Abstract
The present study aimed to investigate the occurrence of caffeine residues in the Nile River according to drainage of treated wastewater at Assiut, Egypt, and the effects of physicochemical parameters and zinc on its concentration. Four different sites were selected to perform the study: S, wastewater treatment plant (WWTP) canal (source site); J, a junction site between WWTP canal and the Nile; R, a reference site in the Nile before J site; and A, a site located after J site in the Nile. Water and sediment samples were collected in Summer 2022 and Winter 2023. Caffeine and Zn concentrations and physicochemical parameters were measured in the collected samples. The caffeine concentrations in water samples ranged from 5.73 to 53.85 μg L−1 at S in winter and summer, respectively, while those in sediment ranged from 0.14 mg kg−1 at R in winter to 1.54 mg kg−1 at S in summer. Caffeine and Zn concentrations were higher in summer samples. The Water Quality Index (WQI) of the collected samples recorded the lowest values in winter season at S and J sites. The study found that caffeine and zinc concentrations are positively correlated with water temperature and conductivity, while negatively correlated with pH. The association between caffeine and Zn highlights the environmental impact of heavy metals and pharmaceutical residues, and stresses the need for future research on these interactions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many chemicals and compounds that are not controlled yet are becoming increasingly harmful to the quality of freshwater ecosystems, such as pharmaceuticals (PhACs) and personal care products (PCPs) (Ortúzar et al. 2022). Given the growing use of PhACs in human and animal medicine (Ebele et al. 2020), active chemical residues in ocean, surface water, groundwater and even drinking water are becoming more common (Ebele et al. 2020; Hawash et al. 2023). Recently, the continuous discharge of pharmaceutical and personal care products (PPCPs) in natural waters have received a lot of interest (Korekar et al. 2020; Mohammad and Abd El-wakeil 2021) and it was comprehensively discovered in the worldwide environment (Hawash et al. 2023).
Pharmaceutical residues are chemical composites that contaminate waterways and soil, producing severe difficulties for environmental health and non-target organisms (Mohammad et al. 2021). Furthermore, through food chains, some plants can carry these chemicals from the soil to animals and humans (Tiwari et al. 2017; Mohammad et al. 2021). Water pollution by PhACs has been a concern since the 1990s (Doerr-MacEwen and Haight 2006), with findings revealing the presence of pharmaceutical chemicals and their metabolites in the ng L−1 to mg L−1 range in freshwater environments. PhACs pass in the aquatic environment via public effluents, and detoxification methods in wastewater treatment plants (WWTPs) appear insufficient to control them. The consistent outflow immediately affects the aquatic habitat, giving it pseudo constancy (Hernando et al. 2006).
Caffeine is a central nervous system stimulant (Asghar et al. 2018). As a common ingredient of caffeinated food and beverages (e.g. chocolate, coffee, cocoa, tea, dairy desserts and soft drinks), caffeine is the most extensively used medication in the world, accounting for cold remedies, analgesics, stimulants and illegal narcotics (Kosma et al. 2014), as well as one of the most representative pharmaceutical residue pollutants detected in the water environment (Pires et al. 2016). Concentrations (ng L−1) of caffeine were detected in surface water in various regions of the world as well as in tap/drinking water (Hawash et al. 2023). Lv et al. (2019) detected high levels of caffeine (607.85 ng L−1) in drinking water in China. However, it was discovered that locations out of human impact, like Antarctica, are affected by caffeine pollution (Li et al. 2020a). Abdallah et al. (2019) determined the presence of 30 PPCPs, including caffeine, in the effluent of five WWTPs and five surface water samples collected (7 to 54 ng L−1) in Egypt’s Assiut Governorate. Caffeine concentrations in the environment vary from 2 to 1600 ng L−1, with higher levels recorded for estuary and coastal waters (Korekar et al. 2020; Li et al. 2020b). Li et al. (2020b) referred to caffeine as an indicator for anthropogenic pollution as well as a source-specific indicator for wastewater in surface waters.
Despite the fact that caffeine has outstanding removal efficiency during wastewater treatment, the amount of caffeine that enters the water is substantially greater than that which is degraded (Zhu et al. 2013). Furthermore, caffeine residue in the aquatic environment is exceptionally stable, with a reported half-life of 100–240 days (Hillebrand et al. 2012). Caffeine is thus classed as an emerging pollutant, a drug that is not currently monitored (USEPA 2016), but should be included in future laws because it is commonly found and can impair aquatic biota like algae, plankton, benthos and fish (Stuart et al. 2012). Caffeine’s continual presence in aquatic habitats creates interest in studying its occurrence, concentration and bioaccumulation in freshwater ecosystem.
Heavy metals are severe pollutants in the environment due to their toxicity, persistence, pervasive nature and non-biodegradability (Pekey 2006; Wu et al. 2016) and have a variety of adverse sub-lethal and deadly impacts on aquatic organisms (Peters et al. 1997). Additionally, heavy metal pollution can have a disastrous impact on the recipient environment’s biological balance and a variety of aquatic creatures (Vosyliene and Jankaite 2006). Heavy metals pose a threat due to their inability to biodegrade and their ability to accumulate in soil, where they are transferred to organisms that live in water via nutrition or respiration, and then bioaccumulation of higher-level organisms (Gedik and Boran 2013). Heavy metal pollution is primarily caused by human activities like mining, smelting and foundries, as well as leaching from sources like landfills, waste dumps and roadworks. Secondary sources include agricultural use and natural factors like volcanic activity and erosion (Briffa et al. 2020).
One of the most important heavy metals is zinc (Zn), which is the second most abundant trace metal in the human body after iron. Zn is an essential trace element that plays an important role in the growth and development of animals and humans (Read et al. 2019). Although Zn is necessary for life, it is hazardous in large amounts (Taylor et al. 1982) and, through bioaccumulation processes, might harm creatures at higher trophic levels in the food chain, leading to significant impacts on human life and ecosystems (Ip et al. 2007). Zhou et al. (2016a) showed that bioaccumulation of Zn in the human body caused by a heavy metal–contaminated diet can impair the immune system and disrupt the high-density lipoprotein. In the pharmaceutical sector, zinc compounds are utilised as components in commodities such as vitamin supplements, diaper rash ointments, sunblock, deodorants and antidandruff shampoos (McComb et al. 2014). As the whole world is grappling with coronavirus disease 2019 (COVID-19) pandemic and the lack of clinically effective therapies, attention is shifting to different ways of strengthening the immune system. Zn has produced a lot of excitement as one of the promising applicants to reduce the harshness of COVID-19 infection. Zn, a well-known anti-inflammatory and antioxidant mineral contained in diet, is currently being employed in numerous studies against COVID-19 (Pal et al. 2021). Therefore, world’s zinc production is still increasing. This essentially means that more zinc enters to environment. Zn concentrations in many freshwaters are increased by domestic and industrial waste or other sources (Taylor et al. 1982). Zn is considered as one of the utmost extensively known pollutants in the aquatic ecosystems (Brinkman and Johnston 2012).
WWTPs are considered a main input path for micropollutants into aquatic environments (Korekar et al. 2020; Li et al. 2020b). The primary objective of the present study was to investigate the occurrence and concentrations of caffeine residues and Zn in the Nile River according to drainage of WWTPs at Assiut Province, Egypt. Also, the study aimed to assess some physicochemical parameters (air/water temperature, pH, electric conductivity, total dissolved salts, turbidity, dissolved oxygen, organic matter, phosphorus, nitrate and ammonia) and their interaction on caffeine and Zn concentrations during extreme summer and winter seasons. Additionally, the studied physicochemical parameters were used to assess and calculate Water Quality Index (WQI) at investigated sites during studied seasons.
Materials and methods
Sampling
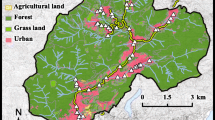
The collection of water and sediment samples was carried out on the 31st of July 2022 (summer) and 2nd of February 2023 (winter) from Assiut Province, Egypt (27° 14′ N, 31° 11′ E). Samples were collected in triplicates. Sediment samples were stored in plastic cases, while water samples were kept in 1.5-L polyethylene bottles. The samples were kept cold by being placed in an icebox filled with ice prior to reaching the laboratory for analysis. Four different sites were selected to perform the present study (Fig. 1): site 1 (S), a treated wastewater canal (source of pollution) where treated water is discharged from wastewater treatment plant effluent (Arab El-Madabegh WWTP); site 2 (J), a junction site where the water of the wastewater treatment canal meets with water flow of the Nile; site 3 (R), a reference site in the Nile River before the junction site; and site 4 (A), a site located after the junction point in the Nile River.
Maps showing the study sites at Assiut Governorate, Egypt. S, source-of-pollution site, where treated water is discharged from wastewater treatment plant effluent (Arab El-Madabegh WWTP); J, junction site where the water of the wastewater treatment canal meets with water flow of the Nile; R, reference site in the Nile River before the junction site; A, site located after the junction point in the Nile River
Physicochemical parameters
Physicochemical investigations of samples were carried out in accordance with the protocols outlined in the American Public Health Association (APHA 2017). During sampling, air temperature/water temperature (AT/WT, °C), water pH and electrical conductivity (Cond, μS cm−1) were in situ using an Eutech instrument (EcoScan pH 6). A total dissolved solid (TDS, ppm) was measured by a digital TDS handheld meter (hold). Transparency (turbidity (Turb), cm) was assessed by a white/black Secchi disc (20 cm in diameter). Dissolved oxygen (DO, mg L−1) was determined by using a Mic portable water quality meter (model 98725). In Central Laboratory for Chemical Analysis, Faculty of Agriculture, Assiut University, sediment organic matter (OM, %) was determined according to Ben-Dor and Banin (1989). Water phosphate (PO4, mg L−1), nitrate (NO3, mg L−1) and ammonia (NH4, mg L−1) were determined using a spectrophotometer according to APHA-AWWA-WPCF (1989). Hanna Instruments set kits of PO4, NO3 and NH4 were used to perform the analyses. Concentrations of Zn in water and sediment samples were determined by using the inductively coupled plasma emission spectrometer (iCAP 6200) according to Jackson (1974). The wavelength used for the detection and measurement of Zn was 213.856 nm. The limit of quantification (LOQ) was 0.0034 ppm with 98.78% confidence level. The precision of the Zn concentration was validated by repeating every sample three times.
Determination of caffeine concentration
Caffeine concentrations in sediment and water samples were determined in Multidisciplinary Research Center of Excellence, Assiut University (MIRCE), by means of a single flow-through UV multiparameter sensor (a multiparameter-responding flow-through system with solid-phase UV spectrophotometric detection (a multiparameter optosensor)) (Vidal et al. 2003). The accuracy of caffeine detection was calculated with triple measurements. The linearity of the calibration range was between 0.2 and 2 ppm. The concentration reached the detection zone and was measured at 273 nm. The LOQ was 0.1 ppm with 99.9% confidence level.
Determination of WQI
WQI used refers to the CCMEWQI method (CCME 2001) and was settled to estimate the overall water quality status of investigated samples. Ten water quality parameters were used. These parameters are temperature (°C), pH, conductivity (μS cm−1), dissolved oxygen (mg L−1), total dissolved solids (mg L−1), nitrate (mg L−1), ammonium (mg L−1), phosphate (mg L−1), turbidity (nephelometric turbidity units (NTU)) and Zn concentration (mg L−1). It is worth mentioning that the turbidity Secchi disc transparency measurements were converted into standard NTU according to Baughman et al. (2015). The minimum Egyptian standards for the water quality of the Nile River according to Law 48/1982 (EEAA 1999) were used for WQI calculation. There are five classes of the WQI to describe water quality as excellent, good, fair, marginal and poor when the value of the WQI lies between 95 and 100, between 80 and 94, between 65 and 79, between 45 and 64, and between 0 and 44, respectively.
Statistical analysis
Excel Office 2013, IBM SPSS Statistics (version 20) and PAST4 program performed data summary and analysis. Two-way analysis of variance (ANOVA) was applied to investigate for significant differences of physicochemical parameters between the investigated sites and seasons followed by the Duncan test to determine pairwise differences between means. Pearson correlation and stepwise multiple regression were used to consider association between the investigated physicochemical parameters and caffeine and Zn concentrations. The PAST4 program was used to perform the distance-based two-way permutational multivariate analysis of variance (PERMANOVA) to investigate the influence of physicochemical parameters on collected samples followed by PERMANOVA pairwise tests to verify the significance of the differences among samples. After standardizing the collected data, a hierarchical cluster and principal component analyses (PCA) of the mean values of investigated physicochemical parameters and caffeine and Zn concentrations were applied.
Results
Physicochemical parameters
Table 1 shows the summarized results of physicochemical parameters at the study sites during the investigated seasons. Statistical result showed that the differences among sites were significant for all investigated parameters (p < 0.05), whereas the differences between seasons were non-significant in the case of transparency and dissolved oxygen and significant for the rest of parameters (Supplementary material 1). The mean temperature of the collected water samples at the four sites ranged from 19.43 °C in winter to 30.43 °C in summer, while air temperature changes ranged from 15.57 °C in winter to 38.29 °C in summer. Water temperatures at S and J sites showed statistically significant higher values than those at R and A sites (Fig. 2).
The pH of water samples in the study ranged from 6.28 to 8.23 in summer at S site and in winter at R site, respectively (Table 1). Water pH was in the alkaline range at R and A sites while it was slightly acidic in S and J sites (Fig. 2). The mean EC of the analysed water samples at the four sites fluctuated between 40 and 46 μS cm−1. The TDS concentration of the collected water samples was within the range between 131.33 and 561.00 ppm (Table 1). The S site recorded the highest values for both conductivity and TDS followed by the J site (Fig. 2). DO concentrations showed variable results according to site and season. In summer, DO ranged from 1.08 to 6.87 mg L−1 at J and R sites, respectively. In winter, DO ranged from 1.33 to 6.70 mg L−1 at S and R sites, respectively (Table 1). DO concentrations were significantly higher at R and A sites than at S and J sites (Fig. 2).
The highest sediment OM value was 23.52 in winter at S site, and the lowest was 1.94 in summer at R site (Table 1). OM was significantly higher at S site than at J, A and R sites (Fig. 2). The concentration levels of PO4 of the collected water samples at four sites ranged from 0.10 to 7.43 mg L−1, while the level of NO3 ranged between 13.86 and 63.00 mg L−1. NH4 concentrations showed variable results according to site and season. In summer, NH4 ranged from 9.15 to 32.84 mg L−1 at R and S sites, respectively. In winter, NH4 ranged from 21.90 to 54.36 mg L−1 at A and J sites, respectively (Table 1). PO4, NO3 and NH4 levels were significantly higher at S and J sites than at A and R sites (Fig. 2).
Caffeine and Zn concentrations in water and sediment
The present results of caffeine and Zn concentrations in water and sediment for the study sites during the investigated seasons are represented in Table 2. Both caffeine and zinc showed fluctuations among study samples. Statistical results showed that these variations were significant among sites and seasons in case of water and sediment caffeine and sediment Zn while the variation of Zn in water was non-significant (Supplementary material 2).
The variations in water caffeine (WCaf) ranged between 23.72 μg L−1 at R site and 53.85 μg L−1 at S site in summer, while in winter, the maximum value was recorded at both J and A sites (8.13 μg L−1), and the minimum value was recorded at S site (5.73 μg L−1). The values of sediment caffeine (SCaf) ranged from 0.14 mg kg−1 at R site during winter to 1.54 mg kg−1 at S site during summer (Table 2). S site caffeine in both water and sediment showed significantly higher concentrations, in contrast to R site which showed the lowest concentration (Fig. 3).
The mean water zinc (WZn) of studied sites ranged from 0.08 mg L−1 at S and J sites in winter to 0.22 mg L−1 at A site in winter, while sediment zinc concentrations ranged between 28.59 mg kg−1 at J site in winter and 155.02 mg kg−1 at J site in summer (Table 2). The differences in WZn concentrations were statistically non-significant, while sediment zinc (SZn) concentrations at S, J and A sites was significantly higher than that at R site (Fig. 3).
The extent of differences between collected samples
Two-way PERMANOVA for investigated physicochemical parameters and caffeine and Zn concentrations in water and sediment of the studied sites and seasons indicated that there are significant differences among sites (F = 21.815, p = 0.0001) seasons (F = 54.107, p = 0.0001) and interactions (F = 3.638, p = 0.0012) (Table 3). Pairwise tests indicated that S and J sites differ significantly from R and A sites; however, there were no significant differences between S and J sites and between R and A sites (Table 4). At a Euclidean distance of 5.5, the results classified the collected samples into three clusters. The first cluster consisted of winter samples from S and J sites. The second cluster consisted of summer samples from S and J sites. The third cluster consisted of samples from A and R sites in both winter and summer seasons (Fig. 4).
Water Quality Index
Figure 5 shows the WQI for the collected samples. The quality of water for the collected samples ranged from good to marginal quality. Samples collected from R and A sites during summer had a good quality (WQI = 83.9% and 84.7%, respectively). Winter samples from R (76.3%) and A (76.9%) sites had a fair quality. On the other hand, the quality of water samples from S (47.5% and 55.4%) and J (46.3% and 56.1%) sites (during winter and summer seasons, respectively) was marginal quality.
Effect of physicochemical parameters on caffeine and Zn concentrations
The correlations between the studied physicochemical parameters and caffeine and Zn concentrations in water and sediment are illustrated in Table 5. WCaf had a strong negative correlation with pH (r = − 0.688) and a strong positive correlation with AT, WT and Cond (r = 0.804, r = 0.898 and r = 0.857, respectively). SCaf had a strong positive correlation with AT, WT, Cond and WCaf (r = 0.853, r = 0.879, r = 0.869 and r = 0.885, respectively), whereas SCaf had a strong negative correlation with pH (r = − 0.0623). WZn showed a negative correlation with NO3 and NH4 (r = − 0.439 and r = − 0.430, respectively). SZn presented a weak negative relation with pH (r = − 0.476) and a positive relation with AT, WT, Cond, WCaf and SCaf (r = 0.433, r = 0.526, r = 0.513, r = 0.548 and r = 0.548, respectively).
Stepwise multiple regression was applied to select the most effective factors that have a significant impact on zinc and caffeine (Table 6). The results indicated that caffeine concentrations in water and sediment are correlated and mainly effected by temperature. WZn showed a negative correlation with NO3 while SZn had a positive correlation with SCaf and OM. The equations illustrating these relations are presented in Table 6. PCA revealed a direct correlation between WCaf and SCaf, WT, Cond, AT, WZn, Turb, DO and pH. However, there was a negative correlation between WCaf and SZn, PO4, TDS, OM, NH4 and NO3 (Fig. 6).
Principal component analysis (PCA) for the physicochemical parameters and caffeine and Zn concentrations in water and sediment at study sites during summer and winter seasons (after standardizing the collected data). Notation of variables: AT, air temperature (°C); WT, water temperature (°C); pH, water pH; Cond, conductivity (μS cm−1); TDS, total dissolved solids (ppm); Turb, turbidity (cm); DO, dissolved oxygen (mg L−1); OM, organic matter (%); PO4, phosphate (mg L−1); NO3, nitrate (mg L−1); NH4, ammonia (mg L−1); WCaf, water caffeine (μg L−1); SCaf, sediment caffeine (mg kg−1); WZn, water zinc (mg L−1); SZn, sediment zinc (mg kg−1)
Discussion
Studies on the presence of caffeine in surface waters have been published around the world. There are only two publications on caffeine residues in the freshwater environment in Africa (Li et al. 2020a; Hawash et al. 2023). The present results demonstrated that caffeine concentrations in surface water samples from the Nile River ranged from 7.13 μg L−1 (R site during winter) to 40.32 μg L−1 (A site during summer). Abdallah et al. (2019) recorded that caffeine concentration in the surface water of the Nile River and El-Ebrahimiya Canal in Assiut City ranged between 7 and 54 ng L−1. This indicates that caffeine concentration increased over time. Also, the recorded concentrations are relatively higher than those recorded in other rivers and streams around the world. For example, caffeine was detected at quantities ranging from 0.006 to 0.250 g L−1 in Swiss lakes and rivers (Poiger et al. 2003). Caffeine concentrations ranged from 0.014 to 0.907 μg L−1 in samples collected upstream from Canadian Avon, Cornwallis and Annapolis rivers (Ghoshdastidar et al. 2015). Česen et al. (2019) reported that caffeine concentrations in Slovenian and Croatian Sava River ranged from 0.370 to 1.39 μg L−1. The caffeine concentrations in the urban rivers in China ranged from 0.066 to 8.571 μg L−1 (Zhou et al. 2016b). On the other hand, caffeine was observed in high levels in the Henares River, Spain (0.475 to 0.515 mg L−1) (Martínez Bueno et al. 2011); Lebanon rivers (10.2 mg L−1) (Mokh et al. 2017); the UK rivers (which reached 23.778 mg L−1) (Ebele et al. 2017); and Jundiaí River, Brazil (19.30 mg L−1) (de Sousa et al. 2014).
The present study demonstrated that caffeine concentrations of treated wastewater at the S site (effluents of Arab El-Madabegh WWTP, Assiut) ranged from 5.73 to 53.85 μg L−1. This range of concentration is higher than the concentration of caffeine recorded in 2019 for other effluents of WWTP in Assiut City which ranged between 70 and 1739 ng L−1 (Abdallah et al. 2019). A similar increasing caffeine concentration in effluent of WWTP over time was recorded for Canadian WWTP effluent. In 2006, caffeine was detected in Canadian WWTP effluent at concentrations ranging from 0.0017 to 1.244 μg L−1 (Hua et al. 2006); however, in 2015, it ranged from 0.013 to 115.141 μg L−1 (Ghoshdastidar et al. 2015). A relatively high concentration of caffeine was detected in WWTP effluent all over the world. For examples, caffeine concentrations in effluents of WWTPs reached 13 mg L−1 in Kuwait (Smith et al. 2015), 18 mg L−1 in Germany (Bahlmann et al. 2012), 28.8 mg L−1 in western Saudi Arabia (Alidina et al. 2014) and 34.2 mg L−1 in England (Baker and Kasprzyk-Hordern 2013). Mijangos et al. (2018) discovered the highest quantity of caffeine (66 mg L−1) in the effluent of a WWTP in Spain’s Urdaibai Estuary (Gernika).
The current results showed that caffeine concentrations in sediment reached 1.54 mg kg−1 at source site during summer. This indicated that caffeine was more concentrated in the sediment than in the water. According to Zhao et al. (2013), sediment functions as a sink, accumulating chemicals that may be discharged back into the aquatic environment. In fact, comparable analytical data from river sediment remains scarce. Martín et al. (2010) detected caffeine at concentrations of 7.21 μg kg−1 in sediment of the Guadiamar River in Seville, Southern Spain. Also, caffeine (659 ng/g) was detected in sediment from the Msunduzi River, South Africa (Matongo et al. 2015). On the other hand, caffeine pollution levels in coastal and marine ecosystems have been determined through sediment and water analysis (Nodler et al. 2014). Caffeine concentrations in coastal sediments have been documented in a few studies, with values ranging from 1.90 to 12.20 ng/g in Spain (Maranho et al. 2015) and from 0.31 to 23.4 ng/g in Brazil (Beretta et al. 2014).
The water Zn concentrations in the investigated sites ranged from 0.08 mg L−1 (S and J sites) to 0.22 mg L−1 (A site). Moreover, Zn concentration of sediments ranged between 28.59 and 155.02 mg kg−1 at J site in winter and summer seasons, respectively. The levels of Zn in studied samples often exceeded CCME (2007) guidelines for aquatic life. Samples collected from A site showed a relatively higher concentration than those from other sites which may be related to the effect of fertilizer plant emissions at Manqabad close to it. Mohamed et al. (2014) showed that the dust, fumes and gases that come out from fertilizer plants containing heavy metals affect the nearby area where soil samples collected near phosphate fertilizer production plants were found to be substantially enriched in heavy metals.
Examination of seasonal variations revealed that caffeine and Zn concentrations in water and sediments were higher during summer season at all studied sites. Seasonal variation in the concentrations of PPCPs (Ebele et al. 2020) and heavy metals (Hussein et al. 2006; Ololade et al. 2023) has been reported. It seems that the changes in human activities among different seasons might underlie the variation in caffeine and Zn existence from season to another. It is worth mentioning that Egyptian government uses the high dam to rationalize water during the winter season when crops do not need much irrigation water, also to implement many maintenance, disinfection and industrial works in the Nile River and its branches. According to Jagoda et al. (2015), greater caffeine residue concentrations were observed in Rudawa River water in Poland during the summer than in the fall, which was attributed to increased human agricultural activities and recreation in this area during the summer. Similarly, in Greece, the daylight hours increase throughout the summer, causing the caffeine content to rise to 9.48 mg L−1 in the summer compared to 1.93 mg L−1 in the winter (Kosma et al. 2014).
The maximal caffeine concentration was reported to be 44.6 mg L−1 in dry weather flow but 32.9 mg L−1 in wet weather flow in research done in Spain (Del Río et al. 2013). In addition, the median caffeine content in Guanajuato, Mexico, was 31.1 mg L−1 during the dry season, compared to 12.4 mg L−1 during the rainy season (Estrada-Arriaga et al. 2016). Caffeine residue levels in the influent of a WWTP in Greece peaked in winter at a mean concentration of 4.97 mg L−1, according to Papageorgiou et al. (2016), since caffeinated food and beverages were predominantly consumed by the public during this season. Caffeine concentration increased from 0.051 to 0.857 g L−1 in the Annapolis Royal STP effluent, while it decreased from 0.910 to 0.077 g L−1 in the Digby STP effluent in a study conducted in Canada (Ghoshdastidar et al. 2015), indicating possible seasonal variation in treatment efficiency or caffeine consumption, considering population change due to visitors. Ebele et al. (2020) showed that there is little information available on the variables influencing seasonal fluctuation of PPCPs in surface water and groundwater around the world. They illustrated that seasonal variations in concentrations of PPCPs could be related to numerous aspects distinctive to the investigated local environment, such as dilution by agricultural activities, rainfall and local community medicinal usage habits. It seems that the precise relationship between caffeine pollution levels and seasonal human inputs is currently unknown.
Concerning the physicochemical parameters, the present results indicate spatial and seasonal variations among investigated samples. These differences plus the variations in the amount of caffeine and zinc indicated that characteristics of water from S and J sites are significantly different from R and A sites. According to these differences, water samples from R and A sites during both study seasons cluster in one group while summer samples and winter samples from S and J sites were separated into two groups (Fig. 3). Overall, S and J sites have higher values of water temperature, Cond, Turb, OM, PO4, NO3, NH4, caffeine concentrations and Zn in sediment. In contrast, they have lower values of pH and DO. This indicated the effect of discharging of treated wastewater in the Nile River. Many previous studies indicated the effect of wastewater on environmental characteristics of surface water (Abdel-Satar et al. 2017; Berger et al. 2017; Onwona Kwakye et al. 2021; Rahman et al. 2021; Abdo et al. 2022; Tanjung et al. 2022). The microbial respiration as well as decomposing activities in wastewater consume part of the dissolved oxygen, affecting the pH value reflected in the changes of the other parameters like increasing of values of nutrients and NH4 (Onwona Kwakye et al. 2021; Abdo et al. 2022). Another factor that contributed to a low level of DO reality to the greater water temperature during the summer season (Table 1) was reduction due to the inverse connection between dissolved oxygen and temperature (Ice 2008). The relatively high concentrations of nitrates at all investigated sites may relate to runoff or leakage from artificial fertilizers, and water from a wastewater treatment plant, in addition to sludge, animal dung and septic tank waste (Zeidan 2017).
The current results show that water temperature, Cond and OM values still meet the quality standards for aquatic life at all study samples. On the contrary, WZn and NH4 concentrations have exceeded the quality standards in all samples. Likewise, samples from S and J sites having turbidity, DO and PO4 do not meet Egyptian standards for the water quality of the Nile River (EEAA 1999) in both study seasons. During summer season, S and J sites have pH values lower than the quality standards, while during winter, they have NO3 concentration higher than the quality standards. Only during summer, TDS has exceeded the standard for water of S site. These variations of physicochemical parameters led to that WQI of the collected samples ranged between good quality (water from R and A sites during summer) and marginal quality (water from S and J sites during summer and winter seasons). The relatively low value of WQI for the collected sample may relate to the high NH4 and Zn concentrations which related to the wastewater. Abdel-Satar et al. (2017) concluded that unceasing discharge of contaminants, generally heavy metals and nutrients, impacted the river health and reduced their self-purification capability, which affected the usability of Nile water for a variety of applications. The lowest WQI values were recorded in S (47.5%) and J (46.3%) sites during winter season. This is related to a drop in the Nile flow level in winter (as mentioned before), which tends to concentrate the ions. According to Abdelmageed et al. (2022), the Nile water level decreased by about 2.5 m in winter. They illustrated that increasing pollution caused by the Nile River water level declines has become Egypt’s primary issue, particularly with the building of the Grand Ethiopian Renaissance Dam (Abdel-Satar et al. 2017). On the other side, seasonal variations in natural processes such as temperature affect the quality of water in river and cause different features for altered seasons (Vega et al. 1998).
The current statistical analyses indicated that caffeine concentration in water and sediment, as well as Zn concentration in sediment, is positively correlated with WT and Cond and negatively with pH. Bethke et al. (2023) conducted a literature study on the impact of temperature, pH and combination stresses on pharmaceutical toxicity to organisms in aquatic ecosystems. They explained that the bioavailability of such micropollutants is tightly dependent on temperature and pH (Puckowski et al. 2016). According to Shiller and Boyle (1985), acidification and decreasing pH of river water may result in a great increase in dissolved Zn. In addition, temperature is also known to affect other factors such as pH, conductivity, dissolved gases and different alkalinity profiles (Beniwal et al. 2021). Berger et al. (2017) revealed that caffeine concentration is correlated with electrical conductivity as well as nitrite, nitrate and ammonium in German rivers. Rahman et al. (2021) observed that temperature is substantially correlated with pH in Bangladesh’s Turag River. They concluded that due to the wide variety of temperature tolerance in aquatic life, water temperature may not be as essential in pure water in contrast to polluted water, where temperature can have a significant impact on other factors.
The obtained results revealed that accumulation of Zn in sediment is highly related to organic matter. Similarly, Ololade et al. (2023) found that all examined elements including Zn are positively linked with sediment organic content in rivers in Southwestern Nigeria, whereas Hahn et al. (2018) demonstrated that organic matter in sediment has a significant impact on heavy metal absorption from the overlaying surface water. The present study showed a strong positive association between caffeine and Zn concentrations in sediments. This synergistic interaction poses an important environmental impact of heavy metals and pharmaceutical residues, as well as their bioavailability as a joint concern to the environment and human health. This emphasises the need of researching for such interactions in future research.
Conclusion
The present results indicated that the discharge of treated wastewater in the Nile River is a significant source of caffeine and Zn in the environment. It seems that conventional WWTPs are incapable of removing caffeine and Zn completely. The caffeine and Zn residue levels in WWTP effluents peaked in the summer season, which showed a seasonal difference in treatment efficiency or caffeine consumption. The consumption of caffeine and Zn is expected to increase with the increase in the human population and activities. The variations in human social activities between seasons may impact anthropogenic inputs of caffeine and Zn in the environment, explaining the fluctuation in caffeine and Zn occurrence from season to season. However, the precise relationship between caffeine and Zn pollution levels and seasonal human inputs remains unclear. The decrease in Nile flow level during the winter season caused an increase in ion concentration in water, resulting in relatively lower WQI values for the collected samples in winter. The current results indicated that caffeine concentration in water and sediment is positively correlated with water temperature, conductivity and Zn concentration in sediment and negatively correlated with pH. As a result of the synergistic interaction between caffeine and Zn concentrations in the environment, great concerns should be raised about heavy metals and pharmaceutical residues having a significant environmental impact, which is a problem for ecological safety and human health. More attention should be paid to investigating such relationships in future research.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Abdallah MA, Nguyen KH, Ebele AJ, Atia NN, Ali HR, Harrad S (2019) A single run, rapid polarity switching method for determination of 30 pharmaceuticals and personal care products in waste water using Q-Exactive Orbitrap high resolution accurate mass spectrometry. J Chromatogr A 1588:68–76. https://doi.org/10.1016/j.chroma.2018.12.033
Abdelmageed AA, Ellah RG, Abdel-Satar AM, Gawad SS, Khalifa N et al (2022) Evaluation of the ecological health and food chain on the shores of four River Nile Islands, Egypt. Environ Monit Assess 194(4):309. https://doi.org/10.1007/s10661-022-09959-w
Abdel-Satar AM, Ali MH, Goher ME (2017) Indices of water quality and metal pollution of Nile River, Egypt. Egypt J Aquat Res 43(1):21–29. https://doi.org/10.1016/j.ejar.2016.12.006
Abdo MH, Ahmed H, Helal M, Fekry M, Abdelhamid A (2022) Water Quality Index and environmental assessment of Rosetta Branch Aquatic System, Nile River, Egypt. Egypt J Chem 65(4):321–331. https://doi.org/10.21608/EJCHEM.2021.92605.4405
Alidina M, Hoppe-Jones C, Yoon M, Hamadeh AF, Li D, Drewes JE (2014) The occurrence of emerging trace organic chemicals in wastewater effluents in Saudi Arabia. Sci Total Environ 478:152–162. https://doi.org/10.1016/j.scitotenv.2014.01.093
American Public Health Association (APHA) (2017) Standard methods for examination of water and wastewater, 23rd edn. American Public Health Association
APHA-AWWA-WPCF, American Public Health Association, American Water Works Association and Water Pollution Control Federation (1989) Standard methods for the examination of water and wastewater
Asghar MA, Zhu Q, Sun S, Shuai Q (2018) Suspect screening and target quantification of human pharmaceutical residues in the surface water of Wuhan, China, using UHPLC-Q-Orbitrap HRMS. Sci Total Environ 635:828–837. https://doi.org/10.1016/j.scitotenv.2018.04.179
Bahlmann A, Carvalho JJ, Weller MG, Panne U, Schneider RJ (2012) Immunoassays as high-throughput tools: monitoring spatial and temporal variations of carbamazepine, caffeine and cetirizine in surface and wastewaters. Chemosphere 89:1278–1286. https://doi.org/10.1016/j.chemosphere.2012.05.020
Baker DR, Kasprzyk-Hordern B (2013) Spatial and temporal occurrence of pharmaceuticals and illicit drugs in the aqueous environment and during wastewater treatment: new developments. Sci Total Environ 454:442–456. https://doi.org/10.1016/j.scitotenv.2013.03.043
Baughman CA, Jones BM, Bartz KK, Young DB, Zimmerman CE (2015) Reconstructing turbidity in a glacially influenced lake using the Landsat TM and ETM+ surface reflectance climate data record archive, Lake Clark, Alaska. Remote Sens 7(10):13692–13710. https://doi.org/10.3390/rs71013692
Ben-Dor E, Banin A (1989) Determination of organic matter content in arid-zone soils using a simple “loss-on-ignition” method. Commun Soil Sci Plant Anal 20(15-16):1675–1695. https://doi.org/10.1080/00103628909368175
Beniwal VD, Kumari R, Jain S (2021) Physico-chemical parameter: an indicator of water pollution. J Environ Res 5(5):7857
Beretta M, Britto V, Tavares TM, da Silva SM, Pletsch AL (2014) Occurrence of pharmaceutical and personal care products (PPCPs) in marine sediments in the Todos os Santos Bay and the north coast of Salvador, Bahia, Brazil. J Soils Sediments 4:1278–1286. https://doi.org/10.1007/s11368-014-0884-6
Berger E, Haase P, Kuemmerlen M, Leps M, Schaefer RB, Sundermann A (2017) Water quality variables and pollution sources shaping stream macroinvertebrate communities. Sci Total Environ 587:1–10. https://doi.org/10.1016/j.scitotenv.2017.02.031
Bethke K, Kropidłowska K, Stepnowski P, Caban M (2023) Review of warming and acidification effects to the ecotoxicity of pharmaceuticals on aquatic organisms in the era of climate change. Sci Total Environ 877:162829. https://doi.org/10.1016/j.scitotenv.2023.162829
Briffa J, Sinagra E, Blundell R (2020) Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6(9):e04691. https://doi.org/10.1016/j.heliyon.2020.e04691
Brinkman SF, Johnston WD (2012) Acute toxicity of zinc to several aquatic species native to the Rocky Mountains. Arch Environ Contam Toxicol 62:272–281. https://doi.org/10.1007/s00244-011-9698-3
CCME (2001) Canadian water quality guidelines for the protection of aquatic life: CCME Water Quality Index 1.0 user’s manual. Canadian Council of Ministers of the Environment, Winnipeg
CCME (2007) For the protection of aquatic life 2007. In: Canadian Environmental Quality Guidelines, 1999. Canadian Council of Ministers of the Environment, 1999, Winnipeg
Česen M, Ahel M, Terzić S, Heath DJ, Heath E (2019) The occurrence of contaminants of emerging concern in Slovenian and Croatian wastewaters and receiving Sava River. Sci Total Environ 650:2446–2453. https://doi.org/10.1016/j.scitotenv.2018.09.238
de Sousa DN, Mozeto AA, Carneiro RL, Fadini PS (2014) Electrical conductivity and emerging contaminant as markers of surface freshwater contamination by wastewater. Sci Total Environ 484:19–26. https://doi.org/10.1016/j.scitotenv.2014.02.135
Del Río H, Suárez J, Puertas J, Ures P (2013) PPCPs wet weather mobilization in a combined sewer in NW Spain. Sci Total Environ 449:189–198. https://doi.org/10.1016/j.scitotenv.2013.01.049
Doerr-MacEwen NA, Haight ME (2006) Expert stakeholders’ views on the management of human pharmaceuticals in the environment. Environ Manag 38:853–866. https://doi.org/10.1007/s00267-005-0306-z
Ebele AJ, Abdallah MA, Harrad S (2017) Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg Contam 3:1–16. https://doi.org/10.1016/j.emcon.2016.12.004
Ebele AJ, Oluseyi T, Drage DS et al (2020) Occurrence, seasonal variation and human exposure to pharmaceuticals and personal care products in surface water, groundwater and drinking water in Lagos State, Nigeria. Emerg Contam 6:124–132. https://doi.org/10.1016/j.emcon.2020.02.004
EEAA, Egyptian Environmental Affairs Agency, (1999) The Arab Republic of Egypt: Initial National Communication on Climate Change under the United Nations Framework Convention on Climate Change.
Estrada-Arriaga EB, Cortés-Muñoz JE, González-Herrera A, Calderón-Mólgora CG et al (2016) Assessment of full-scale biological nutrient removal systems upgraded with physico-chemical processes for the removal of emerging pollutants present in wastewaters from Mexico. Sci Total Environ 571:1172–1182. https://doi.org/10.1016/j.scitotenv.2016.07.118
Gedik K, Boran M (2013) Assessment of metal accumulation and ecological risk around Rize Harbor, Turkey (southeast Black Sea) affected by copper ore loading operations by using different sediment indexes. Bull Environ Contam Toxicol 90:176–181. https://doi.org/10.1007/s00128-012-0894-2
Ghoshdastidar AJ, Fox S, Tong AZ (2015) The presence of the top prescribed pharmaceuticals in treated sewage effluents and receiving waters in Southwest Nova Scotia, Canada. Environ Sci Pollut Res 22:689–700. https://doi.org/10.1007/s11356-014-3400-z
Hahn J, Opp C, Evgrafova A, Groll M, Zitzer N, Laufenberg G (2018) Impacts of dam draining on the mobility of heavy metals and arsenic in water and basin bottom sediments of three studied dams in Germany. Sci Total Environ 640:1072–1081. https://doi.org/10.1016/j.scitotenv.2018.05.295
Hawash HB, Moneer AA, Galhoum AA, Elgarahy AM et al (2023) Occurrence and spatial distribution of pharmaceuticals and personal care products (PPCPs) in the aquatic environment, their characteristics, and adopted legislations. J Water Process Eng 52:103490
Hernando MD, Mezcua M, Fernández-Alba AR, Barceló D (2006) Environmental risk assessment of pharmaceutical residues in waste water effluents, surface waters and sediments. Talanta 69:334–342. https://doi.org/10.1016/j.jwpe.2023.103490
Hillebrand O, Nodler K, Licha T, Sauter M, Geyer T (2012) Caffeine as an indicator for the quantification of untreated wastewater in karst systems. Water Res 46:395–402. https://doi.org/10.1016/j.watres.2011.11.003
Hua WY, Bennett ER, Maio XS, Metcalfe CD, Letcher RJ (2006) Seasonality effects on pharmaceuticals and s-triazine herbicides in wastewater effluent and surface water from the Canadian side of the upper Detroit River. Environ Toxicol Chem: Int J 25(9):2356–2365. https://doi.org/10.1897/05-571r.1
Hussein MA, Obuid-Allah AH, Mohammad AH, Scott-Fordsmand JJ, Abd El-Wakeil KF (2006) Seasonal variation in heavy metal accumulation in subtropical population of the terrestrial isopod, Porcellio laevis. Ecotoxicol Environ Saf 63(1):168–174. https://doi.org/10.1016/j.ecoenv.2005.01.005
Ice GG (2008) Stream temperature and dissolved oxygen. In: Hydrological and biological responses to forest practices: the Alsea Watershed Study. Springer New York, New York, NY, pp 37–54
Ip CC, Li XD, Zhang G, Wai OW, Li YS (2007) Trace metal distribution in sediments of the Pearl River Estuary and the surrounding coastal area, South China. Environ Pollut 147(2):311–323. https://doi.org/10.1016/j.envpol.2006.06.028
Jackson ML (1974) Soil chemical analysis: advanced course, 2nd edn Published by the author. University of Wisconsin, Madison, Wisc., USA
Jagoda A, Zukowski W, Dabrowska B (2015) Investigations of the presence of caffeine in the Rudawa River, Krakow, Poland. Environ Monit Assess 187:1–12. https://doi.org/10.1007/s10661-015-4760-7
Korekar G, Kumar A, Ugale C (2020) Occurrence, fate, persistence and remediation of caffeine: a review. Environ Sci Pollut Res 27:34715–34733. https://doi.org/10.1007/s11356-019-06998-8
Kosma CI, Lambropoulou DA, Albanis TA (2014) Investigation of PPCPs in wastewater treatment plants in Greece: occurrence, removal and environmental risk assessment. Sci Total Environ 466:421–438. https://doi.org/10.1016/j.scitotenv.2013.07.044
Li S, He B, Wang J, Liu J, Hu X (2020b) Risks of caffeine residues in the environment: necessity for a targeted ecopharmacovigilance program. Chemosphere 243:125343. https://doi.org/10.1016/j.chemosphere.2019.125343
Li S, Wen J, He B, Wang J, Hu X, Liu J (2020a) Occurrence of caffeine in the freshwater environment: implications for ecopharmacovigilance. Environ Pollut 263:114371. https://doi.org/10.1016/j.envpol.2020.114371
Lv J, Zhang L, Chen Y, Ye B, Han J, Jin N (2019) Occurrence and distribution of pharmaceuticals in raw, finished, and drinking water from seven large river basins in China. J Water Health 17(3):477–489. https://doi.org/10.2166/wh.2019.250
Maranho LA, Moreira LB, Baena-Nogueras RM, Lara-Martín PA et al (2015) A candidate short-term toxicity test using Ampelisca brevicornis to assess sublethal responses to pharmaceuticals bound to marine sediments. Arch Environ Contam Toxicol 68:237–258. https://doi.org/10.1007/s00244-014-0080-0
Martín J, Santos JL, Aparicio I, Alonso E (2010) Multi-residue method for the analysis of pharmaceutical compounds in sewage sludge, compost and sediments by sonication-assisted extraction and LC determination. J Sep Sci 33(12):1760–1766. https://doi.org/10.1002/jssc.200900873
Martínez Bueno MJ, Ucles S, Hernando MD, Fernandez-Alba AR (2011) Development of a solvent-free method for the simultaneous identification/quantification of drugs of abuse and their metabolites in environmental water by LC-MS/MS. Talanta 85:157–166. https://doi.org/10.1016/j.talanta.2011.03.051
Matongo S, Birungi G, Moodley B, Ndungu P (2015) Pharmaceutical residues in water and sediment of Msunduzi River, KwaZulu-natal, South Africa. Chemosphere 134:133–140. https://doi.org/10.1016/j.chemosphere.2015.03.093
McComb J, Alexander TC, Han FX, Tchounwou PB (2014) Understanding biogeochemical cycling of trace elements and heavy metals in estuarine ecosystems. J Bioremed Biodegr 5:1000–1148. https://doi.org/10.4172/2155-6199.1000e148
Mijangos L, Ziarrusta H, Ros O, Kortazar L et al (2018) Occurrence of emerging pollutants in estuaries of the Basque Country: analysis of sources and distribution, and assessment of the environmental risk. Water Res 147:152–163. https://doi.org/10.1016/j.watres.2018.09.033
Mohamed TA, Mohamed MAK, Rabeiy R, Ghandour MA (2014) Application of pollution indices for evaluation of heavy metals in soil close to phosphate fertilizer plant, Assiut, Egypt. Assiut Univ Bull Environ Res 17(1):45–55
Mohammad W, Mohammed T, Abd El-Wakeil KF, Hassan MM (2021) Effects of combined treatment of cadmium and oxytetracycline on the terrestrial isopod Porcellio leavis. Braz J Biol 82:246979. https://doi.org/10.1590/1519-6984.246979
Mohammad WA, Abd El-Wakeil KF (2021) Effects of dietary coffee on feeding parameters and growth of terrestrial isopod, Porcellio laevis. Egyptian Academic Journal of Biological Sciences, B. Zoology 13(2):173–183. https://doi.org/10.21608/EAJBSZ.2021.203498
Mokh S, El Khatib M, Koubar M, Daher Z, Al IM (2017) Innovative SPE-LC-MS/MS technique for the assessment of 63 pharmaceuticals and the detection of antibiotic-resistant-bacteria: a case study natural water sources in Lebanon. Sci Total Environ 609:830–841. https://doi.org/10.1016/j.scitotenv.2017.07.230
Nodler K, Voutsa D, Licha T (2014) Polar organic micropollutants in the coastal environment of different marine systems. Mar Pollut Bull 85:50–59. https://doi.org/10.1016/j.marpolbul.2014.06.024
Ololade IA, Apata A, Oladoja NA, Alabi BA, Ololade OO (2023) Appraisal of river sediments in southwestern Nigeria with a special focus on trace metals: occurrence, seasonal variation, sources, and health risks. Acta Ecol Sin. https://doi.org/10.1016/j.chnaes.2023.08.004
Onwona Kwakye M, Peng FJ, Hogarh JN, Van den Brink PJ (2021) Linking macroinvertebrates and physicochemical parameters for water quality assessment in the lower basin of the Volta River in Ghana. Environ Manag 68:928–936. https://doi.org/10.1007/s00267-021-01535-1
Ortúzar M, Esterhuizen M, Olicón-Hernández DR et al (2022) Pharmaceutical pollution in aquatic environments: a concise review of environmental impacts and bioremediation systems. Front Microbiol 13:869332. https://doi.org/10.3389/fmicb.2022.869332
Pal A, Squitti R, Picozza M, Pawar A et al (2021) Zinc and COVID-19: basis of current clinical trials. Biol Trace Elem Res 199:2882–2892. https://doi.org/10.1007/s12011-020-02437-9
Papageorgiou M, Kosma C, Lambropoulou D (2016) Seasonal occurrence, removal, mass loading and environmental risk assessment of 55 pharmaceuticals and personal care products in a municipal wastewater treatment plant in Central Greece. Sci Total Environ 543:547–569. https://doi.org/10.1016/j.scitotenv.2015.11.047
Pekey H (2006) The distribution and sources of heavy metals in Izmit Bay surface sediments affected by a polluted stream. Mar Pollut Bull 52:1197–1208. https://doi.org/10.1016/j.marpolbul.2006.02.012
Peters EC, Gassman NJ, Firman JC, Richmond RH, Power EA (1997) Ecotoxicology of tropical marine ecosystems. Environ Toxicol Chem: Int J 16:12–40. https://doi.org/10.1002/etc.5620160103
Pires A, Almeida Â, Calisto V, Schneider RJ et al (2016) Long-term exposure of polychaetes to caffeine: biochemical alterations induced in Diopatra neapolitana and Arenicola marina. Environ Pollut 214:456–463. https://doi.org/10.1016/j.envpol.2016.04.031
Poiger T, Buser HR, Müller MD, Balmer ME, Buerge IJ (2003) Occurrence and fate of organic micropollutants in the environment: regional mass balances and source apportioning in surface waters based on laboratory incubation studies in soil and water, monitoring, and computer modeling. Chimia 57(9):492–498. https://doi.org/10.2533/000942903777678920
Puckowski A, Mioduszewska K, Łukaszewicz P, Borecka M et al (2016) Bioaccumulation and analytics of pharmaceutical residues in the environment: a review. J Pharm Biomed Anal 127:232–255. https://doi.org/10.1016/j.jpba.2016.02.049
Rahman A, Jahanara I, Jolly YN (2021) Assessment of physicochemical properties of water and their seasonal variation in an urban river in Bangladesh. Water Sci Eng 14(2):139–148. https://doi.org/10.1016/j.wse.2021.06.006
Read SA, Obeid S, Ahlenstiel C, Ahlenstiel G (2019) The role of zinc in antiviral immunity. Adv Nutr 10(4):696–710. https://doi.org/10.1093/advances/nmz013
Shiller AM, Boyle E (1985) Dissolved zinc in rivers. Nature 317(5):49–52. https://doi.org/10.1038/317049a0
Smith AJ, McGowan T, Devlin MJ, Massoud MS et al (2015) Screening for contaminant hotspots in the marine environment of Kuwait using ecotoxicological and chemical screening techniques. Mar Pollut Bull 100(2):681–688. https://doi.org/10.1016/j.marpolbul.2015.08.043
Stuart M, Lapworth D, Crane E, Hart A (2012) Review of risk from potential emerging contaminants in UK groundwater. Sci Total Environ 416:1–21. https://doi.org/10.1016/j.scitotenv.2011.11.072
Tanjung RHR, Yonas MN, Suwito MHK et al (2022) Analysis of surface water quality of four rivers in Jayapura regency, Indonesia: CCME-WQI approach. J Ecol Eng 23(1):73–82. https://doi.org/10.12911/22998993/143998
Taylor MC, Demayo A, Taylor KW (1982) Effects of zinc on humans, laboratory and farm animals, terrestrial plants, and freshwater aquatic life. Crit Rev Environ Sci Technol 12:113–181. https://doi.org/10.1080/10643388209381696
Tiwari B, Sellamuthu B, Ouarda Y, Drogui P et al (2017) Review on fate and mechanism of removal of pharmaceutical pollutants from wastewater using biological approach. Bioresour Technol 224:1–12. https://doi.org/10.1016/j.biortech.2016.11.042
USEPA (2016). United States Environmental Protection Agency, Contaminants of emerging concern including pharmaceuticals and personal care products. http://www.epa.gov
Vega M, Pardo R, Barrado E, Deban L (1998) Assessment of seasonal and polluting effects on the quality of river water by exploratory data analysis. Water Res 32(12):3581–3592. https://doi.org/10.1016/S0043-1354(98)00138-9
Vidal AD, Barrales PO, Díaz AM (2003) Simultaneous determination of paracetamol, caffeine and propyphenazone in pharmaceuticals by means of a single flow through UV multiparameter sensor. Mikrochim Acta 141:157–163. https://doi.org/10.1007/s00604-002-0938-0
Vosyliene MZ, Jankaite A (2006) Effect of heavy metal model mixture on rainbow trout biological parameters. Ekologia 4:12–17
Wu X, Cobbina SJ, Mao G, Xu H, Zhang Z, Yang L (2016) A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ Sci Pollut Res 23:8244–8259. https://doi.org/10.1007/s11356-016-6333-x
Zeidan BA (2017) Groundwater degradation and remediation in the Nile Delta Aquifer. The Nile Delta:159–232
Zhao JL, Zhang QQ, Chen F, Wang L et al (2013) Evaluation of triclosan and triclocarban at river basin scale using monitoring and modeling tools: implications for controlling of urban domestic sewage discharge. Water Res 47(1):395–405. https://doi.org/10.1016/j.watres.2012.10.022
Zhou H, Yang WT, Zhou X, Liu L, Gu JF, Wang WL, Liao BH (2016a) Accumulation of heavy metals in vegetable species planted in contaminated soils and the health risk assessment. Int J Environ Res Public Health 13(3):289. https://doi.org/10.3390/ijerph13030289
Zhou H, Ying T, Wang X, Jianbo L (2016b) Occurrence and preliminarily environmental risk assessment of selected pharmaceuticals in the urban rivers, China. Sci Rep 6:34928. https://doi.org/10.1038/srep34928
Zhu S, Chen H, Li J (2013) Sources, distribution and potential risks of pharmaceuticals and personal care products in Qingshan Lake basin, Eastern China. Ecotoxicol Environ Saf 96:154–159. https://doi.org/10.1016/j.ecoenv.2013.06.033
Acknowledgements
We are very grateful to Dr. Omar, H., NIOF, Alexandria, Egypt; Dr. Mahdy, A., Faculty of Science, Al-Azhar University; Dr. Omer, M., NIOF, Red Sea, Egypt; and Dr. Abdelhafez, M., Wadi El Gemal National Park, Egypt, for their efforts in sampling during this study.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This work is funded by the Egyptian Academy of Scientific Research and Technology, Scientists for Next Generation (FRM-SGO-CYCL#8).
Author information
Authors and Affiliations
Contributions
Nouran A. I. Tawfik contributed to collecting and analysing the data and drafting the article. Zienab A. El-Bakary contributed to conception and design of the work and contributed to follow-up writing of the draft. Khaleid F. Abd El-Wakeil contributed to conception and design of the work and statistical analysis of the data, and critically revised the article for important intellectual content. All authors contributed in writing, reading and approving the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. All authors voluntarily agreed to participate in this research study.
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ester Heath
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 24 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tawfik, N.A.I., El-Bakary, Z.A. & Abd El-Wakeil, K.F. Determination of caffeine in treated wastewater discharged in the Nile River with emphasis on the effect of zinc and physicochemical factors. Environ Sci Pollut Res 31, 28124–28138 (2024). https://doi.org/10.1007/s11356-024-32918-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-32918-6