar average removal efficiencies of TSS were achieved at each stage (92.9 ± 1.1% and 90.4 ± 1.2% at stage 1 and stage 2, respectively). The average turbidity in the influent was 209 ± 9 NTU, while it was 3.5 ± 0.1 NTU in the effluent at Stage 1. At stage 2, the turbidity of the influent was 236 ± 9 NTU, and it reduced to 5.4 ± 0.3 NTU in the effluent. Based on particle size distribution, d50 in the influent was 2011 ± 206 nm at Stage 1, while it was 2851 ± 268 nm at stage 2. d50 in the effluent decreased to 414 ± 35 nm and 581 ± 27 nm at the end of the operation at stage 1 and stage 2, respectively (Fig. 4).
Granule formation and structure
Physical and chemical characteristics of granules
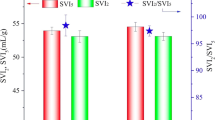
Average TSS concentrations in the AGS process were similar during feeding with only raw wastewater, and the mixture of the raw wastewater and FW (8077 ± 159 mg/L at stage 1 and 7916 ± 171 mg/L at stage 2) (Fig. 5). VSS/TSS ratio was 63 ± 3% at stage 1 and 61 ± 2% at stage 2. The SVI parameter, which is measured after 5 min, 10 min, and 30 min of the settling (SVI5, SVI10, and SVI30, in order), is an indicator of sludge settleability. The ratio of SVI30/SVI5 and SVI30/ SVI10 was close to 1 in this study, indicating a fast settling of well-granulated sludge property (Ekholm et al. 2022). Excess sludge from the AGS process exhibited excellent settleability with SVI30 value of 54 ± 3 mL/g at stage 1, and 48 ± 3 mL/g at stage 2. At each stage, SVI30 values for the AGS process consistent were with the literature (30–67 mL/g) (Bengtsson et al. 2018). The ratio of SVI30/SVI5 was calculated to be 0.81 ± 0.04 and 0.87 ± 0.06 at stage 1 and stage 2, respectively (Table 3). Normalized CST was 1.03 ± 0.04 s/g TSS/L at stage 1 and 0.96 ± 0.06 s/g TSS/L for stage 2. Following the normalized CST, it could be inferred that the dewaterability and filterability characteristics of the excess sludge from the AGS process at stage 2 were better than those at stage 1. Higher d50 values of the excess sludge were obtained at stage 2 (92 ± 11 μm) compared to stage 1 (80 ± 7 μm) (Table 3; Fig. S1). A key factor for the denotation of nitrogen removal efficiency is the size of an aerobic granule (Bathe et al. 2005). Small granules allow oxygen to penetrate inner layers, while big granules can include anoxic/anaerobic zones to complete denitrification (Nguyen Quoc et al. 2021). An increase in the ratio of COD/TN in the influent from 6.98 (stage 1) to 9.65 (stage 2) led to an increase in both granule size and capacity of denitrification. A similar finding was found by Kosar et al. (2023) that the ratio of nitrifiers to heterotrophs could rise and, denitrifiers could be dominated by typical aerobic heterotrophs due to the lack of available carbon in the influent and hence, the growth of granules might be hampered by a lack of adequate anoxic/anaerobic volume in the granule core. Sufficient carbon can be provided for denitrifiers by increasing the COD/TN ratio (Kosar et al. 2023).
Aerobic granules typically range in diameter from 0.2 to 3 mm. (Bengtsson et al. 2018). Zhou et al. (2016) found that the optimum size of the granular range is 0.7–1.9 mm to enhance nitrogen removal efficiency. At stage 1, a mean granule diameter of 1.42 mm was obtained. The granules (91%) were in a range of 0.5–1.0 mm. However, the granule size had more variation at stage 2. Mean granule diameter of 1.54 mm was obtained at stage 2, and only 74% of granules were in a range of 0.5–1 mm. The granules (22%) were in a range of 1–2 mm, and the remaining granules had diameter > 2 mm at stage 2 (Fig. 6a). Better TN removal efficiency achieved at stage 2, compared to stage 1, might be associated with that more granules were in the range of 1–2 mm at stage 2. The physical characteristics of the aerobic granules are given in Fig. 6b. Granule densities ranged between 1043 and 1069 kg/m3 at both stages in this study. This finding was in agreement with the results of van den Berg et al. (2022) in which granule densities were in the range of 1005–1070 kg/m3. Settling velocities of 19.1–27.4 m/h, 34.3–37.5 m/h, and 48.7–49.2 m/h were found for granules in the size range of 0.5–1 mm, 1–2 mm, and > 2 mm, respectively. Feng et al. (2021) found that settling velocities ranged between 25 and 70 m/h. In this study, the IC of the aerobic granules at stage 1 and stage 2 was found 3.53 ± 0.39% and 2.41 ± 0.23%, respectively. Granules having low IC values represent higher strength compared to those that have higher IC values (Ghangrekar et al. 1996).
Morphology of granules
The surface of the granules was imaged by ESEM/EDX at the end of each stage (Fig. 7; Fig. S2). Surface of the granules was remarkably different at stage 2 compared to stage 1. The structure of granules obtained at stage 2 was smoother than those at stage 1. EDX analysis was utilized to describe the elemental composition of the granules. Based on the EDX results, elements such as O, C, and N were the main components in the granules at each stage, forming more than 90% of the elements on the granule surface. A relatively high percentage of C (stage 1: 53.2%; stage 2: 48.0%) and N (stage 1: 8.2%; stage 2: 7.2%) could be associated with the high organic content of the influent. The availability of minerals promotes the aggregation of microorganisms, which contributes to the aerobic granulation process. Divalent and trivalent metal ions act as a bridging mechanism between negatively charged ions on the cell surface which positively impact the aggregation of the granules (Li et al. 2014). Mg and Ca have a considerable impact on augmenting the granulation process (Jiang et al. 2003; Ren et al. 2008). Moreover, Al and Fe are essential in developing more compact and settleable granules (Li et al. 2014). Othman et al. 2013 showed that aerobic granule structure was positively affected by Si, which also supported the strength of the matured granules.
Similar peaks on the FTIR spectra were observed at each stage, which suggested that the surface of aerobic granules had similar functional groups (Fig. 8). The peaks at 3276 cm−1 in the spectrum showed stretching of the O–H stretching of the amide groups, which indicated the presence polysaccharide in the samples (Liu et al. 2015). The peak at 2924 cm−1 was associated with aliphatic C-H stretching (Isik et al. 2019). The structure of the protein was illustrated by the presence of amide groups. Amides I (stretching of C = O and C–N bonds) corresponded to the peak at 1634 cm−1. Amides II (deformation of N–H and C = N bonds) corresponded to peaks at 1538 cm−1 (Isik et al. 2020). The peaks at 1416 cm−1 represented Amides III (C–N stretching) (Abdelrahman et al. 2022). The peak 1027 cm−1 indicated the presence of C–OH stretching, representing polysaccharides (Allen et al. 2004; Low et al. 2021). These results of FTIR analysis illustrated the presence of polysaccharides-like and protein-like substances in the aerobic granules, which was expected since EPS likely accumulates on the surface of the aerobic granules.
EPS, which are gel-like materials secreted either by cells or formed as a result of cell lysis, plays a key function in the growth of microorganisms. Thus, the interaction between EPS and microbial cells has an important impact on the formation of excess sludge from the AGS process (Flemming et al. 2007; Chen et al. 2010; Ni 2013). The results of FTIR highlighted EPS presence at each stage. PN and PS contents are indicated in Fig. 9. PN increased from 51.7 ± 0.8 mg/g VSS to 62.6 ± 1.9 mg/g VSS, while PS slightly increased from 14.7 ± 0.5 mg/g VSS to 16.4 ± 0.3 mg/g VSS at stage 2. Accordingly, the PN/PS ratio was 3.5 ± 0.1 and 3.8 ± 0.2 at stage 1 and stage 2, respectively. The increased EPS amount might be mainly made up of the increase in the production of PN at stage 2. A higher PN/PS ratio provides better granulation (Zhang et al. 2017). Relatively stable PS content at each stage indicated that FW addition did not substantially alter the PS content in the aerobic granule. This finding is consistent with the study of Liang et al. (2021), in which FW addition contributed to the PN content rather than PS content. Consequently, FW addition to an AGS system might increase the protein-based EPS content.
Biochemical methane potential
Digestibility of the excess sludge from the AGS process was evaluated via the BMP test (Fig. 10). The BMP of the PC was 368 ± 13 mL CH4/g VS, on average. It was reported by Holliger et al. (2021) that the BMP of PC should be in the range of 340 and 395 mL CH4/g VS, which was fulfilled in this study. The highest BMP value was obtained with the mixture of the excess sludge from the AGS process and FW, which was 312 ± 8 mL CH4/g VS. Co-digestion of FW provides higher methane yield and accelerates methane production rate because of a synergistic effect that balances in nutrients and improves biodegradation (Prabhu and Mutnuri 2016). Co-digestion of FW with sewage sludge could enhance methane production by 35–48% (Fitamo et al. 2016). A higher BMP value was obtained from the co-digestion of FW with the excess sludge from the AGS process than the other scenarios. This result could be linked with the high COD/TN ratio in FW, resulting in an enhancement of nutrient balance. BMP of the excess sludge from the AGS process fed with the mixture of wastewater and FW (195 ± 17 mL CH4/g VS) was slightly higher than BMP of excess sludge from the AGS process fed with solely wastewater (173 ± 16 mL CH4/g VS) (Fig. 10a). Guo et al. (2020a) found that the BMP value for the excess sludge from an AGS process was 232 ± 11 CH4/g VS. Higher BMP value observed in the study of Guo et al. (2020a) compared to our study might be related with the higher COD concentration in the excess sludge (71.3 ± 0.4 g/L), which was around 8 g/L in our study (Table 2).
The modified Gompertz model was used to determine methane production since different methane production rates and lag phases were observed. The methane production curves were well-fitted with the modified Gompertz model (R2 > 0.98 for all curves) (Fig. 10a). The excess sludge from the AGS process fed with solely wastewater had the lowest Rm (4.2 ± 0.5 mL CH4/g VS day) and the highest λ (4.0 ± 0.1 day). The kinetics of the excess sludge from the AGS process fed with the mixture of wastewater and FW had Rm and λ of 5.4 ± 0.3 mL CH4/g VS day and 4.3 ± 0.1 days, respectively. The shortest lag phase was observed during the mixture of the excess sludge from the AGS process and FW digestion (2.9 ± 0.1 day) (Fig. 10b).
Mass balance
A mass balance for the COD parameter was set up to have a better understanding of the fate of the carbonaceous organic matter (Fig. 11). AGS system fed with solely wastewater had a COD removal efficiency (83%), whereas the COD removal efficiency of the AGS system fed with the mixture of wastewater and FW was 85%. AGS system fed with solely wastewater redirected less COD to sludge for anaerobic digestion since 60.8% of COD was used during the metabolic activities which were converted to CO2. Similarly, it was 62.7% for the AGS system fed with the mixture of wastewater and FW. COD (44–61%) is transformed into CO2 for the AGS process in the literature (Jahn et al. 2019; Guo et al. 2020b). Based on the COD mass balance, only 7.6% of COD could be converted into methane gas if the excess sludge from the AGS system fed with solely wastewater was digested. The excess sludge from the AGS system fed with the mixture of wastewater and FW could recover 9.1% COD from wastewater, for subsequent conversion into methane gas. Moreover, 23.5% of COD could be converted into methane gas if the excess sludge from the AGS process and FW are co-digested.
Conclusions
In this study, the impact of co-treatment of wastewater and FW on the treatment performance, and granule morphology was assessed, and a BMP assay was conducted to determine the methane potential of mono- and co-digestion of the excess sludge from the AGS process. Better nutrient removal efficiencies and granule strength were achieved in the AGS process co-treating of the wastewater and FW (stage 2) compared to the AGS process treating solely wastewater (stage 1). The highest methane yield was obtained for excess sludge from the AGS system and FW mixture with the value of 312 ± 8 mL CH4/g VS. Both the addition of FW to wastewater and the mixture of the excess sludge from the AGS process and FW could enhance energy content by adding more biodegradable matter, which can increase energy recovery potential in anaerobic digestion. Hence, co-digestion of FW with the excess sludge from the AGS process can be a beneficial approach for maximizing energy recovery for the treatment of municipal wastewater.
Data availability
No supplementary information.
References
Abdelrahman AM, Ozgun H, Dereli RK, Isik O, Ozcan OY, van Lier JB, Ozturk I, Ersahin ME (2021) Anaerobic membrane bioreactors for sludge digestion: current status and future perspectives. Crit Rev Environ Sci Technol 51(18):2119–2157
Abdelrahman AM, Aras MF, Cicekalan B, Fakioglu M, Cingoz S, Basa S, Guven H, Ozgun H, Ozturk I, Koyuncu I, van Lier JV, Volcke EIP, Ersahin ME (2022) Primary and A-sludge treatment by anaerobic membrane bioreactors in view of energy-positive wastewater treatment plants. Bioresour Technol 351:126965
Allen MS, Welch KT, Prebyl BS, Baker DC, Meyers AJ, Sayler GS (2004) Analysis and glycosyl composition of the exopolysaccharide isolated from the floc-forming wastewater bacterium Thauera sp. MZ1T. Environ Microbiol 6(8):780–790
APHA (2017) Standard methods for examination of water and wastewater, 23rd edn. American Public Health Association, Washington
Barrios-Hernández ML, Buenaño-Vargas C, García H, Brdjanovic D, van Loosdrecht MC, Hooijmans CM (2020) Effect of the co-treatment of synthetic faecal sludge and wastewater in an aerobic granular sludge system. Sci Total Environ 741:140480
Bathe S, de Kreuk, MK, McSwain BS, Schwarzenbeck N (2005) Aerobic granular sludge. In: Water and environmental management series, IWA Publishing, London
Bengtsson S, de Blois M, Wilen BM, Gustavsson D (2018) Treatment of municipal wastewater with aerobic granular sludge. Crit Rev Environ Sci Technol 48(2):119–166
Beun J, van Loosdrecht MCM, Heijnen JJ (2002) Aerobic granulation in a sequencing batch airlift reactor. Water Res 36:702–712
Bolzonella D, Pavan P, Battistoni P, Cecchi F (2003) The under-sink garbage grinder: a friendly technology for the environment. Environ Technol 24(3):349–359
Bolzonella D, Battistoni P, Susini C, Cecchi F (2006) Anaerobic co-digestion of waste activated sludge and OFMSW: the experiences of Viareggio and Treviso plants (Italy). Water Sci Technol 53:203–211
Cabbai V, Ballico M, Aneggi E, Goi D (2013) BMP tests of source selected OFMSW to evaluate anaerobic codigestion with sewage sludge. Waste Manage 33:1626–1632
Chen H, Zhou S, Li T (2010) Impact of extracellular polymeric substances on the settlement ability of aerobic granular sludge. Environ Technol 31(14):1601–1612
Cicekalan B, Kosar S, Cingoz S, Eyit N, Ersahin ME, Ozgun H (2023) Techno-economic and environmental assessment of different municipal wastewater treatment systems. J Water Process Eng 53:103822
Coma M, Verawaty M, Pijuan M, Yuan Z, Bond PL (2012) Enhancing aerobic granulation for biological nutrient removal from domestic wastewater. Biores Technol 103(1):101–108
de Bruin LMM, de Kreuk MK, Van Der Roest HFR, Uijterlinde C, van Loosdrecht MCM (2004) Aerobic granular sludge technology: an alternative to activated sludge? Water Sci Technol 49(11–12):1–7
de Castro TM, Torres DGB, Arantes EJ, de Carvalho KQ, Passig FH, Christ D, Gotardo JT, Gomes SD (2020) Anaerobic co-digestion of industrial landfill leachate and glycerin: methanogenic potential, organic matter removal and process optimization. Environ Technol (united Kingdom) 41(20):2583–2593
de Kreuk MK, Heijnen JJ, van Loosdrecht MCM (2005) Simultaneous COD, nitrogen, and phosphate removal by aerobic granular sludge. Biotechnol Bioeng 90:761–769
de Kreuk MK, Kishida N, van Loosdrecht MCM (2007) Aerobic granular sludge-state of the art. Water Sci Technol 55(8–9):75–81
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Ekholm J, Persson F, de Blois M, Modin O, Pronk M, van Loosdrecht MCM, Suarez C, Gustavsson DJI, Wilén BM (2022) Full-scale aerobic granular sludge for municipal wastewater treatment-granule formation, microbial succession, and process performance. Environ Sci: Water Res Technol 8(12):3138–3154
Ersahin ME (2018) Modeling the dynamic performance of full-scale anaerobic primary sludge digester using Anaerobic Digestion Model No.1 (ADM1). Bioprocess Biosyst Eng 41:1539–1545
Ersahin ME, Ozgun H, Tao Y, van Lier JB (2014) Applicability of dynamic membrane technology in anaerobic membrane bioreactors. Water Res 48:420–429
Felz S, Al-Zuhairy S, Aarstad OA, van Loosdrecht MCM, Lin YM (2016) Extraction of structural extracellular polymeric substances from aerobic granular sludge. J vis Exp 115:e54534
Feng C, Lotti T, Canziani R, Lin Y, Tagliabue C, Malpei F (2021) Extracellular biopolymers recovered as raw biomaterials from waste granular sludge and potential applications: a critical review. Sci Total Environ 753:142051
Filer J, Ding HH, Chang S (2019) Biochemical methane potential (BMP) assay method for anaerobic digestion research. Water 11(5):921
Fitamo T, Boldrin A, Boe K, Angelidaki I, Scheutz C (2016) Co-digestion of food and garden waste with mixed sludge from wastewater treatment in continuously stirred tank reactors. Biores Technol 206:245–254
Flemming HC, Neu TR, Wozniak DJ (2007) The EPS matrix: the “house of biofilm cells.” J Bacteriol 189(22):7945–7947
Franca RD, Pinheiro HM, van Loosdrecht MC, Lourenço ND (2018) Stability of aerobic granules during long-term bioreactor operation. Biotechnol Adv 36(1):228–246
Frolund B, Griebe T, Nielsen PH (1995) Enzymatic activity in the activated sludge floc matrix. Appl Microbiol Biotechnol 43:755–761
Ghangrekar M, Asolekar SR, Ranganathan KR, Joshi SG (1996) Experience with UASB reactor start-up under different operating conditions. Water Sci Technol 34(5–6):421–428
Giesen A, Thompson A (2013) Aerobic granular biomass for cost-effective, energy efficient and sustainable wastewater treatment. 7th European Waste Water Management Conference, Wakefield, United Kingdom
Guo D, Zhang X, Shi Y, Cui B, Fan J, Ji B, Yuan J (2020a) Microalgal-bacterial granular sludge process outperformed aerobic granular sludge process in municipal wastewater treatment with less carbon dioxide emissions. Environ Sci Pollut Res 28:13616–13623
Guo H, van Lier JB, de Kreuk M (2020b) Digestibility of waste aerobic granular sludge from a full-scale municipal wastewater treatment system. Water Res 173:115617
Guven H, Ozgun H, Ersahin ME, Dereli RK, Sinop I, Ozturk I (2019) High-rate activated sludge processes for municipal wastewater treatment: the effect of food waste addition and hydraulic limits of the system. Environ Sci Pollut Res 26(2):1770–1780
Guven H, Ersahin ME, Ozgun H, Ozturk I, Koyuncu I (2023) Energy and material refineries of future: wastewater treatment plants. J Environ Manage 329:117130
Holliger C, Astals S, de Laclos HF, Hafner SD, Koch K, Weinrich S (2021) Towards a standardization of biomethane potential tests: a commentary. Water Sci Technol 83(1):247–250
Iqbal A, Zan F, Siddiqui MA, Nizamuddin S, Chen G (2022) Integrated treatment of food waste with wastewater and sewage sludge: energy and carbon footprint analysis with economic implications. Sci Total Environ 825:154052
Isik O, Abdelrahman AM, Ozgun H, Ersahin ME, Demir I, Koyuncu I (2019) Comparative evaluation of ultrafiltration and dynamic membranes in an aerobic membrane bioreactor for municipal wastewater treatment. Environ Sci Pollut Res 26(32):32723–32733
Isik O, Hudayarizka R, Abdelrahman AM, Ozgun H, Ersahin ME, Demir I, Koyuncu I (2020) Impact of support material type on performance of dynamic membrane bioreactors treating municipal wastewater. J Chem Technol Biotechnol 95(9):2437–2446
Jahn L, Svardal K, Krampe J (2019) Nitrous oxide emissions from aerobic granular sludge. Water Sci Technol 80(7):1304–1314
Jiang HL, Tay JH, Liu Y, Tiong-Lee Tay S (2003) Ca2+ augmentation for enhancement of aerobically grown microbial granules in sludge blanket reactors. Biotech Lett 25(2):95–99
Khan SJ, Visvanathan C, Jegatheesan V, BenAim R (2008) Influence of mechanical mixing rates on sludge characteristics and membrane fouling in MBRs. Sep Sci Technol 43(7):1826–1838
Kim H, Kim J, Ahn D (2021) Effects of carbon to nitrogen ratio on the performance and stability of aerobic granular sludge. Environ Eng Res 26(1):1–8
Kosar S, Isik O, Akdag Y, Gulhan H, Koyuncu I, Ozgun H, Ersahin ME (2022a) Impact of seed sludge characteristics on granulation and performance of aerobic granular sludge process. J Clean Prod 363:132424
Kosar S, Isik O, Cicekalan B, Gulhan H, Kurt ES, Atli E, Basa S, Ozgun H, Koyuncu I, van Loosdrecht MCM, Ersahin ME (2022b) Impact of primary sedimentation on granulation and treatment performance of municipal wastewater by aerobic granular sludge process. J Environ Manage 315:115191
Kosar S, Isik O, Cicekalan B, Gulhan H, Cingoz S, Yoruk M, Ozgun H, Koyuncu I, van Loosdrecht MCM, Ersahin ME (2023) Coupling high-rate activated sludge process with aerobic granular sludge process for sustainable municipal wastewater treatment. J Environ Manage 325:116549
Li J, Ding LB, Cai A, Huang GX, Horn H (2014) Aerobic sludge granulation in a full-scale sequencing batch reactor. Biomed Res Int 2014:1–13
Liang J, Luo L, Li D, Varjani S, Xu Y, Wong JW (2021) Promoting anaerobic co-digestion of sewage sludge and food waste with different types of conductive materials: performance, stability, and underlying mechanism. Biores Technol 337:125384
Liu Y, Tay J (2004) The effects of extracellular polymeric substances on the formation and stability of biogranules. Appl Microbiol Biotechnol 65:143–148
Liu Y, Chang S, Defersha FM (2015) Characterization of the proton binding sites of extracellular polymeric substances in an anaerobic membrane bioreactor. Water Res 78:133–143
Low WP, Din MFM, Chang FL, Moideen SNFB, Lee YY, Krishnan S (2021) Development of microbial biofilms on cellulosic fibers for organic matter removal in river water treatment. Desalin Water Treat 224:144–153
Marashlian, N, El-Fadel, M (2005) The effect of food waste disposers on municipal waste and wastewater management. Waste Manag Res 23(1):20–31
Mattsson J, Hedstrom A, Viklander M (2014) Long-term impacts on sewers following food waste disposer installation in housing areas. Environ Technol 35:2643–2651
Mehariya S, Patel AK, Obulisamy PK, Punniyakotti E, Wong JW (2018) Co-digestion of food waste and sewage sludge for methane production: current status and perspective. Biores Technol 265:519–531
Monino P, Jiménez E, Barat R, Aguado D, Seco A, Ferrer J (2016) Potential use of the organic fraction of municipal solid waste in anaerobic co-digestion with wastewater in submerged anaerobic membrane technology. Waste Manag 56:158–165
Monino P, Aguado D, Barat R, Jimenez E, Gimenez JB, Seco A, Ferrer J (2017) A new strategy to maximize organic matter valorization in municipalities: combination of urban wastewater with kitchen food waste and its treatment with AnMBR technology. Waste Manage 62:274–289
Montecchio D, Astals S, Di Castro V, Gallipoli A, Gianico A, Pagliaccia P, Piemonte V, Rossetti S, Tonanzi B, Braguglia CM (2019) Anaerobic co-digestion of food waste and waste activated sludge: ADM1 modelling and microbial analysis to gain insights into the two substrates’ synergistic effects. Waste Manage 97:27–37
Nancharaiah YV, Sarvajith M, Krishna Mohan TV (2019) Aerobic granular sludge: the future of wastewater treatment. Curr Sci 117(3):395–404
Nguyen Quoc B, Armenta M, Carter JA, Bucher R, Sukapanpotharam P, Bryson SJ, Stahl DA, Stensel HD, Winkler MKH (2021) An investigation into the optimal granular sludge size for simultaneous nitrogen and phosphate removal. Water Res 198:117119
Ni BJ, Xie WM, Liu SG, Yu HQ, Wang YZ, Wand G, Dai XL (2009) Granulation of activated sludge in a pilot-scale sequencing batch reactor for the treatment of low-strength municipal wastewater. Water Res 43:751–761
Ni BJ (2013) Formation, characterization and mathematical modeling of the aerobic granular sludge. Springer, New York, NY, USA
Othman I, Anuar AN, Ujang Z, Rosman NH, Harun H, Chelliapan S (2013) Livestock wastewater treatment using aerobic granular sludge. Biores Technol 133:630–634
Prabhu MS, Mutnuri S (2016) Anaerobic co-digestion of sewage sludge and food waste. Waste Manage Res 34(4):307–315
Pronk M, Giesen A, Thompson A, Robertson S, Van Loosdrecht M (2017) Aerobic granular biomass technology: advancements in design, applications and further developments. Water Practice & Technology 12(4):987–996
Ren TT, Liu L, Sheng GP, Liu XW, Yu HQ, Zhang MC, Zhu JR (2008) Calcium spatial distribution in aerobic granules and its effects on granule structure, strength and bioactivity. Water Res 42(13):3343–3352
Sankai T, Ding G, Emori N, Kitamura S, Katada K, Koshio A, Maruyama T, Kudo K, Inamori Y (1997) Treatment of domestic wastewater mixed with crushed garbage and garbage washing water by advanced Gappei-Shori Johkaso. Water Science Technology 36:175–182
Silvestre G, Fernandez B, Bonmatí A (2015) Significance of anaerobic digestion as a source of clean energy in wastewater treatment plants. Energy Convers Management 101:255–262
van den Berg L, Pronk M, van Loosdrecht MCM, de Kreuk MK (2022) Density measurements of aerobic granular sludge. Environ Technol 43:1–11
van Loosdrecht MCM, Nielsen PH, Lopez-Vazquez CM, Brdjaanovic D (2016) Experimental methods in wastewater treatment. IWA Publishing
Wagner J, Weissbrodt DG, Manguin V, da Costa RH, Morgenroth E, Derlon N (2015) Effect of particulate organic substrate on aerobic granulation and operating conditions of sequencing batch reactors. Water Res 85:158–166
Winkler MKH, Le QH, Volcke EIP (2015) Influence of partial denitrification and mixotrophic growth of nob on microbial distribution in aerobic granular sludge. Environ Sci Technol 49:11003–11010
Zan F, Hao T (2020) Sulfate in anaerobic co-digester accelerates methane production from food waste and waste activated sludge. Biores Technol 298:122536
Zhang X, Hu J, Spanjers H, van Lier JB (2014) Performance of inorganic coagulants in treatment of backwash waters from a brackish aquaculture recirculation system and digestibility of salty sludge. Aquac Eng 61:9–16
Zhang D, Li W, Hou C, Shen J, Jiang X, Sun X, Li J, Han W, Wang L, Liu X (2017) Aerobic granulation accelerated by biochar for the treatment of refractory wastewater. Chem Eng J 314:88–97
Zheng YM, Yu HQ, Sheng GP (2005) Physical and chemical characteristics of granular activated sludge from a sequencing batch airlift reactor. Process Biochem 40(2):645–650
Zhou JH, Zhang ZM, Zhao H, Yu HT, Alvarez PJ, Xu XY, Zhu L (2016) Optimizing granules size distribution for aerobic granular sludge stability: effect of a novel funnel-shaped internals on hydraulic shear stress. Bioresour Technol 216:562–570
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This study was financed by the Istanbul Technical University, Scientific Research Projects: Busra Cicekalan (Project No: MDK-2022–43675) and Nastaran Rahimzadeh Berenji (Project No: MYL-2021–43357).
Author information
Authors and Affiliations
Contributions
Busra Cicekakalan: conceptualization, methodology, investigation, formal analysis, writing — original draft, writing — review and editing. Nastaran Rahimzadeh Berenji: investigation, writing — original draft. Muhammed Furkan Aras: writing — original draft Huseyin Guven: investigation, writing — review and editing, supervision, project administration. Ismail Koyuncu: resources, writing — review and editing. Mustafa Evren Ersahin: conceptualization, methodology, investigation, writing — review and editing, supervision, project administration. Hale Ozgun: conceptualization, methodology, investigation, writing — review and editing, supervision, project administration.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interest
The authors declare no competing interests.
Additional information
Responsible Editor: Guilherme Luiz Dotto
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cicekalan, B., Berenji, N.R., Aras, M.F. et al. Impact of food waste addition in energy efficient municipal wastewater treatment by aerobic granular sludge process. Environ Sci Pollut Res 31, 29304–29320 (2024). https://doi.org/10.1007/s11356-024-32997-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-32997-5