Abstract
Due to an increasing reduction of hydrological resources across Mexico and their growing contamination from global warming and anthropogenic activities, this study evaluated water from the perennial Lake Coatetelco (Ca–Mg–HCO3) in tropical central-southern Mexico and groundwater (Ca–Mg–HCO3 and Na–HCO3–Cl) from the surrounding wells for drinking as well as irrigation qualities. Comparison with the WHO guidelines and the estimated water quality indices (DWQI and IWQI) grouped almost all the samples collected after the warm season rainfall in excellent and good categories (DWQI < 100) for drinking, even though fluoride remained > 1.5 mg/L in 50% samples. Except for one groundwater sample, all showed > 25% permeability (classes I and II) in Donnen classification indicating their suitability for irrigation. USSL and Wilcox classifications, however, catalogued some in the high-salinity hazard group and some as doubtful for irrigating regular plants. Samples from about 53% wells were also in high and severe restriction categories of IWQI for the irrigation. Total Hazard Quotient Index (THQI) for estimating the non-carcinogenic risk (HQfluoride > 1) showed that at least one lake water sample and 53% of groundwater might expose the adult and child population to dental and skeletal fluorosis. This water quality assessment data posterior to the rainfall season could be useful as a baseline for both the short- and long-term monitoring in attention to the United Nation’s Sustainable Development Goal 6.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater is the main resource of potable water across the globe as well as of the water used in irrigated agriculture and industrial activity (e.g., Adimalla 2019, 2020; Alarcón-Herrera et al. 2020; Subba Rao et al. 2020). Almost half of the global drinking water and approximately 40% of irrigation water come from the aquifers, and around one-third of the overall population suffers from water shortage every year (Mekonnen and Hoekstra 2016; Abascal et al. 2022). The population growth, aridification caused from the global warming, and the enhancement in industrial activities, including the agriculture, have either polluted or degraded the available water resources by increasing the levels of contaminants (both geogenic and anthropogenic), and thus, augmented the public health threat from this natural resource (e.g., Kimambo et al. 2019; Alarcón-Herrera et al. 2020; Abascal et al. 2022). About 200 million people in more than 25 countries are presently exposed to the risk of dental and skeletal fluorosis, bone fractures, kidney stones, low birth rate and thyroid function, and glucose tolerance as well as low IQ levels from drinking the water with F > 1.5 mg/L (WHO 2017; Duan et al. 2018; Kimambo et al. 2019). Similarly, the exposure to extreme high (> 50 mg/L) nitrate for a longer period leads to adverse health risk, such as methemoglobinaemia, thyroid or cancer, hypertension, diabetes, spontaneous abortion, respiratory tract infection, and change in the immune system (Gupta et al. 2000; Fewtrell 2004; Martínez et al. 2017; Tokazhanov et al. 2020). The interaction with fluoride-rich minerals such as fluorite, mica, amphibole, villiaumite, and topaz present in aquifer lithologies as well as the chemical weathering of volcanic rocks leads to F enrichment in the water bodies (Cronin et al. 2000; Yadav et al. 2021; Alarcón-Herrera et al. 2020). Higher nitrate, however, is a direct consequence of fertilizers as about 60% of water bodies with elevated nitrate occur near the croplands (Singh and Craswell 2021). Hence, the objective of United Nation’s Sustainable Development Goal (SDG) 6 and target 6.4 is to address water scarcity by substantially increasing the water-use efficiency and access to safe water across all sectors by 2030.
Like many other developing countries, Mexico too relies heavily on groundwater for drinking and irrigation, contributing about 39% (i.e., 35, 000 hm3/a) of the total volume consumed from its 653 aquifers, including the 115 overexploited ones present in the semi-arid and arid central and northern regions (CONAGUA 2019). The perennial lakes are also an important source of water in the central Mexico. For example, the largest Lake Chapala (> 1000 km2) can store up to 8126 hm3 of water and smaller lakes like the Lake Tequesquitengo (< 10 km2) has a storage volume of up to 160 hm3. About 20 million people, including 6.5 million children, in Mexico are exposed to water with F above the permissible limit, and about half of them are from the central and northern parts that presently experience prolonged droughts and have volcanic deposits as well as F-bearing limestone as the aquifer lithologies (Reyes-Gómez et al. 2017; Navarro et al. 2017; Roy et al. 2021). Average fluoride content in groundwater is almost half (0.6 mg/L) in regions of Mexico with an annual precipitation above 1000 mm compared to the regions with < 700 mm of average annual rainfall (1.2 mg/L; Alarcón-Herrera et al. 2020). LaFayette et al. (2020) reported up to 15.5 mg/L of F in groundwater from the Independent Basin of central Mexico with abundant andesite. Similarly, Gutierrez and Alarcón-Herrera (2022) reported F above the permissible limit in 36–52% wells of the central and northern Mexico and fluoride up to 28 mg/L in areas with abundant felsic volcanic rocks. In a semi-arid region of south India (< 1000 mm/a precipitation), Adimalla (2019) reported up to 7.1 mg/L of fluoride and up to 440 mg/L of nitrate with about half of the groundwater samples containing contaminants above the maximum permissible limits of the World Health Organization (WHO). The groundwater, in an important agricultural district of northern Mexico at the Comarca Lagunera, has NO3 up to 109 mg/L, and the values above WHO-recommended safe limit of 10 mg/L in 32% wells were mostly from the intensive manure usage and urban sewage (Torres-Martínez et al. 2021). In a previous study of this basin, Calleros et al. (2012) reported a possible health risk for 45% of the 1- to 12-year-old children of this area from methemoglobin, with relatively more threat for male compared to the female population. Fluorosis is more common in Mexico compared to the methemoglobin and the data of national caries survey of 2001 indicated an overall fluorosis prevalence of 27.9% (Betancourt-Lineares et al. 2013). The survey of 2011–2014, however, revealed that the dental fluorosis is increasingly becoming a public health problem not only in the semi-arid and arid regions of central and northern Mexico, but only in the sub-humid to humid central and southern regions. For example, the prevalence level on 12-year-old school-going children in Morelos state increased from 3.2 to 33.7% after a decade with an average 0.28–0.50 teeth/person showing permanent caries (ENCD 2018). This could be due to increase of F in the groundwater of this region. Huízar Álvarez et al. (2016) also have reported up to 1.90 mg/L of F in groundwater and spring water from the Tenextepango area of Morelos state.
Consistent with the global perspectives, the simulations for Mexico indicate significant reduction in average precipitation in different parts from an increase in the global average temperature between 1.5 and 4 °C in the short, middle, and long terms over a century (Arias et al. 2021). Reduced rainfall and warmer conditions, possibly more than the global average, along with the overexploitation would lead to a critical negative annual imbalance and deterioration in the quality and quantity of available water resources. Under different scenarios of emission and adaptation levels, the agricultural productivity in Mexico could decline up to 48% by the end of this century (e.g., Feng et al. 2010). Concurrently, the application of saline water in irrigation would increase the kinematic viscosity and cause low soil permeability. It could also reduce the movement of water from soil to the plant roots, branches, and leaves (e.g., Nagarajan et al. 2010; Zhou et al. 2020). Irrigation with the high alkalinity water generally reduces the soil nutrients (e.g., Sharma et al. 2017). Here, we attempted to evaluate the quality of water from Lake Coatetelco and surrounding groundwater wells located in the central-southern Mexico, with significant agricultural activity, for drinking and irrigation purposes by comparing the physicochemical characteristics with WHO guidelines and estimating the water quality indices such as DWQI and IWQI. The irrigation quality was also assessed using the USSL classification, Wilcox diagram, and Doneen classification. The possible non-carcinogenic health risks from nitrate and fluoride in water samples were evacuated by estimating the hazard quotients for adults (> 21 years) and children (average weight 20 kg).
Study area
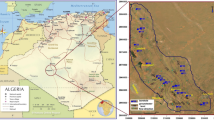
Lake Coatetelco is located at central-south Mexico (Morelos state), and physiographically, it belongs to the Sierra Madre del Sur province (Fig. 1). It has a tropical, warm semi-humid climate with the nearest meteorological station of Miacatlán (Fig. 1) registering an average annual precipitation of ca.1080 mm between 1970 and 2016 CE with > 85% of it occurring in the warmer months of May-to-September and the average annual evaporation of about 1940 mm (source: National Meteorological Service of Mexico or SMN). This perennial lake (length: ca. 2 km and width: ca. 1.5 km) has a shallow water column with a mean depth of < 0.5 m. Over-extraction of groundwater and enhanced erosion in the watershed from increasing urbanization in the recent years has reduced the maximum water depth to < 3 m.
a Map showing Lake Coatetelco (Morelos state) in central-south Mexico and some other sites discussed in the paper. b Samples of water from the shallow lake (n = 3) and groundwater (n = 17) from the surrounding wells used for domestic and agricultural usages were collected after the warm rainfall season
The study area belongs to northwestern part of the unconfined, heterogenous, and anisotropic Zacatepec aquifer (total surface area: about 1279 km2) comprising of about 150-m-thick unconsolidated Cenozoic alluvial deposits as well as sandstone and conglomerates intercalated with volcanic deposits (basalt and andesite) and ignimbrites in the upper part (CONAGUA 2020; Rivera-Carranza et al. 1998). The lower part of this aquifer is represented by secondary permeability in the underlying Mesozoic limestones (Morelos and Cuautla Formations) caused from fracturing and dissolution (Fries 1960; Rivera-Carranza et al. 1998; CONAGUA 2020). The general static levels of this shallow aquifer vary between 5 and 80 m, and it is, however, < 20 m in the study area. Hydraulic transmissivity (4.82–12.1 × 10−3 m2/s) and hydraulic conductivity (0.005–0.14 × 10−3 m2/s) are variable, and it is a surplus aquifer with annual recharge of 85.3 hm3 and extraction of 56.4 hm3 (CONAGUA 2020). Most of the groundwater extracted is used for agricultural activities (30%, 17 hm3) and urban consumption (47.5%, 26.8 hm3), and the industrial usage is limited to 1.5% (0.9 hm3) of the total extraction (CONAGUA 2020).
The superficial phaeozem soil and vertisol are currently used for agricultural activities such as the farming of lemon, papaya, avocado, mango, guava, and banana, as well as the cultivation of dominantly corn and sugarcane (INEGI 1984; García Flores et al. 2019). About 24% of the population of the study area is marginalized and vulnerable due to social deficiencies such as lack of access to food with the main economic activities restricting to agriculture, livestock, and fishing (García Flores et al. 2019).
Sampling, analysis, and evaluation method
A total of 3 water samples (L1–L3) from the shallow water column of Lake Coatetelco and groundwater samples from 17 surrounding bore wells (G1–G17) with water depths below 20 m were collected in Teflon bottles immediately after the warmer rainfall season in October 2020 as the objective was to evaluate the physicochemical characteristics of water resources in their dilute states for the drinking and irrigation quality assessments as well as evaluation of the possible health risks from NO3 and F consumptions (Fig. 1). The study area receives more than 85% of its annual precipitation between May and September, and hence, the samples collected in October represented the water resources in their most dilute conditions. The quality assurance and quality control (QA/QC) procedures ensured the reliability of water samples. In-field QA/QC measures included collection of samples in duplicates and their preservation in refrigeration using ice cubes and ice box from the sampling sites to the laboratory. All the Teflon bottles were pre-cleaned with water from Milli-Q direct water purification system and rinsed thrice in field with water from the sample bore well and lake water before the sample collection. A pair of Teflon bottles with water from the Milli-Q system were used as blanks during the entire expedition and they were measured in laboratory to detect possible contamination which occurred in the field. After measuring the electrical conductivity (EC), total dissolved solids (TDS), and hydrogen ion concentration (pH) with a portable Hanna instrument (HI 98130) in the field, all the samples were transported under 4 °C to the laboratory. The cations (Na+, Ca2+, Mg2+, and K+) and anions (HCO3−, Cl−, SO42−, and NO32−) were analyzed using a Waters liquid chromatograph comprising of a binary pump (Model 1525), auto sampler (Model 717 plus), and conductivity detector (Model 432) after Zamora-Martínez et al. (2016). This ion exchange chromatography has separate ion exchange columns for anions (IC-PAK TM Anion HR, 46 mm × 75 mm, Waters) and cations (Metrosep C6-250/4.0, Metrohm). All samples were filtered through 0.45-micron pore size nylon membranes prior to the analysis and the autosampler was rinsed with type I water to avoid cross contamination between each injection. An ion-selective electrode (HANAA instruments) combined with a PC 700 (Oakton) benchtop pH/conductivity meter analyzed F− concentrations. The quality parameters were controlled by using six different NIST-certified high purity standard reference materials (i.e., IC-4–100, IC-1-A-100, IC-1–3-100, IC-1-A-100, etc.) and using the methods certified under the Mexicana and International norms (ISO-9001 and ISO-17025). Duplicate samples were analyzed after every 10 samples, and the accuracy of chemical parameters was evaluated with the calculation of expanded uncertainty at 95% confidence level and the Ionic Balance Error (IBE = ΣCation − ΣCation/ΣCation + ΣAnion × 100).
Drinking water quality evaluation
Comparison of major ion compositions with World Health Organization guidelines (WHO 2017) was the first-hand filter to evaluate the drinking suitability. Additionally, we evaluated overall drinking water quality index (DWQI) by assessing the similarities of physicochemical parameters with WHO limits through a mathematical computation and assigning different weights (wi) and relative weights (Wi) based on their influences considered from Yi et al. (2019), Marghade (2020), Verma et al. (2020), and Wu et al. (2020). Critical water quality parameters such as TDS, Cl, SO4, NO3, and F were allotted the maximum weight of 5, and HCO3 was assigned the minimum weight of 1 (e.g., Vasanthavigar et al. 2010; Tiwari et al. 2017). Other parameters such as pH (3), Ca (3), Mg (3), Na (4), and K (2) were assigned weights varying between 2 and 4 (Verma et al. 2020).
The relative weight (Wi) of each parameter (pH: 0.073; TDS: 0.122; HCO3: 0.024; Cl: 0.122; SO4: 0.122; NO3: 0.122; F: 0.122; Ca: 0.073; Mg: 0.073; Na: 0.098; K: 0.049) was calculated using the following equation:
where wi is the weight of each parameter and n is the number of parameters. A quality rating scale (qi) for each parameter was calculated by dividing the concentration in each water sample with relative standard concentration recommended by the WHO (2017) guidelines and multiplied by 100:
where qi is the quality rating scale, Ci is concentration of each chemical parameter in each water sample in mg/L, and Si is the standard for each chemical parameter in mg/L according to the WHO (2017) guidelines.
Finally, the DWQI was estimated using the value of SIi (= wi × qi) in the following equation:
where SIi is the sub-index of ith parameter, qi is the rating based on concentration of ith parameter, and n is the number of parameters. It switched the water quality parameters of all the lake and groundwater samples into codes to assess their suitability for drinking by separating them into five different categories from excellent (DWQI > 50) to unsuitable (DWQI > 300).
Irrigation water quality evaluation
Multiple indices such as total hardness (TH), salinity hazard, sodium adsorption ratio (SAR), residual sodium carbonate (RSC), sodium percentage (Na%), Kelley ratio (KR), magnesium ratio (MR), potential salinity (PS), and permeability index (PI) evaluated the irrigation water quality of both the lake and groundwater samples (e.g., Richards 1954; Doneen 1962, 1964; Kelley 1963; Ragunath 1987). Additionally, the overall irrigation quality was also evaluated by calculating the irrigation water quality index (IWQI; e.g., Rana et al. 2018; Sharma et al. 2020).
Total hardness (TH) index determined accumulation of divalent calcium and magnesium ions (in meq/L) in water samples by using the equation of Ragunath (1987):
Salinity hazard considered the values of EC for classifying the sample into five different categories between excellent (EC < 250 µS/cm) and unsuitable (EC > 3000 µS/cm). Sodium adsorption ratio (SAR) considered the concentrations of Na with respect to Ca and Mg (in meq/L) as per Richards (1954):
Residual sodium carbonate (RSC) evaluated the water quality by considering the concentrations of carbonate and bicarbonate ions against the proportions of calcium and magnesium ions (in meq/L) in the formula of Eaton (1950):
The index of Na percentage (Na %) determined irrigation water quality from concentrations (meq/L) of all dissolved cations in the formula of Doneen (1962):
Kelley ratio (KR) evaluated the ratio of concentrations of Na against Ca and Mg (in meq/L) using the formula of Kelley (1963):
Magnesium ratio (MR) represented the concentrations of Mg against the contents of both Ca and Mg (in meq/L) as per Paliwal (1972):
The potential salinity (PS) index considered chloride ion concentration and half of sulfate concentration in meq/L as per Doneen (1964):
Finally, the permeability index (PI) assessed suitability of water for irrigation by evaluating water flux efficiency (permeability) in soil using the concentrations of Na, Ca, Mg, and HCO3 in meq/L by following Doneen (1964):
All these indices separated the water samples into different categories such as safe and unsafe or suitable or unsuitable classes. IWQI determined the overall suitability of water quality for irrigation as follows (e.g., Rana et al. 2018; Sharma et al. 2020):
where wi represents normalized weight of the ith parameter (EC: 0.211; Na: 0.204; HCO3: 0.202; Cl: 0.194; SAR: 0.189) and qi represents the quality of ith parameter (between 0 and 100, Table 1) determined using the following equation:
where qmax represents the highest value of qi for each class, xij is the value of each parameter, xinf represents the minimum value of the class to which the indicator belongs, qimap is the class amplitude, and xamp represents class amplitude in which the properties belong. IWQI values grouped the water samples into five different categories based on their suitability for irrigation with IWQI of 85–100 representing the water samples of no restriction for irrigation and IWQI of 0–40 representing water samples of severe restriction.
Health risk assessment
The possible adverse health impacts on men, women, and children were assessed following the recommendation of United States Environmental Protection Agency (USEPA 2004). The intake of both NO3 and F through drinking was considered here as the main pathway and their non-carcinogenic risks were estimated by calculating the average daily exposure dose (DE) through ingestion of lake and groundwater (mg/kg/day) using the formula of USEPA (2004) like the studies of Adimalla et al. (2018), Karunanidhi et al. (2020a), and Subba Rao et al. (2020):
where CP is pollutant concentration (mg/L), IR is ingestion rate per unit time (adult male and female: 3.229 L/day and child: 1.006 L/day; USEPA analysis 2019 data at 95th percentile (ATSDR 2023)), ED is exposure duration (male: 64; female; 67; children: 12 years; Subba Rao et al. 2020), and EF is exposure frequency (365 days/year; Subba Rao et al. 2020). We considered the average body weight (AB) in the context of Mexico as 74 kg for adult male, 68 kg for adult female, and 20 kg for a child. AE is the average age exposure time (adult male: 23,360; adult female: 24,455; children: 4380 days; Subba Rao et al. 2020).
The hazard quotient (HQ) was applied to evaluate risks from fluoride and nitrate in water samples using the equation:
where HQ is the hazard quotient for nitrate and fluoride, which is a measure of non-carcinogenic chronic hazard, and RfD represents the reference dose for chronic oral exposure (mg/kg/day) to both the pollutants (NO3: 1.60; F: 0.06 mg/kg/day; Adimalla 2019; Alarcón-Herrera et al. 2020). Finally, the Total Hazard Quotient Index (THQI) of non-carcinogenic risk was evaluated by adding the values of HQnitrate and HQflouride:
The samples with HQ and THQI above 1 may cause non-carcinogenic risk and they are considered inappropriate for ingestion. Samples with HQ and THQI below 1 are considered safe for consumption (e.g., Narsimha and Rajitha 2018; He et al. 2019).
Results and discussion
Hydrochemistry
Lake water (n = 3): They are slightly acidic to almost neutral with pH varying between 5.9 and 7.3 (average: 6.7; Table 2). EC changes between 294 and 536 μS/cm (average: 454 μS/cm) and TDS fluctuates between 147 and 269 mg/L (average: 227 mg/L). All the samples have TDS < 1000 mg/L, and they are categorized as fresh (e.g., Freeze and Cherry 1979). Among the cations, Na is more abundant (52–53.8 mg/L; average: 52.9 mg/L) than the almost comparable K (22.8–28.5 mg/L; average: 25.2 mg/L), Ca (21.2–26.1 mg/L; average: 22.9 mg/L), and Mg (21.3–21.9 mg/L; average: 21.7 mg/L). Among the major anions, the concentration of HCO3 (256.8–310.2 mg/L; average: 276.5 mg/L) is more compared to SO4 (36.3–39.2 mg/L; average: 38.2 mg/L), and Cl shows an abundance of 14.2–18 mg/L (average: 15.6 mg/L). NO3 remains below the detection limit (< 0.75 mg/L) in all the samples and F fluctuates between 0.3 and 4.3 mg/L (average: 1.8 mg/L; Table 2).
Groundwater (n = 17): Some samples (n = 5) are slightly acidic, and the rest are almost neutral with pH between 5.8 and 7.3 (average: 6.8). The acidic nature of some samples may be due to abundant volcanic deposits constituting the aquifer and degradation of organic carbon in the soil layers. EC remains heterogeneous and higher compared to the lake water (481–2030 μS/cm; average: 926.6 μS/cm). TDS of 242–1020 mg/L (average: 463.6 mg/L) classifies one groundwater sample as slightly brackish (TDS > 1000 mg/L) and the rest as fresh (TDS < 1000 mg/L; Freeze and Cherry 1979). Among the cations, Na is more abundant (20.5–308.7 mg/L; average: 103.6 mg/L) than Ca (34.3–75 mg/L; average: 53.3 mg/L), Mg (12–64.2 mg/L; average: 28.3 mg/L), and K (3.6–46.8 mg/L; average: 13.5 mg/L). Among the major anions, the concentration of HCO3 (285.4–1102.2 mg/L; average: 559.2 mg/L) is more compared to SO4 (18.7–198.6 mg/L; average: 69.5 mg/L) and Cl (4.3–90.3 mg/L; average: 27.9 mg/L). NO3 remains heterogenous with below the detection limit (< 0.75 mg/L) in some samples (n = 6) and up to 29.5 mg/L (average: 8.7 mg/L) in others (n = 11). F fluctuates between 0.1 and 8.7 mg/L with an average of 1.9 mg/L.
Rock-water interaction
Lake water (n = 3): Cation (Ca, Mg, and Na + K) and anion (HCO3, SO4, and Cl) abundances in the trilinear diagram of Piper (1944) grouped the samples in Ca-Mg-HCO3 water type (Fig. 2). The distributions of cation classified the lake water as no dominant type, indicating interactions with variable watershed geology, and the anions grouped the samples as dominant HCO3 type due to the importance of carbonic acid (H2CO3) in weathering reactions (Fig. 2). The major influence of rock-water interaction compared to precipitation and evaporation on the hydrochemistry of lake water was mirrored by comparing the ratio of Na + K/(Na + K + Ca) against TDS and the ratio of Cl/(Cl + HCO3) against TDS in Gibbs (1970) diagrams (Fig. 3). Both the Piper and Gibbs plots suggest that the interaction with catchment lithologies controlled the lake water chemistry. Abundant HCO3 in the lake water reflected the interaction with watershed lithologies such as limestone and volcanic deposits (basalt and andesite) as well as non-carbonate rocks such as the alluvial deposits (e.g., Fries 1960; Rivera-Carranza et al. 1998; CONAGUA 2020). Additionally, the oxidation of organic matter present in the topsoil might have also contributed HCO3 through the CO2 dissolution in water. The weathering of volcanic deposits such as basalt and andesite produces water dominated by HCO3, Ca, and Mg, similar to Ca-Mg-HCO3 type of water from Lake Coatetelco (Eugster and Hardie 1978).
Groundwater (n = 17): The diamond grid in Piper trilinear diagram (Fig. 2) reflects heterogeneity of ion concentrations and classifies some groundwater samples as Na-HCO3-Cl water type (n = 3) and the rest as Ca-Mg-HCO3 (n = 14). All the samples are of dominant HCO3 type in the anion triangle and fluctuate between Ca and Na + K water types in the cation triangle (Fig. 2). The samples with dominant bicarbonate and Ca mirror the interactions with limestone in deeper part of the aquifer, and the samples with no dominant cation as well as dominant Na + K reflect the intercalations with sedimentary (sandstone and conglomerate) and volcanic deposits constituting the upper part of aquifer lithology (e.g., Fries 1960; Rivera-Carranza et al. 1998; CONAGUA 2020). The Gibbs diagrams (Fig. 3) reflect the interaction of groundwater with catchment lithologies with lower Na + K/(Na + K + Ca) values indicating interaction with limestone and higher Na + K/(Na + K + Ca) values representing more interaction with the silicate-bearing (volcanic) deposits (e.g., Marandi and Shand 2018). In the sample with higher Cl/Cl + HCO3 (n = 8), the irrigation return flow possibly enriched Cl and the Piper diagram grouped some of them in the Na-HCO3-Cl facies (e.g., Nagarajan et al. 2010). These wells with Cl between 57.1 and 90.3 mg/L are present at the northern lake margin with more agricultural activity.
Drinking water quality
Comparison with WHO guideline
Lake water (n = 3): Physical parameters remain within the recommendations of WHO (2017), except for the pH of one sample (Table 2). The sample with pH of 5.9 is more acidic compared to the suggested range (pH: 6.5–8.5). With respect to the major ions, all samples (100%) have K above the permissible limit for drinking (> 12 mg/L) and HCO3 of only one sample (33%) exceeds the allowable content (> 300 mg/L). The basic sources of potassium are silicate minerals, i.e., K-feldspars, muscovite, and clay mineral (kaolinite) in the alluvium as well as abundant volcanic deposits in watershed, which are dissolved by the low pH (carbonic acid) water flowing into the lake. The acidic nature of inflowing water is mirrored by pH < 7 in two out of the three samples. More bicarbonate than the permissible limit for drinking in one sample could be from the interaction of inflowing water with organic-rich soil layers and additionally from higher dissolution of limestone in the watershed (e.g., Venkatramanan et al. 2017; Chung et al. 2020).
Concentrations of Ca (< 75 mg/L), Mg (< 50 mg/L), Na (< 200 mg/L), Cl (< 250 mg/L), and SO4 (< 250 mg/L) of all samples remain within the permissible limits (Table 2). NO3 concentrations remain below the permissible limit of WHO (50 mg/L) and Mexican norms (42 mg/L) in all the samples. About 33% samples have fluoride above the recommended limit of 1.5 mg/L and the remaining 66% samples contain fluoride within the WHO (2017) guidelines. The samples show possibility to generate dental caries (< 0.5 mg/L, 33%), beneficial for human health (0.6–1.5 mg/L, 33%), and vulnerability of dental and skeletal fluorosis (> 2 mg/L, 33%) (e.g., Adimalla 2020).
Groundwater (n = 17): All the samples have pH within the permissible limit for drinking. Only one sample (6%) with TDS of 1020 mg/L and EC of 2030 μS/cm has physical characteristics above the WHO-recommended values (Table 2). About 82% samples (n = 12) have HCO3 above the permissible limit (300 mg/L). Similarly, 29% (n = 5) samples have K (12 mg/L) and 12% (n = 2) samples have Mg (50 mg/L) and Na (200 mg/L) exceeding the permissible values. More bicarbonate than the permissible limit in most of the samples shows the effect of limestone dissolution (e.g., Venkatramanan et al. 2017; Chung et al. 2020). The sources of higher potassium and magnesium in some samples are chemical weathering of both the felsic and mafic silicate minerals like K-feldspar, and amphibole present in the volcanic deposits by the low pH groundwater.
Concentrations of Ca (< 75 mg/L), Cl (< 250 mg/L), and SO4 (< 250 mg/L) of all the samples are within the permissible limits (Table 2). NO3 concentrations remain below the permissible limit of WHO (50 mg/L) as well as the Mexican norms (42 mg/L). About 53% samples (n = 9) have fluoride above the recommended limit of 1.5 mg/L and 47% samples (n = 8) might be responsible for dental and skeletal fluorosis (> 2 mg/L; Adimalla 2020). The other samples can be grouped as possible trigger to dental caries (< 0.5 mg/L, 47%; Adimalla 2020). Higher fluoride in more than half of the groundwater samples reflects possible weathering of fluoride-bearing minerals present in the limestone and volcanic deposits through the rock-water interactions. Carrillo-Rivera et al. (2002) also reported up to 3.7 mg/L of F in the groundwater of San Luis Potosi state and Reyes-Gómez et al. (2017) observed up to 6 mg/L of fluoride in the groundwater of Chihuahua state. Both the regions with volcanic geology showed F in groundwater comparable to the groundwater in surroundings of the Lake Coatetelco (up to 8.7 mg/L). Several wells of previous studies in central and northern Mexico as well as the present evaluation in central-south Mexico showed F exceeding the WHO-recommended value of 1.5 mg/L.
Drinking water quality index
The groundwater extracted through shallow borewells as well as the lake water are presently used for drinking as well as other domestic usages. The drinking water quality index (DWQI) grouped them into five categories such as excellent (< 50), good (50–100), poor (100–200), very poor (200–300), and unsuitable (> 300) (Table 3). In general, most of the lake water as well as groundwater samples are in excellent and good categories.
Lake water (n = 3): About 67% (n = 2) samples (DWQI: 36.6–37.3) are grouped in excellent category and one sample (33%) with DWQI of 68.1 is in the good category even though K in all the samples and F in one sample remain above the WHO permissible limits (Table 3).
Groundwater (n = 17): DWQI values (28.5–159.9) are heterogenous compared to the lake water. About 41% samples (n = 7) are in excellent category and 53% (n = 9) are in good category (Table 3). Only one sample (6%) is in poor category, and it has the highest values of F (8.7 mg/L), TDS (1020 mg/L), and Na (308.7 mg/L) among all the lake and groundwater samples.
Irrigation water quality
Indices
The lake water is moderately hard (67%, TH: 75–150) and hard (33%, TH: 150–300) (e.g., Sawyer and McCarty 1967). Among the groundwater samples, about 77% are hard, 17% are very hard (TH > 300), and 6% are moderately hard (Table 4). The estimation of salinity and alkalinity hazards through indices such as RSC, SAR, Na%, KR, MR, PS, and PI evaluated the suitability of both lake water and groundwater for irrigation (e.g., Eaton 1950; Richards 1954; Doneen 1962, 1964; Kelley 1963; Sawyer and McCarty 1967; Paliwal 1972). RSC represents the amount of carbonate (NaCO3 and NaHCO3) present in the irrigation water and its higher values (RSC > 2.5) reflect an increase in sodium adsorption. Irrigation with more saline water alters the osmotic pressure and affects the crop yield (e.g., Thorne and Peterson 1954; Trivedy and Goel 1984). It increases the kinematic viscosity and prevents water from reaching the branches and leaves of plants by decreasing the permeability coefficient. High salinity water also induces the accumulation of salt in soil, and the high sodium concentration leads to enhancement in soil alkalinity. It reduces the stability of soil structure and affects the extension of plant roots and movement of water through the soil. SAR estimated the potential of Na accumulation in soil through the Na-enriched irrigation water with low SAR (< 10) values reflecting the better water percolation time (Richards 1954). In the US Regional Salinity Laboratory (USSL) diagram, SAR values grouped all the lake and groundwater as low sodium hazard (S1), suggesting their suitability for all types of soils and crops (Fig. 4). EC (Wilcox 1955), however, divided the samples between low salinity hazard (C1) and high salinity hazard (C3). The samples in low salinity hazard are suitable for irrigating all plants, the samples in medium salinity hazard are suitable for salt-tolerant plants, and the samples with high salinity hazards are unsuitable for irrigation (Table 4). The Wilcox diagram (Na% vs. EC) classified the samples in categories varying from excellent to doubtful for irrigation (Fig. 5, e.g., Wilcox 1955). Similarly, the permeability index (PI) vs. total concentration (meq/L) in the Doneen (1964) classification divided the samples between class I with > 75% of permeability and class III with < 25% of permeability to assess the irrigation suitability (Fig. 6).
Lake water (n = 3): Based on the salinity hazard (EC: 250–750 in μS/cm, good) and SAR (< 10, excellent), all the lake water samples are suitable for irrigation. RSC (1.25–2.50) and Na% (40–60) ranges also group them in acceptable and permissible categories (Table 4). The indices of Kelly ratio (KR), potential salinity (PS), and permeability (PI) categorized all as suitable, safe, and good for irrigation. These samples in C2S1 field of USSL suggest moderate salinity hazard and low sodium hazard (C2S1), and all of them can be used to irrigate crops without considering any special measure (Fig. 4). They are also excellent-to-good for irrigation in the Wilcox diagram (Fig. 5). The Doneen diagram classified all these samples under class I with > 75% of permeability, indicating their suitability for irrigation (Fig. 6).
Groundwater (n = 17): The individual indices as well as classification diagrams show the heterogeneity of groundwater. The salinity hazard divides the samples in good (EC: 250–750 in μS/cm, 47%) and permissible (EC: 750–2250 μS/cm, 53%) categories. SAR (< 10) classifies all of them (100%) as excellent for irrigation (Table 4). Based on RSC (> 2.50), about 53% of samples (n = 9), however, are unsuitable. Similarly, about 24% (n = 4) are doubtful in Na% (60–80) index (Table 4). The Kelly ratio (KR < 1) classifies 59% (n = 10) samples as suitable, the potential salinity (PS < 3) groups 88% (n = 15) samples as safe, and the permeability index (PI) groups 47% (n = 8) as good and 53% (n = 9) as fair for irrigation. In the USSL classification, about 71% samples (n = 12) with low and medium salinity hazards (C1S1 and C2S1) can be used to irrigate crops without considering any special measure, and 29% samples (n = 5) with high salinity hazard (C3S1) are generally suitable for salt-tolerant plants (Fig. 4). Irrigation with the high salinity hazard water might affect crop growth through osmotic effects and nutritional disorders (e.g., Läuchli and Epstein 1990). Irrigation with the water of this nature needs special consideration for salinity control and mechanism to improve the soil permeability and should be considered only to irrigate salt-tolerant crops under favorable drainage conditions. The Wilcox diagram also classifies 71% of groundwater samples (n = 12) as excellent-to-good (n = 10) and good-to-permissible (n = 2). The remaining 29% samples are in the permissible-to-doubtful (n = 4) and doubtful-to-unsuitable (n = 1) categories for irrigation (Fig. 5). They are also in the excellent-to-good groups for irrigation in the Wilcox diagram. The Doneen diagram classifies 59% samples (n = 11) under class I with > 75% of permeability and 35% (n = 6) in class II with 25–75% permeability, indicating their suitability for irrigation (Fig. 6). One sample, in class 3 with < 25% permeability, is unsuitable for irrigation.
Irrigation water quality index (IWQI)
Estimation of IWQI assessed the overall quality for irrigation, and demarcation of the most suitable wells. It divides the groundwater samples into five different classes such as severe restriction (< 40), high restriction (40–55), moderate restriction (55–70), low restriction (70–85), and no restriction (85–100) (Table 5).
Lake water (n = 3): All have almost similar and homogenous IWQI between 64.3 and 64.4 (average: 78) and they are in the group of moderate restriction for irrigation. There are no sample in the high to severe restriction categories (Table 5). The lake water should be used for irrigating soils with moderate to high permeability with special salinity control practices to minimize salt accumulation.
Groundwater (n = 17): The samples with variable IWQI (38.8–68.6; average: 54.5) are in the categories of moderate restriction (47%, n = 8), high restriction (47%, n = 8), and severe restriction (6%, n = 1). The groundwater samples are relatively less suitable for irrigation compared to the lake water. It could be used for irrigating soils with light texture or moderate permeability to avoid the effects of salt leaching. The salt sensitivity plants should be avoided in this region and the soil texture can be modified by changing the sand to clay ratio.
Health risk assessment
The exposure through ingestion of lake water and groundwater with elevated concentrations of NO3 could cause methemoglobinemia (commonly known as blue baby syndrome in infants; Adimalla et al. 2018; Karunanidhi et al. 2020b) and higher F might trigger both dental and skeletal fluorosis (e.g., Kimambo et al. 2019; Alarcón-Herrera et al. 2020). We computed non-carcinogenic risks for the population of different age groups residing around the Lake Coatetelco from ingestion of nitrate and fluoride in water by considering an adult male with an average weight of 74 kg, adult female with an average weight of 68 kg, and a child with average weight of 20 kg in Table 6. The estimated Health Quotients (HQnitrate and HQfluoride) for individuals suggest absence of any risk from NO3 in both lake and groundwater. However, the HQfluoride shows potential non-carcinogenic concerns and possible effect in order of child > adult female > adult male. In the lake water samples, the HQnirate values for different age groups could not be computed as nitrate remained below the detection limit. However, the HQfluoride values for adult male (0.19–3.11; avg: 1.29), adult female (0.21–3.39; avg: 1.40), and child (0.22–3.59, avg: 1.48) suggest relatively higher but similar risks for child and adult female compared to the adult male in 33% of the samples (HQ > 1). In the groundwater samples, the HQnitrate outcome for adult male (0.04–0.81; avg: 0.37), adult female (0.05–0.88; avg: 0.40), and child (0.04–0.74; avg: 0.34) remained < 1 and did not show any immediate health risk. Negative correlation (r = − 0.62) between NO3 and Cl in groundwater and nitrate remaining below the detection limit (< 0.75 mg/L) in most of the samples with Cl above 20 mg/L rules out the domestic sewage and farm manures as the dominant sources of nitrate (e.g., Mahlknecht et al. 2008). The synthetic nitrogenous fertilizers (e.g., ammonium nitrate) used in the agricultural fields near the groundwater wells and lake surroundings possibly contributed nitrate. The groundwater samples with NO3 < 10 mg/L generally show natural conditions, with minimal human interferences around the lake, and the samples with > 10 mg/L of nitrate (n = 7) around the Miacatlán village indicate anthropogenic impact possibly from synthetic fertilizers on a part of the shallow aquifer (Fig. 7, e.g., Marghade et al. 2015; Subba Rao et al. 2020). Unlike the groundwater of Monterrey City, the effects of sewage leakage and soil organic nitrogen were minimal on groundwater around the Lake Coatetelco (e.g., Torres-Martinez et al. 2020). All the samples of this study, however, remained below the WHO (< 50 mg/L) norms and did not pose any immediate health risk to the surrounding population (e.g., Adimalla et al. 2018).
HQfluoride values, however, showed possible risk of dental and skeletal fluorosis from consumption of 53% of the groundwater samples for male (0.09–6.35; avg: 1.37), female (0.10–6.91; avg: 1.49), and child (0.11–7.32; avg: 1.57). In the present study, HQfluoride for non-carcinogenic risk exceeds 1 in 33% of lake water samples and 53% of groundwater samples with respect to men, women, and child, respectively (Table 6). The child population of this region is more exposed to the health threat compared to the adults due to their relatively lighter body weight, similar to the observations of other studies in India and China (Narsimha and Rajitha 2018; Zhang et al. 2018; Karunanidhi et al. 2020b). Total Health Index (THQI) values (> 1) were influenced mainly by HQfluoride and they indicate that 53% of the groundwater samples could be avoided for drinking as they are unsafe or pose high risk for men, women, and children (USEPA 2019). The samples beyond the allowable limit of HQ are located at the wells of northern (n = 3) lake margin and southern (n = 3) lake margin (i.e., Coatetelco village) as well as the wells (n = 3) in surroundings of the Miacatlán village (Fig. 8).
The data of national caries survey of 2001 indicated an overall fluorosis prevalence of 27.9% in Mexico with the lowest values in Morelos state (3.2%, the study area) and the highest in Durango state (88.8%, Betancourt-Lineares et al. 2013). Only about 11.2% of 12-year-olds lacked dental fluorosis in the semi-arid Durango state, whereas nearly 96.8% of the same age group did not show dental fluorosis in Morelos state (Betancourt-Lineares et al. 2013). The survey of 2011–2014, however, revealed that the dental fluorosis is increasingly becoming a public health problem in the sub-humid to humid central and southern states. The prevalence level on the same age group school-going children in Morelos increased to 33.7% after a decade with an average 0.28–0.50 teeth/student showing permanent caries (ENCD 2018). The community fluorosis index for the children and adolescents increased from < 0.4 to > 2.5 (Betancourt-Lineares et al. 2013; ENCD 2018). This could be due to increasing concentration of F in the groundwater of this region. Similar to above 1.5 mg/L of F in both lake and groundwater from Coatetelco, Huízar Álvarez et al. (2016) reported up to 1.90 mg/L of F in groundwater and spring water of Tenextepango area of Morelos (about 40 km, east of Coatetelco). Higher fluoride in 50% of the total samples of this study could be from weathering of fluoride-bearing minerals present in the limestone as well as volcanic deposits through the rock-water interaction. Minerals such as fluorite might have contributed fluorine to the lake and groundwater, and it needs further evaluation and research (e.g., Narsimha and Rajitha 2018; Adimalla and Li 2018). However, the sustainable development of water resources and supply of safe drinking water in this region are the immediate necessities in order to protect the consuming population from exposure to chronic fluorosis, and both can be achieved by considering some of the measures, such as (a) regular quality monitoring, (b) generation of maps indicating vulnerable parts of the aquifer and public awareness programs about water-borne diseases and their preventions, and (c) identification of possible sources and measure of prevention through implementation of aquifer recharge technique through the check dam constructions and recharge pits to reduce the concentration levels of pollutants.
Conclusions
Physicochemical characteristics of 20 water samples from the Lake Coatetelco in the tropical central-south Mexico and groundwater from the surrounding wells collected immediately after the wet season were evaluated to determine the suitability for drinking and irrigation as well as to demarcate the samples that might cause health risk for adults and children from the ingestion of nitrate and fluoride, with an aim to provide a baseline data for comparison and monitoring of water quality in the short and long terms. More specifically:
-
(i)
In the trilinear diagram of Piper, the lake water was grouped as Ca-Mg-HCO3 and the groundwater samples represented facies varying between Na-HCO3-Cl and Ca-Mg-HCO3, indicating the heterogenous watershed lithologies, i.e., limestone and the siliciclastic volcanic deposits as well as alluvium. The Gibbs plots indicated the dominant influence of rock-water interaction on water chemistry. The irrigation return flow possibly enriched Cl in some of the groundwater samples characterized by high Cl/Cl + HCO3.
-
(ii)
In the lake water, the physical parameters mostly remain within the WHO recommendations. K in all samples and HCO3 in one sample remained above the permissible limits. NO3 remained below the permissible limit of WHO (50 mg/L) and Mexican norms (42 mg/L) in all the samples, and fluoride in one sample was above 1.5 mg/L. The drinking water quality index (DWQI), however, grouped them in excellent and good categories. In the groundwater samples, NO3 remained below the permissible limit of WHO and Mexican norms but shows influence of synthetic nitrogenous fertilizers in wells near the agricultural fields. Fluoride was above the recommended limit of 1.5 mg/L in 53% samples (n = 9) showing the possible risk of fluorosis. DWQI demarcated 94% samples in excellent and good categories for drinking and the sample in poor category has the highest F (8.7 mg/L), TDS (1020 mg/L), and Na (308.7 mg/L).
-
(iii)
In the lake water, the indices of salinity hazard and SAR categorized all as suitable for irrigation. The USSL diagram classified them as C2S1 (moderate salinity hazard) and C2S1 (low sodium hazard). The Doneen classification suggested their suitability for irrigation with > 75% of permeability. IWQI, however, grouped all of them as moderate restriction for irrigation. In the groundwater samples, RSC demarcated about 53% samples as unsuitable and Na% categorized about 24% as doubtful for irrigation. The USSL classification helped to demarcate the 71% samples (12 wells), with low and medium salinity hazards (C1S1 and C2S1) for irrigation without any special measure, and 29% samples (5 wells) with high salinity hazard (C3S1) suitable for only salt-tolerant plants. The Doneen diagram divided these samples based on their permeabilities.
-
(iv)
The estimation of non-carcinogenic risks from nitrate and fluoride ingestion for adult male (74 kg), adult female (68 kg), and a child with average weight of 20 kg through Health Quotients (HQ) indicated absence of any risk from NO3. HQfluoride, however, shows risks in the order of child > adult female > adult male. About 33% of lake water samples and 53% of groundwater samples show possible non-carcinogenic risk with respect to adults and children. Higher fluoride in 50% of the total samples of this study could be from weathering of fluoride-bearing minerals in the limestone as well as volcanic deposits through the rock-water interaction. Results of this study indicating F > 1.5 mg/L in both lake and groundwater are in congruence with the data of national caries surveys suggesting almost tenfold increase in fluorosis prevalence from 3.2 to 33.7% on the 12-year-old school-going children in Morelos over a decade between 2001 and 2011–2014.
Data availability
Data used in this study are included and the raw data will be provided upon request.
References
Abascal E, Gómez-Coma L, Ortiz I, Ortiz A (2022) Global diagnosis of nitrate pollution in groundwater and review of removal technologies. Sci Total Environ 810:152233
Adimalla N (2019) Groundwater quality for drinking and irrigation purposes and potential health risks assessment: A case study from semi-arid region of South India. Expos Health 11:109–123
Adimalla N (2020) Controlling factors and mechanism of groundwater quality variation in semiarid region of South India: an approach of water quality index (WQI) and health risk assessment (HRA). Environ Geochem Health 42(6):1725–1752
Adimalla N, Li P (2018) Occurrence, health risks, and geochemical mechanisms of fluoride and nitrate in groundwater of the rockdominant semi-arid region, Telangana State, India. Hum Ecol Risk Assess: An Int J 25:81–103
Adimalla N, Li P, Qian H (2018) Evaluation of groundwater contamination for fluoride and nitrate in semi-arid region of Nirmal Province, South India: a special emphasis on human health risk assessment (HHRA). Hum Ecol Risk Assess Int J 25(5):1107–1124
Alarcón-Herrera MT, Martin-Alarcon DA, Gutiérrez M, Reynoso-Cuevas L, Martín-Domínguez A, Olmos-Márquez MA, Bundschuh J (2020) Co-occurrence, possible origin, and health-risk assessment of arsenic and fluoride in drinking water sources in Mexico: Geographical data visualization. Sci Total Environ 698:134168
Arias PA, Bellouin N, Coppola E, Jones RG et al (2021) Technical Summary. In: Masson-Delmotte V, Zhai P, Pirani A et al (eds) Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom and New York, pp 33–144
ATSDR (Agency for Toxic Substances and Disease Registry) (2023) Exposure dose guidance for water ingestion. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service. https://www.atsdr.cdc.gov/pha-guidance/resources/ATSDR-EDG-Drinking-Water-Ingestion-508.pdf
Betancourt-Lineares A, Irigoyen-Camacho ME, Mejía-González A, Zepeda-Zepeda M, Sánchez-Pérez L (2013) Prevalencia de fluorosis dental en localidades mexicanas ubicadas en 27 estados y el D.F. a seis años de la publicación de la Norma Oficial Mexicana para la fluoruración de la sal. Rev Invest Clin 65:237–247
Calleros EY, Alarcón MT, Pérez R, Cueto JA, Moran J, Sanín LH (2012) Evaluación de riesgo sistémico y niveles de metahemoglobina en niños que consumen agua contaminada por nitratos. Ingeniería 16(3):183–194 ((In Spanish))
Carrillo-Rivera JJ, Cardona A, Edmunds WM (2002) Use of abstraction regime and knowledge of hydrogeological conditions to control high-fluoride concentration in abstracted groundwater: San Luis Potosı basin, Mexico. J Hydrol 261(1–4):24–47
Chung SY, Rajesh R, Venkatramanan S, Selvam S, Ranganathan PC, Oh YY, Hussam EE (2020) Processes and characteristics of hydrogeochemical variations between unconfined and confined aquifer systems: a case study of the Nakdong River Basin in Busan City, Korea. Environ Sci Pollut Res 27:10087–10102
CONAGUA (2019) Estadísticas del Agua en México 2019. Comisión Nacional del Agua, Ciudad de México (In Spanish). https://files.conagua.gob.mx/conagua/publicaciones/Publicaciones/EAM_2019.pdf
CONAGUA (2020) Actualización de la disponibilidad media anual de agua en el acuífero Zacatepec (1703), Estado de Morelos. Subdirección General Técnica Genercia de Aguas Subterráneas. Comisión Nacional del Agua, Ciudad de México. https://sigagis.conagua.gob.mx/gas1/Edos_Acuiferos_18/morelos/DR_1703.pdf
Cronin SJ, Manoharan V, Hedley MJ, Loganathan P (2000) Fluoride: A review of its fate, bioavailability, and risks of fluorosis in grazed-pasture systems in New Zealand. N Z J Agric Res 43(3):295–321
Doneen LD (1962) The influence of crop and soil on percolating water. In: Proceedings of the 1961 biennial conference on groundwater recharge pp 156–163
Doneen LD (1964) Notes on Water Quality in Agriculture published as a Water Science and Engineering Paper 4001, Department of Water Science And Engineering, University of California
Duan Q, Jiao J, Chen X, Wang X (2018) Association between water fluoride and the level of children’s intelligence: a dose–response meta-analysis. Public Health 154:87–97
Eaton FM (1950) Significance of carbonates in irrigation waters. Soil Sci 69(2):123–134
ENCD (2018) Informe Ejecutivo 2011–2014. Encuesta Nacional de Caries y Fluorosis Dental, Centro Nacional de Programas de Preventivos y Control de Enfermidades. Secrretaria de Salud (In Spanish). https://www.gob.mx/cms/uploads/attachment/file/422450/Informe_de_Caries_Dental__Encuesta_Nacional_de_Caries_y_Fluorosis_Dental_2011-2014_1.pdf. Accessed 22 Feb 2023
Eugster HP, Hardie LA (1978) Saline lakes. In: Lerman A (ed) Lakes: chemistry, geology, physics. Springer-Verlag, New York, pp 237–293
Feng SH, Krueger AB, Oppenheimer M (2010) Linkages among climate change, crop yields and Mexico-US cross-border migration. PNAS 107:14257–14262
Fewtrell L (2004) Drinking-water nitrate, methemoglobinemia, and global burden of disease: a discussion. Environ Health Perspect 112:1371–1374
Freeze RA, Cherry JA (1979) Groundwater. Prentice Hall, Englewood Cliffs
Fries C (1960) Geología del Estado de Morelos y de partes adyacentes de México y Guerrero, región central meridional de México. Boletín del Instituto de Geología, Universidad Nacional Autónoma de México
García Flores A, Monroy Martínez R, Colín Bahena H, Pino Moreno JM (2019) Plantas y animales con valor de uso alimentario en los huertos tradicionales de Coatetelco, Morelos, México. Revista Científica Agroecosistemas 7(2):79–86
Gibbs RJ (1970) Mechanism controlling world water chemistry. Science 170:1088–1090
Gupta SK, Gupta RC, Gupta AB, Seth AK, Bassin JK, Gupta A (2000) Recurrent acute respiratory tract infections in areas with high nitrate concentrations in drinking water. Environ Health Perspect 108:363–366
Gutierrez M, Alarcón-Herrera MT (2022) Fluoride in groundwater in the north-central region of Mexico and its possible origin. Revista Internacional De Contaminación Ambiental 38:54307
He X, Wu J, He S (2019) Hydrochemical characteristics and quality evaluation of groundwater in terms of health risks in Luohe aquifer in Wuqi County of the Chinese Loess Plateau, northwest China. Hum Ecol Risk Assess Int J 25:32–51
Huízar Álvarez R, Carrillo Rivera JJ, Juárez F (2016) Fluoruro en el agua subterránea: niveles, origen y control natural en la región de Tenextepango, Morelos, México. Investigaciones Geográficas 90:40–58 ((In Spanish))
INEGI (1984) Carta Edafológica Cuernavaca E14-5. Instituto Nacional de Estadística Geografía e Informática, Scale 1: 250, 000 (In Spanish). https://datos.gob.mx/busca/dataset/conjunto-de-datos-vectoriales-de-la-carta-edafologica-1-250-000-serie-lmorelos/resource/2873a568-6f02-4b21-9282-ca4302610d73
Karunanidhi D, Aravinthasamy P, Subramani T, Balakumar KG, Chandran NS (2020a) Health threats for the inhabitants of a textile hub (Tiruppur region) in southern India due to multipath entry of fluoride ions from groundwater. Ecotoxicol Environ Saf 204:111071
Karunanidhi D, Aravinthasamy P, Roy PD, Praveenkumar RM, Prasanth K, Selvapraveen S, Prasanth K, Selvapraveen S, Thowbeekrahman A, Subramani T, Srinivasamoorthy K (2020b) Evaluation of non-carcinogenic risks due to fluoride and nitrate contaminations in a groundwater of an urban part (Coimbatore region) of south India. Environ Monit Assess 192:1–16
Kelley WP (1963) Use of saline irrigation water. Soil Sci 95(6):385–391
Kimambo V, Bhattacharya P, Mtalo F, Mtamba J, Ahmad A (2019) Fluoride occurrence in groundwater systems at global scale and status of defluoridation–state of the art. Groundw Sustain Dev 9:100223
LaFayette GN, Knappett PS, Li Y, Loza-Aguirre I, Polizzotto ML (2020) Geogenic sources and chemical controls on fluoride release to groundwater in the Independence Basin, Mexico. Appl Geochem 123:104787
Läuchli A, Epstein E (1990) Plant responses to saline and sodic conditions. Agric Salin Assess Manag 71:113–137
Mahlknecht J, Horst A, Hernández-Limón G, Aravena R (2008) Groundwater geochemistry of the Chihuahua City region in the Rio Conchos Basin (northern Mexico) and implications for water resources management. Hydrol Process 22(24):4736–4751
Marandi A, Shand P (2018) Groundwater chemistry and the Gibbs Diagram. Appl Geochem 97:209–212
Marghade D (2020) Detailed geochemical assessment & indexing of shallow groundwater resources in metropolitan city of Nagpur (western Maharashtra, India) with potential health risk assessment of nitrate enriched groundwater for sustainable development. Geochemistry 80:125627
Marghade D, Malpe DB, Subba Rao N (2015) Identification of controlling processes of groundwater quality in a developing urban area using principal component analysis. Environ Earth Sci 74:5919–5933
Martínez J, Ortiz A, Ortiz I (2017) State-of-the-art and perspectives of the catalytic and electrocatalytic reduction of aqueous nitrates. Appl Catal B 207:42–59
Mekonnen MM, Hoekstra AY (2016) Four billion people facing severe water scarcity. Sci Adv 2:e1500323
Meireles ACM, Andrade EMD, Chaves LCG, Frischkorn H, Crisostomo LA (2010) A new proposal of the classification of irrigation water. Revista Ciência Agronômica 41:349–357
Nagarajan R, Rajmohan N, Mahendran U, Senthamilkumar S (2010) Evaluation of groundwater quality and its suitability for drinking and agricultural use in Thanjavur city, Tamil Nadu, India. Environ Monit Assess 171:289–308
Narsimha A, Rajitha S (2018) Spatial distribution and seasonal variation in fluorid enrichment in groundwater and its associated human health risk assessment in Telangana State, South India. Hum Ecol Risk Assess Int J 24:2119–2132
Navarro O, González J, Júnez-Ferreira HE, Bautista CF, Cardona A (2017) Correlation of arsenic and fluoride in the groundwater for human consumption in a semiarid region of Mexico. Procedia Eng 186:333–340
Paliwal KV (1972) Irrigation with saline water. Water Technology Centre, Indian Agricultural Research Institute 2: 198
Piper AM (1944) A graphical procedure in the geochemical interpretation of water analysis. Trans Am Geophys Union 25:914–928
Ragunath HM (1987) Groundwater. Wiley Eastern Ltd, New Delhi
Rana R, Ganguly R, Gupta AK (2018) Indexing method for assessment of pollution potential of leachate from non-engineered landfill sites and its effect on ground water quality. Environ Monit Assess 190:46
Rawat KS, Singh SK, Gautam SK (2018) Assessment of groundwater quality for irrigation use: a peninsular case study. Appl Water Sci 8:1–24
Reyes-Gómez VM, Gutiérrez M, Nájera-Haro B, Núñez-López D, Alarcón-Herrera MT (2017) Groundwater quality impacted by land use/land cover change in a semiarid region of Mexico. Groundw Sustain Dev 5:160–167
Richards LA (1954) Diagnosis and improvement of saline and alkali soils Agricultural Handbook. USDA and IBH Pub. Coy Ltd, New Delhi
Rivera-Carranza E, De la Teja-Segura MA, Miranda-Huerta A, Lemus-Bustos O, Motolinía-García O, León-Ayala V, Moctezuma-Salgado MD (1998) Carta Geológica-Minera Cuernavaca, estados de Morelos, Puebla, Guerrero, Estado de México y Oaxaca. Servicio Geológico Mexicano, Scala 1(250):000 ((In Spanish))
Roy PD, Selvam S, Gopinath S, Lakshumanan C, Muthusankar G, Quiroz-Jiménez JD, Zamora-Martínez O, Venkatramanan S (2021) Hydro-geochemistry-based appraisal of summer-season groundwater from three different semi-arid basins of northeast Mexico for drinking and irrigation. Environ Earth Sci 80(16):529
Sawyer CN, McCarty PL (1967) Chemistry for sanitary engineers. Mc-Graw Hill, New York
Sharma DA, Rishi MS, Keesari T (2017) Evaluation of groundwater quality and suitability for irrigation and drinking purposes in southwest Punjab, India using hydrochemical approach. Appl Water Sci 7:3137–3150
Sharma A, Ganguly R, Kumar Gupta A (2020) Impact Assessment of Leachate Pollution Potential on Groundwater: An Indexing Method. J Environ Eng 146:05019007
Singh B, Craswell E (2021) Fertilizers and nitrate pollution of surface and ground water: an increasingly pervasive global problem. SN Appl Sci 3:518
Subba Rao N, Sunitha B, Sun L, Spandana BD, Chaudhary M (2020) Mechanisms controlling groundwater chemistry and assessment of potential health risk: a case study from South India. Geochemistry 80(4):125568
Thorne DW, Peterson HB (1954) Irrigated soils. Soil Sci 78(5):406
Tiwari AK, Nota N, Marchionatti F, De Maio M (2017) Groundwater-level risk assessment by using statistical and geographic information system (GIS) techniques: a case study in the Aosta Valley region, Italy. Geomatics, Nat Hazards Risk 8:1396–1406
Tokazhanov G, Ramazanova E, Hamid S, Bae S, Lee W (2020) Advances in the catalytic reduction of nitrate by metallic catalysts for high efficiency and N2 selectivity: a review. Chem Eng J 384:123252
Torres-Martínez JA, Mora A, Knappett PS, Ornelas-Soto N, Mahlknecht J (2020) Tracking nitrate and sulfate sources in groundwater of an urbanized valley using a multi-tracer approach combined with a Bayesian isotope mixing model. Water Res 182:115962
Torres-Martínez JA, Mora A, Mahlknecht J, Daesslé LW, Cervantes-Avilés PA, Ledesma-Ruiz R (2021) Estimation of nitrate pollution sources and transformations in groundwater of an intensive livestock-agricultural area (Comarca Lagunera), combining major ions, stable isotopes and MixSIAR model. Environ Pollut 269:115445
Trivedy RK, Goel PK (1984) Chemical and Biological Methods for Water Pollution Studies. Environmental Publications, Pune
USEPA (2004) Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part E). http://www.epa.gov/oswer/riskassessment/ragse/pdf/introduction.pdf. Accessed 22 Feb 2023
USEPA (2019) Guidelines for Human Exposure Assessment. U.S. Environmental Protection Agency (EPA/100/B-19/001). Washington, D.C.: Risk Assessment Forum, U.S. EPA. https://www.epa.gov/risk/guidelines-human-exposure-assessment
Vasanthavigar M, Srinivasamoorthy K, Rajiv Ganthi R, Vijayaraghavan K, Sarma VS (2010) Characterisation and quality assessment of groundwater with a special emphasis on irrigation utility: Thirumanimuttar sub-basin, Tamil Nadu, India. Arab J Geosci 5:245–258
Venkatramanan S, Chung SY, Selvam S, Lee SY, Elzain HE (2017) Factors controlling groundwater quality in the Yeonjegu District of Busan City, Korea, using the hydrogeochemical processes and fuzzy GIS. Environ Sci Pollut Res 24(30):23679–23693
Verma A, Yadav BK, Singh NB (2020) Hydrochemical monitoring of groundwater quality for drinking and irrigation use in Rapti Basin. SN Appl Sci 2:1–15
WHO (2017) Guidelines for Drinking Water Quality: Fourth Edition Incorporating the First Addendum. World Health Organization, Geneva. https://www.who.int/publications/i/item/9789241549950. Accessed 22 Feb 2023
Wilcox LV (1955) Classification and use of irrigation waters. USDA Circular 969:19
Wu J, Zhang Y, Zhou H (2020) Groundwater chemistry and groundwater quality index incorporating health risk weighting in Dingbian County, Ordos basin of northwest China. Geochemistry 80(4):125607
Yadav M, Singh G, Jadeja RN (2021) Fluoride Contamination in Groundwater, Impacts, and Their Potential Remediation Techniques. In: Madhav S, Singh P (eds) Groundwater Geochemistry: Pollution and Remediation Methods. Wiley Blackwell, Oxford, UK, pp 22–41
Yi FH, Chen L, Yan F (2019) The health risk weighting model in groundwater quality evaluation. Hum Ecol Risk Assess: An Int J 25(8):2089–2097
Zamora-Martínez O, Montaño-Hilario JM, Galindo-Zavala VB, Siebe-Grabach C, Prado-Pano BL (2016) Determinación simultánea de cationes mayoritarios en muestras de agua residual por medio de cromatografía de iones con detección conductimétrica. Revista Internacional De Contaminación Ambiental 32(3):293–301 ((In Spanish))
Zhang Y, Wu J, Xu B (2018) Human health risk assessment of groundwater nitrogen pollution in Jinghui canal irrigation area of the loess region, northwest China. Environ Earth Sci 77(7):273
Zhou Y, Li P, Xue L, Dong Z, Li D (2020) Solute geochemistry and groundwater quality for drinking and irrigation purposes: Acase study in Xinle City North China. Geochemistry 80(4):125609
Acknowledgements
PDR acknowledges financial support from the Support Program for Research and Technological Innovation Projects of DGAPA-UNAM through the proposal PAPIIT-IN101121. OG acknowledges Posgrado en Ciencias del Mar y Limnología (UNAM) and a scholarship from CONACyT (928976) for the doctoral study. The authors are thankful to Olivia Zamora Martínez (laboratory of ion chromatography, LANGEM) for the major ion analysis. The suggestions and comments from both the anonymous reviewers and editor are thankfully acknowledged.
Funding
Supported by Program for Research and Technological Innovation Projects of DGAPA-UNAM through proposal PAPIIT-IN101121.
Author information
Authors and Affiliations
Contributions
Priyadarsi D. Roy: conceptualization, investigation, writing—original draft, review and editing, project administration, supervision and funding acquisition. Oscar Agesandro García-Arriola: investigation and writing—original draft, review and editing. S. Selvam: data curation, investigation, writing—review and editing. Irma Gabriela Vargas-Martínez: investigation and data processing. José Luis Sánchez Zavala: investigation and data processing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Xianliang Yi
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Roy, P.D., García-Arriola, O.A., Selvam, S. et al. Evaluation of water from Lake Coatetelco in central-south Mexico and surrounding groundwater wells for drinking and irrigation, and the possible health risks. Environ Sci Pollut Res 30, 115430–115447 (2023). https://doi.org/10.1007/s11356-023-30488-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-30488-7