Abstract
Electrical and electronic equipment reaching the end of its useful life is currently being disposed of at such an alarmingly high pace that raises environmental concerns. Together with other potentially dangerous compounds, electronic waste contains the rare-earth element gadolinium (Gd), which has already been reported in aquatic systems. Additionally, the vulnerability of aquatic species to this element may also be modified when climate change related factors, like increase in temperature, are taken into consideration. Thus, the present study aimed to evaluate the toxicity of Gd under a scenario of increased temperature in Mytilus galloprovincialis mussels. A multi-biomarker approach and Gd bioaccumulation were assessed in mussels exposed for 28 days to 0 and 10 μg/L of Gd at two temperatures (control – 17 °C; increased – 22 °C). Results confirmed that temperature had a strong influence on the bioaccumulation of Gd. Moreover, mussels exposed to Gd alone reduced their metabolism, possibly to prevent further accumulation, and despite catalase and glutathione S-transferases were activated, cellular damage seen as increased lipid peroxidation was not avoided. Under enhanced temperature, cellular damage in Gd-exposed mussels was even greater, as defense mechanisms were not activated, possibly due to heat stress. In fact, with increased temperature alone, organisms experienced a general metabolic depression, particularly evidenced in defense enzymes, similar to the results obtained under Gd-exposure. Overall, this study underlines the importance of conducting environmental risk assessment taking into consideration anticipated climate change scenarios and exposures to emerging contaminants at relevant environmental concentrations.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
The rapid technological expansion, particularly since the industrial revolution, has resulted in an enhanced social demand for electrical and electronic equipment (EEE). Consequently, an increase in generated electronic waste (e-waste) can be anticipated, due not only to a rise in EEE production, but also to a shorter lifetime of these items, reduced maintenance options, and a fast-paced replacement lifestyle (Perkins et al. 2014). Moreover, since most e-waste is incorrectly managed, disposed of, or even recycled, there is a growing concern about the release of potentially hazardous associated substances into the environment, affecting aquatic systems and their organisms (Abalansa et al. 2021; Forti et al. 2020; Widmer et al. 2005). Among these substances, rare-earth elements (REEs) are valuable and crucial components of EEE. Their importance relies on their unusual optical and magnetic characteristics, as well as their high reactivity, which allows them to be used in numerous applications (Gupta and Bose 1989; Reisman et al. 2013).
Gadolinium (Gd) in particular, is a REE employed in a variety of products, including microwaves, TV tubes, compact discs, alloys, and optical components. (Rogowska et al. 2018). Furthermore, Gd is extensively applied in the medical field for magnetic resonance imaging (MRI) as a contrast agent (Kümmerer and Helmers 2000). Contrast agents are made as chelate molecules, which are resistant to metabolic processes and can be easily excreted (Bellin 2006; Brünjes and Hofmann 2020). Due to this property, it is released unaltered in the aquatic environment and it is difficult to remove in waste-water treatment plants (WWTPs) (Künnemeyer et al. 2009; Migaszewski and Gałuszka 2016). Gd has been reported to occur in aquatic systems mainly because of anthropogenic sources derived from Gd-based contrast agents (Bau and Dulski 1996; Kulaksız and Bau 2011, 2013; Telgmann et al. 2013). That is, effluents from associated WWTPs discharge up to 200-1100 μg/L of Gd into South Africa’s rivers, while environmental concentrations ranged from 0.001-0.004 µg/L in South Africa’s and Germany’s rivers; and up to 0.181 µg/L Gd has been detected in surface waters worldwide (Rogowska et al. 2018). In Finland’s streams, Gd concentrations ranged between 0.032-207 μg/L due to the leaching of REEs from acid sulfate agriculture soils (Åström 2001). Anthropogenic Gd has also been detected in marine systems with concentrations ranging from 0.00064 up to 0.027 μg/L in San Frascisco Bay in the United States of America and from 0.14 to 0.41 μg/L at submarine outfalls from Brazil, associated with high industrialization and medical use (Hatje et al. 2016; Pedreira et al. 2018). Given the prevalence of this REE in the environment, it is essential to know its potential threat to aquatic wildlife.
Gadolinium has also been detected in several aquatic organisms, including freshwater clams Corbicula fluminea and mussels Dreissena rostiformis bugensis at concentrations between 0.004-0.110 μg/g dry weight (DW) and 0.01-0.123 μg/g DW, respectively (Merschel and Bau 2015; Pereto et al. 2020; Perrat et al. 2017) as well as in the marine scallop Pecten maximus at concentrations up to 0.002 μg/g DW (Le Goff et al. 2019). In recent years, this element has also been reported to cause neurotoxicity and alterations in the metabolic capacity and oxidative stress status of marine mussels, Mytilus galloprovincialis (Andrade et al. 2022b; Henriques et al. 2019; Trapasso et al. 2021). Other bivalves have also experienced alterations in the oxidative stress condition after Gd accumulation, including freshwater clams (C. fluminea and Spisula solida) and mussels (D. rostriformis bugensis and D. polymorpha) (Figueiredo et al. 2022; Hanana et al. 2017; Perrat et al. 2017). Moreover, Gd has disrupted the embryonic development of Paracentrotus lividus, Arbacia lixula, Heliocidaris tuberculata and Centrostephanus rodgersii sea urchins (Martino et al. 2017).
In addition to these former impacts, it is likely that abiotic factors may also have an influence on aquatic organisms and change their susceptibility to this element. One of them is climate change, undoubtedly linked to the significant increase in population and industrialization witnessed in the last century. In particular, the global mean surface temperature is expected to increase from 1.0 °C up to 5.7 °C at the end of this century depending on different CO2 emission scenarios (IPCC 2022). This temperature increase has already been shown to trigger a variety of physiological and cellular alterations in marine bivalves (Coppola et al. 2017; Pirone et al. 2019; Rahman et al. 2019; Velez et al. 2017). However, the responses of organisms exposed to combined abiotic factors may differ from when exposed to isolated ones. For instance, a rise in temperature may affect both the chemical speciation and bioavailability of pollutants, as well as the organisms’ susceptibility to pollutants (Noyes et al. 2009). However, in the case of emerging pollutants like REEs, this knowledge is still very scarce. As a first step in toxicity evaluation, biomarkers are often employed to assess the effects of natural and anthropogenic factors (Lomartire et al. 2021), and oxidative stress-related parameters can be regarded as early warning biochemical signals with a potential impact at higher biological levels.
Taking all the above-mentioned factors into account, the present study evaluated the biochemical changes induced in the mussel M. galloprovincialis when exposed to increased temperature and to an environmentally relevant concentration of Gd, but also the interaction between these two stressors. After a standard 28-days experimental assay (e.g., ASTM 2000; USEPA and USACE 1991; 1998), the biochemical responses of mussels associated with metabolism, oxidative stress, and neurotoxicity were investigated aiming to assess the influence of temperature on the toxic effects caused by Gd.
Methodology
Sampling and experimental conditions
Adult specimens of M. galloprovincialis with similar size (length: 54.4 ± 2.3 mm; width: 34.1 ± 2.6 mm) were collected at Mira’s channel in Ria de Aveiro coastal lagoon (considered an uncontaminated site at the northwest of Portugal) during low tide in November 2019. After collection, the organisms were transported to the laboratory and allowed to depurate and acclimate for 14 days. During this period, half of the organisms were under control conditions of temperature (17.0 ± 1.0 °C), salinity (30 ± 1), and pH (8.0 ± 0.1) matching those at the sampling location, whereas the other half were under the same conditions for the first week but gradually acclimated (1 °C increase per day) until reaching the targeted higher temperature (22.0 ± 1.0 °C) in the second week. The temperature was maintained constant in air-conditioned rooms. Organisms were kept in synthetic seawater (deionized water with artificial salt, Tropic Marin® SEA SALT) under constant aeration and a natural photoperiod. The synthetic seawater was renewed twice in the first week and once in the second, with the physical water conditions being re-established. Organisms were fed with AlgaMac Protein Plus (Aquafauna BioMarine®, CA, USA) at a concentration of 150,000 cells/mussel per day after the first five days of arrival and from then on, every other day.
After this acclimation period, mussels were divided into different aquaria filled with 3 L of synthetic seawater, with five individuals per aquarium and three aquaria per treatment (fifteen organisms per treatment). To evaluate Gd stability over a week period under tested treatments, positive controls—two other aquaria per treatment at the same conditions but with no mussels—were also considered. Temperature conditions during the first two weeks of acclimation were maintained over 28 days with the temperature targeted at 17.0 ± 1.0 °C to half of the organisms or 22.0 ± 1.0 °C to the other half. The high temperature of 22.0 °C was within the annual range of average temperatures (13.4–22.9 °C) in Ria de Aveiro (Coelho et al. 2014; Santos et al. 2009; Velez et al. 2015) and the global surface temperature increase projected by the IPCC in 2100 (IPCC 2022). At each experimental temperature, half of the mussels were exposed to 10 μg/L of Gd and the other half in the absence of Gd. This concentration choice was based on former bivalve studies (Hanana et al. 2017; Perrat et al. 2017) and findings from freshwater aquatic systems (Åström 2001; Olías et al. 2005; Rogowska et al. 2018). It is worth noting that literature information on Gd concentration in seawater is limited and although it is generally reported as lower, the existence of local sources of Gd contamination, resulting in higher concentrations cannot be discarded. An initial stock solution of 15 mg/L of Gd was prepared from a 10,000 mg/L commercial solution (Inorganic Ventures) with ultrapure H2O, and a pre-determined volume was added to each aquarium to achieve the desired concentration. A total of four treatments were tested: 1) non-contaminated organisms under control temperature (17 °C); 2) contaminated organisms under control temperature (Gd 17 °C); 3) non-contaminated organisms under increased temperature (22 °C); 4) contaminated organisms under increased temperature (Gd 22 °C).
Throughout the 28-day exposure period, mussels were fed with the AlgaMac preparation three times per week. At the end of each week, synthetic seawater was replaced and conditions (temperature, salinity, pH, and Gd concentration) re-established. To confirm the actual concentrations, samples of seawater were collected from each aquarium immediately after spiking with Gd (i.e., after adding the contaminant to water). Seawater samples were also obtained from the positive controls collected immediately after spiking, and after 24, 48, 72, and 144 h to confirm the element’s stability throughout one-week.
Organisms were sampled from the respective aquaria after the exposure period and frozen immediately with liquid nitrogen before being transported and maintained at -80 °C. No mortality was recorded during the experiment. For biochemical analyses, three frozen mussels per aquarium (nine per treatment) were considered, with the whole tissue manually homogenized with liquid nitrogen using a mortar and pestle. The homogenized tissue correspondent to each mussel was separated into five aliquots of 0.5 g fresh weight (FW) each and stored at -80 °C until further analysis. The remaining tissue of three mussels per treatment (of different aquaria) was freeze-dried and used for Gd quantification.
Gadolinium quantification in seawater and mussel’s soft tissue
For gadolinium quantification, seawater samples were analyzed using an inductively coupled plasma mass spectrometer (Thermo ICP-MS XSeries) equipped with a Burgener nebulizer, after being diluted 20 times and acidified with HNO3 2 % (for obtaining pH < 2).
For soft tissue, 200 mg of freeze-dried samples were weighted in Teflon vessels, followed by 1 mL of HNO3 65 % (v/v), 2 mL of H2O2 and 1 mL of ultrapure H2O, being afterwards transferred to a CEM MARS 5 microwave. The microwave-aided acid digestion was performed by increasing the temperature to 170 °C for 15 min and leaving the samples at this temperature for another additional 5 min. Following the cooling of the vessels, samples were put in polyethylene flasks, which were then filled with ultrapure water to a final level of 25 mL and stored at room temperature until measurement with ICP-MS. Blanks (microwaved vessels without sample), duplicates, and the certified reference material BCR-668 (mussel tissue; 13.0 ± 0.6 μg/Kg of Gd) were used for quality control. In digested blanks, the concentration of Gd was below the methods’ limit of quantification and recovery values from the certified reference material (3 replicates) varied between 95-119 %, confirming the reliable performance of the digestion and quantification protocols.
The LOQ was 0.02 μg/L for both water and tissues (corresponding to the value of the lowest standard concentration used in the calibration curve).
Biological responses: biochemical parameters
Biological responses were evaluated at a whole organism level through the analysis of different biochemical parameters. As indicators of metabolic capacity, the electron transport system (ETS) activity, glycogen (GLY), and total protein (PROT) levels were examined. The activities of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione reductase (GR) were selected as oxidative stress defense mechanisms and the biotransformation enzymes such as glutathione S-transferases (GSTs) and carboxylesterases (CbEs) were considered as additional detoxification mechanisms. Lipid peroxidation (LPO) and protein carbonylation (PC) levels were used to evaluate oxidative damage and inhibition of acetylcholinesterase (AChE) activity as a sign of neurotoxicity. Nine samples per treatment (three mussels per aquaria and three aquaria per treatment) were analyzed in duplicate and considered for each parameter. Samples were extracted through homogenization with a respective buffer (ratio of 1:2, w/v) using TissueLyzer II (Qiagen) set at frequency 20 1/s for 90 sec and centrifugation at 4 °C during 20 min with the supernatant being collected at the end. Most of the parameters (GLY, PROT, SOD, CAT, GR, GSTs, CbEs, PC, and AChE) were extracted using phosphate buffer 50 mmol/L containing 1 mmol/L ethylenediaminetetraacetic acid, 1 % (v/v) Triton X-100 and 1 mmol/L dithiothreitol at pH 7.0 with samples being centrifuged at 10,000 g. For LPO, samples were extracted with 20 % (w/v) trichloroacetic acid and equally centrifuged at 10,000 g. In the case of ETS, samples were extracted with 100 mmol/L Tris-HCl with 15 % (w/v) polyvinylpyrrolidone, 0.153 mmol/L magnesium sulfate and 0.2 % (v/v) Triton X-100 at pH 8.5 and centrifuged at 3,000 g. Each biochemical parameter was measured using the methods outlined in Andrade et al. (2021).
Data analyses
Bioconcentration factor
The bioconcentration factor (BCF) was calculated using the Arnot and Gobas (2006) equation, which was defined as the ratio of total Gd concentration in mussels' tissue to total Gd concentration measured in water after spiking.
Statistical and multivariate analyses
A non-parametric permutational analysis of variance with a one-factor design (Gd concentration and temperature) was used through the PERMANOVA add-on in PRIMER v6 (Anderson et al. 2008) for hypothesis testing of the biochemical data (n=3, corresponding to 3 aquaria) obtained for each parameter measured (ETS, GLY, PROT, SOD, CAT, GR, GSTs, CbEs, LPO, PC, and AChE). The data set was subjected to a square root transformation, and a resemblance matrix was created using the Euclidean distance, since it is sensitive to different units. The Euclidean distance data matrix was assessed individually using type III sums of squares and unrestricted permutation of raw data (9999 permutations). The null hypothesis tested of “mussels exposed to different treatments (17 °C, Gd 17 °C, 22 °C and Gd 22 °C) showed no significant differences among them” was considered. Significant differences (p < 0.05) in the figures were represented by different letters.
An ordination analysis by Principal Coordinates (PCO) was also performed using all the biochemical data. For this, the Euclidean distance similarity matrix was simplified by using the different treatments (17 °C, Gd 17 °C, 22 °C, Gd 22 °C) to calculate the distance between centroids. Vectors relating to biochemical descriptors were placed as new variables superimposed on the PCO graph using the Spearman correlation with a correlation > 75 %, considering its robustness to outliers and non-normally distributed data often included in biochemical data.
Integrated biomarker response
Data obtained from the biochemical parameters were combined using the integrated biomarker response index version 2 (IBRvs2), based on Beliaeff and Burgeot (2002) and modified by Sanchez et al. (2013), to illustrate the overall biochemical responses in mussels under the different treatments. This was achieved by comparing the deviation between biochemical parameters from each treatment (Gd 17 °C; 22 °C; Gd 22 °C) to those determined in the control (17 °C). To minimize variance before calculating the IBR index, a log transformation (Yi) was initially used, where Yi = log (Xi/X0), with Xi standing for individual biomarker data from exposure treatment and X0 for mean reference data from the control treatment. The general mean (μ) and standard deviation (σ) of Yi were considered, and Yi was then standardized using the formula Zi = (Yi - μ) / σ. Then, to establish a basal line centered on 0 and reflect the variations of the parameters along this line, the biomarker deviation index (A = Zi - Z0) was estimated. IBRvs2 was then determined to equal IBRvs2 = |A|.
For the IBRvs2 calculations, all the reported biochemical parameters were considered. The biomarker deviation index findings were shown as a star plot, with the area outside of the REF line (the line that corresponds to 17 °C treatment used as reference data) representing biomarker induction and the area inside the REF line representing biomarker inhibition. In the graphical representation, the obtained IBRvs2 values are also shown with higher values indicating higher biochemical responsiveness of the treated mussels. Values were analyzed in the context of the overall responses given by the final IBRvs2 model.
Results
Gadolinium concentration in seawater and mussel’s tissue
The concentration of Gd in non-contaminated treatments was consistently below the limit of quantification (LOQ < 0.02 μg/L). The concentrations of Gd in the seawater soon after spiking were 9.70 ± 0.19 μg/L at control temperature and 10.10 ± 0.55 μg/L at increased temperature, both close to the targeted nominal concentration (10 μg/L) with a maximum deviation of 3 %.
Water samples aimed to test the stability over time of Gd in seawater were collected from the aquaria at 0, 24, 48, 72, 144, and 168 h after Gd spiking. Under both tested temperatures (Table 1), the coefficients of variations were below 15 % for each treatment, being the concentration of Gd confirmed stable over at least one week and demonstrating that temperature did not influence the bioavailability of this element.
Gadolinium was detected in all mussel tissues, independently of the treatment. Non-contaminated mussels had values of 0.015 ± 0.002 μg/g dry weight (DW) at control temperature and 0.045 ± 0.012 μg/g DW at increased temperature scenario. In Gd-exposed mussels the concentrations were 0.126 ± 0.008 μg/g DW at control temperature (17 °C) and 0.150 ± 0.008 μg/g DW at 22 °C, with significant differences among treatments (Table 2). At increased temperature, the BCF (15.6 ± 0.5 L/Kg) was significantly higher than values found at the control temperature treatment (13.0 ± 0.6 L/Kg) (Table 2).
Biological responses
Metabolic capacity
The ETS activity did not differ significantly among treatments (Fig. 1A).
A: Electron transport system (ETS); B: Glycogen (GLY) content; C: Protein (PROT) content in Mytilus galloprovincialis exposed to different temperatures (17 °C and 22 °C) in the absence and presence (0 and 10 μg/L) of Gd for 28 days. Results are means with standard deviations. Significant differences (p < 0.05) among all four treatments are identified with different lowercase letters. FW represents the mussels’ fresh weight measured in g
Regarding GLY content, mussels exposed to Gd showed about 1.5-1.9 times significantly higher content than non-contaminated ones, regardless of the test temperature (Fig. 1B). Non-contaminated mussels kept at elevated temperature showed between 1.6-2.4 times significantly lower GLY content compared with values observed in the other treatments (Fig. 1B).
The PROT content was shown to be 1.1 times significantly higher in mussels exposed to Gd under increased temperature in comparison to the ones exposed to Gd at 17 °C or non-contaminated mussels at 22 °C (Fig. 1C).
Antioxidant and biotransformation enzymes
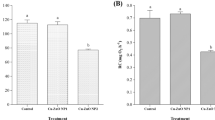
Concerning SOD activity, non-exposed mussels at 22 °C showed 1.7-2.2 times significantly lower activity in comparison to the ones at 17 °C (Fig. 2A).
A: Superoxide dismutase (SOD) activity; B: Catalase (CAT) activity; C: Glutathione reductase (GR) activity in Mytilus galloprovincialis exposed to different temperatures (17 °C and 22 °C) in the absence and presence (0 and 10 μg/L) of Gd for 28 days. Results are means with standard deviations. Significant differences (p < 0.05) among all four treatments are identified with different lowercase letters. FW represents the mussels’ fresh weight measured in g
The CAT activity displayed a similar pattern to SOD, with significant differences (1.4 times at 17 °C and 2.9 times at 22 °C) between contaminated and non-contaminated mussels at both temperatures (Fig. 2B).
The activity of GR showed no significant differences among treatments (Fig. 2C).
Regarding GSTs activity, at control temperature, mussels exposed to Gd exhibited 1.6 times significantly higher activity than non-contaminated ones. Non-contaminated mussels subjected to 22 °C exhibited between 1.6-2.7 times significantly lower GSTs activity compared to values from all the other treatments (Fig. 3A).
A: Glutathione S-transferases (GSTs) activity; B: Carboxylesterases (CbEs) activity in Mytilus galloprovincialis exposed to different temperatures (17 °C and 22 °C) in the absence and presence (0 and 10 μg/L) of Gd for 28 days. Results are means with standard deviations. Significant differences (p < 0.05) among all four treatments are identified with different lowercase letters. FW represents the mussels’ fresh weight measured in g
In terms of CbEs activity, although the pattern of response was similar to GSTs, only non-contaminated mussels at increased temperature had their activity significantly reduced (1.6-2.4 times) compared to the other treatments (Fig. 3B).
Oxidative damage
The LPO levels significantly increased between 1.7 and 4 times in mussels exposed to Gd independently of the temperature tested compared to their non-contaminated counterpart, being those Gd-exposed at 22 °C the ones with the highest LPO levels (Fig. 4A).
A: Lipid peroxidation (LPO) levels; B: Protein carbonylation (PC) levels in Mytilus galloprovincialis exposed to different temperatures (17 °C and 22 °C) in the absence and presence (0 and 10 μg/L) of Gd for 28 days. Results are means with standard deviations. Significant differences (p < 0.05) among all four treatments are identified with different lowercase letters. FW represents the mussels’ fresh weight measured in g
Concerning PC levels, non-contaminated mussels maintained at 22 °C showed significantly lower (1.3-1.4 times) values with respect to the ones under control temperature regardless of Gd presence (Fig. 4B).
Neurotoxicity
The activity of AChE was 1.6-1.7 times significantly lower in non-contaminated mussels exposed to 22 °C in comparison to the ones contaminated at 17 and 22 °C (Fig. 5).
Acetylcholinesterase (AChE) activity in Mytilus galloprovincialis exposed to different temperatures (17 °C and 22 °C) in the absence and presence (0 and 10 μg/L) of Gd for 28 days. Results are means with standard deviations. Significant differences (p < 0.05) among all four treatments are identified with different lowercase letters. FW represents the mussels’ fresh weight measured in g
Multivariate analysis
The first principal component (PCO1) of the PCO analysis (Fig. 6) represented 81 % of the total variance among treatments, it primarily separated contaminated mussels at the lowest temperature (Gd 17 °C) on the positive side of the axis from uncontaminated mussels at the increased temperature on the negative side (22 °C). The second component, PCO2 explained 14.2 % of the total variance distinguishing mussels exposed to Gd and increased temperature (Gd 22 °C) on the positive side from the ones exposed to Gd at control temperature (Gd 17 °C) on the negative side. The biochemical descriptors superimposed on the PCO1 revealed a strong positive correlation (p > 0.75) among all parameters with the exception of PROT and the contaminated organisms under control temperature (Gd 17 °C). This was a direct result of increased activities and levels at this treatment and the lower activities of these parameters in higher temperature (22 °C) which was on the opposite side of the axis. As for PCO2, the organisms under the combination of increased temperature and exposure to Gd, showed an especially strong positive correlation with PROT (p = 0.8) with higher values at this treatment, while also negatively correlating with the antioxidant enzymes, ETS and PC (p = -0.8).
Centroids ordination diagram (PCO) based on the tested conditions and biochemical markers measured in Mytilus galloprovincialis. The following conditions are presented: 17 °C and 22 °C (non-contaminated organisms under different temperatures of 17 °C and 22 °C) and Gd 17 °C and Gd 22 °C (organisms exposed to 10 μg/L of Gd under different temperature of 17 °C and 22 °C). Spearman correlation vectors are superimposed as supplementary variables, namely biochemical data (r > 0.75): ETS = Electron Transport System, PROT = Protein, SOD = Superoxide Dismutase, CAT = Catalase, GSTs = Glutathione S-Transferases, CbEs = Carboxylesterases, LPO = Lipid Peroxidation, PC = Protein Carbonylation, AChE = Acetylcholinesterase
Integrated biomarker response
In IBRvs2, the greater values (IBRvs2 = 15) reached were those seen in uncontaminated mussels at the increased temperature (22 °C), indicating a greater impact at this temperature. The star plot clearly illustrated that this treatment resulted in lower values for all biochemical indicators, which contributed to this high score (Fig. 7). The results also showed that Gd-exposed organisms at both temperatures (Gd 17 °C and Gd 22 °C) scored similar IBRvs2 values (IBRvs2 = 8 and 6, respectively). As determining features, variations in LPO showed to be a common factor being especially relevant at the increased temperature treatment (Table 3). Nevertheless, Gd-exposed organisms at control temperature showed a slightly higher IBRv2 value associated to PROT, GLY and GSTs as these parameters were more determinant (higher inhibition or increase) at this treatment (Table 3). Between Gd treatments at the two tested temperatures, most of the enzymes analyzed showed a greater positive contribution, indicating a higher level of influence, under the control condition (17°C) (Table 3).
Integrated Biomarker Response (IBRvs2) index considering all biochemical parameters used on Mytilus galloprovincialis after exposure to different temperatures (17 °C and 22 °C) in the absence and presence (0 and 10 μg/L) of Gd for 28 days. The biomarker quantified for uncontaminated organisms under 17 °C were considered for the reference line (REF). ETS = Electron Transport System; GLY = Glycogen; PROT = Protein; SOD = Superoxide Dismutase; CAT = Catalase; GR = Glutathione Reductase; GSTs = Glutathione S-Transferases; CbEs = Carboxylesterases; LPO = Lipid Peroxidation; PC = Protein Carbonylation; AChE = Acetylcholinesterase
Discussion
Concentration of Gd in mussels under different temperature scenarios
The present findings demonstrated the capacity of mussels to accumulate Gd in their tissues after 28 days of water-borne exposure to 10 μg/L of Gd. The accumulation of Gd has already been documented in other bivalves. For instance, the freshwater clam Corbicula fluminea and the mussel D. rostriformis bugensis, exposed to Gd concentrations of 1 and 10 μg/L for 28 days, exhibited tissue concentrations ranging from 0.002 to 0.078 μg/g DW (Perrat et al. 2017). The freshwater mussels Dreissena polymorpha exposed to Gd at 10, 50, 250, and 1250 μg/L also for 28 days resulted in Gd tissue concentrations of approximately 0.43, 1.35, 3.18 and 44.97 μg/g wet weight (WW), respectively (Hanana et al. 2017). Comparing the values obtained in the present study with those from other bivalves under similar Gd concentrations and taking in account that values reported in WW are generally lower than in DW, it becomes evident that M. galloprovincialis exhibits a higher accumulation capacity than C. fluminea and D. rostriformis bugensis, but lower than D. polymorpha. This discrepancy may be due not only to the different ionic strengths of the two environments (freshwater vs. seawater) but also to species-dependent factors. The present results are also in accordance with the study conducted by Henriques et al. (2019), in which the same mussel species showed Gd concentrations lower than 0.38 μg/g DW (below the limit of detection) after 28 days of exposure to 15 μg/L. Furthermore, at higher concentrations of 30, 60 and 120 μg/L, the species displayed concentrations of 0.44, 0.81 and 2.5 μg/g DW, respectively, confirming its bioaccumulation capacity. The present study further reflected an increased accumulation potential and BCF values at increased temperature. Similarly, the 28-day study of Mubiana and Blust (2007) with Mytilus edulis described a positive correlation between the accumulation of non-essential metals (cadmium and lead) and temperature. The authors attributed this trend to enhanced solubility and kinetics at higher temperatures. It was also noted that increased temperature enhanced the activity of free metal ions, which are more bioavailable. Moreover, the intensified chemical reactions and diffusion rates further facilitate uptake. In our particular case on determining the influence of temperature on Gd accumulation, it is necessary not to pinpoint a singular determining factor as bioaccumulation can also be influenced by species, tissue and experimental conditions. For instance, and unlike our observations, Figueiredo et al. (2022) recently demonstrated that Gd accumulation in the clam Spisula solida, following 7 days of exposure, was not influenced by water temperature. This discrepancy might stem from the different exposure durations (7 vs. 28 days). Longer exposure periods to increased temperature could induce more changes in mussels’ physiology, affecting Gd uptake rates and mechanisms for regulating its accumulation over time. Considering these divergent responses and the broader context of global warming, it is imperative to comprehensively assess organisms’ responses from the interplay between metals such as Gd and warming.
Metabolic capacity
The difference between energy consumption and available energy reserves serves as a valuable indicator of overall organisms’ health (De Coen and Janssen 2003). Environmental modifications are known to alter organisms’ energy metabolism, reallocating energy resources toward cellular mechanisms to cope with stress rather than supporting critical functions like growth, reproduction, and development (Sokolova et al. 2012). In the current study, electron transport system (ETS) activity, considered a measure of organisms’ metabolic capacity, remained consistent across treatments despite variations in energy reserves. Specifically, Gd-exposed mussels exhibited elevated glycogen (GLY) content in comparison to the uncontaminated counterpart, irrespective of temperature. This may suggest an adaptative effort to conserve energy expenditure in the presence of Gd. A similar trend was observed in M. galloprovincialis exposed to Gd (15-120 μg/L), with increased GLY reserves paralleled with decreased metabolic capacity (Henriques et al. 2019; Trapasso et al. 2021). This strategy was attributed to potential physiological adjustments, including reduced filtration rate and prolonged valve closure, aimed at preventing Gd accumulation. This response was also observed in mussels exposed to the REE lanthanum (La), reinforcing the hypothesis of metabolic maintenance and/or depression under mild stress conditions (Pinto et al. 2019). Still, more research is necessary in bivalves to determine the specific mechanisms adopted to mitigate metal accumulation, such as reductions in filtration and respiration rates. The high PROT reserves exhibited in contaminated mussels at 22 °C suggests an effort to limit protein expenditure (to safeguard functional proteins' vital survival and performance). Simultaneously, an enhanced PROT synthesis may be speculated to possibly repair or replace damaged proteins. The strategy of accumulating energy reserves under stressful conditions has been previously documented in M. galloprovincialis at increased temperature and arsenic presence (Coppola et al. 2018), as well as in response to 17 alpha-ethinylestradiol (EE2) exposure coupled with elevated temperature (Lopes et al. 2022). Nevertheless, while this adaptative mechanism may compromise immediate metabolic performance, its potential long-term consequences on crucial physiological functions like reproduction and growth warrant further consideration.
Antioxidant and biotransformation enzymes
In response to adverse environmental conditions, organisms may experience an accumulation of reactive oxygen species (ROS), prompting the activation of antioxidant defenses to mitigate ROS excess and prevent cellular damage (Regoli and Giuliani 2014). In the present study, at the control temperature, the presence of Gd increased the antioxidant capacity of mussels, suggesting that this metal stimulates the activation of this defense mechanism, possibly due to an elevation in ROS production. The enhanced antioxidant capacity was also formerly observed in M. galloprovincialis exposed to Gd (15-60 μg/L) (Henriques et al. 2019; Trapasso et al. 2021). In the present study, at 22 °C, Gd-exposed mussels exhibited greater antioxidant capacity compared to their non-contaminated counterpart, in line with the results observed at 17 °C between non-contaminated and Gd contaminated mussels. However, at higher temperature (22 °C), both Gd-exposed mussels and non-contaminated ones displayed reduced antioxidant capacity in comparison to those at the lowest ambient temperature (17 °C), indicating that elevated temperature alone may compromise antioxidant defenses. This trend is consistent with former findings by Andrade et al. (2022a) and Morosetti et al. (2020), who demonstrated that bivalves subjected to increased temperature or the combined effect of warming and metal contamination (e.g., La, mercury (Hg) and cerium (Ce) oxide nanoparticles) displayed diminished defense capacity.
Organisms may favor the conversion of a wide range of organic contaminants through phase I (carboxylesterases; CbEs) and/or phase II biotransformation enzymes (Glutathione S-transferases; GSTs), facilitating their excretion from the cells (Regoli and Giuliani 2014; Yan 2014). These enzymes, mainly involved in detoxification processes, are also susceptible to be modulated by metal contamination (Dobritzsch et al. 2020; Hauser-Davis et al. 2021). Similarly to the trend observed in antioxidant responses, mussels exposed to Gd increased their biotransformation capacity (GSTs and CbEs) in comparison to the non-contaminated counterpart. Notably, at 22 °C, both reported activities were lower than those observed at 17 °C for the non-exposed groups. Once more, Gd exposure induced the detoxification capacity in mussels (Gd-exposed vs. non-contaminated mussels), with temperature being a modulating factor (17 °C vs. 22 °C). Previous studies have already demonstrated that elevated temperature can negatively impact GSTs activity. For instance, Morosetti et al. (2020) indicated a reduction in GSTs activity in M. galloprovincialis mussels at increased temperature, either alone or in combination with Hg and Ce oxide nanoparticles. Similarly, Lopes et al. (2022) found decreased GSTs activity in mussels exposed to EE2 under increased temperature. Nevertheless, an increase of GSTs activity (which also possesses antioxidant properties) in the presence of Gd can be attributed to heightened oxidative stress derived from a rise in ROS due to metal exposure. In the same mussel species, Henriques et al. (2019) reported enhanced GSTs activity at various concentrations of Gd (ranging from 15 to 120 μg/L). The same observation was described in the digestive gland of the freshwater mussel D. rostriformis bugensis after 7 days of exposure to 10 μg/L of Gd (Perrat et al. 2017), further supporting the modulation of GSTs in the presence of Gd. Both studies noted that this increase in GSTs activity reached a peak and subsequently declined, potentially associated to a concentration threshold or a 28-day exposure duration, indicating a link to Gd accumulation. A plausible explanation for this phenomenon lies in the ionic radius of Gd, comparable to that of divalent Ca2+. Consequently, Gd may function as a calcium channel blocker (Sherry et al. 2009) as it can block Ca2+-dependent enzymes such as GSTs, among others (Rogosnitzky and Branch 2016). This explanation could rationalize the observed enzymatic response during co-exposure to warming and Gd. Notably, the decline in both GSTs and CbEs activities with increased temperature might compromise mussels’ defensive mechanisms, thereby amplifying their susceptibility to additional stressors.
Oxidative damage
Lipid peroxidation (LPO) and protein carbonylation (PC) can arise when defense mechanisms are unable to effectively counteract ROS (Lushchak 2011; Pisoschi et al. 2021). In the current study, Gd was found to cause LPO, revealing that: i) at the control temperature, the efforts to prevent further Gd accumulation and the activation of antioxidant and biotransformation enzymes were insufficient; ii) at 22 °C, the ineffective defense enzymes and metabolic capacity maintenance contributed to the greatest lipid damage. Similarly, M. galloprovincialis exposed to concentrations ranging from 30 to 120 μg/L of Gd exhibited signs of cellular damage, regardless of the activation of several antioxidant and biotransformation enzymes (Henriques et al. 2019; Trapasso et al. 2021). In clams C. fluminea, LPO occurred after 7 days of exposure to 10 μg/L of Gd. The inefficiency of defense mechanisms leading to elevated LPO levels in M. galloprovincialis has been noted under increased temperature and various contaminants, including Hg (Coppola et al. 2017), diclofenac (Freitas et al. 2019) and rutile titanium dioxide (TiO2, Leite et al. 2020b). Nonetheless, neither Gd nor temperature induced protein oxidation, suggesting that lipids are more susceptible to ROS excess. While PC can result directly from amino acid side chain oxidation involving metals and hydrogen peroxide, it is also possible that PC arising indirectly from lipid-derived aldehydes is more prevalent (Grimsrud et al. 2008). Additionally, cells have mechanisms such as the induction of heat shock proteins (HSPs) in response to metal-induced and heat stress, playing a protective role in maintaining protein structure integrity (see Fabbri et al. 2008). Thus, our observation suggests that lipids are more vulnerable than proteins to Gd exposure and increased temperature. Previous studies have similarly demonstrated that M. galloprovincialis experienced LPO while PC levels remain stable when exposed to various contaminants such as anatase TiO2 (Leite et al. 2020a) and multi-walled carbon nanotubes (Andrade et al. 2019), further supporting this hypothesis.
Neurotoxicity
The inhibition of AChE activity has been mainly used as an indicator of the toxic effects of organophosphorus and other pesticides (English and Webster 2012). However, in marine invertebrates, metals and nanoparticles can also inhibit this activity (Brown et al. 2004; De Marchi et al. 2018; Perić et al. 2017). In the present study, the presence of Gd did not result in the inhibition of AChE activity at any temperature. Also, Henriques et al. (2019) reported unchanged AChE activity under a similar Gd concentration of 15 μg/L in M. galloprovincialis. Yet, at higher Gd concentrations (30, 60 and 120 μg/L), AChE inhibition was observed. Nevertheless, in the present study, the rise of temperature alone led to reduced AChE activity, aligning with decreased metabolic performance (as indicated by low antioxidant and detoxifying enzymatic activities) under this condition. This observation is likely connected to the overall metabolic depression due to heat stress.
Multivariate analysis and integrated biomarker response
The choice of a battery of biochemical parameters to evaluate the impact of chemical contaminants and other environmental stressors is highly recommended as it allows for a comprehensive and general assessment of their effects, providing a more accurate and detailed understanding of their impact on biological systems; however, the selection of multiple parameters can complicate the identification of patterns and difficult the interpretation of their relationships. Additionally, the benefits on the use of whole tissue, as opposed to specific organs, not only enables a broader spectrum of biomarkers to be evaluated and enhances cost-effectiveness, but also yields a more comprehensive and integrated understanding of the organism's overall health. To overcome drawbacks, indexes, such as IBR, aid to comprehensively assess the overall impacts of multiple stressors and simplify data interpretation. In this context, several authors have endorsed the use of the IBR model to evaluate risks in bivalves (e.g., Andrade et al. 2023; Cao et al. 2022; Chahouri et al. 2022; Khan et al. 2021). In the present study, the IBRvs2 model indicated that the rise in temperature led to depression of most biomarkers. This outcome aligns with the notion of metabolic depression of the defense enzymes analyzed, likely serving as a strategy to mitigate heat stress and prevent cellular damage. This can be further supported by the PCO analysis, which indicated stronger negative impacts on most of the biomarkers under this increased temperature. Nevertheless, organisms exposed to Gd regardless of the temperature, exhibited a lower IBRvs2 score, reflecting a contrasting trend in the deployment of defenses mechanisms. Notably, the Gd-only exposure treatment displayed a slightly higher index value, as there was an activation of the defense mechanisms due to Gd presence. In the case of organisms simultaneously exposed to Gd and increased temperature (co-exposure), the activation of defense mechanisms was countered by slight inhibition due to heat stress. In both scenarios, the IBRvs2 index was significantly affected by the elevated LPO values, particularly evident in organisms subjected to increased temperature. This observation supports the notion of Gd’s impact, especially in the context of warming. Both IBRvs2 and PCO models revealed that the defense enzymes contribution was more pronounced for Gd-exposed organisms under control conditions than in the context of increased temperature. This finding highlights how warming influences the activation of these enzymes, ultimately leading to heightened cellular damage.
Conclusions
The consequences of Gd exposure under increased temperature on the mussel M. galloprovincialis were reported in here for the first time, demonstrating its toxicological effects particularly under warming conditions. Mussels exposed to Gd-alone reduced their metabolic performance possibly to prevent further Gd accumulation. However, even though certain defenses were activated, their effectiveness fell short on preventing cellular damage. Furthermore, because the defensive mechanisms were compromised at a higher temperature, the cellular damage was accentuated in Gd-exposed organisms. As a direct consequence of warming, mussels experienced metabolic depression to avoid heat stress and consequent cellular damage. This study highlights the risks that Gd can pose to coastal organisms in a global warming scenario, and consequently, the need to further investigate REEs exposures in the context of climate change-related stressors.
References
Abalansa S, El Mahrad B, Icely J, Newton A (2021) Electronic Waste, an Environmental Problem Exported to Developing Countries: The GOOD, the BAD and the UGLY. Sustainability 13(9):5302. https://doi.org/10.3390/su13095302
Anderson M, Gorley RN, Clarke RK (2008) Permanova+ for Primer: Guide to Software and Statistical Methods. Primer-e, Plymouth, pp 1–214
Andrade M, De Marchi L, Pretti C, Chiellini F, Morelli A, Figueira E, Rocha RJM, Soares AMVM, Freitas R (2019) The impacts of warming on the toxicity of carbon nanotubes in mussels. Mar Environ Res 145:11–21. https://doi.org/10.1016/j.marenvres.2019.01.013
Andrade M, Soares AMVM, Solé M, Pereira E, Freitas R (2021) Salinity influences on the response of Mytilus galloprovincialis to the rare-earth element lanthanum. Sci Total Environ 794:148512. https://doi.org/10.1016/j.scitotenv.2021.148512
Andrade M, Soares AMVM, Solé M, Pereira E, Freitas R (2022a) Do climate change related factors modify the response of Mytilus galloprovincialis to lanthanum? The case of temperature rise. Chemosphere 307(Part 2):135577. https://doi.org/10.1016/j.chemosphere.2022.13557
Andrade M, Soares AMVM, Solé M, Pereira E, Freitas R (2022b) Will climate changes enhance the impacts of e-waste in aquatic systems? Chemosphere 288(Part 1):132264. https://doi.org/10.1016/j.chemosphere.2021.132264
Andrade M, Soares AMVM, Solé M, Pereira E, Freitas R (2023) Threats of Pollutants Derived from Electronic Waste to Marine Bivalves: The Case of the Rare-Earth Element Yttrium. Environ Toxicol Chem 42(1):166–177. https://doi.org/10.1002/etc.5508
Arnot JA, Gobas FA (2006) A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ Rev 14(4):257–297. https://doi.org/10.1139/a06-005
ASTM (2000) Standard Guide for Determination of the Bioaccumulation of Sediment-Associated Contaminants by Benthic Invertebrates. ASTM E1688-19. West Conshohocken, PA
Åström M (2001) Abundance and fractionation patterns of rare earth elements in streams affected by acid sulphate soils. Chem Geol 175:249–258. https://doi.org/10.1016/S0009-2541(00)00294-1
Bau M, Dulski P (1996) Anthropogenic origin of positive gadolinium anomalies in river waters. Earth Planet Sci Lett 143:245–255. https://doi.org/10.1016/0012-821X(96)00127-6
Beliaeff B, Burgeot T (2002) Integrated biomarker response: a useful tool for ecological risk assessment. Environ Toxicol Chem 21:1316–1322. https://doi.org/10.1002/etc.5620210629
Bellin MF (2006) MR contrast agents, the old and the new. Eur J Radiol 60(3):314–323. https://doi.org/10.1016/j.ejrad.2006.06.021
Brown RJ, Galloway TS, Lowe D, Browne MA, Dissanayake A, Jones MB, Depledge MH (2004) Differential sensitivity of three marine invertebrates to copper assessed using multiple biomarkers. Aquat Toxicol 66:267–278. https://doi.org/10.1016/j.aquatox.2003.10.001
Brünjes R, Hofmann T (2020) Anthropogenic gadolinium in freshwater and drinking water systems. Water Res 182:115966. https://doi.org/10.1016/j.watres.2020.115966
Cao R, Zhang Y, Ju Y, Wang W, Xi C, Liu W, Liu K (2022) Exacerbation of copper pollution toxicity from ocean acidification: A comparative analysis of two bivalve species with distinct sensitivities. Environ Pollut 293:118525. https://doi.org/10.1016/j.envpol.2021.118525
Chahouri A, Agnaou M, Hanaoui ME, Yacoubi B, Moukrim A, Banaoui A (2022) Assessment of seasonal and spatial variation responses of integrated biomarkers in two marine sentinel bivalve species: Agadir Bay (Southern of Morocco). Mar Pollut Bull 174:113179. https://doi.org/10.1016/j.marpolbul.2021.113179
Coelho JP, Pato P, Henriques B et al (2014) Long-term monitoring of a mercury contaminated estuary (Ria de Aveiro, Portugal): the effect of weather events and management in mercury transport. Hydrol Process 28:352–360. https://doi.org/10.1002/hyp.9585
Coppola F, Almeida Â, Henriques B, Soares AMVM, Figueira E, Pereira E, Freitas R (2017) Biochemical impacts of Hg in Mytilus galloprovincialis under present and predicted warming scenarios. Sci Total Environ 601-602:1129–1138. https://doi.org/10.1016/j.scitotenv.2017.05.201
Coppola, F., Almeida, Â., Henriques, B., Soares, A.M.V.M, Figueira, E., Pereira, E., Freitas, R., 2018. Biochemical responses and accumulation patterns of Mytilus galloprovincialis exposed to thermal stress and Arsenic contamination. Ecotoxicol Environ Saf 147, 954-962. https://doi.org/10.1016/j.ecoenv.2017.09.051.
De Coen WM, Janssen CR (2003) The missing biomarker link: relationships between effects on the cellular energy allocation biomarker of toxicant-stressed Daphnia magna and corresponding population characteristics. Environ Toxicol Chem 22:1632–1641. https://doi.org/10.1002/etc.5620220727
De Marchi L, Neto V, Pretti C, Figueira E, Chiellini F, Morelli A, Soares AMVM, Freitas R (2018) Toxic effects of multi-walled carbon nanotubes on bivalves: comparison between functionalized and nonfunctionalized nanoparticles. Sci Total Environ 622(623):1532–1542. https://doi.org/10.1016/j.scitotenv.2017.10.031
Dobritzsch D, Grancharov K, Hermsen C, Krauss G-J, Schaumlöffel D (2020) Inhibitory effect of metals on animal and plant glutathione transferases. J Trace Elem Med Biol 57:48–56. https://doi.org/10.1016/j.jtemb.2019.09.007
English BA, Webster AA (2012) Chapter 132 – acetylcholinesterase and its inhibitors. D. Robertson, I. Biaggioni, G. Burnstock, P.A. Low, J.F.R. Paton (Eds.), Primer on the Autonomic Nervous System (Third edition), Academic Press (2012), pp. 631-633
Fabbri E, Valbonesi P, Franzellitti S (2008) HSP expression in bivalves. Invertebr Surviv J 5(2):135–161
Figueiredo C, Grilo TF, Oliveira R, Ferreira IJ, Gil F, Lopes C, Brito P, Ré P, Caetano M, Diniz M, Raimundo J (2022) Gadolinium ecotoxicity is enhanced in a warmer and acidified changing ocean as shown by the surf clam Spisula solida through a multibiomarker approach. Aquat Toxicol 253:106346. https://doi.org/10.1016/j.aquatox.2022.106346
Forti V, Baldé CP, Kuehr R, Bel G (2020) The Global E-waste Monitor 2020: Quantities, flows and the circular economy potential. United Nations University (UNU)/United Nations Institute for Training and Research (UNITAR) – co-hosted SCYCLE Programme, International Telecommunication Union (ITU) & International Solid Waste Association (ISWA), Bonn/Geneva/Rotterdam
Freitas R, Coppola F, Costa S, Pretti C, Intorre L, Meucci V, Soares AMVM, Solé M (2019) The influence of temperature on the effects induced by Triclosan and Diclofenac in mussels. Sci Total Environ 663:992–999. https://doi.org/10.1016/j.scitotenv.2019.01.189
Grimsrud PA, Xie H, Griffin TJ, Bernlohr DA (2008) Oxidative Stress and Covalent Modification of Protein with Bioactive Aldehydes. J Biol Chem 283(32):21837–21841. https://doi.org/10.1074/jbc.R700019200
Gupta CK, Bose DK (1989) Process technology — rare and refractory metals. Bull Mar Sci 12:381–405. https://doi.org/10.1007/BF02747144
Hanana H, Turcotte P, André C, Gagnon C, Gagné F (2017) Comparative study of the effects of gadolinium chloride and gadolinium – based magnetic resonance imaging contrast agent on freshwater mussel, Dreissena polymorpha. Chemosphere 181:197–207. https://doi.org/10.1016/j.chemosphere.2017.04.073
Hatje V, Bruland KW, Flegal AR (2016) Increases in anthropogenic gadolinium anomalies and rare earth element concentrations in San Francisco Bay over a 20 year record. Environ Sci Technol 50:4159–4168. https://doi.org/10.1021/acs.est.5b04322
Hauser-Davis RA, Lavradas RT, Monteiro F, Rocha RCC, Bastos FF, Araújo GF, Júnior SFS, Bordon IC, Correia FV, Saggioro EM, Saint’Pierre TD, Godoy JM (2021) Biochemical metal accumulation effects and metalloprotein metal detoxification in environmentally exposed tropical Perna perna mussels. Ecotoxicol Environ Saf 208:111589. https://doi.org/10.1016/j.ecoenv.2020.111589
Henriques B, Coppola F, Monteiro R, Pinto J, Viana T, Pretti C, Soares A, Freitas R, Pereira E (2019) Toxicological assessment of anthropogenic Gadolinium in seawater: Biochemical effects in mussels Mytilus galloprovincialis. Sci Total Environ 664:626–634. https://doi.org/10.1016/j.scitotenv.2019.01.341
IPCC, 2022. Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. H.-O. Pörtner, D.C. Roberts, M. Tignor, E.S. Poloczanska, K. Mintenbeck, A. Alegría, M. Craig, S. Langsdorf, S. Löschke, V. Möller, A. Okem, B. Rama (eds.). Cambridge University Press. Cambridge University Press, Cambridge, UK and New York, NY, USA, pp. 3056. https://doi.org/10.1017/9781009325844
Khan FU, Chen H, Gu H, Wang T, Dupont S, Kong H, Shang Y, Wang X, Lu W, Hu M, Wang Y (2021) Antioxidant responses of the mussel Mytilus coruscus co-exposed to ocean acidification, hypoxia and warming. Mar Pollut Bull 162:111869. https://doi.org/10.1016/j.marpolbul.2020.111869
Kulaksız S, Bau M (2011) Anthropogenic gadolinium as a microcontaminant in tap water used as drinking water in urban areas and megacities. Appl Geochem 26:1877–1885. https://doi.org/10.1016/j.apgeochem.2011.06.011
Kulaksız S, Bau M (2013) Anthropogenic dissolved and colloid/nanoparticle-bound samarium, lanthanum and gadolinium in the Rhine River and the impending destruction of the natural rare earth element distribution in rivers. Earth Planet Sci Lett 362:43–50. https://doi.org/10.1016/j.epsl.2012.11.033
Kümmerer K, Helmers E (2000) Hospital effluents as a source of gadolinium in the aquatic environment. Environ Sci Technol 34(4):573–577. https://doi.org/10.1021/es990633h
Künnemeyer J, Terborg L, Meermann B, Brauckmann C, Möller I, Scheffer A, Karst U (2009) Speciation Analysis of Gadolinium Chelates in Hospital Effluents and Wastewater Treatment Plant Sewage by a Novel HILIC/ICP-MS Method. Environ Sci Technol 43(8):2884–2890. https://doi.org/10.1021/es803278n
Le Goff S, Barrat J, Chauvaud L, Paulet Y, Gueguen B, Salem DB (2019) Compound-specific recording of gadolinium pollution in coastal waters by great scallop. Sci Rep Nat 9:8015. https://doi.org/10.1038/s41598-019-44539-y
Leite C, Coppola F, Monteiro R, Russo T, Polese G, Lourenço MAO, Silva MRF, Ferreira P, Soares AMVM, Pereira E, Freitas R (2020a) Biochemical and histopathological impacts of rutile and anatase (TiO2 forms) in Mytilus galloprovincialis. Sci Total Environ 719:134886. https://doi.org/10.1016/j.scitotenv.2019.134886
Leite C, Coppola F, Monteiro R, Russo T, Polese G, Silva MRF, Lourenço MAO, Ferreira P, Soares AMVM, Pereira E, Freitas R (2020b) Toxic impacts of rutile titanium dioxide in Mytilus galloprovincialis exposed to warming conditions. Chemosphere 252:126563. https://doi.org/10.1016/j.chemosphere.2020.126563
Lomartire S, Marques JC, Gonçalves AMM (2021) Biomarkers based tools to assess environmental and chemical stressors in aquatic systems. Ecol Indic 122:107207. https://doi.org/10.1016/j.ecolind.2020.107207
Lopes J, Coppola F, Soares AMVM, Meucci V, Pretti C, Polese G, Freitas R (2022) How temperature rise will influence the toxic impacts of 17 α-ethinylestradiol in Mytilus galloprovincialis? Environ. Res. 204 (Part C), 112279. https://doi.org/10.1016/j.envres.2021.112279.
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101(1):13–30. https://doi.org/10.1016/j.aquatox.2010.10.006
Martino C, Bonaventura R, Byrne M, Roccheri M, Matranga V (2017) Effects of exposure to gadolinium on the development of geographically and phylogenetically distant sea urchins species. Mar Environ Res 128:98–106. https://doi.org/10.1016/j.marenvres.2016.06.001
Merschel G, Bau M (2015) Rare earth elements in the aragonitic shell of freshwater mussel Corbicula fluminea and the bioavailability of anthropogenic lanthanum, samarium and gadolinium in river water. Sci Total Environ 533:91–101. https://doi.org/10.1016/j.scitotenv.2015.06.042
Migaszewski ZM, Gałuszka A (2016) The use of gadolinium and europium concentrations as contaminant tracers in the Nida River watershed in south-central Poland. Geol Q 60:67–76. https://doi.org/10.7306/gq.1241
Morosetti, B., Freitas, R., Pereira, E., Hamza, H., Andrade, M., Coppola, F., Maggioni, Della Torre, C., 2020. Will temperature rise change the biochemical alterations induced in Mytilus galloprovincialis by cerium oxide nanoparticles and mercury? Environ Res 188, 109778. https://doi.org/10.1016/j.envres.2020.109778.
Mubiana VK, Blust R (2007) Effects of temperature on scope for growth and accumulation of Cd, Co, Cu and Pb by the marine bivalve Mytilus edulis. Mar Environ Res 63(3):219–235. https://doi.org/10.1016/j.marenvres.2006.08.005
Noyes PD, McElwee MK, Miller HD, Clark BW, Van Tiem LA, Walcott KC, Erwin KN, Levin ED (2009) The toxicology of climate change: Environmental contaminants in a warming world. Environ Int 35(6):971–986. https://doi.org/10.1016/j.envint.2009.02.006
Olías M, Cerón JC, Fernández I, De la Rosa J (2005) Distribution of rare earth elements in an alluvial aquifer affected by acid mine drainage: the Guadiamar aquifer (SW Spain). Environ Pollut 135(1):53–64. https://doi.org/10.1016/j.envpol.2004.10.014
Pedreira RMA, Pahnke K, Böning P, Hatje V (2018) Tracking hospital effluent-derived gadolinium in Atlantic coastal waters off Brazil. Water Res 145:62–72. https://doi.org/10.1016/j.watres.2018.08.005
Pereto C, Coynel A, Lerat-Hardy A, Gourves PY, Schäfer J, Baudrimont M (2020) Corbicula fluminea: a sentinel species for urban Rare Earth Element origin. Sci Total Environ 732:138552. https://doi.org/10.1016/j.scitotenv.2020.138552
Perić L, Nerlović V, Žurga P, Žilić L, Ramšak A (2017) Variations of biomarkers response in mussels Mytilus galloprovincialis to low, moderate and high concentrations of organic chemicals and metals. Chemosphere 174:554–562. https://doi.org/10.1016/j.chemosphere.2017.01.138
Perkins DN, Drisse MNB, Nxele T, Sly PD (2014) E-Waste: A Global Hazard. Ann Glob Health 80(4):286–295. https://doi.org/10.1016/j.aogh.2014.10.001
Perrat E, Parant M, Py J-S, Rosin C, Cossu-Leguille C (2017) Bioaccumulation of gadolinium in freshwater bivalves. Environ Sci Pollut Res 24:12405–12415. https://doi.org/10.1007/s11356-017-8869-9
Pinto J, Costa M, Leite C, Borges C, Coppola F, Henriques B, Monteiro R, Russo T, Di Cosmo A, Soares AMVM, Polese G, Pereira E, Freitas R (2019) Aquat Toxicol 211:181–192. https://doi.org/10.1016/j.aquatox.2019.03.017
Pirone G, Coppola F, Pretti C, Soares AMVM, Solé M, Freitas R (2019) The effect of temperature on Triclosan and Lead exposed mussels. Comp Biochem Physiol B Biochem Mol Biol 232:42–50. https://doi.org/10.1016/j.cbpb.2019.02.007
Pisoschi AM, Pop A, Iordache F, Stanca L, Predoi G, Serban AI (2021) Oxidative stress mitigation by antioxidants - An overview on their chemistry and influences on health status. Eur J Med Chem 209:112891. https://doi.org/10.1016/j.ejmech.2020.112891
Rahman MA, Henderson S, Miller-Ezzy P, Li XX, Qin JG (2019) Immune response to temperature stress in three bivalve species: Pacific oyster Crassostrea gigas, Mediterranean mussel Mytilus galloprovincialis and mud cockle Katelysia rhytiphora. Fish Shellfish Immunol 86:868–874. https://doi.org/10.1016/j.fsi.2018.12.017
Regoli F, Giuliani ME (2014) Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar Environ Res 93:106–117. https://doi.org/10.1016/j.marenvres.2013.07.006
Reisman D, Weber R, Mckernan J, Northeim C (2013) Rare earth elements: a review of production, processing, recycling, and associated environmental issues. United States Environmental Protection Agency, Cincinnati, OH EPA/600/R-12/572
Rogosnitzky M, Branch S (2016) Gadolinium-based contrast agent toxicity: a review of known and proposed mechanisms. BioMetals 29:365–376. https://doi.org/10.1007/s10534-016-9931-7
Rogowska J, Olkowska E, Ratajczyk W, Wolska L (2018) Gadolinium as a new emerging contaminant of aquatic environments. Environ Toxicol Chem 37(6):1523–1534. https://doi.org/10.1002/etc.4116
Sanchez W, Burgeot T, Porcher J-M (2013) A novel “Integrated Biomarker Response” calculation based on reference deviation concept. Environ Sci Pollut Res 20:2721–2725. https://doi.org/10.1007/s11356-012-1359-1
Santos AL, Mendes C, Gomes NCM, Henriques I, Correia A, Almeida A, Cunha  (2009) Short-term variability of abundance, diversity and activity of estuarine bacterioneuston and bacterioplankton. J Plankton Res 31:1545–1555. https://doi.org/10.1093/plankt/fbp083
Sherry AD, Caravan P, Lenkinski RE (2009) Primer on gadolinium chemistry. J Magn Reason Imaging 30:1240–1248. https://doi.org/10.1002/jmri.21966
Sokolova IM, Frederich M, Bagwe R, Lannig G, Sukhotin AA (2012) Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar Environ Res 79:1–15. https://doi.org/10.1016/j.marenvres.2012.04.003
Telgmann L, Sperling M, Karst U (2013) Determination of gadolinium-based MRI contrast agents in biological and environmental samples: a review. Anal Chim Acta 764:1–16. https://doi.org/10.1016/j.aca.2012.12.007
Trapasso G, Coppola F, Queirós V, Henriques B, Soares AMVM, Pereira E, Chiesa S, Freitas R (2021) How Ulva lactuca can influence the impacts induced by the rare earth element Gadolinium in Mytilus galloprovincialis? The role of macroalgae in water safety towards marine wildlife. Ecotoxicol Environ Saf 215:112101. https://doi.org/10.1016/j.ecoenv.2021.112101
Velez C, Figueira E, Soares AMVM, Freitas R (2015) Spatial distribution and bioaccumulation patterns in three clam populations from a low contaminated ecosystem. Estuar Coast Shelf Sci 155:114–125. https://doi.org/10.1016/j.ecss.2015.01.004
Velez C, Figueira E, Soares AMVM, Freitas R (2017) Effects of seawater temperature increase on economically relevant native and introduced clam species. Mar Environ Res 123:62–70. https://doi.org/10.1016/j.marenvres.2016.11.010
USEPA, USACE (1991) Evaluation of Dredged Material Proposed for Ocean Disposal–Testing Manual. Water: Dredged Material Management EPA 503/8-91/001. EP
USEPA, USACE, 1998. Evaluation of material proposed for discharge to waters of the U.S. –Testing Manual (Inland Testing Manual). EPA 823/B-98/O04. Washington,DC: U.S. Environmental Protection Agency
Widmer R, Oswald-Krapf H, Sinha-Khetriwal D, Schnellmann M, Böni H (2005) Global perspectives on e-waste. Environ Impact Assess Rev 25(5):436–458. https://doi.org/10.1016/j.eiar.2005.04.001
Yan B (2014) Carboxylesterases. Philip Wexler (Ed), Encyclopedia of Toxicology (Third Edition), Academic Press, pp. 695-698. https://doi.org/10.1016/B978-0-12-386454-3.00109-3
Acknowledgements
Madalena Andrade benefited from PhD grant (SFRH/BD/143422/2019) funded by National Funds through the Portuguese Science Foundation (Fundação para a Ciência e a Tecnologia, FCT). Thanks are due for the financial support to REQUIMTE and CESAM (UIDB/50006/2020 and UIDP/50017/2020+UIDB/50017/2020+LA/P/0094/2020, respectively). The authors also acknowledge to the RED RIESCOS - Evaluación de los Efectos de los Contaminantes Emergentes en Organismos Acuáticos y sobre la Salud Humana, from the Programa Iberoamericano de Ciencia y Tecnología para el Desarrollo (CYTED) with reference 419RT0578 and the institutional support of the ‘Severo Ochoa Centre of Excellence’ accreditation (CEX2019-000928-S).
Funding
Open access funding provided by FCT|FCCN (b-on). Madalena Andrade benefited from PhD grant (SFRH/BD/143422/2019) funded by National Funds through the Portuguese Science Foundation (Fundação para a Ciência e a Tecnologia, FCT). Thanks are due for the financial support to REQUIMTE and CESAM (UIDB/50006/2020 and UIDP/50017/2020+UIDB/50017/2020+LA/P/0094/2020, respectively). The authors also acknowledge to the RED RIESCOS - Evaluación de los Efectos de los Contaminantes Emergentes en Organismos Acuáticos y sobre la Salud Humana, from the Programa Iberoamericano de Ciencia y Tecnología para el Desarrollo (CYTED) with reference 419RT0578 and the institutional support of the ‘Severo Ochoa Centre of Excellence’ accreditation (CEX2019-000928-S).
Author information
Authors and Affiliations
Contributions
Madalena Andrade; Writing - Original Draft, Investigation, Formal analysis. Amadeu M.V.M. Soares; Funding acquisition. Montserrat Solé; Supervision, Writing – review & editing. Eduarda Pereira: Conceptualization, Supervision, Funding acquisition, Writing – review & editing. Rosa Freitas: Conceptualization, Supervision, Writing – review & editing, Funding acquisition.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
All the authors have approved the manuscript for publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Cinta Porte
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Andrade, M., Soares, A.M.V.M., Solé, M. et al. Gadolinium accumulation and its biochemical effects in Mytilus galloprovincialis under a scenario of global warming. Environ Sci Pollut Res 30, 116120–116133 (2023). https://doi.org/10.1007/s11356-023-30439-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-30439-2