Abstract
Agricultural wastes are potential sustainable adsorbents since they are available in large quantities, are low-cost, and may require little or no treatment, in some cases. In this study, several fruit peels, such as banana, orange, and pomegranate, were collected from local markets and prepared by a simple and eco-friendly method and used as natural adsorbents for the removal of both anionic (Reactive Red 120 (RR120), Reactive Black 5 (RB5), Remazol Brilliant Blue R (RBBR)) and cationic Methylene Blue (MB) dyes found in wastewaters. Many industries, such as leather and textiles, can release huge amounts of synthetic dyes into the wastewater during dyeing processes. These are one of the most important pollutants of water pollution as they cause enormous damage to the water body and also affect the health of organisms due to their toxicity and carcinogenicity. The search for a sustainable and at the same time efficient material for the removal of a wide variety of dyes is the innovation of this work. These peels were prepared by washing, drying, grinding, and finally sieving, under natural sustainable conditions. Porosometry (BET analysis), FTIR, SEM/EDS, and XRD techniques were used to characterize the fruit peels before and after the adsorption process. Factors affecting the adsorption of dyes (adsorbent dosage, pH solution, initial concentration of dyes, contact time, and temperature) were investigated. According to the results, in terms of the effectiveness of fruit peels as (natural) adsorbent materials, for anionic dyes, 5.0–6.0 g/L of banana or orange dry peels was sufficient to remove near or even more than 90% anionic dyes at pH 2.0, and 4.0 g/L was sufficient to remove 98% of cationic MB dye at pH 9.0. Similar amount of pomegranate peels had lower efficiency for anionic dyes (50–70%), while cationic MB was still efficiently removed (98%) at pH 9.0. Moreover, the adsorption process in all cases was found to better fit to pseudo-second-order model, in comparison to pseudo-first-order model. According to isotherms, Freundlich model fitted better in some cases to the equilibrium data, while the Langmuir model in others. Finally, this study demonstrates the viability of reusing the banana, orange, and pomegranate peel adsorbents for eight, four, and five cycles, showing a gradual reduction of around 50% of their effectiveness.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The access to clean water is vital for the well-being of humanity. Unfortunately, in many developing countries, over 1 billion of people is denied access to drinking water (Thillainayagam et al. 2022; Zhao et al. 2022). The pollution of industrial wastewater effluents, such as leather and textiles, represents a significant environmental challenge (Tolkou and Zouboulis 2020), particularly due to the presence of highly hazardous substances, such as synthetic dyes, which have the potential to cause carcinogenic effects (Mahmoudabadi et al. 2019), containing some dangerous constitutes, i.e., benzidine, 4-aminobiphenyl, and 4-aminoazobenzene, found in food colorant substances (Ghalkhani et al. 2022). This problem is more evident in countries that still dominate dye industry such as China, Malaysia, Bangladesh, Thailand, and Indonesia (Islam and Dey 2015; Li et al. 2023). This type of pollution mainly originates from industrial wastewater that contains a substantial concentration of pollutants. There are three types of dyes: non-ionic, cationic, and anionic. All of them are soluble in water and can pose harm even in small amounts (Panda et al. 2021). The dyes can be classified as natural or synthetic, with synthetic dyes being used more often and being available in a wide range of colors. Synthetic dyes are further categorized based on their specific applications, such as reactive, direct, disperse, basic, and vat dyes. They can also be classified based on their chemical structure and different functional groups, such as azo, anthraquinone, sulfur, oxazine, phthalocyanine, and triarylmethane dyes (Fobiri 2022; Yeow et al. 2021).
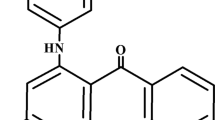
Reactive Red 120, denoted hereafter as RR120 (C44H24Cl2N14O20S6Na6) (Fig. 1a) and Reactive Black 5, denoted hereafter as RB5 (C26H21N5Na4O19S6) (Fig. 1c), are anionic azo dyes, being widely used in the textile industry to color cellulose-based fibers like cotton and rayon. These dyes have the unique property of forming a strong chemical bond with the fibers, creating a covalent linkage. However, the presence of synthetic azo dyes, including reactive dyes, in industrial processes like dyeing and textile production poses a significant risk to water resources. These dyes have a long persistence in aquatic environments and can potentially exhibit harmful effects such as mutagenicity and carcinogenicity (H. Saroyan et al. 2019).
Remazol Brilliant Blue R, denoted hereafter as RBBR (C22H16N2Na2O11S3) (Fig. 1d), is an anionic (reactive) dye with anthraquinonic groups, being soluble in water. Anthraquinonic dyes are known for their vibrant and long-lasting colors, making them desirable for dyeing applications (Yeow et al. 2021).
Methylene blue, denoted hereafter as MB (C16H18ClN3S) (Fig. 1b) is a type of basic dye, extensively used in industries such as dyeing, printing, and pharmaceuticals. Cationic dyes are attracted to materials’ negatively charged surfaces, allowing them to form strong bonds (Kubra et al. 2021). However, it has been found to have detrimental effects on the central nervous system, leading to the development of various skin conditions. Consequently, the presence of MB in freshwater reservoirs is related with a potential hazard to both aquatic organisms and human beings (Dey et al. 2017).
Over the years, numerous approaches based on physical, chemical, and biological processes have been established to address the issue of dye removal from wastewater (Dai et al. 2018). A wide range of techniques have been employed to eliminate dye pollutants, including chemical oxidation (Saroyan et al. 2019), coagulation and flocculation (Ihaddaden et al. 2022), electrocoagulation (Saad et al. 2022; Taheri 2022), adsorption (Tolkou et al. 2023), membrane separation (Semiz 2020), ozonation (Ikhlaq et al. 2021), and fungal decolorization (Saad et al. 2022; Taheri 2022). Among these methods, adsorption is a highly popular, simple, and cost-effective technique for dye removal (Zhu et al. 2018).
Currently, there is a wide variety of commercially available adsorbents designed for various applications, such as zeolites (Ikhlaq et al. 2021), chitosan (Rostamian et al. 2022; Zeng et al. 2023), magnetic nanocomposites (Panda et al. 2021), graphene oxide (H. Saroyan et al. 2019), and activated carbon (Nizam et al. 2021). Activated carbon is highly effective in removing organic pollutants, and its properties, such as its porous texture and functional groups, are important for dye adsorption (Ndagijimana et al. 2021). However, the economic viability of activated carbon may be a limitation despite its extensive use. As a result, researchers have been investigating alternative sources of adsorbents, such as agricultural wastes that provide a cost-effective and sustainable solution for dye removal from wastewater (Zazycki et al. 2018). These agricultural wastes that have already been used as adsorbents are from shells (such as coconut (Tolkou et al. 2022), roots (Alcea rosea (Mahmoudabadi et al. 2019)), nuts (areca (Sukla Baidya and Kumar 2021)), fruit peels (i.e.. peels from banana (Mondal and Kar 2018), orange (Munagapati et al. 2019), watermelon (Krueger et al. 2019)), and leaves (cassava (Chairunnisa and Lisafitri 2020)). Some of them have been chemically treated to enhance their effectiveness, such as orange peels (Munagapati et al. 2019). Therefore, it is necessary to prepare more sustainable and cost-effective adsorbents using agricultural wastes with low cost.
Banana peels are often discarded in juice factories and fruit shops. Bananas are part of the Musaceae family, which consists of several hybrid varieties within the Musa genus (Hikal et al. 2022). Banana peels are low-cost materials and contain significant amounts of dietary fibers and phenolic compounds (Vu et al. 2018). Orange peels, which are numerous, inexpensive, and widely available materials abandoned at fresh fruit stalls and juice stores, may be employed as applied economic adsorbents for the removal of various contaminants from aqueous solutions, such as dyes and heavy metal ions. This type of peels contains cellulose, lignin, pectin (galacturonic acid), hemicelluloses, and chlorophyll pigments (Ayala et al. 2021). These constituents possess various functional groups, such as amide, carboxyl, and hydroxyl groups, which can play a significant role in the effective removal of dyes from wastewaters (Munagapati et al. 2019). Pomegranate peels are plentiful, cost-effective, and readily accessible materials derived from agricultural wastes. They have been effectively employed as affordable adsorbents for the separation of phenols, phenolic compounds, chromium(VI), nickel, and crude oil from simulated wastewater (Drinić et al. 2020; Ramadan et al. 2021). This low-cost material has proven its potential in efficiently removing these pollutants, showcasing its viability as a cost-effective solution for wastewater treatment (Saigl and Ahmed 2021).
The present study focuses on the removal of four different dyes (RR120, RB5, RBBR, and MB), representing both the anionic and cationic dye categories, from wastewaters by adsorption. As adsorbents, banana, orange, and pomegranate peels were used, washed and dried, and applied without further chemical modification. The aim of this study was to prepare natural adsorbents derived from fruit peels comprising environmental-friendly and affordable materials for the effective removal of dyes. In many studies, banana, orange, and pomegranate peels have been used as adsorbents for dye removal (Ben-Ali 2021; Daud et al. 2019; Kapoor et al. 2022; Michael-Igolima et al. 2023; Munagapati et al. 2018). However, in these studies, the materials were applied after chemical modification. In this work, the focus was on the development of cost-effective adsorbents due to their more economical and less invasive way of preparation. Therefore, the novelty of the present study is clear because these peels were prepared and used without further chemical modification, and in addition, the combination of these adsorbents used and the examined dye removal has not been studied, yet. The research examined the influence of various factors, initial pH value, adsorbent dosage, initial dye concentration, and contact time. The natural adsorbents used were analyzed for their structural and morphological properties using techniques such as BET, FTIR, and SEM/EDS. Kinetic and isotherm models were also analyzed to understand and evaluate the adsorption process, while thermodynamic and regeneration studies were conducted.
Materials and methods
Materials
Various locally available and low-cost materials, i.e., banana, orange, and pomegranate fruits, were collected from the local markets and used as tentative absorbents to remove dye from aqueous solutions. The peels of those fruits usually are thrown as wastes. Two (reactive) anionic dyes, which are commercially known as Reactive Red 120 (RR120) (dye content, ≥ 90%) and Reactive Black 5 (RB5) with a purity of ≥ 50% (supplied by Kahafix), one anthraquinone dye, i.e., Remazol Brilliant Blue R (RBBR) with 95% purity, as well as a cationic one known as Methylene Blue (MB) ≥ 95%, were purchased from Sigma-Aldrich-Merck KGaA, Darmstadt, Germany, and used as adsorbates. It is noted that the purity of the dyes was included in all calculations. The chemical structure and formula of these commercially available dyes are shown in Fig. 1. Stock dye aqueous solutions of 1000 mg/L were prepared by dissolving 1 g of dye in distilled water. The solution pH, where it was necessary, was adjusted by using HCl 37% v/v (supplied by Panreac) or NaOH (pellets, ACS reagent, ≥ 97.0%, supplied by Sigma-Aldrich). Finally, for the regeneration experiments, alkali solutions of 1.0 M NaOH were used.
Preparation of natural adsorbents
Fruit peels were collected before being thrown away as wastes and processed using a green approach. Hence, a big amount of peels of banana, orange, and pomegranate were then extensively washed carefully with deionized water to remove any dirt or impurity. Selected peels are then air-dried at room temperature for 3 days before sieved and oven-dried at 100 °C (373 K) for 2 h. After drying, the collected fruit peels were grinded in small pieces by a domestic mixer into powder form (75–125 μm) in order to be used in adsorption experiments. The weight of the resulting powder of each natural adsorbent (Appendix 1) was recorded and then sealed in a plastic bag and stored in a cold room (Azamzam et al. 2022; Ben-Ali 2021; Shaharom 2019). At this point, it should be noted that the amount of sorbent produced was sufficient for all experiments and characterizations.
Characterization techniques of adsorbents
Several characterization techniques were applied to study the surface of the adsorbents before and after the adsorption experiments. Among these techniques, scanning electron microscopy (SEM) (Jeol JSM-6390 LV, Japan scanning electron microscop)/ EDS, Fourier transform infrared spectroscopy (FT-IR, PerkinElmer, New York, NY, USA), and Brunauer, Emmett and Teller (BET) analysis software and X-ray diffraction (XRD) (Bruker D8 FOCUS, CuKα, λ = 0.154 nm) were applied. Characterization measurements were conducted in several samples, and the results were found to be repeatable and in agreement with all other non-shown measurements. Therefore, here are presented representative results.
Analytical determinations of dye concentration
After each adsorption experiment, water samples were collected from the supernatant of each falcon tube, then filtered through a nylon membrane filter (0.45 μm) and stored for immediate determination. The initial and residual dye concentration was determined by corresponding the absorbance taken from a UV–Vis spectrophotometer (WTW Spectroflex 6100, Weilheim, Germany), to the standard curves of each dye, at different wavelengths depending on the dye. Thus, the relative λmax were 515, 603, 593, and 664 nm for RR120, RB5, RBBR, and MB, respectively (Arya et al. 2020a; Kuang et al. 2020; Samarghandy et al. 2011; Travlou et al. 2013). For the repeatability of experiments and precision of measurements of the obtained results, the relevant experiments were repeated in triplicate, and in addition, the measurement of each sample was taken three times. Data were expressed as trace element mean values ± standard deviation.
Adsorption experiments
The efficiency of banana, orange, and pomegranate peels as adsorbents for RR120, RB5, RBBR, and MB removal was investigated through a series of single-component experiments. Falcon tubes were used, each containing 10 mL of RB5 dye solution with varying initial concentrations needed. The temperature was kept constant throughout the experiments, and the mixture was stirred at 80 rpm using a Trayster overhead shaker and Loopster rotator. Throughout the experiments, various experimental factors were adjusted individually, while ensuring that all other parameters remained unchanged. These factors included pH levels (ranging from 2.0 to 9.0); initial RR120, RB5, RBBR, and MB dye concentrations (ranging from 5 to 1000 mg/L); adsorbent doses (ranging from 1 to 7 g/L, according to preliminary experiments and achieved by adding 0.01–0.07 g to the working volume of 10 mL); and contact time (ranging from 5 to 1440 min (24 h)). Following the adsorption process, samples were collected from the tubes and passed through a 0.45-μm nylon filter. The filtrate was then used for subsequent analyses. The average values of triplicate experiments were used as experimental data points. The percentage removal (R (%)) of RR120, RB5, RBBR, and MB dyes was calculated from the following equation (Eq. 1):
where C0 represents the initial RR120, RB5, RBBR, and MB concentrations in mg/L and Cf represents the final dye concentrations after the experimental process, calculated in mg/L.
To determine the adsorption capacity of the adsorbents, Qe in mg/g was calculated using Eq. 2 as follows:
where Ce indicates the RR120, RB5, RBBR, and MB concentration (mg/L) at equilibrium, V indicates the volume of dye solutions (L), and m indicates the mass of each adsorbent added (g).
Equilibrium experiments
In the isothermal experiments, a fixed amount of banana, orange, and pomegranate peel adsorbents (in g) was added to 15-mL falcon tubes containing 10 mL of RR120, RB5, RBBR, and MB dye solutions. The concentrations of each dye solution ranged from 5 to 1000 mg/L, by keeping constant the initial pH value and the temperature, at 24 h as contact time. The data collected from these experiments were analyzed using the commonly used and easiest to apply experimentally Langmuir and Freundlich isotherm models, as the Langmuir model is the foundation upon which much of adsorption theory is built and therefore provides a useful conceptual basis for understanding the process (Kalam et al. 2021). The Langmuir and Freundlich models are expressed mathematically by Eqs. 3 and 4, respectively.
where Qe represents the equilibrium concentrations of the adsorbates in the solid phase relative to the equilibrium concentrations in the liquid phase, measured in mg/g. Qm represents the maximum adsorption capacity, which depicts the theoretical capacity of a monolayer, measured in mg/g. KL represents the energy associated with the adsorption of RR120, RB5, RBBR, and MB dyes, measured in L/mg. KF ((mg/g)∙(L/mg)1/n) constant related to the adsorption capacity, and 1/n constant associated with the adsorption intensity or the surface heterogeneity.
According to the Langmuir theory, adsorption occurs when the adsorbate molecules form monolayer on the surface of the adsorbent, without interacting with each other. This theory also supposes that the adsorbent has a limited adsorption capacity (Qm), representing the maximum amount of adsorbate that can be adsorbed under equilibrium conditions.
Otherwise, the Freundlich model establishes a relationship between the equilibrium concentrations of dyes (mg/L) and the adsorption capacity of the adsorbent, represented by Qe (mg/g). Freundlich model is an empirical model based on adsorption on a heterogeneous surface that is valid only up to a certain concentration and assumes the formation of multilayer and heterogeneous systems (Arami et al. 2005; Jaroniec 1975).
Kinetic experiments
The dye removal capacity of peels adsorbents also depends on the contact time. Kinetic experiments were carried out at optimum pH values, constant temperature, and different contact times (0–210 min). The kinetic study investigated the adsorption of RR120, RB5, RBBR, and MB dyes using both the pseudo-first-order (PFO) (Eq. 5) and pseudo-second-order (PSO) (Eq. 6) models. These models are mainly applied in many adsorption studies (Revellame et al. 2020). The obtained kinetic parameters were utilized to estimate the adsorption process and identify suitable rate expressions for potential reaction mechanisms. These analyses were conducted to enhance the understanding of banana, orange, and pomegranate peels onto dye adsorption.
where parameters Qt and Qe represent the amount of RR120, RB5, RBBR, and MB dyes adsorbed (in mg/g) at a given time t (min) and at equilibrium, respectively. k1 (L/min) and k2 (g/mg∙min) constants correspond to the rates of adsorption for the pseudo-first-order (PFO) and pseudo-second-order (PSO) models, respectively. t denotes the duration of the contact time in minutes.
Thermodynamics
Temperature is a variable that affects the adsorption process, and the relative effect on dye adsorption was observed at different temperatures, such as 298, 308, 318, and 338 K, applying the optimum dosage and pH value for 90 min as contact time. The change of Gibbs free energy (ΔG°, kJ/mol), enthalpy (ΔH°, kJ/mol), and entropy (ΔS°, kJ/mol·K) is considered to thermodynamically evaluate the adsorption process and tentatively characterize its nature. Consequently, to calculate the thermodynamic parameters, four different temperatures (298, 308, 318, and 338 K) were conducted and the following equations (Smith 1916–2009, 1959) were applied:
where KC is the thermodynamic constant, R is the universal gas constant (8.314 J/mol·K), and T is the temperature (K), while Cs (mg/L) is the amount adsorbed on solid at equilibrium and Ce indicates the RR120, RB5, RBBR, and MB concentration (mg/L) at equilibrium (Kuang et al. 2020; Munagapati et al. 2019). ΔG° was given from Eq. 8, and the values of ΔH° and ΔS° were calculated from the slope and intercept of the plot of ln(Kc) versus 1/T (Eq. 10).
Results and discussion
Characterizations
Physical properties
BET analysis was carried out to determine the surface area (m2/g), pore diameter (nm), and total pore volume (cm3/g) of the adsorbents, by using Barrett-Joyner-Halenda (BJH) analysis. The relative values are listed in Table 1, and as shown, banana peels have a larger surface area and total pore volume (73.2 m2/g and 0.231 cm3/g, respectively) relative to that of orange peels (63.2 m2/g and 0.195 cm3/g) and of pomegranate peels (35.9 m2/g and 0.109 cm3/g). On the other hand, regarding the diameter of the mean pores, it was found that all adsorbent materials present similar values, i.e., 1.613, 1.618, and 1.615 nm.
Scanning electron microscopy and EDS analysis
The SEM micrographs of banana, orange, and pomegranate peels before and after the adsorption process were taken and illustrated in Fig. 2. As shown, banana peels (Fig. 2a) and pomegranate peels (Fig. 2b) exhibited highly heterogeneous and irregular porous structure which supports the adsorption of RR120 (Fig. 2d–f), RB5 (Fig. 2g–i), RBBR (Fig. 2j–l), and MB (Fig. 2m–o). After adsorption, peels appear to have rough surface with wider pores (Munagapati et al. 2018), as the pores were packed with dyes (Arami et al. 2005). Moreover, the SEM image of orange peels (Fig. 2b) shows a cleaner and smoother surface and less porous structure, compared to that of banana and pomegranate peels; this may exhibit less adsorption capacity due to the reduced number of active adsorption sites available for dye binding (Michael-Igolima et al. 2023). After adsorption of MB on orange peels (Fig. 2n), a change in microstructure and morphology of the surface is observed, in comparison with the adsorption of RR120 (Fig. 2e), RB5 (Fig. 2h), and RBBR (Fig. 2k). Similar structural divergences have been reported in previous studies (Stavrinou et al. 2018). This gives interest for the continuation of the research and the correlation of these findings with the adsorption capacity of these materials.
Furthermore, EDS analysis of adsorbents before and after dye adsorption is conducted, and the results are presented in Fig. 3 and Table 2. EDS can be used to determine which chemical elements are present in a sample and also to estimate their relative abundance. In addition, EDS analysis after adsorption was determined in order to check if there is any modification in the percentage of elements on the surface of the materials, which possibly actively participating in the removal of the dye. As shown, C, Cl, K, and O elements were recorded on the surface of banana, orange, and pomegranate peels (46.45, 0.97, 3.46, and 49.13%; 56.01, 0.19, 0.88, and 42.92%; and 49.77, 0.27, 1.11, and 48.85%, respectively), confirming the homogeneous distribution of these elements on the surface. After the adsorption, a greater percentage of carbon content and the appearance of sulfur are observed, which are possibly due to the additional C and S that resulted from the structure of the adsorbed dyes (see Fig. 1). After adsorption, a decrease or in some cases disappearance of the percentage of K is observed, possibly related to the removal and binding of the dye especially in the case of banana peels as the 3.46% before adsorption, set to almost zero (or values closed to that) after adsorption of RR120, RBBR, and MB and to 0.1% of RB5.
Adsorbed dye distribution maps on a banana, b orange, and c pomegranate peels, before and after adsorption: d banana_RR120; e orange_RR120; f pomegranate_RR120; g banana_RB5; h orange_ RB5; i pomegranate_RB5; j banana_RBBR; k orange_RBBR; l pomegranate_RBBR; m banana_MB; n orange_MB; and o pomegranate_MB, by SEM/EDS analysis
FTIR analysis
The FTIR spectra of banana, orange, and pomegranate peels before and after adsorption of RR120, RB5, RBBR, and MB, in the range of 4000–400 cm−1, were determined and compared to get information about the potential interactions between the adsorbent and each dye separately. These comparative spectra are shown in details in Fig. 4. Specifically, in Fig. 4a, the three adsorbents are compared in terms of their peaks in the FTIR spectrum before the adsorption of the dyes, while in Fig. 4b–e, the absorptions after the adsorption of RR120, RB5, RBBR, and MB, respectively, are presented and compared. In addition, Fig. 4f–h compare the effect of banana, orange, and pomegranate peels separately in this four dyes. The widespread band presented in all diagrams, around 3272 cm−1 matching to the stretching vibration of O–H groups and amine (–NH) functional groups (Al-Tabakha et al. 2021). The peak at 2920 cm−1 is accredited to aliphatic acids C–H stretching vibrations. The peak appeared at 1735 cm−1 may be due to the stretching vibrations of C = O due to the bonds of carboxylic groups (–COOH, –COOCH3) (Yang et al. 2007). The peaks at around 1630 and 1590 cm−1 correspond to skeletal aromatic vibrations of C = C in lignin (Gonultas and Candan 2018). Furthermore, the peak at 1432 cm−1 is attributed to phenol –COO– symmetric vibrations. Moreover, the peak at 1100 cm−1 may be attributed to the primary OH group in lignin or hemicellulose, regarding the composition of the peels (Mahrous et al. 2022). Additionally, the band at 1428 cm−1 is assigned to the C − H bending vibration, which forms the basic structure of lignocellulosic material, and the stretching at around 1011 cm−1 could confirm the existence of C–OH and C–OR groups found in carbohydrates (Michael-Igolima et al. 2023).
In addition, several other peaks that appeared after the adsorption are characteristic due to the structure of the dyes. Hence, at 1589 cm−1, the peak is relative to N − H bending vibrations of amine, at 1490 cm−1 relative to N = N stretching vibrations of azide; at 1247 cm−1, there is a peak regarding the stretching vibration of –S = O group and at 1153 cm−1 for stretching vibration of the –C − N. Moreover, the peak at 1028 cm−1 is characteristic for the stretching vibrations of alcoholic C–O, at 828 cm−1 for the aromatic C–H bending vibrations, and at 695 cm−1 may attributed to –S–O stretching (Munagapati et al. 2019). Consequently, the presence of hydroxyl and amine groups on the adsorbent surface is showing.
Several bands presented changes, i.e., at 1630 cm−1 and at 2928 cm−1, after adsorption of dyes. In addition, especially for banana and pomegranate peels, there is a new peak appeared that is not found in spectrums before adsorption. In addition, at 1476, 836, and 689 cm−1, new peaks appeared (Fig. 4b) indicating the formation of bonds between the peels and RR120 dye molecules (Munagapati et al. 2019). Therefore, it can be concluded that the hydroxyl and amine functional groups may play an important role in the adsorption of RR120 on the surface of the peels (Munagapati et al. 2019). Moreover, the spectra of the RB5 dye (Fig. 4c) showed similar characteristics and small changes in their positions and intensity occurred. These results specified the involvement of several functional groups (hydroxyl, amine, and carboxyl) in the adsorption of RB5 on the surface of the peels possibly due to weak electrostatic interactions or Van der Waals forces (Kapoor et al. 2022; Munagapati et al. 2018).
In addition, in Fig. 5d, at 1037 cm−1, the S = O stretching vibration is shown from sulfonic (–SO3−) groups (Sangar et al. 2019) that exist at RBBR structure (Tsoutsa et al. 2023). Some peaks in the spectra of the peels disappeared, decreased, or shifted after adsorption, possibly due to the adsorption of RBBR dye on their surface (Ahmad et al. 2020). Specifically, the peak at 1037 cm−1, due to S = O for –SO3−, particularly when orange peels are used as adsorbent, appears and was not presented before.
Finally, the FTIR spectrum of banana, orange, and pomegranate peels after MB dye adsorption (Fig. 4e) did not show much difference from the spectrum before adsorption. In addition to this, some small shifts in the peaks can be detected, which may provide evidence that the acidic functional groups, such as –COOH, are responsible for the binding of MB cationic dye molecules (Jawad et al. 2021).
XRD analysis
The diffraction of X-ray (XRD) was used in this study to further validate the structure of peels. Figure 5 shows the patterns of banana, orange, and pomegranate peels. All the samples have a broad peak centered at 2θ = 14° which corresponds to the (101), at 2θ = 17° to (111), and at 2θ = 23° to (002) carbon reflection (less intense regarding banana peels). These peaks can be attributed to the Miller indices of the crystalline cellulose (JCPDS #03–0829) (Li et al. 2018; Padmanabhan et al. 2022). As expected for organic adsorbents, banana, orange, and pomegranate peels have an amorphous structure as the broad peak in the 2θ 20–25ο range is indicative of the amorphous carbon present in such adsorbents (Çatlıoğlu et al. 2021; Hashem et al. 2023; Tadesse et al. 2023).
Batch adsorption experiments
Effect of adsorbent’s dose
Batch experiments were conducted to investigate the prospect of banana, orange, and pomegranate peels for the effective removal of RR120, RB5, RBBR, and MB dyes from wastewaters. In the experiments carried out, the initial concentration of each dye was 100 mg/L, at an indicative pH of 2.0 according to the literature (Devi and Prabhakar 2020; Mengelizadeh and Pourzamani 2020; Tsoutsa et al. 2023), a temperature of 298 K, and a contact time of 24 h, with the addition of different doses of the natural adsorbents in the range of 1.0–7.0 g/L. The relevant results are shown in Fig. 6, and as expected, in all cases, the dye removal rate increased as the dosage increased and more adsorption sites appeared on the adsorbent surface.
More specifically, when banana peels were used as adsorbent, as shown in Fig. 6a, 4.0 g/L was required to remove 80% of RR120 dye, a rate that increased to 90% with 6.0 g/L and to 94% with 7.0 g /L. In addition, for the removal of RB5, the 86% was removed by 5.0 g/L and 94% by 6.0 g/L. In the case of RBBR, by applying 3.0 g/L banana peels, the 90% of dye was removed, reaching the 100% with 5.0 g/L. Finally, for MB with 6.0 g/L, the relative rate reached 94%. In Fig. 6b, the effect of orange peel dosage is illustrated, and as depicted, in general, this adsorbent is less effective than banana peels. For instance, with 7.0 g/L of the adsorbent, the relative removal rates reached up to 92, 90, 94, and only 48% for RR120, RB5, RBBR, and MB, respectively.
However, in the case of using pomegranate peels as adsorbent (Fig. 6c), when applied to RR120, RB5, and RBBR dyes, the removal rates were found to be low, i.e., 58, 59, and 75%, respectively, even with 7.0 g/L. On the other hand, in the case of MB, pomegranate peels proved to be very effective even at lower doses, indicating that even fewer active sites on the adsorbent surface are capable of removing MB. This will be of interest in the continuation to be related to the pHPZC of the material. Particularly, with the addition of only 2.0 g/L, 80% of the initial dye concentration was removed, a percentage that increased to 93% with 6.0 g/L and to 95% with 7.0 g/L.
Moreover, according to Fig. 7, where the effect of the adsorbents’ dose on each dye is analyzed separately and at the same time these materials are compared in terms of their effectiveness, the relative efficiency order emerged as follows:
-
RR120: banana > orange > pomegranate peels (Fig. 7a)
-
RB5: banana > orange > pomegranate peels (Fig. 7b)
-
RBBR: banana > orange > pomegranate peels (Fig. 7c)
-
MB: pomegranate > banana > orange peels (Fig. 7d)
Taking into account all the above that appeared from the analysis of Figs. 6 and 7, for the further study of the effectiveness of the adsorbent materials, the following doses were chosen:
-
Banana peels: 6.0 g/L for RR120; 6.0 g/L for RB5; 5.0 g/L for RBBR; and 4.0 g/L for MB.
-
Orange peels: 6.0 g/L for RR120, RB5, RBBR, and MB.
-
Pomegranate peels: 6.0 g/L for RR120, RB5, RBBR, and MB.
A cost analysis was conducted for the preparation of peels as adsorbents (Appendix 1). As shown, for using 6.0 g/L as indicative dosage, the cost was around 0.196 €/L for banana peels (final working volume is 10 mL = 0.0020 €/experiment), 0.129 €/L (0.0013 €/experiment) for orange peels, and 0.277 €/L (0.0028 €/experiment) for pomegranate peels. Hence, these are low-cost adsorbents prepared from waste fruit peels.
Effect of initial solution pH
One of the most important factors that can affect the adsorption performance is the effect of the initial solution pH. Therefore, the effect of pH on the adsorption capacity of RR120, RB5, RBBR, and MB dyes onto banana (Fig. 8a), orange (Fig. 8b), and pomegranate (Fig. 8c) peels was studied in various pH conditions in the range of 2.0 to 9.0 at 298 K, at a constant initial dye concentration of 100 mg/L, and adsorbent dosage according to the results achieved in the previous section for each dye and adsorbent. The obtained results are shown in Fig. 8 and will be further analyzed below.
In addition, the point of zero charge (pHpzc) of each material is a very important parameter for obtaining information about the surface charge of the adsorbent, as it is the value at which the surface charge of the material becomes neutral. At solution pH below pHpzc, the adsorbent surface is positively charged and can attract negatively charged ions, while at solution pH values greater than the pHpzc, the surface is negatively charged and attracts cations. In this study, pHpzc was determined by measuring it over a pH range of 2.0–10.0 ± 0.1, according to pH drift method (Tai et al. 2020). Hence, as shown in Fig. 9, the calculated pHpzc values were 6.06, 4.09, and 3.33 for banana, orange, and pomegranate peels, respectively.
Determination of pHpzc of natural adsorbents using pH drift method (Tai et al. 2020)
Based on the above, the effect of pH is further discussed for the removal of these dyes, taking also in account the results obtained from Fig. 8. Hence, regarding the adsorption of RR120 dye, in all materials, it is shown that it is significantly dependent on the pH of the solution. In general, the removal percentage of RR120 decreased from 90 to 31% by using banana peels, from 88 to 31% by orange peels, and from 51 to 22% by using pomegranate peels, with increasing pH solution from 2.0 to 9.0. Thus, the maximum removal rate was reached at 2.0; therefore, this pH was chosen for further adsorption experiments. According to literature, RR120 has water-soluble sulphonate (R-SO3−) groups (Fig. 1a) (Munagapati et al. 2019), leaving the dye molecule with a net negative charge, as shown in the following Reaction (11):
By comparing the pHpzc values of banana (6.06), orange (4.09), and pomegranate (3.33) peels with the optimum pH of RR120 solution, an explanation can be given, as the positive surface charge of the peels attracts the negative charged RR120 and enhances uptake. However, at higher pH values, the abundance of hydroxylic ions competes with RR120 and ionic repulsions between the negatively charged surface and the RR120 molecule resulting in lower RR120 adsorption, as the surface of all peels lacks of exchangeable anions at higher pH values (Bazrafshan et al. 2013; Munagapati et al. 2019), due to the loss of protons in a basic solution.
In the case of RB5 dye, the percentage removal was also decreased with the increase of pH. Particularly, from the high removal rate of 94% at pH 2.0 to 45% at pH 9.0, when the banana peels were used as the adsorbent (Fig. 8a), this is evident. Similarly, to banana peels, when orange peels (Fig. 8b) or pomegranate peels (Fig. 8c) are used, there is also a decrease in dye removal rates as pH increases. RB5 is also a reactive dye with sulphonate (R-SO3−) groups (Fig. 1c), and for this reason, an identical reaction takes place (Reaction (12)):
At higher pH, the electrostatic repulsion between the negatively charged surface of the adsorbents and the anionic ions of the RB5 dye causes a significant drop in dye removal. Therefore, the maximum dye removal by adsorption on banana, orange, and pomegranate peels was achieved at pH 2.0, at which the surface of the adsorbents and the functional groups of the dye behave in a relative manner. Thus, at acidic pH 2.0 (a value lower than any pHpzc determined), the surfaces of the materials are positively charged, and with the sulfonic group (–SO3−) of the surface of RB5, a strong electrostatic attraction is expected. On the other hand, the lower adsorption capacity in the alkaline condition (pH 9.0) is due to the competition between hydroxyl ions (OH−) and the negatively charged dye ions for the adsorption sites (Devi and Prabhakar 2020; Mengelizadeh and Pourzamani 2020; Tsoutsa et al. 2023).
RBBR dye is also a reactive anthraquinone dye with sulphonate (R-SO3−) groups (Fig. 1d), which upon its dissolution in water behaves in the same way (Reaction (13)):
Similar to previous results in the literature (Arya et al. 2020b; Ozturk and Silah 2020; Tolkou et al. 2023), pH 2.0 also found to be optimum for the removal of RBBR dye, as shown in Fig. 8. The results for all adsorbents used indicate that the adsorption efficiency decreased as solution pH increased, and at pH 2.0, banana peels had the highest removal rate at 100%, orange peels at 91%, and pomegranate peels at 70%. Under acidic conditions, strong electrostatic interactions occur between the cationic groups (ΝΗ3+) of adsorbents and the anionic groups of RBBR dyes. Under alkali conditions at pH 9.0, the adsorption efficiency significantly decreased to 58, 51, and 41% for banana, orange, and pomegranate peels, respectively, due to competitions between anionic RBBR dye ions and hydroxyl ions (Arya et al. 2020b; Yeow et al. 2021). Therefore, electrostatic attraction between the positively charged surface of banana, orange, and pomegranate peels at pH 2.0 and negatively charged RR120, RB5, and RBBR reactive dye molecules may be favored as the principal adsorption mechanism. Nevertheless, in Fig. 8, mainly in the case of banana and orange peels, a slight increase in the removal of anionic dyes is most likely observed above the pHpzc value of each material. This fact is possibly attributable to the presence of some positively charged dye groups (ΝΗ2+) that interact with the negatively charged surface of adsorbents.
On the other hand, MB is a cationic dye (Fig. 1b), and it was also chosen in this study because of its strong adsorption on solid adsorbents as found in the literature (Atay 2018). MB occurs in the singly protonated form (MBH2+) in water due to the protonation of the dimethylamino groups (Minamoto et al. 2021). As derived from the pH drift method, the pHpzc of banana peels was 6.06, of orange peels 4.09, and of pomegranate peels 3.33. As illustrated in Fig. 8, with the increase of pH value, an increase in MB dye removal was observed, for instance from 65 and 43% at pH 2.0, to 98% and 94% at pH 9.0 by using banana and orange peels, respectively. This could be due to the fact that the pH 9.0 of the solution was greater than the pHpzc of the adsorbents, so the surface charge was negative, while in an acidic conditions, the positively charged surface of the adsorbent seemed to limit the adsorption of the cationic dye (Khuluk et al. 2019). Hence, an increase in electrostatic attraction between positively charged dye and negatively charged adsorbent obtained at pH 9.0. However, when the pomegranate peels were used as the adsorbent, it was observed that the removal rates of MB were high, at all pH values, with percentages ranging from 93% at pH 2.0 to 97% at pH 9.0. This is most likely due to the low pHpzc displayed by pomegranate peels (3.33), and its surface charge changes to anionic at sufficiently acidic values, and therefore, from this value and above, electrostatic attractions are observed, between the anionic adsorbent and cationic MB dye ions.
Taking into account all the above, Table 3 summarizes the optimal conditions obtained during the study of the adsorption of the four dyes on the surface of the three natural adsorbents examined in this study. These conditions will be used in the following process evaluation experiments.
Effect of initial dye concentration
The effect of the initial concentration of RR120, RB5, RBBR, and MB on the adsorption efficiency of banana (Fig. 10a), orange (Fig. 10b), and pomegranate (Fig. 10c) peels was studied by changing the initial dyes’ concentration between 5 and 1000 mg/L and by appliyng the optimum selected pH values and dosages, as resulted in Table 3, for each dye solution and adsorbent. The data showed that there was a steady increase in adsorption capacity with the increase in dye concentration and that could be attributed to the fact that the driving force provided by the increased solute concentration which is sufficient to overcome the mass transfer resistance between the solid and liquid phases (Inglezakis et al. 2020). Therefore, adsorption will be enhanced with higher initial concentration (Banerjee and Chattopadhyaya 2017). The results were found to be in full agreement with the literature (Arya et al. 2020a; Moussavi and Mahmoudi 2009).
Effect of initial dye concentration onto a banana, b orange, and c pomegranate peel adsorption; C0 5–1000 mg/L, T = 298 K, contact time 24 h, dose and pH according to Table 3 results
In particular, the adsorption capacity of banana peels (Fig. 10a) was found to increase remarkably from 1.1 to 119 mg/g for MB (pH 9.0 and 4.0 g/L) and from 0.8 to 60 mg/g for RR120 (pH 2.0 and 6.0 g/L), RB5 (pH 2.0 and 6.0 g/L), and RBBR (pH 2.0 and 5.0 g/L). In the case of orange peels (Fig. 10b), the adsorption capacity for all the dye solutions showed a lower increase ranging from 0.7 to 45 mg/g. Finally, the second most effective material after banana peels, the pomegranate peels, also showed an impressive increase in capacity with increasing initial concentration, especially of MB from 0.7 to 102 mg/g (pH 9.0 and 6.0 g/L), as shown in Fig. 10c. For RR120, the relative variations in adsorption capacity were from 0.5 to 58 mg/g (pH 2.0 and 6.0 g/L), for RB5 (pH 2.0 and 6.0 g/L) from 0.7 to 35 mg/g, and for RBBR (pH 2.0 and 6.0 g/L) from 0.8 to 34 mg/g, as initial solution concentration increases.
Effect of contact time
In Fig. 11, the effect of contact time on the adsorption of RR120, RB5, RBBR, and MB on banana (Fig. 11a), orange (Fig. 11b), and pomegranate (Fig. 11c) peels is presented. The effect was examined in the range of 5 to 1440 min (24 h) and in almost all cases the 240 min (4 h) of reaction determined as the optimal contact time for further batch experiments (in which the equilibrium achieved), indicating a rapid distribution and surface adsorption.
Effect of contact time on dye removal with a banana, b orange, and c pomegranate peels; C0 100 mg/L, T = 298 K, contact time 24 h, dose and pH according to Table 3 results
Specifically, for MB removal, it was found that the adsorption was very quick when banana and pomegranate peels were used as the 90% of the dye was removed in the first 20–30 min, and after 24 h, the relative percentage was 99%. By using orange peels, 90 min is required for the 90% removal.
In addition, for the RB5 dye up to 90 min, there is an immediate adsorption of the dye on banana peels, as the 85% was removed (Fig. 11a). When using orange and pomegranate peels, the respective removal rates decrease and the required contact time increases (Fig. 11b, c). Furthermore, for RBBR, 85, 80, and 60% removal rates were achieved in the first 90 min for banana, orange, and pomegranate peels, respectively, reaching 100% with the use of banana after 360 min (6 h) up to 1440 min (24 h) where equilibrium occurred.
In the case of RR120 dye, more time (150 min) is required to remove the 75% of the color with banana peels and only 50% with pomegranate peels, with the latter being the highest percentage removed even after 24 h. Relative adsorption on orange peels was somewhat faster and more efficient in the first 90 min (80% removal), as shown in Fig. 11b.
Adsorption isotherms
Langmuir and Freundlich isotherm models were used in this study for the evaluation of the equilibrium adsorption data of RR120, RB5, RBBR, and MB onto banana, orange, and pomegranate peels at the optimum conditions. The obtained experimental data were fitted to those models, and the resulting non-linear plots are shown in Fig. 12, while Table 4 summarizes the relative equilibrium constants and coefficients. According to the results, the adsorption in some of the adsorbent materials and dyes fitted better the Freundlich and in some others the Langmuir isotherm model based on the values of the determination coefficient (R2). It should be noted here that the Freundlich adsorption isotherm model is valid for heterogeneous surfaces and large value of n indicates a strong interaction between the surface of the adsorbent and dyes (Arya et al. 2020a). On the other hand, Langmuir model suggests that monolayer adsorption of the dye on the homogeneous surface of the adsorbent takes place (Moussavi and Mahmoudi 2009).
Langmuir and Freundlich isotherm models for the adsorption of dyes onto a, b banana, c, d orange, and e, f pomegranate peels. C0 5–1000 mg/L, T = 298 K, contact time 24 h, dose and pH according to Table 3 results
In more details, regarding RR120 dye solution, it was found that the adsorption on all three examined adsorbents fitted better to Langmuir model, displaying a higher R2 (0.9644, 0.9554, and 0.9836 for banana, orange, and pomegranate peels, respectively), exhibiting a maximum adsorbent capacity, on the homogeneous surface of the adsorbent, of 58.24 mg banana/g, 36.95 mg orange/g, and 91.29 mg pomegranate/g. The latter is also in agreement with the literature for RR120 dye and its better correlation with Langmuir model (Navaei et al. 2019).
On the other hand, in the case of RB5, the results showed a better correlation to Freundlich model when orange (R2 = 0.9711) and pomegranate (R2 = 0.9948) are used and to Langmuir model in the case of banana peels (R2 = 0.9835). The RBBR dye, which belongs to the same category as RB5, followed the Langmuir model on orange peels with a higher R2 = 0.9659, but the Freundlich model on banana and pomegranate peels (R2 = 0.9065 and 0.9876, respectively). That could be attributed to the groups NH3+ existing on the dye (Fig. 1d), and have a strong affinity with the cationic exchange sites of banana peels (Arya et al. 2020a).
Finally, for MB dye solution, Freundlich model was better fitted in the case of orange peels used as adsorbent (R2 = 0.9399). Langmuir model was dominant in the case of banana (R2 = 0.9673) and pomegranate (R2 = 0.8961) peels, relative to literature (Hurairah et al. 2020), indicating a very high adsorption capacity, i.e., 112.35 mg banana/g and 98.08 mg pomegranate/g.
Consequently, it could be assumed that monolayer adsorption of the dye on the homogeneous surface of the adsorbent (Langmuir model) took place in the cases of RR120 on banana, orange, and pomegranate peels, of RB5 on banana peels, of RBBR on orange peels, and of MB on banana and pomegranate peels. On the contrary, multilayer adsorption with strong interactions between the heterogeneous surface of the adsorbent and dyes (Freundlich model) occurred in the cases of RB5 on orange and pomegranate peels, of RBBR on banana and pomegranate peels, and of MB on orange peels.
Adsorption kinetics
Adsorption kinetics is used to understand the rate of adsorption and the mechanism that controls the whole process. In order to interpret the kinetics of the adsorption of RR120, RB5, RBBR, and MB on banana (Fig. 13a), orange (Fig. 13b), and pomegranate (Fig. 13c) peel adsorbents, the pseudo-first-order (PFO) and the pseudo-second-order (PSO) kinetic models were used. According to the results, the adsorption of all the dyes examined on the surface of all the tested adsorbents was found to better fit to pseudo-second-order model, by comparing the R2 values (> 0.93) presented in Table 5. It should be clarified that the corresponding R2 values obtained during the application of the pseudo-first-order model were < 0.9, and for this reason, the relevant parameters are not shown in this paper. PSO model and its non-linear forms are described in Fig. 13 and Table 5. Moreover, the kinetic parameters showed that almost all Qe,cal values calculated from Eq. 6 were comparable to the experimental values obtained from the adsorption studies of RR120, RB5, RBBR, and MB on banana, orange, and pomegranate peels. For instance in the case of MB dye, the R2 was the highest (0.9957) when banana peels were used as the adsorbent, and the relative Qe,cal and Qe,exp (calculated from Eq. 2) were 23.96 and 24.6 mg/g. Therefore, the adsorption kinetic study showed that the adsorption was closer most likely to chemisorption (Arya et al. 2020a; Tsoutsa et al. 2023).
Kinetic pseudo-second-order models for the adsorption of dyes onto a banana, b orange, and c pomegranate peels adsorption; C0 100 mg/L, T = 298 K, dose and pH according to Table 3 results
Thermodynamics
Figure 14 depicts the variation of Qe (mg/g) with temperature in thermodynamic experiments. It is evident that as the temperature increases, the adsorption capacity also tends to increase. The improved adsorption and uptake efficiency of the dyes on the particle surface at higher temperatures indicate an endothermic nature of the adsorption process. The increase in Qe at higher temperatures could be attributed either to the chemical interaction between the dye molecules and the adsorbents or to the formation of new adsorption sites on the surface of the particles (Parimelazhagan et al. 2022).
Effect of temperature on dye removal with a banana, b orange, and c pomegranate peels; C0 100 mg/L, contact time 90 min, dose and pH according to Table 3 results
According to thermodynamics for banana peels, the ∆H° and ∆S° values were derived by examining the ln(Kc) versus 1/T plot (Lima et al. 2019), exhibiting high correlation coefficients of R2 = 0.953, 0.991, 0.974, and 0.927 for RR120, RB5, RBBR, and MB, respectively. As shown in Table 6, the adsorption process for each dye resulted in negative ∆G° values. This suggests that the removal of dyes through adsorption took place spontaneously and without the need for additional energy from an external source. The adsorption process of banana peels in RR120, RB5, RBBR, and MB was found to be endothermic due to the values of ∆H° (31.299 kJ/mol, 4.471 kJ/mol, 61.539 kJ/mol, and 16.497 kJ/mol, respectively). The positive ∆S° values observed for RR120 (0.1097 kJ/mol∙K), RB5 (0.0290 kJ/mol∙K), RBBR (0.2190 kJ/mol∙K), and MB (0.0792 kJ/mol∙K) are results of the enhanced disorder or randomness of the adsorbates at the interface between the solids and the solutes during the adsorption processes (Rao 2017).
For the orange peels, the relative plot showed strong correlation coefficients of R2 = 0.958, 0.996, 0.964, and 0.966 for RR120, RB5, RBBR, and MB, respectively. As presented in Table 6, the adsorption process for each dye exhibited negative ∆G° values, indicating that the removal of dyes through adsorption occurred spontaneously without requiring additional external energy. The adsorption process of orange peels in RR120, RB5, RBBR, and MB was determined to be endothermic based on the respective ∆H° values of 16.510 kJ/mol, 11.443 kJ/mol, 33.052 kJ/mol,, and 5.215 kJ/mol. The positive ∆S° values observed were for RR120 0.667 kJ/mol∙K, for RB5 0.0467 kJ/mol∙K, for RBBR 0.1229 kJ/mol∙K, and for MB 0.0348 kJ/mol∙K, and were attributed to the increased disorder or randomness of the adsorbates at the interface between the solid and solute during the adsorption process. Due to the adsorbed species, the adsorbed solvent molecules are displaced and thus gain more translational entropy than is lost from the adsorbed ions/molecules, thus enhancing the appearance of randomness in the system (Saha and Chowdhury 2011).
Additionally, based on the identical graph, the values of ∆H° (39.807 kJ/mol for RR120, 3.066 kJ/mol for RB5, 42.479 kJ/mol for RBBR, and 9.345 kJ/mol for MB) and ∆S° (0.1312 kJ/mol∙K for RR120, 0.0104 kJ/mol∙K for RB5, 0.1462 kJ/mol∙K for RBBR, and 0.0601 kJ/mol∙K for MB) derived from the application of pomegranate peels were obtained, with an R2 of 0.993 for RR120, 0.965 for RB5, 0.993 for RBBR, and 0.996 for MB, as presented in Table 6. As it referred above, the positive entropy values indicate the heightened disorder or randomness of the adsorbates at the interface between solids and solutes during the adsorption processes, while the positive enthalpy values signify an endothermic reaction and the values of the free energy Gibbs (∆G0) were found to be negative showing that the adsorption took place spontaneously (Sun et al. 2021).
Regeneration study
Regeneration studies were performed to investigate the reusability of banana, orange, and pomegranate peels in the removal of RB5 dye from wastewater. RB5 dye is one of the most widespread synthetic reactive dyes used in the dyeing industry as it is used to dye mainly several cellulosic fibers, such as cotton, wool, and nylon (El Bouraie and El Din 2016), and for this reason, it was chosen indicatively to study the regeneration and reusability of the peels. Therefore, each cycle was conducted under similar conditions, involving an initial RB5 concentration of 100 mg/L and an adsorbent dosage of 6.0 g/L at pH 2.0 ± 0.1 for each adsorbent for approximately 90 min. After the completion of the first cycle, the particles utilized in the process underwent a treatment using a 1 M NaOH solution. Following the treatment, the particles rinsed with distilled water to remove any remaining traces of the base. An appropriate duration was chosen so that it was possible to reuse the adsorbents in subsequent cycles, ensuring their effectiveness and maintaining their ability to remove RB5 from the wastewater.
As shown in Fig. 15, the regenerated banana peels could be effectively reused for 8 cycles in removing RB5, following treatment with 1 M NaOH. The percentage of RB5 removal was approximately 85% during the initial (first) cycle, after 90 min of treatment, gradually declining to around 35% by the eighth cycle. The orange peels found to be effective as adsorbent for RB5 removal after 4 cycles of reusability. During the first cycle, 74% of RB5 was removed, but this percentage gradually decreased to around 28% by the fourth cycle. The pomegranate peels regenerated up to 5 cycles with removal percentage of RB5 dye in the first cycle being 48% and in the fifth cycle 24%. In summary, it is clear the viability of reusing the banana, orange, and pomegranate peel adsorbents for 8, 4, and 5 cycles in the effective removal of RB5 from wastewater, showing a reduction of around 50% loss of their effectiveness.
Mixtures of dyeing solutions
The ability of banana, orange, and pomegranate peels to simultaneously remove basic cationic dyes (MB) and anionic dyes (RR120, RB5, and RBBR) in a mix solution containing 100 mg/L of each dye was investigated. The conditions applied were pH 2.0 ± 0.1, dose 6.0 g/L, and T = 298 K. The comparative results of natural adsorbents on dye removal are presented in Fig. 16, separately and together in solution. MB occurs in the single protonated form (MBH2+) in water (Minamoto et al. 2021), and the anionic dyes contain the sulfonic groups (dye-SO3−) (Munagapati et al. 2019). In addition, as derived from the pH drift method, the pHpzc of banana peels was 6.06, of orange peels 4.09, and of pomegranate peels 3.33. Thus, at pH 2.0, the surfaces of the materials are positively charged.
As shown in Fig. 16, MB adsorption on banana and orange peels increased (from 94 to 96 and 43 to 87%, respectively) in the presence of anionic dyes (synergistic effect), but a competitive effect was determined, mainly between RB5 and RBBR with MB, resulting in a decrease in their respective percentages (from 94 to 65% for RB5 on banana and from 87 to 17% on orange, and for RBBR from 100 to 16% and from 91 to 2%, respectively), a phenomenon found also in the literature (Shirazi et al. 2019). In the case of pomegranate peels, the removal percentage of MB did not differ much in the presence of the negative dyes, but the competitive (antagonism) effect was intense for RB5 and RBBR. The RR120 dye exhibited similar results in mix solution.
Comparison with recent literature
The adsorption efficiency of the fruit peels used in this study for the removal of several dye solutions is compared with some related materials published in the recent literature. Various parameters used for this comparison and the findings are presented in Table 7. As depicted, in the case of RR120 dye, in addition to banana, orange, and pomegranate peels, other peels have been also used, such as those of cactus or modified orange peels, e.g., with quaternary amine. In all cases, acidic conditions proved to be more effective (pH 2 or 3), as well as the adsorbent dose used was 6.0 g/L. Only in the case that the peels were modified, the dose was lower (2.0 g/L) and the adsorption capacity was high. Variation existed mainly in the initial concentration of the dye (Munagapati et al. 2019).
In the case of RB5 removal, not many peel applications were found in the literature. Similar results to the present research, regarding the adsorption capacity and rate, were also found using potato peels, but in this case, a much higher dose of adsorbent (20 g/L) and a lower initial dye concentration (50 g/L) were used (Samarghandy et al. 2011).
In contrast, more variety in the use of peels as adsorbents was found in the literature for RBBR removal, for example, pineapple leaf, lime peel, and ambarella peel. What emerged from their comparison with the materials of this research is that they showed a lower adsorption capacity, only 7–9 mg/g compared to 35–45 mg/g when using the proposed materials, and in fact in the case of ambarella peel (Chong and Hadibarata 2021), a high dose was used and low initial concentration, offering only 58% removal.
Finally, for the removal of MB, banana and pomegranate peels were also used in the literature, along with the use of mango peels. The following are worth noting: first, the banana peels used in the present study proved to be very effective in yielding high adsorption capacity (112.4 mg/g) at basic pH values and furthermore a very low dose (4.0 g/L) was used, especially compared to other research found in the literature that also used banana peels (Dey et al. 2017). In that case, 20 mg/L was used with 50 mg/L initial dye concentration and only 23 mg/g uptake.
In conclusion, the materials used in this research (banana, orange, and pomegranate peels) proved to be effective in agreement with the literature. In addition, these are materials that promote the sustainability, economic use, and reuse of food waste, and in fact, as proposed in this study, by increasing the drying of the peels in air conditions, instead of extensive drying in an oven, the energy costs for the synthesis of the materials are reduced.
Conclusions
In this study, banana, orange, and pomegranate peels were collected from local markets and prepared by a proposed simple and environmentally friendly method, promoting the sustainability, economic use, and reuse of food waste and applied as natural adsorbents for the removal of both anionic (RR120, RB5 and RBBR) and cationic (MB) dyes from wastewater. The application of such processed materials for the simultaneous removal of a wide variety of dyes is an innovative solution presented in this work. According to the characterizations from BET analysis, banana peels have the largest surface area and total pore volume (73.2 m2/g and 0.231 cm3/g, respectively).
Regarding the results, the optimum conditions found to be for banana peels: pH 2.0, 6.0 g/L for RR120 (90%) and RB5 (94%); 5.0 g/L for RBBR (100%); and pH 9.0, 4.0 g/L for MB (98%); orange peels: pH 2.0, 6.0 g/L for RR120, RB5, RBBR (88, 87, and 91%, respectively) and pH 9.0, 4.0 g/L for MB (94%); and pomegranate peels: pH 2.0, 6.0 g/L for RR120, RB5, RBBR (51, 59, and 69% respectively), and pH 9.0, 6.0 g/L for MB (98%). Moreover, the adsorption process in all cases was found to better fit to pseudo-second-order model, and according to isotherms, Freundlich model fitted better in some cases and the Langmuir model in others. Thermodynamics indicated that the adsorption was endothermic and that took place spontaneously, in all cases. According to regeneration experiments, this study demonstrates the viability of reusing the banana, orange, and pomegranate pee adsorbents for eight, four, and five cycles, showing a reduction of around 50% of their effectiveness. Moreover, the ability of banana, orange, and pomegranate peels to simultaneously remove cationic and anionic dyes in a mix solution was investigated. It was found that the MB adsorption on banana and orange peels increased in the presence of anionic dyes (synergistic effect).
In conclusion, the outcomes of the present study suggest that fruit peels can be used as sustainable natural adsorbents, for the removal both of cationic and anionic dyes from wastewater samples and that the simultaneous removal of dyes from mixtures should be further examined and analyzed. The fact that anionic dyes show a minimum of removal efficiency at specific pH values is a phenomenon that will concern our group at future studies.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ahmad MA, Eusoff MA, Oladoye PO, Adegoke KA, Bello OS (2020) Statistical optimization of Remazol Brilliant Blue R dye adsorption onto activated carbon prepared from pomegranate fruit peel. Chem Data Collect 28:100426. https://doi.org/10.1016/j.cdc.2020.100426
Al-Tabakha MM, Khan SA, Ashames A, Ullah H, Ullah K, Murtaza G, Hassan N (2021) Synthesis, characterization and safety evaluation of sericin-based hydrogels for controlled delivery of acyclovir. Pharmaceuticals 14. https://doi.org/10.3390/ph14030234
Arami M, Limaee NY, Mahmoodi NM, Tabrizi NS (2005) Removal of dyes from colored textile wastewater by orange peel adsorbent: equilibrium and kinetic studies. J Colloid Interface Sci 288:371–376. https://doi.org/10.1016/j.jcis.2005.03.020
Arya MC, Bafila PS, Mishra D, Negi K, Kumar R, Bughani A (2020a) Adsorptive removal of Remazol Brilliant Blue R dye from its aqueous solution by activated charcoal of Thuja orientalis leaves: an eco-friendly approach. SN Appl Sci. 2. https://doi.org/10.1007/s42452-020-2063-2
Arya MC, Bafila PS, Mishra D, Negi K, Kumar R, Bughani A (2020b) Adsorptive removal of Remazol Brilliant Blue R dye from its aqueous solution by activated charcoal of Thuja orientalis leaves: an eco-friendly approach. SN Appl Sci 2. https://doi.org/10.1007/s42452-020-2063-2
Atay UA (2018) Removal of methylene blue by using biosolid. Glob. NEST J. 8, 113–120. https://doi.org/10.30955/gnj.000351
Ayala JR, Montero G, Coronado MA, García C, Curiel-Alvarez MA, León JA, Sagaste CA, Montes DG (2021) Molecules characterization of orange peel waste and valorization to obtain reducing sugarshttps://doi.org/10.3390/molecules26051348
Azamzam AA, Rafatullah M, Yahya EB, Ahmad MI, Lalung J, Alam M, Siddiqui MR (2022) Enhancing the efficiency of banana peel bio-coagulant in turbid and river water treatment applications. Water 14:2473. https://doi.org/10.3390/w14162473
Banerjee S, Chattopadhyaya MC (2017) Adsorption characteristics for the removal of a toxic dye, tartrazine from aqueous solutions by a low cost agricultural by-product. Arab J Chem 10:S1629–S1638. https://doi.org/10.1016/j.arabjc.2013.06.005
Bazrafshan E, Mostafapour FK, Hosseini AR, Raksh Khorshid A, Mahvi AH (2013) Decolorisation of reactive red 120 dye by using single-walled carbon nanotubes in aqueous solutions. J Chem 2013. https://doi.org/10.1155/2013/938374
Ben-Ali S (2021). Application of raw and modified pomegranate peel for wastewater treatment: a literature overview and analysis. Int J Chem Eng 2021. https://doi.org/10.1155/2021/8840907
Çatlıoğlu F, Akay S, Turunç E, Gözmen B, Anastopoulos I, Kayan B, Kalderis D (2021) Preparation and application of Fe-modified banana peel in the adsorption of methylene blue: process optimization using response surface methodology. Environ Nanotechnology, Monit Manag 16. https://doi.org/10.1016/j.enmm.2021.100517
Chairunnisa Lisafitri Y (2020) Preliminary studies of cassava leaves’ ability to remove dyes from water. J Math Fundam Sci 52, 66–80https://doi.org/10.5614/j.math.fund.sci.2020.52.1.5
Chong SN, Hadibarata T (2021) Adsorption of phenol red and remazol brilliant blue r by coconut shells (Cocos nucifera) and ambarella peels (spondias dulcis). Biointerface Res Appl Chem 11, 8564–8576. https://doi.org/10.33263/BRIAC111.85648576
Dai L, Zhu W, He L, Tan F, Zhu N, Zhou Q, He M, Hu G (2018) Calcium-rich biochar from crab shell: an unexpected super adsorbent for dye removal. Bioresour Technol 267:510–516. https://doi.org/10.1016/j.biortech.2018.07.090
Daud ND, Puzi SM, Khalijah S, Rozi M (2019) New application of pomegranate peel waste : the decontamination of toxic methylene blue dye from textile wastewater. J Eng Res Educ 11:45–58
Devi SUMA, Prabhakar P (2020) Removal of Reactive Black 5 Dye from water by adsorption Onto carbon adsorbent prepared from a natural bio waste Bauhinia Racemosa Fruit Pods 39, 346–352.
Dey MD, Ahmed M, Singh R, Boruah R, Mukhopadhyay R (2017) Utilization of two agrowastes for adsorption and removal of methylene blue: kinetics and isotherm studies. Water Sci Technol 75:1138–1147. https://doi.org/10.2166/wst.2016.589
Drinić Z, Mudrić J, Zdunić G, Bigović D, Menković N, Šavikin K (2020) Effect of pomegranate peel extract on the oxidative stability of pomegranate seed oil. Food Chem 333:127501. https://doi.org/10.1016/j.foodchem.2020.127501
El Bouraie M, El Din WS (2016) Biodegradation of Reactive Black 5 by Aeromonas hydrophila strain isolated from dye-contaminated textile wastewater. Sustain Environ Res 26:209–216. https://doi.org/10.1016/j.serj.2016.04.014
Fobiri GK (2022) Synthetic dyeapplication in textiles: a review on the efficacies and toxicities involved. Text Leather Rev 5, 180–198. https://doi.org/10.31881/TLR
Gebrezgiher M, Kiflie Z (2020) Utilization of cactus peel as biosorbent for the removal of reactive dyes from textile dye effluents. J Environ Public Health 2020. https://doi.org/10.1155/2020/5383842
Ghalkhani M, Zare N, Karimi F, Karaman C, Alizadeh M, Vasseghian Y (2022) Recent advances in Ponceau dyes monitoring as food colorant substances by electrochemical sensors and developed procedures for their removal from real samples. Food Chem Toxicol 161:112830. https://doi.org/10.1016/J.FCT.2022.112830
Gonultas O, Candan Z (2018) Chemical characterization and ftir spectroscopy of thermally compressed eucalyptus wood panels. Maderas Cienc y Tecnol 20:431–442. https://doi.org/10.4067/S0718-221X2018005031301
Hashem A, Chukwunonso, Aniagor O, Fikry M, Taha GM, Sayed, Badawy M (2023) Characterization and adsorption of raw pomegranate peel powder for lead (II) ions removal 25, 2087–2100. https://doi.org/10.1007/s10163-023-01655-2
Hikal WM, Said-Al Ahl HAH, Bratovcic A, Tkachenko KG, Sharifi-Rad J, Kačániová M, Elhourri M, Atanassova M (2022) Banana peels: a waste treasure for human being. evidence-based complement. Altern Med 2022. https://doi.org/10.1155/2022/7616452
Hurairah SN, Lajis NM, Halim AA (2020) Methylene blue removal from aqueous solution by adsorption on Archidendron jiringa seed shells. J Geosci Environ Prot 08:128–143. https://doi.org/10.4236/gep.2020.82009
Ihaddaden S, Aberkane D, Boukerroui A, Robert D (2022) Removal of methylene blue (basic dye) by coagulation-flocculation with biomaterials (bentonite and Opuntia ficus indica). J Water Process Eng 49:102952. https://doi.org/10.1016/j.jwpe.2022.102952
Ikhlaq A, Zafar M, Javed F, Yasar A, Akram A, Shabbir S, Qi F (2021) Catalytic ozonation for the removal of reactive black 5 (RB-5) dye using zeolites modified with CuMn2O4/gC3N4 in a synergic electro flocculation-catalytic ozonation process. Water Sci Technol 84:1943–1953. https://doi.org/10.2166/wst.2021.404
Inglezakis VJ, Balsamo M, Montagnaro F (2020) Liquid-solid mass transfer in adsorption systems—an overlooked resistance? Ind Eng Chem Res 59:22007–22016. https://doi.org/10.1021/acs.iecr.0c05032
Islam A, Dey S (2015) A review on textile wastewater characterization in Bangladesh. Resour Environ 5:15–44. https://doi.org/10.5923/j.re.20150501.03
Jaroniec M (1975) Adsorption on heterogeneous surfaces: the exponential equation for the overall adsorption isotherm. Surf Sci 50:553–564. https://doi.org/10.1016/0039-6028(75)90044-8
Jawad AH, Abdulhameed AS, Hanafiah MAKM, Alothman ZA, Khan MR, Surip SN (2021) Numerical desirability function for adsorption of methylene blue dye by sulfonated pomegranate peel biochar: modeling, kinetic, isotherm, thermodynamic, and mechanism study. Korean J Chem Eng. 38:1499–1509. https://doi.org/10.1007/s11814-021-0801-9
Kalam S, Abu-Khamsin SA, Kamal MS, Patil S (2021) Surfactant adsorption isotherms: a review. ACS Omega 6:32342–32348. https://doi.org/10.1021/acsomega.1c04661
Kapoor RT, Rafatullah M, Siddiqui MR, Khan MA, Sillanpää M (2022) Removal of Reactive Black 5 dye by banana peel biochar and evaluation of its phytotoxicity on tomato. Sustain. 14. https://doi.org/10.3390/su14074176
Khuluk RH, Rahmat A, Buhani Suharso (2019) Removal of Methylene blue by adsorption onto activated carbon from coconut shell (Cocous Nucifera L.). Indones. J Sci Technol. 4, 229–240. https://doi.org/10.17509/ijost.v4i2.18179
Krueger MD de S, Volkmann AC, Rainert KT (2019) Removal of textile dye Remazol Brilliant Blue Reactive (RBBR) using fibers of Citrullus lanatus (watermelon) and Cocos nucifera (green coconut) as adsorbent material. Rev. Eletrônica em Gestão, Educ e Tecnol Ambient. 23, 5. https://doi.org/10.5902/2236117038526
Kuang Y, Zhang X, Zhou S (2020) Adsorption of methylene blue in water onto activated carbon by surfactant modification. Water (switzerland) 12:1–19. https://doi.org/10.3390/w12020587
Kubra KT, Salman MS, Hasan MN (2021) Enhanced toxic dye removal from wastewater using biodegradable polymeric natural adsorbent. J Mol Liq 328:115468. https://doi.org/10.1016/j.molliq.2021.115468
Li F, Guo Z, Mao L, Feng J, Huang J, Tao H (2023) Impact of textile industries on surface water contamination by Sb and other potential toxic elements: a case study in Taihu Lake Basin, China. Int J Environ Res Public Health 20. https://doi.org/10.3390/ijerph20043600
Li Z, Guo K, Lin L, He W, Zhang L, Wei C (2018) Comparison of physicochemical properties of starches from flesh and peel of green banana fruit. Molecules 23:1–15. https://doi.org/10.3390/molecules23092312
Lima EC, Hosseini-Bandegharaei A, Moreno-Piraján JC, Anastopoulos I (2019) A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption. J Mol Liq 273:425–434. https://doi.org/10.1016/j.molliq.2018.10.048
Mahmoudabadi TZ, Talebi P, Jalili M (2019) Removing disperse red 60 and reactive blue 19 dyes removal by using Alcea rosea root mucilage as a natural coagulant. AMB Express 9. https://doi.org/10.1186/s13568-019-0839-9
Mahrous SS, Galil EAA, Mansy MS (2022) Investigation of modified orange peel in the removal of Cd2+, Co2+ and Zn2+ from wastewater. J Radioanal Nucl Chem 331:985–997. https://doi.org/10.1007/s10967-021-08166-0
Mengelizadeh N, Pourzamani H (2020) Adsorption of Reactive Black 5 dye from aqueous solutions by carbon nanotubes and its electrochemical regeneration process. Heal Scope 9. https://doi.org/10.5812/jhealthscope.102443
Michael-Igolima U, Abbey SJ, Ifelebuegu AO, Eyo EU (2023) Modified orange peel waste as a sustainable material for adsorption of contaminants. Materials (Basel). 16. https://doi.org/10.3390/ma16031092
Minamoto C, Fujiwara N, Shigekawa Y, Tada K, Yano J, Yokoyama T, Minamoto Y, Nakayama S (2021) Effect of acidic conditions on decomposition of methylene blue in aqueous solution by air microbubbles. Chemosphere 263:128141. https://doi.org/10.1016/j.chemosphere.2020.128141
Mondal NK, Kar S (2018) Potentiality of banana peel for removal of Congo red dye from aqueous solution: isotherm, kinetics and thermodynamics studies. Appl Water Sci. 8. https://doi.org/10.1007/s13201-018-0811-x
Moussavi G, Mahmoudi M (2009) Removal of azo and anthraquinone reactive dyes from industrial wastewaters using MgO nanoparticles. J Hazard Mater 168:806–812. https://doi.org/10.1016/j.jhazmat.2009.02.097
Munagapati VS, Wen JC, Pan CL, Gutha Y, Wen JH (2019) Enhanced adsorption performance of Reactive Red 120 azo dye from aqueous solution using quaternary amine modified orange peel powder. J Mol Liq 285:375–385. https://doi.org/10.1016/j.molliq.2019.04.081
Munagapati VS, Yarramuthi V, Kim Y, Lee KM, Kim DS (2018) Removal of anionic dyes (Reactive Black 5 and Congo Red) from aqueous solutions using banana peel powder as an adsorbent. Ecotoxicol Environ Saf 148:601–607. https://doi.org/10.1016/j.ecoenv.2017.10.075
Navaei A, Yazdani M, Alidadi H, Dankoob M, Bonyadi Z, Dehghan A, Hosseini A (2019) Biosorption of reactive red 120 dye from aqueous solution using saccharomyces cerevisiae: Rsm analysis, isotherms and kinetic studies. Desalin Water Treat 171:418–427. https://doi.org/10.5004/dwt.2019.24780
Ndagijimana P, Liu X, Xu Q, Lai D, Wang G, Pan B, Wang Y (2021) Cassava flour extracts solution to induce gelatin cross-linked activated carbon-graphene oxide composites: the adsorption performance of dyes from aqueous media. Environ Adv. 5. https://doi.org/10.1016/j.envadv.2021.100079
Nizam NUM, Hanafiah MM, Mahmoudi E, Halim AA, Mohammad AW (2021) The removal of anionic and cationic dyes from an aqueous solution using biomass-based activated carbon. Sci Rep 11:1–17. https://doi.org/10.1038/s41598-021-88084-z
Ozturk G, Silah H (2020) Adsorptive removal of Remazol Brilliant Blue R from water by using a macroporous polystyrene resin: isotherm and kinetic studies. Environ Process 7:479–492. https://doi.org/10.1007/s40710-020-00429-4
Padmanabhan SK, Lionetto F, Nisi R, Stoppa M, Licciulli A (2022) Sustainable production of stiff and crystalline bacterial cellulose from orange peel extract. Sustain. 14. https://doi.org/10.3390/su14042247
Panda SK, Aggarwal I, Kumar H, Prasad L, Kumar A, Sharma A, Vo DVN, Van Thuan D, Mishra V (2021) Magnetite nanoparticles as sorbents for dye removal: a review, Environmental Chemistry Letters. Springer International Publishing. https://doi.org/10.1007/s10311-020-01173-9
Parimelazhagan V, Yashwath P, Arukkani Pushparajan D, Carpenter J (2022) Rapid removal of toxic Remazol Brilliant Blue-R dye from aqueous solutions using juglans nigra shell biomass activated carbon as potential adsorbent: optimization, isotherm, kinetic, and thermodynamic investigation. Int J Mol Sci 23:1–33. https://doi.org/10.3390/ijms232012484
Phawachalotorn C, Suwanpayak N (2021) The efficiency of mangosteen peel for dye removal. J. Phys. Conf. Ser. 1719. https://doi.org/10.1088/1742-6596/1719/1/012061
Rahmat NA, Ali AA, Salmiati Hussain N, Muhamad MS, Kristanti RA, Hadibarata T (2016) Removal of Remazol Brilliant Blue R from aqueous solution by adsorption using pineapple leaf powder and lime peel powder. Water Air Soil Pollut. 227. https://doi.org/10.1007/s11270-016-2807-1
Ramadan HS, Mobarak M, Lima EC, Bonilla-Petriciolet A, Li Z, Seliem MK (2021) Cr(VI) adsorption onto a new composite prepared from Meidum black clay and pomegranate peel extract: experiments and physicochemical interpretations. J Environ Chem Eng 9:105352. https://doi.org/10.1016/j.jece.2021.105352
Rao SN (2017) Adsorption, Interface Science and Technologyhttps://doi.org/10.1016/B978-0-12-801970-2.00005-7
Revellame ED, Fortela DL, Sharp W, Hernandez R, Zappi ME (2020) Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: a review. Clean Eng Technol 1:100032. https://doi.org/10.1016/j.clet.2020.100032
Rostamian M, Hosseini H, Fakhri V, Talouki PY, Farahani M, Gharehtzpeh AJ, Goodarzi V, Su CH (2022) Introducing a bio sorbent for removal of methylene blue dye based on flexible poly(glycerol sebacate)/chitosan/graphene oxide ecofriendly nanocomposites. Chemosphere 289:133219. https://doi.org/10.1016/j.chemosphere.2021.133219
Saad MS, Kai OB, Wirzal MDH (2022) Process modelling and techno economic analysis for optimal design of integrated electrocoagulation-membrane system for dye removal in wastewater. Chemosphere 306:135623. https://doi.org/10.1016/j.chemosphere.2022.135623
Saha P, Chowdhury S (2011) Insight into adsorption thermodynamics. Thermodynamicshttps://doi.org/10.5772/13474
Saigl ZM, Ahmed AM (2021) Separation of rhodamine b dye from aqueous media using natural pomegranate peels. Indones. J Chem. 21, 212–224. https://doi.org/10.22146/ijc.58592
Samarghandy MR, Hoseinzade E, Taghavi M, Hoseinzadeh S (2011) Biosorption of reactive black 5 from aqueous solution using acid-treated biomass from potato peel waste. BioResources 6:4840–4855
Sangar SK, Lan CS, Razali SM, Farabi MSA, Taufiq-Yap YH (2019) Methyl ester production from palm fatty acid distillate (PFAD) using sulfonated cow dung-derived carbon-based solid acid catalyst. Energy Convers Manag 196:1306–1315. https://doi.org/10.1016/j.enconman.2019.06.073
Saroyan Arampatzidou A, Voutsa D, Lazaridis NK, Deliyanni EA (2019) Activated carbon supported MnO 2 for catalytic degradation of reactive black 5. Colloids Surfaces A Physicochem. Eng Asp. 566, 166–175https://doi.org/10.1016/j.colsurfa.2019.01.025
Saroyan H, Kyzas GZ, Deliyanni EA (2019) Effective dye degradation by graphene oxide supported manganese oxide. Processes 7. https://doi.org/10.3390/pr7010040
Semiz L (2020) Removal of reactive black 5 from wastewater by membrane filtration. Polym Bull 77:3047–3059. https://doi.org/10.1007/s00289-019-02896-8
Shaharom MS (2019) Potential of Orange Peel As a Coagulant. Infrastruct. Univ. Kuala Lumpur Res J. Vol.7 7, 63–72.
Shirazi EK, Metzger JW, Fischer K, Hassani AH (2019) Simultaneous removal of a cationic and an anionic textile dye from water by a mixed sorbent of vermicompost and Persian charred dolomite. Chemosphere 234:618–629. https://doi.org/10.1016/j.chemosphere.2019.05.224
Smith 1916-2009, J.M. (Joseph M., 1959. Introduction to chemical engineering thermodynamics. Second edition. New York : McGraw-Hill, 1959
Stavrinou A, Aggelopoulos CA, Tsakiroglou CD (2018) Exploring the adsorption mechanisms of cationic and anionic dyes onto agricultural waste peels of banana, cucumber and potato: Adsorption kinetics and equilibrium isotherms as a tool. J Environ Chem Eng 6:6958–6970. https://doi.org/10.1016/j.jece.2018.10.063
Sukla Baidya K, Kumar U (2021) Adsorption of brilliant green dye from aqueous solution onto chemically modified areca nut husk. South African J Chem Eng 35:33–43. https://doi.org/10.1016/j.sajce.2020.11.001
Sun Z, Qu K, Cheng Y, You Y, Huang Z, Umar A, Ibrahim YSA, Algadi H, Castañeda L, Colorado HA, Guo Z (2021) Corncob-derived activated carbon for efficiently adsorption dye in sewage. ES Food Agrofor. 61–74. https://doi.org/10.30919/esfaf473
Tadesse MG, Kasaw E, Lübben JF (2023) Valorization of banana peel using carbonization: potential use in the sustainable manufacturing of flexible supercapacitors. Micromachines 14. https://doi.org/10.3390/mi14020330
Taheri M (2022) Techno-economical aspects of electrocoagulation optimization in three acid azo dyes’ removal comparison. Clean Chem Eng 2:100007. https://doi.org/10.1016/j.clce.2022.100007
Tai MH, Saha B, Streat M (2020) Determination of point zero charge (PZC) of homemade charcoals of Shorea Robusta (Sakhuwa) and Pinus Roxburghii (Salla). Int J Eng Res Technol 9:153–155
Thillainayagam BP, Nagalingam R, Saravanan P (2022) Batch and column studies on removal of methylene blue dye by microalgae biochar. Biomass Convers. Biorefinery. https://doi.org/10.1007/s13399-022-03038-3
Tolkou AK, Mitropoulos AC, Kyzas GZ (2023) Removal of anthraquinone dye from wastewaters by hybrid modified activated carbons. Environ Sci Pollut Res 30:73688–73701. https://doi.org/10.1007/s11356-023-27550-9
Tolkou AK, Trikalioti S, Makrogianni O, Xanthopoulou M, Deliyanni EA, Katsoyiannis IA, Kyzas GZ (2022) Chromium(VI) removal from water by lanthanum hybrid modified activated carbon produced from coconut shells. Nanomaterials 12:1–19. https://doi.org/10.3390/nano12071067
Tolkou AK, Zouboulis AI (2020) Application of composite pre-polymerized coagulants for the treatment of high-strength industrial wastewaters. Water (Switzerland) 12. https://doi.org/10.3390/W12051258
Travlou NA, Kyzas GZ, Lazaridis NK, Deliyanni EA (2013) Graphite oxide/chitosan composite for reactive dye removal. Chem Eng J 217:256–265. https://doi.org/10.1016/j.cej.2012.12.008
Tsoutsa EK, Tolkou AK, Katsoyiannis IA, Kyzas GZ (2023) Composite activated carbon modified with AlCl3 for the effective removal of Reactive Black 5 Dye from wastewaters. J Compos Sci 7:224. https://doi.org/10.3390/jcs7060224
Vu HT, Scarlett CJ, Vuong QV (2018) Phenolic compounds within banana peel and their potential uses: a review. J Funct Foods 40:238–248. https://doi.org/10.1016/j.jff.2017.11.006
Yang H, Yan R, Chen H, Lee DH, Zheng C (2007) Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 86:1781–1788. https://doi.org/10.1016/j.fuel.2006.12.013
Yeow PK, Wong SW, Hadibarata T (2021) Removal of azo and anthraquinone dye by plant biomass as adsorbent—a review. Biointerface Res Appl Chem. 11, 8218–8232. https://doi.org/10.33263/BRIAC111.82188232
Zazycki MA, Godinho M, Perondi D, Foletto EL, Collazzo GC, Dotto GL (2018) New biochar from pecan nutshells as an alternative adsorbent for removing reactive red 141 from aqueous solutions. J Clean Prod 171:57–65. https://doi.org/10.1016/j.jclepro.2017.10.007
Zeng X, Zhang G, Wen J, Li X, Zhu J, Wu Z (2023) Simultaneous removal of aqueous same ionic type heavy metals and dyes by a magnetic chitosan/polyethyleneimine embedded hydrophobic sodium alginate composite: performance, interaction and mechanism. Chemosphere 318:137869. https://doi.org/10.1016/j.chemosphere.2023.137869
Zhao Z, Yang Y, Xu L, Qiu Z, Wang Z, Luo Y, Du K (2022) Amino acid-doped polyaniline nanotubes as efficient adsorbent for wastewater treatment. J Chem 2022. https://doi.org/10.1155/2022/2041512
Zhu Y, Yi B, Yuan Q, Wu Y, Wang M, Yan S (2018) Removal of methylene blue from aqueous solution by cattle manure-derived low temperature biochar. RSC Adv 8:19917–19929. https://doi.org/10.1039/c8ra03018a
Acknowledgements
Authors would like to thank the Control and Research Laboratory of Prefecture of Central Macedonia, Thessaloniki, Greece, for providing the research facilities to carry out this research work, and this research work was financially supported by the Greek Ministry of Development and Investments (General Secretariat for Research and Technology) through the research project “Research-Create-Innovate,” with the topic “Development of an integration methodology for the treatment of micropollutants in wastewaters and leachates coupling adsorption, advanced oxidation processes and membrane technology” (Grant no: T2EDK-04066). The authors thank Mr. Dimitrios G. Trikkaliotis for taking some characterization measurements.
Funding
Open access funding provided by HEAL-Link Greece.
Author information
Authors and Affiliations
Contributions
Athanasia K. Tolkou: investigation, methodology, writing—original draft, and supervision. Eleftheria K. Tsoutsa: investigation and formal analysis. George Z. Kyzas: conceptualization, resources, formal analysis, validation, visualization, and supervision. Ioannis A. Katsoyiannis: conceptualization, resources, formal analysis, validation, visualization, and supervision.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
We undertake and agree that the manuscript submitted to your journal has not been published elsewhere and has not been simultaneously submitted to other journals.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tolkou, A.K., Tsoutsa, E.K., Kyzas, G.Z. et al. Sustainable use of low-cost adsorbents prepared from waste fruit peels for the removal of selected reactive and basic dyes found in wastewaters. Environ Sci Pollut Res 31, 14662–14689 (2024). https://doi.org/10.1007/s11356-024-31868-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-31868-3