Abstract
Fine particulate matter (PM2.5) can enter the human body in various ways and have adverse effects on human health. Human lungs and eyes are exposed to the air for a long time and are the first to be exposed to PM2.5. The “liquid immersion exposure method” has some limitations that prevent it from fully reflecting the toxic effects of particulate matter on the human body. In this study, the collected PM2.5 samples were chemically analyzed. An air–liquid interface (ALI) model with a high correlation to the in vivo environment was established based on human lung epithelial cells (A549) and immortalized human corneal epithelial cells (HCE-T). The VITROCELL Cloud 12 system was used to distribute PM2.5 on the cells evenly. After exposure for 6 h and 24 h, cell viability, apoptosis rate, reactive oxygen species (ROS) level, expression of inflammatory factors, and deoxyribonucleic acid (DNA) damage were measured. The results demonstrated significant dose- and time-dependent effects of PM2.5 on cell viability, cell apoptosis, ROS generation, and DNA damage at the ALI, while the inflammatory factors showed dose-dependent effects only. It should be noted that even short exposure to low doses of PM2.5 can cause cell DNA double-strand breaks and increased expression of γ-H2AX, indicating significant genotoxicity of PM2.5. Increased abundance of ROS in cells plays a crucial role in the cytotoxicity induced by PM2.5 exposure These findings emphasize the significant cellular damage and genotoxicity that may result from short-term exposure to low levels of PM2.5.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With urbanization and industrialization on the rise, environmental pollution, particularly air pollution, has escalated worldwide. This pressing issue has garnered increasing attention due to its detrimental impact on the human body. In 2013, the International Agency for Research on Cancer (IARC) listed atmospheric particulates as the first category of carcinogens. Fine particulate matter (PM2.5) has a smaller size, a larger specific surface area, and a greater ability to adsorb harmful organic matter and heavy metals, which is more detrimental to human health compared to inhalable particles (PM10) (Deng et al. 2013; Deng et al. 2014). China is one of the countries most affected by PM2.5, with 1.27 million premature deaths due to PM2.5 pollution each year (Pang et al. 2020; Wang et al. 2017). Epidemiological studies have provided evidence that once PM2.5 enters the human body, it can reach the deep alveoli in the lungs and penetrate the alveolar area. Subsequently, these particles may potentially be absorbed by the bloodstream and disseminated throughout the body, thereby contributing to the development of various respiratory and cardiovascular diseases in humans (Bengalli et al. 2013). It can also penetrate the blood–brain barrier and placental barrier, causing harm to the human nervous system and reproductive system (Wang et al. 2022). Furthermore, recent research has brought to light the detrimental effects of PM2.5 on the surface of the eyes, thereby contributing to the emergence of various eye conditions (Yang et al. 2019). Studies have shown that PM2.5 can deposit on the eyelids and eyelashes (Muruganandam et al. 2023). Short-term or long-term exposure to an environment with excessive air quality can cause eye irritation (eye itching, tears, etc.) and lead to diseases such as dry eye disease, conjunctivitis, and pterygium. Numerous studies have shown that the health effects caused by PM2.5 are closely related to its composition. PM2.5 is composed of a complex blend of water-soluble ions, carbonaceous compounds, organic substances, heavy metals, and other elements. Among these, water-soluble ions are a significant constituent of PM2.5 and have the potential to impact cell viability, lactic dehydrogenase levels (LDH), and reactive oxygen species (ROS) (Zou et al. 2016). Heavy metals, such as chromium (Cr), arsenic (As), lead (Pb), and cadmium (Cd), have been found to have detrimental effects on human health due to their carcinogenic and teratogenic properties. Additionally, they can accumulate within the body, leading to harm to the reproductive system (Mukherjee et al. 2022; Sui et al. 2020; Zhang et al. 2016). Polycyclic aromatic hydrocarbons (PAHs) can lead to oxidative stress, inflammatory reactions, and deoxyribonucleic acid (DNA) damage in cells. Studies have indicated that short-term exposure to high concentrations of PAHs can lead to diarrhea and eye discomfort, and long-term exposure can cause damage to the human immune system and lungs (Abdel-Shafy and Mansour 2016).
Due to the substantial time and cost associated with conventional in vivo inhalation and animal experiments, there is a heightened interest in utilizing in vitro methods to evaluate the toxicity of PM2.5. Currently, most of the in vitro models for evaluating PM2.5 toxicity are the “liquid immersion exposure method,” which directly uses the medium to expose PM2.5 to cells to assess the cytotoxicity of PM2.5. However, it is important to note that this method has certain limitations. For example, it demonstrates poor repeatability and may result in changes in particle size due to the accumulation of PM2.5, thereby potentially inadequately reflecting the toxicity of particulate matter to the human body (Bihari et al. 2008). Brownian motion of particles in the medium prevents particles from settling on cells completely, which may underestimate the cytotoxicity of particles (Muehlfeld et al. 2008). Another method of cell exposure is the air–liquid interface (ALI) method, which allows cells to grow at the interface between air and liquid through the use of a Transwell chamber. Compared with traditional in vitro exposure, ALI exposure can avoid the problems encountered in traditional exposure experiments. It can make direct contact between particles and cells, which is more in line with the real state of cells in the body (Guenette et al. 2022). Our previous studies have also demonstrated that PM2.5 has better dispersion and deposition properties at the ALI model (Chen et al. 2024). Studies have shown that when cells are cultured at the ALI, they develop highly differentiated functions and structures (Aufderheide 2005). These findings underscore the significance of investigating the toxic effects of PM2.5 on both the respiratory and ocular systems, employing advanced research methods like the ALI exposure model.
In this study, a comprehensive analysis was conducted on the chemical composition of the collected PM2.5 samples. To simulate exposure scenarios in real life, human lung epithelial cells (A549) and immortalized human corneal epithelial cells (HCE-T) were exposed to different doses of PM2.5 at the ALI. Following exposures of 6 and 24 h, several key cytotoxic indicators were assessed, including cell viability, ROS levels, cell apoptosis rate, expression of inflammatory factors, and DNA damage. The primary objective of our research was to evaluate the dose- and time-response relationships concerning the cellular toxicity of PM2.5 on both the respiratory and ocular systems at the ALI. It is crucial for assessing the potential risks associated with PM2.5 exposure and improving the understanding of its impact on the respiratory and ocular systems.
Materials and methods
Collection of particulate matter
The PM2.5 samples were collected at Nanjing University of Information Science & Technology (N 32° 15′ 11″, E 118° 42′ 49″), and a total of 80 samples were collected from December 2021 to November 2022. The samples were collected on quartz microfiber filters (Whatman, UK). The collection process involved the use of a large-flow sampler (Jinshida, Qingdao, China), which was set to operate at a flow rate of 1050 L/min. To prepare the quartz microfiber filters for use, they underwent a process of baking in a muffle furnace at a temperature of 450 ℃ for a duration of 8 h. The purpose of this procedure was to effectively remove any organic matter present on the filters. The filters were weighed both before and after sampling and were put into a desiccator for 24 h of drying and balancing before weighing. The accuracy of the balance used for weighing is one in 100,000, and the filters should be weighed three times with an error not exceeding 0.05 mg. The collected quartz microfiber filters were cut into pieces, and ultrasonic extraction was carried out in ultrapure water for 20 min and repeated 3 times. After ultrasound treatment, the suspension was filtered using 8 layers of sterile gauze. The filtered solution was then transferred into a freeze-drying machine and subjected to a 1-week process to obtain the PM2.5 samples. The samples were sealed in centrifugal tubes and stored in a − 20 ℃ refrigerator away from light for later use.
Composition analysis of PM2.5
The PM2.5 samples were placed in the centrifuge tubes, sonicated with ultrapure water, and then filtered through a 0.22 μm filter. The content of water-soluble ions in the samples was determined by an ion chromatograph (ICS-3000, ICS-2000, Dionex, Sunnyvale, CA, USA). The organic carbon (OC) and elemental carbon (EC) in PM2.5 samples were determined using a carbon analyzer (Sunset Model 4, Sunset Laboratory Inc., Forest Grove, OR, USA). After 65% HNO3 was added to the particles for microwave digestion, an inductively coupled plasma mass spectrometry was used to determine the concentration of heavy metals in samples (ICP-MS, Agilent Technologies, USA). The content of PAHs in samples was determined by a gas chromatography-mass spectrometer (GC–MS, Agilent Technologies, USA). PM2.5 samples added with CH2Cl2 and internal standard solution were sonicated, filtered, and rotary evaporated. 200 μL of CH2Cl2 was then added to fix the volume, and a total of 16 PAHs were detected in the samples. The standard curves of all measured substances displayed excellent linearity (r2 > 0.999). The recovery rates of all the measured substances fell within the acceptable range of 100 ± 15%.
Cell culture
A549 cells were obtained from Stem Cell Bank, Chinese Academy of Sciences. A549 cells were cultured in RPMI-1640 medium (Bio-Channel, China), which was supplemented with 10% fetal bovine serum (FBS, BIOAGRIO SCIENCE, China). HCE-T cells were obtained from Wuhan Punosai Life Technology Co., LTD. HCE-T cells were cultured in the DMEM/F12 medium (Bio-Channel, China), which was supplemented with 10% FBS, 5 μg/mL Insulin (Beyotime, China), and 10 ng/mL human epidermal growth factor (EGF, BIOAGRIO SCIENCE, China). The cell culture was maintained in an incubator (Thermo Scientific, MA, USA) at a constant temperature of 37 ℃ and 5% CO2. The supplemented RPMI-1640 and DMEM/F12 medium were further cited as complete cell medium (CCM).
A549 and HCE-T cells were passaged when 80–90% confluent. For all the exposure experiments, 50,000 cells/insert were cultured in 12-well culture plate inserts. These inserts have a surface area of 1.12 cm2 and are equipped with a 0.4 μm pore polyester (PET) membrane (LABSELECT, China). For each well, 0.5 mL of CCM was added to the upper side and 1.5 mL to the basal side for each well. The cells were incubated for 24 h under immersion conditions, and the medium in the upper side was then removed after forming a tight contact cell layer. The cells were incubated for12 h at the ALI until the exposure experiment was conducted.
Cell exposure
The exposure experiments were performed in the VITROCELL Cloud 12 system (VITROCELL SYSTEMS, Waldkirch, Germany); detailed information of which is available on the vendor’s website (www.Vitrocell.com). 4 mL of the basal medium was added to each exposed module of the VITROCELL Cloud 12 system so that the liquid level was consistent with the height of the PET membrane to ensure sufficient contact between medium and cells. The exposure dose of PM2.5 was determined by the cell growth area of the insert, and the deposition mass of PM2.5 on the insert was 25, 50, and 100 μg in this study. The Transwell chamber was put into the exposure module, and the prepared PM2.5 solution was quickly and evenly exposed to the cells using the atomizer (Aeroneb Lab; Aerogen, Galway, Ireland). Complete exposure could be achieved within 5 min. The Cloud 12 system has electric heating, which can maintain 37 ℃ throughout the exposure process to provide a favorable environment for cells to survive. After ALI exposure was complete, the CCM in the basal side was replaced with 1 mL basal medium and placed in an incubator for 6 h or 24 h until cytotoxic indicators were determined.
Detection of cell viability
Cell Counting Kit-8 kit (CCK-8, Beyotime, China) was used to measure cell viability. After exposure, 1 mL of the mixture CCK-8 and the medium (CCK-8 concentration of 10%) was added to each upper chamber and incubated at 37 °C for a duration of 2 h. After the incubation, the mixtures from each chamber were transferred to separate wells in a 96-well plate. The optical density of the samples was measured at a specific wavelength of 450 nm using a Microplate Reader (ID3, SpectraMax, USA), and the results were normalized.
Detection of apoptosis rate
Apoptosis was determined using Annexin V-FITC apoptosis assay kit (Beyotime, China). After exposure, the cells were washed with PBS once, digested with EDTA-free trypsin, and collected into a centrifuge tube. Centrifuge at 1000 r/min for 5 min, resuspend and count 100,000 cells, and centrifuge again. 195 μL of Annexin V-FITC binding solution, 5 μL of Annex V-FITC, and 10 μL of propidium iodide (PI) staining solution were added to suspend the cells. Double-negative group, Annex V-FITC single-staining group, and PI single-staining group were set up for localization of cells. The double-negative group used cells that were not exposed to PM2.5, and the single-stained group used cells from the exposed group. The cells were gently mixed and assayed by flow cytometry (Beckman, CA, USA) at 10,000 cells per assay.
Detection of ROS
To detect ROS levels, the DCFH-DA probe (Sigma, MO, USA) was utilized. DCFH-DA was diluted in the basal medium to a final concentration of 10 μM. In each well, 500 μL of the diluted DCFH-DA solution was added, and the cells were then incubated at 37 ℃ for a duration of 20 min. To ensure the removal of excess DCFH-DA, the cells were washed 2 times with phosphate buffer saline (PBS). Then, digest the cells with trypsin without EDTA, suspend with 500 μL PBS, and transfer to a flow tube. Fluorescence intensity was measured by flow cytometry, with excitation wavelength at 488 nm and emission wavelength at 525 nm. 5000 cells were measured each time and the results were normalized.
Detection of inflammatory factor
TNF-α and IL-6 are inflammatory factors that play a significant role in particulate matter-induced inflammatory damage. TNF-α is a pre-inflammatory response factor that can promote the secretion of inflammatory factor IL-6, and the overexpression of IL-6 is related to the inflammation of cells (Möller and Villiger 2006). The inflammatory factor levels in cell culture medium were determined using an enzyme-linked immunosorbent assay (ELISA) kit (Jiangsu Meimian, China) to judge the immunosorbent damage effects of particulate matter on cells. The optical density at 450 nm was measured using a Microplate Reader, and the results were normalized.
Detection of DNA damage
H2AX is a variant of the histone protein H2A. H2AX phosphorylates when a cell’s DNA double strand breaks. The level of γ-H2AX produced by H2AX phosphorylation reflects the degree of DNA damage. γ-H2AX staining showed green fluorescence. DNA damage was determined by γ-H2AX immunofluorescence assay, using the DNA damage detection kit (Beyotime, China). Finally, images were captured under an inverted fluorescence microscope (NE900, Jiangnan Yong Xin Corporation, China). Fluorescence intensity was quantified using ImageJ (National Institutes of Health, MD, USA) to calculate the mean pixel intensity of nuclear area, ultimately resulting in normalization.
Statistical analysis
In this study, each experiment was repeated a minimum of 3 times, and the experimental data were expressed as mean ± standard deviation (SD). Statistical analysis was performed by using SPSS 26.0 (SPSS Inc., USA). The differences in cytotoxicity between different concentration groups and time groups were analyzed by one-way analysis of variance (ANOVA). p < 0.05 implies statistically significant and p < 0.01 was extremely significant.
Results
Mass concentration analysis of different chemical components in PM2.5
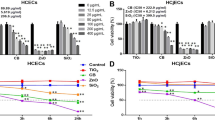
The average mass concentration of PM2.5 collected was 36.96 μg/m3, and the main components of PM2.5 are shown in Fig. 1. Water-soluble ions accounted for 44.29% of the total mass of PM2.5, of which SO42−, NO3−, and NH4+ (SNA) particles account for the highest proportion. Atmospheric carbon comes in the form of OC and EC, both of which are important components of PM2.5. OC and EC account for 11.45% and 1.22% of the total mass of PM2.5. The OC/EC ratio is commonly employed to determine the secondary source contribution to PM2.5. The OC/EC ratio of PM2.5 was 9.39, indicating that the PM2.5 in Jiangbei New Area of Nanjing was dominated by the secondary generation. A total of 32 kinds of metallic elements were determined in PM2.5, accounted for 2.38% of the mass of PM2.5. The content of 16 kinds of PAHs in PM2.5 was also determined, and the PAH content accounted for 0.01‰ of the mass of PM2.5. Although PAHs make up only a small fraction of PM2.5, they are harmful to the human body, causing damage to the respiratory and nervous systems. For more detailed information on water-soluble ions, OCEC, metallic elements, and PAHs, please refer to the Online Resource 1, 2, 3, and 4.
PM2.5 decreases epithelial cell viability
As depicted in Fig. 2, for A549 cells, exposure to PM2.5 for 6 h and 24 h resulted in a significant decrease in cell viability in each dose group compared to the control group. For HCE-T cells, only the 25 μg dose group exposed for 6 h showed no significant decrease in cell viability, while the cell viability in all other dose groups significantly decreased. We observed that as the dose of PM2.5 increased, the inhibitory effect on cell viability became more pronounced, indicating the harmful impact of PM2.5 on cells. Furthermore, at the same exposure time and dose, the cell viability of A549 cells was lower than that of HCE-T cells, with the greatest difference observed at a dose of 100 μg and an exposure time of 6 h, where A549 cell viability was 9.6% lower than that of HCE-T cells, suggesting higher sensitivity of A549 cells to PM2.5 stimulation. With increasing exposure time, A549 and HCE-T cells showed significant differences in cell viability at a dose of 100 μg, while the differences between other exposure groups were not statistically significant. These findings indicate that PM2.5 exhibits greater cytotoxicity on A549 cells compared to HCE-T cells. Exposure to medium and low doses of PM2.5 for 6 h resulted in a decrease in cell viability similar to that caused by exposure for 24 h. However, as the exposure dose increased, longer exposure to PM2.5 had a greater impact on cell viability.
PM2.5 causes apoptosis in epithelial cells
Exposure to PM2.5 typically results in cellular apoptosis and necrosis. The data in Fig. 3 show that the apoptosis rates of both cell types showed a significant dose dependence as the PM2.5 exposure dose increased. In the medium and high-dose exposure groups, the apoptosis rate of cells increased significantly with prolonged exposure duration. In addition, the apoptosis rates of A549 and HCE-T cells were similar at medium and low-dose exposures. When the dose was increased to 100 μg, the difference in apoptosis rates between the two cells gradually increased. The apoptosis rate of A549 cells was higher than that of HCE-T by 4.43% at an exposure time of 6 h. When the exposure time was increased to 24 h, the maximum difference in apoptosis rates of the two cells appeared, with 30.59% for A549 cells and 19.79% for HCE-T cells, which was 10.8% different from each other. The results indicated that the apoptosis rate of A549 cells was higher with the increase of exposure dose.
PM2.5 promotes ROS generation in epithelial cells
Figure 4 shows the level of ROS generation. After exposure to PM2.5, ROS levels showed a significant dose-dependent increase in A549 and HCE-T cells compared to the control. Whether exposed for 6 h or 24 h, HCE-T cells exhibited higher levels of ROS at low doses of PM2.5 exposure, whereas A549 cells exhibited higher levels of ROS as the exposure dose increased. These findings suggest that HCE-T cells are more sensitive to ROS at low doses of PM2.5 exposure, while A549 cells experience more pronounced oxidative damage at high doses. Interestingly, the trend in ROS levels parallels that of apoptosis rates, with the increase of exposure time, the ROS level of cells significantly increases, suggesting that the cells were subjected to more severe oxidative damage with increasing exposure time.
PM2.5 induces inflammatory factor expression in epithelial cells
Current studies have generally shown that the expression level of inflammatory factors increases after oxidative stress occurs in cells (Lugrin et al. 2014). To this end, we determined the expression levels of TNF-α and IL-6 in the cell culture medium. As shown in Fig. 5, for A549 cells, TNF-α level increased significantly with increasing exposure dose after 6 and 24 h of exposure to PM2.5. IL-6 level showed no significant difference only after 6 h of low-dose exposure, and the other exposure groups showed significant difference compared with the control group. For HCE-T cells, after 6 h of exposure, TNF-α level was significantly increased only when exposed to a dose of 100 μg, whereas IL-6 level began to increase significantly at an exposure of 50 μg. After 24 h of exposure, TNF-α and IL-6 levels in HCE-T cells of all dose exposure group were statistically different from those of the control group. The results indicated that the expression levels of TNF-α and IL-6 of the two cells were significantly dose-dependent. Notably, there was no particularly significant difference in the expression of inflammatory factors in the two types of cells with the increase in exposure time, suggesting that inflammation-induced cellular damage occurs already after 6 h of exposure.
PM2.5 causes DNA damage in epithelial cells
In addition, this study also measured the damage of PM2.5 to cellular DNA. DNA double-strand breaks are considered to be the most severe DNA damage. When cellular DNA is damaged, H2AX is phosphorylated to produce γ-H2AX, which fluoresces green when stained. The fluorescence intensity can be calculated to reflect the degree of DNA damage. As shown in Fig. 6, the levels of γ-H2AX increased dose-dependently in both cells at different exposure times. For A549 cells, only the 100 μg dose group showed a significant increase in γ-H2AX levels with increasing exposure time, and no significant differences were observed in the low and medium-dose groups. For HCE-T cells, the extent of γ-H2AX levels after 24 h of exposure to PM2.5 was significantly higher than that after 6 h of exposure. The results indicated that a low dose of PM2.5 could lead to DNA damage in HCE-T cells compared with A549 cells and that HCE-T cells had a more pronounced time dependence of PM2.5-induced DNA damage.
Discussion
Previous studies have shown that the ALI model has good applicability in in vitro toxicology, and compared to traditional immersion exposure, the ALI model can more accurately simulate the real environment of the human body. Currently, the ALI method has been used in numerous studies to investigate the effects of air pollution and particulate matter mixtures on lung cell models (Gonzalez-Rivera et al. 2020; Kaur et al. 2022; Leclercq et al. 2016; Offer et al. 2022; Tomasek et al. 2016). For the human body, long-term exposure of the lungs and eyes system to the atmosphere can easily affect various pollutants in the atmosphere, leading to a series of health risks. Human epithelial cells such as A549 and HCE-T cells serve as the first barrier of the human body, playing a role as a barrier and sentinel. Currently, extensive and profound research has been conducted on the cytotoxic effects of PM2.5 on A549 and HCE-T cells under immersion exposure. However, epithelial cells were exposed to air for a long period of time, and using traditional immersion exposure to evaluate the cytotoxicity of PM2.5 clearly does not meet the requirements. Current research has shown that in traditional immersion exposure, the duration of exposure significantly affects the cytotoxicity of particulate matter, leading to decreased cell activity and increased inflammatory response (Rahmatinia et al. 2022). However, at the ALI, the debate is not unanimous. Loret and Wang et al. pointed out that exposure at the ALI for 3 h is sufficient to observe adverse cellular reactions, including cytotoxicity, oxidative stress, and inflammatory damage, after 24 h of exposure (Loret et al. 2016; Wang et al. 2019). However, Miao et al. found that HCE cells exposed to cigarette extracts exhibited time-dependent cytotoxicity under ALI (Miao et al. 2019). Therefore, exposure time is still crucial for studying the cytotoxicity of PM2.5 at the ALI. Therefore, in this study, a three-dimensional model of human epithelial cells was created using A549 and HCE-T cells, exposing the cells to PM2.5 during ALI. The effects of PM2.5 on human epithelial cell activity, cell apoptosis rate, ROS levels, inflammatory factor expression levels, and DNA damage were measured.
The cell viability results showed that after exposure to PM2.5, the cell viability of A549 and HCE-T cells decreased in a dose- and time-dependent manner. The dose-dependent decrease in cell viability is consistent with other studies, with a dose-dependent decrease in 16HBE cell viability after exposure to PM2.5 for 24 h (Niu et al. 2020). The results of cell apoptosis showed that with the increase of PM2.5 exposure dose and exposure time, the apoptosis rates of both types of cells significantly increased, indicating that PM2.5 has a time- and dose-dependent effect on cell apoptosis. It can be seen that A549 cells exhibited lower cell viability and higher apoptosis rates with increasing PM2.5 exposure dose compared to HCE-T cells. A recent study showed that the apoptosis rate induced by PM2.5 is higher than that of PM10. Shan et al. exposed cells to PM2.5 after treatment with antioxidants (NAC, N-acetylcysteine) and found that NAC significantly reduced the apoptosis rate after PM2.5 exposure, indicating that PM2.5-induced apoptosis is related to the generation of ROS (Shan et al. 2021). Another study suggests that ROS can induce cell apoptosis by participating in signaling pathways such as P53 and Caspase. Liu et al.’s research suggests that oxidative stress can trigger ATM/P53/CDK2 and mitochondrial apoptosis pathways to induce apoptosis in rat spermatocytes (Liu et al. 2019). Qi et al. believe that PM2.5 can induce apoptosis and injury in A549 cells by activating the MAPK/NF-xB/STAT1 pathway (Qi et al. 2019).
Oxidative stress is considered one of the key mechanisms of cell damage, and the possible cause of cell apoptosis is mitochondrial stress induced by oxidative stress, which in turn leads to cell apoptosis (Liu et al. 2019). ROS is a product of normal cellular metabolism. When cells are stimulated and produce excessive ROS, this balance is disrupted, resulting in oxidative damage to the cells. Previous reports have shown that excessive ROS generation plays an important role in PM2.5-induced cytotoxicity (Abbas et al. 2019). To further investigate the cytotoxic mechanism of PM2.5 at the ALI, we measured the ROS generation levels of two types of cells after PM2.5 exposure. Our research results indicate that PM2.5 increases ROS generation, and ROS generation is dose- and time-dependent. HCE-T cells were more sensitive at low doses, and A549 cells exhibited higher ROS levels as the exposed dose increased. As the exposure time increases, the ROS levels of HCE-T cells show greater temporal differences. This is consistent with our previous research findings that an increase in PM2.5 concentration can induce ROS generation in mouse spermatocytes (Ge et al. 2023). Other studies using different cell lines have also shown that exposure to PM2.5 can cause an increase in cell ROS and inflammatory damage (Jia et al. 2023; Jin et al. 2018; Kim et al. 2022; Qiu et al. 2019). It should be noted that previous studies have shown that A549 cells are one of the most heterologous cells in many lung cell models compared to lung tissue and primary lung epithelial cells. Therefore, there may be significant differences in ROS generation and accumulation between A549 cells and in vivo lung tissue (Courcot et al. 2012). In our previous research, we found that water-soluble ions such as SO42−, NO3−, and NH4+ showed a strong positive correlation with ROS (Di et al. 2020). In the PM2.5 used in this study, SO42−, NO3−, and NH4+ accounted for 13.04%, 16.45%, and 5.72%, respectively. They are the main components of water-soluble ions. They are relatively more likely to enter cells, leading to oxidative stress. The carbon components present in PM2.5, such as elemental carbon and organic carbon, can also have cytotoxic effects on cells. Elemental carbon nanoparticles have been shown to induce oxidative stress in cells and trigger an inflammatory response, leading to cell dysfunction, DNA damage, and apoptosis. Recent studies have emphasized the important contribution of metals in oxidative stress, such as nickel (Ni), copper (Cu), Pb, Cd, and As (Crobeddu et al. 2020; Fang et al. 2021). Metals will adsorb on PM2.5 and induce ROS generation when inhaled into the lungs or deposited on the surface of the eyes. Al and Pb have been proven to be related to redox reactions, while zinc (Zn) can inhibit lead-induced oxidative stress (Bondy and Kirstein 1996; Zhang 2023). Some carcinogenic metals, such as Cr and Cd, induce ROS generation, which in turn leads to DNA damage (Lin et al. 2007; Wakeman 2006; Wang et al. 1997). Composite heavy metal exposure can lead to more severe oxidative stress than single heavy metal exposure (Sun et al. 2015). In addition, PAHs have been shown to induce ROS generation in cells (He et al. 2022; Onduka et al. 2017). Although PAHs have a low proportion in PM2.5, they have high toxicity and carcinogenicity. Previous studies have shown that PAHs with high ring numbers have higher toxicity.
In addition to oxidative stress, inflammatory damage is also considered an important mechanism of cell damage and a major precursor to cell apoptosis (Yin et al. 2015). Therefore, we measured TNF-α and IL-6 levels in the supernatant of A549 cells and HCE-T cells. The results showed a significant dose-dependent relationship between TNF-α and IL-6 levels in both types of cells, and A549 cells are more sensitive to the expression of inflammatory factors than HCE-T cells. Meanwhile, A549 cells are more sensitive to the increase in cell apoptosis rate after PM2.5 exposure. According to reports, metals in particulate matter are the main components that induce inflammatory damage, and the release of inflammatory factors is related to the content of Cu and Zn (Deng et al. 2023; Hosseini et al. 2021; Schneider et al. 2020). The water-soluble components in PM2.5 can quickly enter cells and cause cellular inflammatory damage. NH4+ can disrupt cell homeostasis by altering intracellular pH, induce apoptosis, activate cellular inflammatory responses leading to cell damage (Lagadic-Gossmann et al. 2004; Shrode et al. 1997). PM2.5 can trigger endothelial cell activation by upregulating the IL-6 dependent JAK1/STAT3 pathway (Hu et al. 2016). Inflammatory damage can also be induced by activating transcription factors such as NF-kB and AP-1 (Potnis et al. 2013; Xu et al. 2019). These studies indicate that exposure to PM2.5 can induce cellular inflammatory damage and subsequently induce cell apoptosis. Some studies have shown that oxidative damage caused by high exposure to PM2.5 may be a key factor in the high expression of inflammatory factors. ROS is an important messenger that activates various cellular signaling pathways and can activate the expression of inflammatory genes. In addition, PM2.5-induced inflammatory damage can be significantly inhibited by antioxidants, indicating that the release of cytokines requires oxidative stress (Santos et al. 2015). Inconsistent research results demonstrate that the complex components of PM2.5 are important participants in PM2.5-induced inflammatory damage.
Due to the presence of carcinogenic metals and PAHs in PM2.5, studying the genotoxicity of PM2.5 is particularly important. These metals can induce DNA damage and impair DNA repair mechanisms, leading to genomic instability and increased risk of disease (Cavallo et al. 2009). A recent study has shown that γ-H2AX levels appear to increase with increasing cadmium levels in PM samples (Engels et al. 2023). PAHs are highly toxic and carcinogenic (Chen et al. 2021; Yu et al. 2015). PAHs can penetrate cells and produce reactive metabolites that can covalently bind to DNA, resulting in genotoxicity (Bai et al. 2017; Stading et al. 2021). This DNA damage can lead to mutations and lead to the development of cancer. When DNA in a cell is damaged, a phosphorylation event occurs on a histone variant called H2AX, resulting in its transformation into γ-H2AX. The expression level of γ-H2AX can directly reflect the degree of DNA damage and repair. DNA damage can be assessed by detecting γ-H2AX. In this study, we used immunofluorescence to detect DNA damage and found that γ-H2AX expression was significantly increased in A549 and HCE-T cells exposed to PM2.5, suggesting that A549 and HCE-T cells exhibited significant DNA damage at low doses of acute exposure, which became increasingly severe with increasing exposure time and dose. In addition, the DNA-damaging effect of PM2.5 on HCE-T cells showed a greater time dependence than that of A549 cells. Yang et al. found that PM2.5 in six Chinese cities induced γ-H2AX production in human bronchial epithelioid cells (BEAS-2B) (Yang et al. 2016). Borgie et al. collected PM2.5 from rural and urban Lebanon and found that both showed induction of γ-H2AX, and PM2.5 showed significant genotoxicity to cells (Borgie et al. 2015). This also supports our results. ROS-mediated oxidative damage is considered to be one of the important mechanisms of DNA damage in cells. In our research results, we measured ROS and found that PM2.5 induced ROS generation, disrupting the homeostasis of cellular oxidative stress. We observed that HCE-T cells exhibited significant time-dependent effects on the γ-H2AX level compared to A549 cells. This observation is consistent with the generation of ROS. It suggests that HCE-T cells have a higher sensitivity to ROS-induced DNA damage, as reflected by the increased γ-H2AX level over time. Recent studies have also shown that PM2.5 induces DNA double-strand breaks in HCEC and 16HBE cells and increases the expression of repair protein γ-H2AX through ROS accumulation and oxidative stress. Gao et al. found that PM2.5 can cause significant DNA damage in HCECs, and ROS inhibitor NAC can partially eliminate this effect, indicating that PM2.5-induced oxidative stress plays an important role in DNA damage (Gao et al. 2016).
Conclusion
This study showed that PM2.5 induced cytotoxic effects on A549 and HCE-T cells through cell viability, apoptosis rate, oxidative stress, inflammation damage, and genotoxicity at the ALI. The results indicate that PM2.5 has dose-and time-dependent effects on cell viability, apoptosis rate, ROS generation, and DNA damage, while inflammatory factors only exhibit dose-dependent effects. A549 cells were more sensitive to apoptosis and inflammation, while HCE-T cells exhibited rapid expression of ROS and γ-H2AX. Increased abundance of ROS in cells is crucial in PM2.5-induced cytotoxicity. Further research is needed to assess the potential risks to human health of each component of PM2.5 and the exact mechanisms involved and develop effective strategies to mitigate the health risks posed by PM2.5 on humans.
Data availability
Available upon request by contacting the author.
References
Abbas I et al (2019) In vitro evaluation of organic extractable matter from ambient PM(2.5) using human bronchial epithelial BEAS-2B cells: Cytotoxicity, oxidative stress, pro-inflammatory response, genotoxicity, and cell cycle deregulation. Environ Res 171:510–522
Abdel-Shafy HI, Mansour MSM (2016) A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation. Egypt J Pet 25:107–123
Aufderheide M (2005) Direct exposure methods for testing native atmospheres. Exp Toxicol Pathol 57:213–226
Bai H et al (2017) Chronic polycyclic aromatic hydrocarbon exposure causes DNA damage and genomic instability in lung epithelial cells. Oncotarget 8:79034–79045
Bengalli R et al (2013) Effect of nanoparticles and environmental particles on a cocultures model of the air-blood barrier. Biomed Res Int 2013:801214
Bihari, P, et al., (2008) Optimized dispersion of nanoparticles for biological in vitro and in vivo studies. Particle Fibre Toxicol 5. https://doi.org/10.1186/1743-8977-5-14
Bondy SC, Kirstein S (1996) The promotion of iron-induced generation of reactive oxygen species in nerve tissue by aluminum. Mol Chem Neuropathol 27:185–194
Borgie M et al (2015) Genotoxic and epigenotoxic effects of fine particulate matter from rural and urban sites in Lebanon on human bronchial epithelial cells. Environ Res 136:352–362
Cavallo, D, et al., (2009) Direct-oxidative DNA damage and apoptosis induction in different human respiratory cells exposed to low concentrations of sodium chromate. J Appl Toxicol 30. https://doi.org/10.1002/jat.1487
Chen Y-Y et al (2021) Polycyclic aromatic hydrocarbon metabolites and mortality risk in an adult population. Environ Geochem Health 43:37–45
Chen W et al (2024) Acute exposure to seasonal PM2.5 induces toxicological responses in A549 cells cultured at the air-liquid interface mediated by oxidative stress and endoplasmic reticulum stress. Environ Res 248:118283
Courcot E et al (2012) Xenobiotic metabolism and disposition in human lung cell models: comparison with in vivo expression profiles. Drug Metab Dispos 40:1953–1965
Crobeddu B et al (2020) Lung antioxidant depletion: a predictive indicator of cellular stress induced by ambient fine particles. Environ Sci Technol 54:2360–2369
Deng X et al (2013) PM2.5-induced oxidative stress triggers autophagy in human lung epithelial A549 cells. Toxicol in Vitro 27:1762–1770
Deng X et al (2014) Airborne fine particulate matter induces multiple cell death pathways in human lung epithelial cells. Apoptosis 19:1099–1112
Deng H et al (2023) The dysregulation of inflammatory pathways triggered by copper exposure. Biol Trace Elem Res 201:539–548
Di, A, et al., (2020) Chemical characterization of seasonal PM2.5 samples and their cytotoxicity in human lung epithelial cells (A549). Int J Environ Res Public Health 17. https://doi.org/10.3390/ijerph17124599
Engels, SM, et al., (2023) Particulate matter composition drives differential molecular and morphological responses in lung epithelial cells. PNAS Nexus. 3. https://doi.org/10.1093/pnasnexus/pgad415
Fang, Y, et al., (2021) Activation of the ROS/HO-1/NQO1 signaling pathway contributes to the copper-induced oxidative stress and autophagy in duck renal tubular epithelial cells. Science of the Total Environment. 757. https://doi.org/10.1016/j.scitotenv.2020.143753
Gao ZX et al (2016) Assessment of DNA damage and cell senescence in corneal epithelial cells exposed to airborne particulate matter (PM2.5) collected in Guangzhou. China Invest Ophthalmol vis Sci 57:3093–3102
Ge, P, et al., (2023) Chemical characteristics and cytotoxicity to GC-2spd(ts) cells of PM2.5 in Nanjing Jiangbei New Area from 2015 to 2019. Toxics 11. https://doi.org/10.3390/toxics11020092
Gonzalez-Rivera, JC, et al., (2020) RNA oxidation in chromatin modification and DNA-damage response following exposure to formaldehyde. Sci Rep 10. https://doi.org/10.1038/s41598-020-73376-7
Guenette J et al (2022) Establishing an air-liquid interface exposure system for exposure of lung cells to gases. Inhalation Toxicol 34:80–89
He, J, et al., (2022) Environmental dose of 16 priority-controlled PAHs mixture induce damages of vascular endothelial cells involved in oxidative stress and inflammation. Toxicol Vitro 79. https://doi.org/10.1016/j.tiv.2021.105296
Hosseini, R, et al., (2021) Zinc supplementation is associated with a reduction in serum markers of inflammation and oxidative stress in adults: a systematic review and meta-analysis of randomized controlled trials. Cytokine 138. https://doi.org/10.1016/j.cyto.2020.155396
Hu H et al (2016) Fine particulate matter induces vascular endothelial activation via IL-6 dependent JAK1/STAT3 signaling pathway. Toxicol Res 5:946–953
Jia, R, et al., (2023) PM2.5 induce myocardial injury in hyperlipidemic mice through ROS-pyroptosis signaling pathway. Ecotoxicol Environ Safety 254. https://doi.org/10.1016/j.ecoenv.2023.114699
Jin X et al (2018) Mitochondrial damage mediated by ROS incurs bronchial epithelial cell apoptosis upon ambient PM2.5 exposure. J Toxicol Sci 43:101–111
Kaur K et al (2022) Comparison of biological responses between submerged, pseudo-air-liquid interface, and air-liquid interface exposure of A549 and differentiated THP-1 co-cultures to combustion-derived particles. J Environ Sci Health Part a-Toxic/hazardous Substances Environ Eng 57:540–551
Kim DH et al (2022) Particulate matter 2.5 promotes inflammation and cellular dysfunction via reactive oxygen species/p38 MAPK pathway in primary rat corneal epithelial cells. Cutan Ocul Toxicol 41:273–284
Lagadic-Gossmann D et al (2004) Alterations of intracellular pH homeostasis in apoptosis: origins and roles. Cell Death Differ 11:953–961
Leclercq B et al (2016) Differential responses of healthy and chronic obstructive pulmonary diseased human bronchial epithelial cells repeatedly exposed to air pollution-derived PM4. Environ Pollut 218:1074–1088
Lin A-J et al (2007) Oxidative stress and DNA damages induced by cadmium accumulation. J Environ Sci 19:596–602
Liu J et al (2019) Fine particulate matters induce apoptosis via the ATM/P53/CDK2 and mitochondria apoptosis pathway triggered by oxidative stress in rat and GC-2spd cell. Ecotoxicol Environ Saf 180:280–287
Loret T et al (2016) Air-liquid interface exposure to aerosols of poorly soluble nanomaterials induces different biological activation levels compared to exposure to suspensions. Part Fibre Toxicol 13:58
Lugrin J et al (2014) The role of oxidative stress during inflammatory processes. Biol Chem 395:203–230
Miao Q et al (2019) Cigarette smoke induces ROS mediated autophagy impairment in human corneal epithelial cells. Environ Pollut 245:389–397
Möller B, Villiger PM (2006) Inhibition of IL-1, IL-6, and TNF-α in immune-mediated inflammatory diseases. Springer Semin Immunopathol 27:391–408
Muehlfeld C et al (2008) Interactions of nanoparticles with pulmonary structures and cellular responses. American Journal of Physiology-Lung Cellular and Molecular Physiology 294:L817–L829
Mukherjee, AG, et al., (2022) Heavy metal and metalloid-induced reproductive toxicity. Environ Toxicol Pharmacol 92. https://doi.org/10.1016/j.etap.2022.103859
Muruganandam N et al (2023) Meandered and muddled: a systematic review on the impact of air pollution on ocular health. Environ Sci Pollut Res Int 30:64872–64890
Niu, BY, et al., (2020) Effects of DNA damage and oxidative stress in human bronchial epithelial cells exposed to PM(2.5) from Beijing, China, in winter. Int J Environ Res Public Health 17. https://doi.org/10.3390/ijerph17134874
Offer, S, et al., (2022) Effect of atmospheric aging on soot particle toxicity in lung cell models at the air-liquid interface: differential toxicological impacts of biogenic and anthropogenic secondary organic aerosols (SOAs). Environ Health Perspect 130. https://doi.org/10.1289/EHP9413
Onduka T et al (2017) Photo-induced toxicity and oxidative stress responses in Tigriopus japonicus exposed to nitro-polycyclic aromatic hydrocarbons and artificial light. Chemosphere 169:596–603
Pang, Y, et al., (2020) In-vitro human lung cell injuries induced by urban PM2.5 during a severe air pollution episode: variations associated with particle components. Ecotoxicol Environ Safety 206. https://doi.org/10.1016/j.ecoenv.2020.111406
Potnis PA et al (2013) Role of NF-κB in the oxidative stress-induced lung inflammatory response to iron and selenium at ambient levels. Toxicol Res 2:259–269
Qi Z et al (2019) Water soluble and insoluble components of PM(2.5) and their functional cardiotoxicities on neonatal rat cardiomyocytes in vitro. Ecotoxicol Environ Saf 168:378–387
Qiu Y-N et al (2019) PM2.5 induces liver fibrosis via triggering ROS-mediated mitophagy. Ecotoxicol Environ Saf 167:178–187
Rahmatinia T et al (2022) The effect of PM(2.5)-related hazards on biomarkers of bronchial epithelial cells (A549) inflammation in Karaj and Fardis cities. Environ Sci Pollut Res Int 29:2172–2182
Santos CL et al (2015) Lipoic acid and N-acetylcysteine prevent ammonia-induced inflammatory response in C6 astroglial cells: The putative role of ERK and HO1 signaling pathways. Toxicol in Vitro 29:1350–1357
Schneider, T, et al., (2020) The copper/zinc ratio correlates with markers of disease activity in patients with inflammatory bowel disease. Crohn’s Colitis 360. 2, otaa001-otaa001. https://doi.org/10.1093/crocol/otaa001
Shan X et al (2021) PM(2.5) and the typical components cause organelle damage, apoptosis and necrosis: role of reactive oxygen species. Sci Total Environ 782:146785
Shrode LD et al (1997) Role of intracellular pH in proliferation, transformation, and apoptosis. J Bioenerg Biomembr 29:393–399
Stading R et al (2021) Molecular mechanisms of pulmonary carcinogenesis by polycyclic aromatic hydrocarbons (PAHs): implications for human lung cancer. Semin Cancer Biol 76:3–16
Sui S et al (2020) Pollution characteristics and chronic health risk assessment of metals and metalloids in ambient PM2.5 in Licheng District, Jinan. China Environ Geochem Health 42:1803–1815
Sun L et al (2015) Adverse effects of coal combustion related fine particulate matter (PM2.5) on nematode Caenorhabditis elegans. Sci Total Environ 512:251–260
Tomasek, I, et al., (2016) Combined exposure of diesel exhaust particles and respirable Soufriere Hills volcanic ash causes a (pro-)inflammatory response in an in vitro multicellular epithelial tissue barrier model. Particle Fibre Toxicol 13. https://doi.org/10.1186/s12989-016-0178-9
Wakeman TP (2006) ATR regulates hexavalent chromium-induced S-phase checkpoint through phosphorylation of SMC1 [J]. Mutat Res-Genet Toxicol Environ Mutagen 610(1–2):14–20
Wang JF et al (1997) High mobility group proteins 1 and 2 recognize chromium-damaged DNA. Carcinogenesis 18:371–375
Wang J et al (2017) Particulate matter pollution over China and the effects of control policies. Sci Total Environ 584:426–447
Wang R et al (2019) Complex to simple: in vitro exposure of particulate matter simulated at the air-liquid interface discloses the health impacts of major air pollutants. Chemosphere 223:263–274
Wang W et al (2022) Internal exposure and distribution of airborne fine particles in the human body: methodology, current understandings, and research needs. Environ Sci Technol 56:6857–6869
Xu X et al (2019) MAPK/AP-1 pathway activation mediates AT1R upregulation and vascular endothelial cells dysfunction under PM2.5 exposure. Ecotoxicol Environ Saf 170:188–194
Yang L et al (2016) Pro-inflammatory response and oxidative stress induced by specific components in ambient particulate matter in human bronchial epithelial cells. Environ Toxicol 31:923–936
Yang, Q, et al., (2019) Effects of fine particulate matter on the ocular surface: an in vitro and in vivo study. Biomed Pharmacotherapy 117. https://doi.org/10.1016/j.biopha.2019.109177
Yin G et al (2015) Lipid peroxidation-mediated inflammation promotes cell apoptosis through activation of NF-kappaB pathway in rheumatoid arthritis synovial cells. Mediators Inflamm 2015:460310–460310
Yu Y et al (2015) Risk of human exposure to polycyclic aromatic hydrocarbons: a case study in Beijing. China Environmental Pollution 205:70–77
Zhang L et al (2016) Hazard posed by metals and As in PM2.5 in air of five megacities in the Beijing-Tianjin-Hebei region of China during APEC. Environ Sci Pollut Res 23:17603–17612
Zhang H et al (2023) Zinc inhibits lead-induced oxidative stress and apoptosis of ST cells through ROS/PTEN/PI3K/AKT axis. Biol Trace Element Res 202(3):980–989
Zou Y et al (2016) Water soluble and insoluble components of urban PM2.5 and their cytotoxic effects on epithelial cells (A549) in vitro. Environ Pollut 212:627–635
Funding
This work was supported by the National Social Science Foundation of China (Grant numbers: 22176100, 21976094).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Wankang Chen, Pengxiang Ge, and Xiaoming Liu. The first draft of the manuscript was written by Wankang Chen. Writing—reviewing and editing were performed by Minjun Deng, Zhenyu Lu, Zhansheng Yan, and Junfeng Wang. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Diane Purchase
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, W., Ge, P., Deng, M. et al. Toxicological responses of A549 and HCE-T cells exposed to fine particulate matter at the air–liquid interface. Environ Sci Pollut Res 31, 27375–27387 (2024). https://doi.org/10.1007/s11356-024-32944-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-32944-4