Abstract
Post-pandemic inflationist pressures, climate changes and extremes, regional conflicts, and soaring food prices caused the food crisis to increase rapidly worldwide. This global problem directs producers and researchers to use oils used as feedstock in biodiesel production effectively. In this context, it is important to assay the transesterification parameters and conduct new optimization studies to increase biodiesel yield. In this study, methyl ester was produced from hemp oil by transesterification using sodium hydroxide (NaOH). Next, classical optimization study was carried out to determine the effects of catalyst amount, alcohol:oil molar ratio, reaction temperature, and reaction time variables on biodiesel yield. Secondly, the cubic spline mathematical model (CSMM) and polynomial regression mathematical model (PRMM) were applied to the first data of this optimization. Among these optimization methods, the utmost biodiesel yield registered was 96.115% at hemp seed oil (HSO):methanol molar ratio of 5.59:1, catalyst concentration of 0.531 wt%, reaction temperature of 42.5 °C, reaction time of 62.1 min, and agitation intensity of 600 rpm at PRMM. Some vital fuel properties obtained from HSO biodiesels as a result of three optimizations satisfied the EN 14214 standard. The results illustrated that the optimal yields from CSMM and PRMM are 0.765% and 1.065% higher, respectively, according to the maximum efficiency obtained from the classical optimization. The outcomes showed that CSMM and PRMM are cost-effective, easy to handle, and promising new approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Energy is used to meet human needs and enhance the quality of life, and it is one of the most important inputs that determine the economic and social development potential of a country (Tamilalagan et al. 2019). Nowadays, about 86% of the world’s primary energy consumption is from fossil-based sources (Mohr et al. 2015; British Petroleum 2022). However, humanity faces the hazard of depletion of fossil fuels (Srithar et al. 2017). Moreover, it is seen that global climate change and ecological balance have deteriorated due to the greenhouse gases released into the air with the energy production from these fuels (Mateus et al. 2023; Rahman and Islam 2023; Goswami and Kreith 2016). Renewable fuels significantly minimize these disadvantages and reduce foreign-source dependency by using domestic resources (Anani 2020). Biodiesel fuel is in demand owing to its convenient emission and combustion profile, carbon–neutral property, high flash point, and versatile usage. Although there are many methods to reduce the viscosity in crude oil to obtain biodiesel, transesterification is the most preferred method with its advantages (formation of products close to petrodiesel, commercialization potential, mild reaction conditions, renewability, cheap, and high conversion) (Ahmad et al. 2012; Zahan and Kano 2018). Transesterification is forming fatty ester and glycerine by reacting the triglyceride molecule with alcohol in the presence of a catalyst. Methanol and sodium hydroxide is the most chosen alcohol–catalyst binary in the transesterification reaction due to ease of handling, cheap and abundant availability, and high yield (Seffati et al. 2019; Talha and Sulaiman 2016).
Policymakers, environmentalists, and energy producers have shown great interest due to the fact that biodiesel is an environmentally friendly fuel. It is considered that an alternative energy source to fossil fuels, biodiesel is expected to play an impressive role in achieving the Sustainable Development Goals (SDGs). Biodiesel corresponds to some SDGs and supports the Paris Climate Change Agreement (Malla and Bandh 2023). Several reviews have stated that biodiesel and other biofuels contribute specifically to achieving SDG 7 (affordable, sustainable, reliable, and clean energy) and SDG 13 (struggling with climate change). However, to expand the use of biofuels, there is a need to reduce production costs and encourage their commercialization (Nazari et al. 2021). The feedstock used in biodiesel production is selected considering many factors such as local availability, cost, quality, oil yield, fuel performance, renewability, origin, and government support. A wide variety of feedstocks can be evaluated in biodiesel production, such as vegetable (edible and inedible) oils, animal fats, waste oil, and algal oils (Bukkarapu and Krishnasamy 2022; Atabani et al. 2012). Approximately 95% of the feedstock used in biodiesel production is selected from edible oils. However, using these edible oils in biodiesel production has increased the interest in non-edible vegetable oils due to food safety, the gap between supply and demand, and their uneconomical nature (Tran et al. 2021; Demirbas et al. 2016).
Hemp (Cannabis sativa L.) is one of the plants that accompanied humanity, dating back more than 8500 years (Deng et al. 2019). Industrial hemp is an agricultural product preferred in the production of a wide variety of products, including food and beverages, cosmetics and personal care products, nutritional supplements, fabrics and textiles, yarns and spun fibers, paper, construction and insulation materials, resin, pulp, and animal bedding, fuel (Wirtshafter 2004; Johnson 2014). This plant is one of the fastest-growing biomass and aligns with the green future goals societies and governments aim to transition to in the twenty-first century. Also, it is widely adaptable and demands very few pesticides or herbicides (Alcheikh 2015).
Hemp has a significantly developed root system that prevents soil erosion. The stem is green, straight, and blanketed with bristle. The edge of the lance-shaped leaf is jagged, and its surface is rough. The flowers are short prongs, without petioles, and are seated. The seeds vary in diameter depending on the cannabis variety, are egg-shaped, and are between 3 and 4 mm. The weight of the seed increases as it matures and is between 8 and 26 g. The color of the seed coat is mosaic and ranges from brown to dark gray. In practice, the most desirable seed is the one with a dark and bright hue (Strzelczyk et al. 2022). The root, stem, leaf, flower, and seed picture of the hemp plant (Cannabis sativa L.) is illustrated in Fig. 1.
The pictures of hemp (Cannabis sativa L.). a root, b stem, c leaf, d flower, e seed (Strzelczyk et al. 2022)
According to 2021 data from the Food and Agriculture Organization (FAO), the production, area harvested, and yield of hemp seeds are around 5446 t, 10,566 ha, and 5155 hg/ha globally, respectively. For 2021, the country distributions of the annual production amount of hemp seed year were created with mapchart.net and are shown in Fig. 2. Turkey, one of the world’s cannabis producers, has recently increased its investment and support for hemp. The aim of Turkey’s recent hemp policy is to produce and expand hemp production to meet the needs of many different fields. In this context, institutes were established for research and development in cannabis production, a new cannabis variety was developed, and an action plan was made with different ministries and organizations. Yozgat Bozok University, a university in Turkey, received approval from the government as a university specializing in the field of “Industrial Hemp” in 2020 and has started research and development works.
The country distributions of the annual production amount of hemp seed for 2021 year (FAO-Hempseed 2023)
The catalyst type and dosage, free fatty acid and moisture content, alcohol type and quantity, reaction temperature, agitation speed, and reaction time have different degrees of influence on the integrity and efficacy of the transesterification reaction. It is essential to analyze the relations of these parameters and apply the proper optimization method from the point of product efficiency and cost (Elango et al. 2019; Abbah et al. 2016). Also, selecting the optimization method implemented to these parameters is essential. Response surface methodology (Sharma et al. 2022), Taguchi (Jain et al. 2023), artificial intelligence (Said et al. 2023), and Box-Behnken (Nguyen et al. 2023) are preferred methods.
Gupta et al. (2018) evaluated the optimization of hemp (Cannabis sativa L.) oil transesterification variables using the Fresnel lens solar concentrator (FSC) approach and the conventional heating method approach. The reaction variables used are catalyst concentration (0.3–1.2%), alcohol:oil molar ratio (3:1–6:1), reaction time (10–80 min for conventional), and mixing speed (0–400 rpm). The yield of optimum cannabis oil methyl ester was obtained as 97.37% at 4 min reaction time, 4.5:1 methanol:oil molar ratio, 200 rpm mixing speed, and 0.90% catalyst concentration. Stamenković et al. (2015) investigated the optimization of hemp oil methanolysis production: catalyst amount (0.58–1.42 wt.% of the oil), methanol:oil molar ratio (3.5:1–8.5:1), reaction temperature (23.2–56.8 °C), and reaction time (up to 180 min). Biodiesel production process parameters were optimized using RSM and ANN combined with a genetic algorithm (GA). While the highest efficiency was 98.5% (predicted content: 99.8%) with the RSM method, it was determined as 97.5% (predicted content: 97.1%) with the ANN-GA method. Yilbasi et al. (2021) appraised using the Taguchi method (applying an L9 orthogonal design matrix) for process optimization of reaction variables: KOH catalyst concentration (0.6–1.2%), methanol/oil molar ratio (6:1–12:1), reaction time (60–120 min), and reaction temperature (30–60 °C). The optimal reaction conditions for achieving the highest FAME content (96.87%) in 120 min at the KOH loading of 0.9 (by the oil weight) was the methanol-to-oil molar ratio of 12:1 and 45 °C reaction temperature. They also stated that the methanol oil molar ratio was the most influential factor, and the reaction time was the least effective parameter in this study. Finally, Rashid et al. (2016) optimized process parameters in order to get maximum hemp seed oil (HSO) biodiesel using response surface methodology. The investigators optimized the methanol/oil molar ratio (varying from 3:1 to 9:1by steps of 1.5), NaOCH3 concentration (ranging from 0.25 to 1.50 wt% by steps of 0.31wt%), reaction temperature (changing from 45 to 65 °C by steps of 5 °C), reaction time (varying from 30 to 90 min of 15 min.). After all, the maximum Cannabis sativa oil biodiesel yield was determined by 86.01% with 7.5:1 of methanol-to-oil molar ratio, 0.80 wt% of catalyst amount, 65 min of reaction time, and 53 °C of reaction temperature.
Nowadays, food supply security, irrigation shortage, necessary energy from planting to harvesting for seeds, climate change, etc. problems are increasing daily. When these situations are taken into consideration, controlled studies should be carried out in terms of both energy and food safety policies. Feedstock cost is of great importance in biodiesel production. Also, it is essential to analyze the relations of transesterification parameters and apply the proper optimization method from the point of product efficiency and cost. In this study, the biodiesel production yield of new mathematical models was compared with the classical method. The main goal of this study is to provide the highest amount of biodiesel production using new mathematical models that are easy to use, accessible, and understandable. A comparison of the classic method and mathematical models, that are cubic spline mathematical method and polynomial regression mathematical method, for optimizing transesterification of HSO biodiesel has not been informed up to now. Thus, the present study concentrates on optimizing the transesterification of HSO for biodiesel production according to the classical optimization method by using the cubic spline mathematical method and polynomial regression mathematical method to analyze the optimal biodiesel production. At the same time, the current experimental study aims to analyze the impacts of the different process factors, including NaOH concentration, methanol:oil molar ratio, reaction temperature, and reaction time, on the conversion yield of HSO. Subsequently, the fatty acid composition and the elemental analysis of HSO biodiesel were identified. Finally, the physicochemical properties of the biodiesel fuel with the highest efficiency determined by three different optimization techniques were determined and compared using the EN14214 procedure.
Theoretical background
In this study, in addition to the classical method, the cubic spline mathematical method (CSMM) and polynomial regression mathematical method (PRMM) optimization of the transesterification of HSO with methanol in the presence of NaOH were implemented in this work.
CSMM
A series of continuous curves bonded to compose a single continuous curve is named a spline curve. For (xj, yj), j = 0,1, …, n – 1 and xj < xj+1, j = 0,1, …, n considering a given set of data points; generally, m. degree spline f(x) for this dataset is a piecewise polynomial of degree m supplying the undermentioned two conditions (Maindl 2018):
-
1.
For each interval (xj, xj+1) için, it is at j = 0,1, …, n – 1, ≤ m degree.
-
2.
At each node xi and in the range j = 1, …, n − 1, (x0, xn); the spline f(x) and its first m − 1 derivatives are continuous.
Cubic spline interpolation finds a curve connecting data points with three degrees or less. Splines are uniform and continuous polynomials along a given graph and are also continuous first and second derivatives where they converge. In the cubic spline approximation, the approximate cubic polynomials, the function values are such that the first and second derivatives are continuous at the nodes. Therefore, the cubic spline curve is continuous, so the gradient and curvature remain the same everywhere in the whole area. For this reason, the cubic spline provides sufficient smoothness on the approximate curve. A cubic spline polynomial f(x) provides the following three conditions (Gupta 2019):
-
1. [xj-1, xj], 1 ≤ j ≤ n, f(x) is a third-order polynomial at each subinterval.
-
fj(x) = ajx3 + bjx2 + cjx + dj j = 1,2,3,….,n
-
2. Cubic spline values at the nodes are equal to the numerical values of the function at these points.
-
3. The polynomials \(f{\prime}\left(x\right),f^{\prime\prime} \left(x\right){\mathrm{ve}}f{\prime}^{\prime\prime} \left(x\right)\) are continual along the interval (xo, xn).
PRMM
The purpose of regression analysis is to estimate the parameters of the model established between the dependent variable and one or more independent variables and to determine the value of the dependent variable for the observed values of the independent variable (Freedman 2009). In some engineering calculations, the model formed by the data is unsuitable for a linear line. In such cases, using an appropriate curve for the data is necessary. At such times, the polynomial regression mathematical model is an evaluable method. The PRMM can be stated using Eqs. (1) and (2) (Aslan and Eryilmaz 2020):
where y, x, m, e, and a0, a1,..,am present dependent variable, independent variable, polynomial degree, error, and constant coefficients, respectively.
Prediction of fuel properties
Biodiesel fuels with the highest biodiesel efficiency were obtained by determining the optimum values with each optimization method. The fatty acid compositions of these fuels were established via gas chromatography–mass spectrometry (GC–MS). Then, fuel properties of these biodiesels, such as density, kinematic viscosity, heating value, cetane number, flash point saponification number, iodine number, cloud point, cold filter plugging point, pour point, degree of unsaturation, and oxidation stability were estimated using the fatty acid composition values by following equations (Table 1).
Materials and methods
Materials
Hemp (Cannabis sativa L.) seeds
The seeds of the “Narlıdere” type of Cannabis sativa (hemp seed), grown by the farmers of Yahyalı, a district of Turkey, were used. The HSO was obtained through a screw oil press machine using 8-mm nozzles. Moisture and oil contents of the seeds were determined 5.12 ± 0.11 g/100 g and 25.19 ± 0.22 g/100 g, respectively. HSO was left to rest for a few days and was filtered with Whatman filter paper for the suspended oil cake particles to settle to the bottom. Before starting the optimization studies, the titration method designated the free fatty acid content of HSO. The optimization studies applied a one-stage homogeneous catalyst transesterification process since the average FFA value (0.384%) of hemp oil is less than 1%. The average molecular weight of HSO was calculated from the distribution of fatty acid compositions with the Shimadzu single quadrupole GC–MS-QP2010 gas chromatograph–mass spectrometer.
Chemicals and devices
Methanol (99.8%) and NaOH (pellets pure) were procured from Merck (USA). KOH solution (0.1 N) and phenolphthalein indicator (1%) were procured from Norateks Chemical Company (Turkey), and diethyl ether was bought from Tekkim Chemical Company (Turkey). The important device of the study IKA C-MAG HS 7 (Germany) package magnetic stirrer, runs at speeds ranging from 100–1500 rpm, a heating temperature range ranging from 20–500°C, and a temperature step of 0.1°C was used. The weight measurements of the samples were made with a Weightlab LB.WL-603 (Turkey) precision balance has a readability of 0.001 g and a weighing range of up to 600 g.
Transesterification of HSO
Apparatus and experimental method
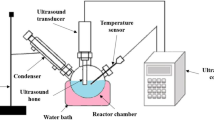
Each trial was carried out with 100 g ± 0.01 of HSO in optimization studies. First, the weighed and noted HSO was poured into a three-neck flat bottom, as shown in Fig. 3. Afterwards, the desired reaction temperature was adjusted, the thermometer was mounted on the magnetic stirrer with heater, and the heating and mixing processes were started. Methanol and NaOH mixed in specific proportions were mixed in a glass container until NaOH dissolved.
When the HSO reached the set temperature, the methanol–NaOH solution was spilt from one neck, and the time was started. The mixing intensity was fixed to 600 rpm for each trial. When the reaction time was complete, the mixture was transferred to a separatory funnel and left to rest for at least 8 h to allow the glycerol to settle to the bottom. The ester was kept in the heater for 1 h without stirring, at a temperature of 70°C (due to the boiling point of methanol) to evaporate unreacted methanol. Then, the ester was separated from glycerol and purified with distilled water. Ester and distilled water temperatures were chosen to be close to each other, between 50 and 55°C. After purification, the wastewater was taken after waiting at least 8 h. As a final process, the drying process was realized at 120°C for 2 h to remove the water in the ester. Some images related to HSO biodiesel production are presented in Fig. 4.
The biodiesel yield at room temperature was calculated according to Eq. (3) (Dhawane et al. 2016). The experimental procedure is depicted in Fig. 5.
Optimization of production parameters
Optimization processes were carried out to reach the maximum ester yield with the help of CSMM and PRMM. Methanol was selected as the alcohol, and NaOH was chosen as the catalyst in optimization studies. The parameters examined using the transesterification method were catalyst concentration, methanol:HSO molar ratio, reaction temperature, and reaction time. Many researchers emphasized that examining these parameters affects transesterification (Miraculas et al. 2018; Hundie and Akuma 2022; Adenuga et al. 2020; Halwe et al. 2021). The parameter data for the optimization processes in this study are illustrated in Table 2.
Optimization processes were started with the catalyst parameter variable and the other parameters fixed. The catalyst concentration varied from 0.4% by weight to 1.0% in 0.2% steps, while the molar ratio, reaction temperature, and reaction time were fixed at 5:1, 60 °C, and 60 min, respectively. In the classical method, the catalyst concentration with the highest efficiency was selected in the biodiesel yields obtained depending on the catalyst concentration change. This value is taken as constant in other transesterification parameter processes. The CSMM approach was applied using the biodiesel yields obtained from each parameter and the numerical variables determined for the relevant parameter. The value obtained with the CSMM application was fixed in other transesterification parameter processes. The PRMM approach pursued a similar tack to CSMM. PRMM and CSMM approaches aim to increase biodiesel production efficiency according to the classical optimization method. The flowchart relevant to using the optimization methods is illustrated in Fig. 6.
Results and discussion
Fatty acid composition
The fatty acid composition of HSO was analyzed by gas chromatography–mass spectrometry (GC–MS) in Yozgat Bozok University Central Laboratory to determine the molecular weight required for the methanol:HSO molar ratio. At the end of each optimization study, biodiesels with the highest yield were produced, and GC–MS determined their fatty acid distributions. The fatty acid compositions of HSO and biodiesels (obtained from classical, CSMM, and PRMM) are shown in Table 3.
The dominant fatty acids in biodiesel produced in three optimization techniques are linoleic acid, oleic acid, and linolenic acid, similar to hemp oil, as shown in Table 2. According to the fatty acid composition, the average molecular weights of the biodiesels obtained by classical, CSMM, and PRMM were calculated as 876.10, 875.98, and 876.07 g/mol, respectively.
Optimization of HSO biodiesel production
The experiments were conducted using classical, CSMM, and PRMM optimisation techniques. The transesterification process was optimized using four parameters: catalyst concentration (0.4–1.0 wt%), methanol:HSO molar ratio(3:1–9:1), reaction temperature (30–60°C), and reaction time (30–75 min.).
Effect of catalyst concentration
The transesterification of the HSO to biodiesel was investigated under various catalyst concentrations, 0.4–1.0%, at an interval of 0.2%, while other conditions were kept fixed: 5:1 molar ratio (methanol:HSO), 60 °C reaction temperature, 60 min reaction time, and stirring at 600 rpm. The effect of catalyst concentration for different optimization conditions is given in Fig. 7. As a result of the experiments, the highest yield was obtained with 94.95% at 0.6 catalyst concentration. These data were used in the classical method in the following reaction conditions. The cubic spline mathematical model and polynomial regression mathematical model were applied to the catalyst concentration and biodiesel yield data. The highest yields and catalyst concentrations were determined at 95.05% to 0.566 wt% and 95.28% to 0.531wt% for CSMM and PRMM, respectively.
It has been determined that the catalysts used at both high and low ratios reduce the efficiency, but there is an optimum point in the middle points. When a large catalyst dosage is utilized, the catalyst reacts with free fatty acids in the oil and form saponification. Conversely, when a low amount of catalyst is preferred, the reaction cannot be completed, and the ester is not formed (Sultana et al. 2014). From Fig. 7, it can be understood that the CSMM and PRMM applications increased biodiesel yield according to classical methods.
Effect of methanol:HSO molar ratio
The alcohol:oil molar ratio is one of the most critical reaction parameters affecting biodiesel yield. Although there is a 3:1 stoichiometric ratio between the oil and alcohol in the transesterification reaction, more stoichiometric ratios are needed to improve the mass transfer between the triglyceride and the alcohol molecule and to increase miscibility (Almasi et al. 2021). Methanol:HSO molar ratios of 3:1, 5:1, 7:1, and 9:1 were studied using 60 °C and 60 min stirring at 600 rpm. Concentrations of catalyst were determined in the previous parameter study. The classical method chose the catalyst concentration corresponding to the highest efficiency as 0.6 wt%. Catalyst concentrations in CSMM and PRMM applications were established as 0.566 wt% and 0.531 wt%, respectively, with the aid of the applied equations. The influence of methanol: HSO molar ratio for different optimization conditions is shown in Fig. 8.
The optimum yields and molar ratios were obtained at 94.95% to 5:1, 95.20% to 5.65:1, and 95.39% to 5.59:1 for classical, CSMM, and PRMM, respectively. In each optimization study, it was concluded that the biodiesel yield decreased at high molar ratios. This decrease in yield may be due to the dissolution of glycerine in excess alcohol. It is not easy to separate the dissolved glycerin from the product (Degfie et al. 2019).
Effect of reaction temperature
The transesterification process can take place at different temperatures. The reaction temperature is active in the reaction rate and biodiesel yield (Pawar et al. 2022). The reaction temperatures used for the study were 30, 40, 50, and 60 °C, with a reaction time of 60 min and agitation intensity of 600 rpm fixed. Catalyst concentrations and methanol:HSO molar ratios corresponding to the highest yield in the previous conditions were retained. For classical, CSMM, and PRMM, the catalyst concentration was 0.6, 0.566, and 0.531 wt.%, respectively; the methanol:HSO molar ratios were chosen as 5:1, 5:65, and 5:59, respectively. The effect of reaction temperature for various optimization conditions is given in Fig. 9.
A decrease in temperatures below and above 40 °C occurred. The optimum yields and reaction temperatures were attained at 95.05% to 40 °C, 95.80% to 43.5 °C, and 96.09% to 42.5 °C for classical, CSMM, and PRMM, respectively.
Effect of reaction time
The methyl ester yield increases as the reaction time increases to a certain point (optimum point). The reaction time beyond this point does not increase the conversion rate due to supporting the back reaction (hydrolysis of esters) (Lakshmana Naik et al. 2015). Aslan and Eryilmaz (2020) stated that 1 h might be appropriate for the optimal reaction time for the ester conversion. The values at the optimum points determined in the previous stages were used as the data of other parameters except for the reaction time. For classical, CSMM, and PRMM, the catalyst concentration was 0.6, 0.566, and 0.531, respectively; the molar ratios of methanol:HSO were chosen as 5:1, 5:65, and 5:59, respectively; the reaction temperature was taken 40, 43.5, 42.5 °C, respectively. The influence of reaction time for classical, CSMM, and PRMM is given in Fig. 10. The yields peaked at around 60 min and then started to decline. The highest yields and reaction times were determined at 95.05% to 60 min, 95.815% to 63.4 min, and 96.115% to 62.1 min for classical, CSMM, and PRMM, respectively.
Optimum transesterification parameter conditions and characterization of biodiesels
The optimal conditions for obtaining the maximum yield for each optimization as a result of three different optimization processes are given in Table 4. When the results are examined, the data obtained from the classical method are compatible with the results of a previously reported study in the literature (Ahmad et al. 2011). While the highest efficiency is obtained with the PRMM method at 1.065% compared to the classical method, this increase is 0.765% in the CSMM method.
The HSO biodiesel produced by the transesterification reaction was characterized to determine its physicochemical properties such as density, kinematic viscosity, calorific value, cetane number, saponification number, iodine number, flash point, cloud point, cold filter plugging point, pour point, unsaturation degree, and oxidation stability and shown in Table 5. According to the fatty acid compositions given in Table 3, the fuel properties of biodiesels were estimated with the aid of the equations given in the subsection “Prediction of fuel properties.”
Table 5 presents that iodine number, acid number, and alkaline–earth content values are higher than the upper limit of the standard value. On the contrary, the cetane number values are lower than the sublimit of the standard value. The higher the number of double bonds, the higher the iodine number. The iodine number is also related to the cetane number. High unsaturation causes the decrease of cetane number in biodiesel (Yesilyurt et al. 2020).
Conclusion
This work optimizes the condition variables using classical, CSMM, and PRMM methods. The results show that parameters other than reaction time have an important effect on biodiesel yield. The maximum biodiesel yield of 96.115% was achieved with NaOH concentration (0.531 wt%), HSO:methanol molar ratio (5.59:1), reaction temperature (42.5 °C), and reaction time (62.1 min) using PRMM method. Compared with the classical method, the biodiesel yield of the CSMM and PRMM methods are increased by 0.765% and 1.065%, respectively. Meanwhile, the different models based on third-degree polynomial equations were generated to reflect the correlation between each optimization parameter and biodiesel yield, with the coefficient of uncertainty (R2) reaching 0.9999 and 1.0000, respectively. All the specified PRMM models showed successfully estimated capability. In this period of the global food crisis, CSMM and PRMM optimization techniques have proven to be good alternatives in biodiesel production.
Author ccontributions
Material preparation, data collection, and analysis were performed by Volkan Aslan. The first draft of the manuscript was written by Volkan Aslan, and conceptualization, methodology, validation, project administration, supervision, writing—review and editing were performed by Volkan Aslan. Volkan Aslan approved the final manuscript.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abbah EC, Nwandikom GI, Egwuonwu CC, Nwakuba NR (2016) Effect of reaction temperature on the yield of biodiesel from neem seed oil. Am J Energy Sci 3(3):16–20
Adenuga AA, Idowu OO, Oyekunle JAO (2020) Synthesis of quality biodiesel from Calophyllum inophyllum kernels through reactive extraction method: Optimization of process parameters and characterization of the products. Renew Energ 145:2530–2537. https://doi.org/10.1016/j.renene.2019.08.035
Agarwal M, Singh K, Chaurasia SP (2010) Prediction of biodiesel properties from fatty acid composition using linear regression and ANN techniques. Indian Chem Eng 52(4):347–361. https://doi.org/10.1080/00194506.2010.616325
Ahmad M, Ullah K, Khan MA, Zafar M, Tariq M, Ali S, Sultana S (2011) Physicochemical analysis of hemp oil biodiesel: a promising non edible new source for bioenergy. Energy Sources a: Recovery Util Environ Eff 33(14):1365–1374. https://doi.org/10.1080/15567036.2010.499420
Ahmad M, Khan MA, Zafar M, Sultana S (2012) Practical handbook on biodiesel production and properties. CRC Press. https://doi.org/10.1201/b12719
Alcheikh A (2015) Advantages and challenges of hemp biodiesel production, Master Thesis. Department of Energy Systems. Faculty of Engineering and Sustainable Development, Gavle University: Gävle, Sweden
Almasi S, Najafi G, Ghobadian B, Jalili S (2021) Biodiesel production from sour cherry kernel oil as novel feedstock using potassium hydroxide catalyst: optimization using response surface methodology. Biocatal Agric Biotechnol 35:102089. https://doi.org/10.1016/j.bcab.2021.102089
Anani N (2020) Renewable Energy Technologies and Resources. Artech House, Boston
Aslan V, Eryilmaz T (2020) Polynomial regression method for optimization of biodiesel production from black mustard (Brassica nigra L.) seed oil using methanol, ethanol, NaOH, and KOH. Energy 209:118386. https://doi.org/10.1016/j.energy.2020.118386
Aslan V (2019) Investigation of fuel properties, motor performances and exhaust emissions of biodiesels used by different alcohols and catalysts in production. Doctoral Dissertation, Department of Mechanical Engineering. Faculty of Engineering-Architecture, Yozgat Bozok University: Yozgat, Turkey
Atabani AE, Silitonga AS, Badruddin IA, Mahlia TMI, Masjuki H, Mekhilef S (2012) A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew Sustain Energy Rev 16(4):2070–2093. https://doi.org/10.1016/j.rser.2012.01.003
Bukkarapu KR, Krishnasamy A (2022) Predicting engine fuel properties of biodiesel and biodiesel-diesel blends using spectroscopy based approach. Fuel Process Technol 230:107227. https://doi.org/10.1016/j.fuproc.2022.107227
British Petroleum (2022) Full report—Statistical review of world energy. British Petroleum: London, UK, 1–70
Cao J, Li H, Xia X, Zou XG, Li J, Zhu XM, Deng ZY (2015) Effect of fatty acid and tocopherol on oxidative stability of vegetable oils with limited air. Int J Food Prop 18(4):808–820. https://doi.org/10.1080/10942912.2013.864674
Degfie TA, Mamo TT, Mekonnen YS (2019) Optimized biodiesel production from waste cooking oil (WCO) using calcium oxide (CaO) nano-catalyst. Sci Rep 9(1):18982. https://doi.org/10.1038/s41598-019-55403-4
Demirbas A (2007) Mathematical relationships derived from biodiesel fuels. Energy Sources a: Recovery Util Environ Eff 30(1):56–69. https://doi.org/10.1080/00908310600626762
Demirbas A, Bafail A, Ahmad W, Sheikh M (2016) Biodiesel production from non-edible plant oils. Energy Explor Exploit 34(2):290–318. https://doi.org/10.1177/0144598716630166
Deng G, Du G, Yang Y, Bao Y, Liu F (2019) Planting density and fertilization evidently influence the fiber yield of hemp (Cannabis sativa L.). Agron 9(7):368. https://doi.org/10.3390/agronomy9070368
Dhawane SH, Kumar T, Halder G (2016) Biodiesel synthesis from Hevea brasiliensis oil employing carbon supported heterogeneous catalyst: optimization by Taguchi method. Renew Energ 89:506–514. https://doi.org/10.1016/j.renene.2015.12.027
Elango RK, Sathiasivan K, Muthukumaran C, Thangavelu V, Rajesh M, Tamilarasan K (2019) Transesterification of castor oil for biodiesel production: process optimization and characterization. Microchem J 145:1162–1168. https://doi.org/10.1016/j.microc.2018.12.039
FAO-Hempseed (2023). Available online: https://www.fao.org/faostat/en/#data/QCL. Accessed 12 Mar 2023
Freedman DA (2009) Statistical models: theory and practice. Cambridge University Press
Goswami DY, Keith F (2016) Global energy systems. In: Goswami DY, Kreith F (eds) Energy Efficiency and Renewable Energy Handbook. CRC Press, Florida, pp 27–56. https://doi.org/10.1201/b18947-7
Gupta RK (2019) Numerical methods: fundamentals and applications. Cambridge University Press
Gupta AR, Jalan AP, Rathod VK (2018) Solar energy as a process intensification tool for the biodiesel production from hempseed oil. Energy Convers Manag 171:126–132. https://doi.org/10.1201/b18947-7
Halwe AD, Deshmukh SJ, Kanu NJ, Gupta E, Tale RB (2021) Optimization of the novel hydrodynamic cavitation based waste cooking oil biodiesel production process parameters using integrated L9 Taguchi and RSM approach. Mater Today: Proc 47:5934–5941. https://doi.org/10.1016/j.matpr.2021.04.484
Hundie KB, Akuma DA (2022) Optimization of biodiesel production parameters from Prosopis julifera seed using definitive screening design. Heliyon 8(2):e08965. https://doi.org/10.1016/j.heliyon.2022.e08965
Jain A, Bora BJ, Kumar R, Sharma P, Deepanraj B, Irshad K, Ravikiran C (2023) Application of hybrid Taguchi L16 and desirability for model prediction and optimization in assessment of the performance of a novel Water Hyacinth biodiesel run diesel engine. Fuel 339:127377. https://doi.org/10.1016/j.fuel.2022.127377
Johnson R. (2014, February). Hemp as an agricultural commodity. Library of Congress Washington DC Congressional Research Service
Lakshmana Naik R, Radhika N, Sravani K, Hareesha A, Mohanakumari B, Bhavanasindhu K (2015) Optimized parameters for production of biodiesel from fried oil. Int Adv Res J Sci Eng Technol 2(6):23. https://doi.org/10.17148/IARJSET.2015.2615
Maindl TI (2018) Introduction to cubic spline interpolation with examples in Python. CreateSpace Independent Publishing
Malla FA, Bandh SA (2023) Biofuels and sustainable development goals. In: Hakeem KR, Bandh SA, Malla FA, Mehmood MA (eds) Environmental sustainability of biofuels: prospects and challenges. Elsevier, United Kingdom, pp 13–26. https://doi.org/10.1016/b978-0-323-91159-7.00010-2
Mateus C, Flor D, Guerrero CA, Córdova X, Benitez FL, Parra R, Ochoa-Herrera V (2023) Anthropogenic emission inventory and spatial analysis of greenhouse gases and primary pollutants for the Galapagos Islands. Environ Sci Pollut Res 1–19. https://doi.org/10.1007/s11356-023-26816-6
Miraculas GA, Bose N, Raj RE (2018) Process parameter optimization for biodiesel production from mixed feedstock using empirical model. Sustain Energy Technol Assess 28:54–59. https://doi.org/10.1016/j.seta.2018.06.004
Mohr SH, Wang J, Ellem G, Ward J, Giurco D (2015) Projection of world fossil fuels by country. Fuel 141:120–135. https://doi.org/10.1016/j.fuel.2014.10.030
Nazari MT, Mazutti J, Basso LG, Colla LM, Brandli L (2021) Biofuels and their connections with the sustainable development goals: a bibliometric and systematic review. Environ Dev Sustain 23(8):11139–11156. https://doi.org/10.1007/s10668-020-01110-4
Nguyen VN, Sharma P, Kumar A, Pham MT, Le HC, Truong TH, Cao DN (2023) Optimization of biodiesel production from Nahar oil using Box-Behnken design, ANOVA and grey wolf optimizer. Int J Renew Energy Dev 12(4):711–719. https://doi.org/10.14710/ijred.2023.54941
Pawar S, Hole J, Bankar M, Channapattana S, Srinidhi C (2022) Studies on Xanthium strumarium L. seed oil: biodiesel synthesis and process optimization. Mater Today: Proc 66:2169–2177. https://doi.org/10.1016/j.matpr.2022.06.024
Rahman MM, Islam ME (2023) The impact of green accounting on environmental performance: mediating effects of energy efficiency. Environ Sci Pollut Res 1–22. https://doi.org/10.1007/s11356-023-27356-9
Ramírez-Verduzco LF, Rodríguez-Rodríguez JE, del Rayo J-J (2012) Predicting cetane number, kinematic viscosity, density and higher heating value of biodiesel from its fatty acid methyl ester composition. Fuel 91(1):102–111. https://doi.org/10.1016/j.fuel.2011.06.070
Ramos MJ, Fernández CM, Casas A, Rodríguez L, Pérez Á (2009) Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour Technol 100(1):261–268. https://doi.org/10.1016/j.biortech.2008.06.039
Rashid U, Bhatti SG, Ansari TM, Yunus R, Ibrahim M (2016) Biodiesel production from Cannabis sativa oil from Pakistan. Energy Sources a: Recovery Util Environ Eff 38(6):865–875. https://doi.org/10.1080/15567036.2013.803179
Said Z, Sharma P, Nhuong QTB, Bora BJ, Lichtfouse E, Khalid HM, Luque R, Nguyen XP, Hoang AT (2023) Intelligent approaches for sustainable management and valorisation of food waste. Bioresour Technol 377:128952. https://doi.org/10.1016/j.biortech.2023.128952
Sarin A, Arora R, Singh NP, Sarin R, Malhotra RK, Kundu K (2009) Effect of blends of Palm-Jatropha-Pongamia biodiesels on cloud point and pour point. Energy 34(11):2016–2021. https://doi.org/10.1016/j.energy.2009.08.017
Seffati K, Honarvar B, Esmaeili H, Esfandiari N (2019) Enhanced biodiesel production from chicken fat using CaO/CuFe2O4 nanocatalyst and its combination with diesel to improve fuel properties. Fuel 235:1238–1244. https://doi.org/10.1016/j.fuel.2018.08.118
Sharma P, Sivaramakrishnaiah M, Deepanraj B, Saravanan R, Reddy MV (2022) A novel optimization approach for biohydrogen production using algal biomass. Int J Hydrog (in Press). https://doi.org/10.1016/j.ijhydene.2022.09.274
Srithar K, Balasubramanian KA, Pavendan V, Kumar BA (2017) Experimental investigations on mixing of two biodiesels blended with diesel as alternative fuel for diesel engines. J King Saud Univ Eng Sci 29(1):50–56. https://doi.org/10.1016/j.jksues.2014.04.008
Stamenković OS, Veličković AV, Kostić MD, Joković NM, Rajković KM, Milić PS, Veljković VB (2015) Optimization of KOH-catalyzed methanolysis of hempseed oil. Energy Convers Manag 103:235–243. https://doi.org/10.1016/j.enconman.2015.06.054
Strzelczyk M, Lochynska M, Chudy M (2022) Systematics and botanical characteristics of industrial hemp Cannabis sativa L. J Nat Fibers 19(13):5804–5826. https://doi.org/10.1080/15440478.2021.1889443
Sultana S, Khalid A, Ahmad M, Zuhairi AA, Teong LK, Zafar M, Hassan FU (2014) The production, optimization, and characterization of biodiesel from a novel source: Sinapis alba L. Int J Green Energy 11(3):280–291. https://doi.org/10.1080/15435075.2013.772520
Talha NS, Sulaiman S (2016) Overview of catalysts in biodiesel production. ARPN J Eng Appl Sci 11(1):439–442
Tamilalagan A, Singaram J, Rajamohan S (2019) Generation of biodiesel from industrial wastewater using oleaginous yeast: performance and emission characteristics of microbial biodiesel and its blends on a compression injection diesel engine. Environ Sci Pollut Res 26(11):11371–11386. https://doi.org/10.1007/s11356-019-04556-w
Tran NN, Tišma M, Budžaki S, McMurchie EJ, Ngothai Y, Morales Gonzalez OM, Hessel V (2021) Production of biodiesel from recycled grease trap waste: a review. Ind Eng Chem Res 60(46):16547–16560. https://doi.org/10.1021/acs.iecr.1c02496
Wirtshafter DE (2004) Ten years of a modern hemp industry. Journal of Industrial Hemp 9(1):9–14. https://doi.org/10.1300/J237v09n01_03
Yesilyurt MK, Cesur C, Aslan V, Yilbasi Z (2020) The production of biodiesel from safflower (Carthamus tinctorius L.) oil as a potential feedstock and its usage in compression ignition engine: a comprehensive review. Renew Sustain Energy Rev 119:109574. https://doi.org/10.1016/j.rser.2019.109574
Yilbasi Z, Yesilyurt MK, Arslan M (2021) The production of methyl ester from industrial grade hemp (Cannabis sativa L.) seed oil: a perspective of Turkey—the optimization study using the Taguchi method. Biomass Convers Biorefin 1–21. https://doi.org/10.1007/s13399-021-01751-z
Zahan KA, Kano M (2018) Biodiesel production from palm oil, its by-products, and mill effluent: A review. Energies 11(8):2132. https://doi.org/10.3390/en11082132
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This study was promoted by the Scientific Research and Projects Unit of Yozgat Bozok University, Turkey, with project number 6602b-MÜH/20–388.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent for publication
Not applicable.
Competing interests
The author declare no competing interests.
Additional information
Responsible Editor: Ta Yeong Wu
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aslan, V. The analysis of classical, polynomial regression and cubic spline mathematical models in hemp biodiesel optimization: an experimental comparison. Environ Sci Pollut Res 31, 9392–9407 (2024). https://doi.org/10.1007/s11356-023-31720-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-31720-0