Abstract
Innovative technologies are needed to enhance access to clean water and avoid waterborne diseases. We investigated the performance of cold atmospheric plasma (CAP), a clean and sustainable approach for microbial inactivation and total organic carbon (TOC) degradation in environmental water. Water matrices played a crucial role in the performance of CAP efficacy; for example, complete removal of ɸX174 from dH2O required 1 min of treatment, while ɸX174 reductions of ~ 2log10 and 4log10 were obtained after 10 min of CAP exposure in river water and wastewater samples, respectively. Similarly, after 10 min of CAP treatment, bacterial concentrations decreased by 3 log10 and 4 log10, in river and wastewater samples, respectively. In contrast, after 30 s of contact time, a 4 log10 reduction of bacteria was accomplished in dH2O. Complete removal of Acanthamoeba from dH2O was found after 30 min of CAP treatment, whereas it was not removed from surface water or wastewater at the same exposure time. Additionally, the approach successfully reduced TOC, and the degradation kinetics of TOC were represented by pseudo-first-order. CAP showed higher rates of TOC degradation in the final effluent of the wastewater treatment plant compared to surface water. The difference in CAP performance between river water and wastewater could be attributed to the bulk structure of humic acids in river water compared to small organic byproducts in the final effluent of WWTP. Overall, the findings reported here support the idea that CAP holds promise as a sustainable solution for controlling pathogens, removing organic water pollution, and integrating with traditional purification processes. Low-cost systems may advance CAP technology and increase its widespread use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water contamination has become an important global concern due to population growth, climate change, industrial expansion, and agricultural expansion. Water pollution and poor sanitation have been associated with the transmission of diseases such as cholera, diarrhea, hepatitis A, typhoid, and poliomyelitis (WHO 2022a).
According to the WHO, 829,000 people die every year from diarrheal disease as a result of poor hand hygiene, poor sanitation, and unsafe drinking water. However, diarrhea is generally avoidable, and if these risk factors were managed, 297,000 infant deaths under the age of five every year could be averted. The discharge of untreated and/or poorly treated wastewater typically has an impact on downstream waterbodies and pollutes groundwater (WHO 2022b).
Conventional treatment methods such as chlorination, UV irradiation, ozonation, and filtration have been widely researched and proven to be successful in removing a wide spectrum of microorganisms (Abdo et al. 2022; Hamza and Abd-Elmaksoud 2023; Shi et al. 2021). These approaches are extensively acknowledged and applied by water treatment plants and have well-established regulatory criteria. However, the technologies used to treat water currently have some limitations. For example, chlorine can react with organic substances, resulting in the production of harmful reactive chlorinated organic compounds for human health. The operation and maintenance of other disinfection agents, including ozone and ultraviolet irradiation, are complex and cannot provide long-term protection when it comes to distribution systems. On the other hand, conventional technologies cannot be applied to treat persistent organic compounds that are soluble in water like pharmaceuticals, halogenated hydrocarbons, aromatic chemicals, and pesticides (Arbuckle et al. 2002).
Advanced oxidation processes (AOPs), including photocatalysis, ozonation, electrochemical treatments, non-thermal processes, Fenton, and photo-Fenton processes, represent emerging and innovative methods for water decontamination. The effectiveness of AOPs in reducing complex and non-biodegradable compounds has attracted significant research attention. In principle, these processes involve introducing electrical, radiation, and/or chemical energy into the reaction zone, which results in the degradation of complex chemicals (Graumans et al. 2020; Mahmoud and El-Liethy 2022). Also, AOPs can be used along with conventional methods such as filtering to remove pollutants that are resistant to chlorine (e.g., bacterial spores and protozoa).
CAP is considered one of the most effective AOP which generates high densities of reactive oxygen species (ROS) and reactive nitrogen species (RNS) due to the high energetic electrons in CAP (Abdel-Wahed et al. 2022; Adamovich et al. 2022). Several reactive species may be formed and categorized into two groups: long-lived species like H2O2, NO2, and NO3, and short-lived species like O, ·NO, ·OH, O2·−, and 1O2. Reactive species have variable reaction rate constants in the gas and liquid phase, and they can be transformed into each other (Fridman et al. 2008). Reactive oxygen and nitrogen species (RONS) are the main components in CAP responsible for the anti-microbial effect, whereas UV photons have just a minimal impact (Nicol et al. 2020).
Generally, plasma exhibits quasineutrality, with relatively similar electrons and ions densities but different temperatures and is typically classified as thermal or non-thermal plasma. Thermal plasma is in thermal equilibrium, which indicates the temperatures of electrons and heavy particles are rather constant, which typically vary in the thousands of Kelvins. CAP are partly ionized gases with electron temperatures in the tens of thousands of degrees Celsius, whereas heavier particles (ions and neutrals) have substantially lower temperatures (Brany et al. 2020; El-Kalliny et al. 2021).
A number of variables can influence the effectiveness of a CAP treatment, such as the gas type, gas flow rate, water flow rate, voltage, frequency, and mode of exposure. Furthermore, internal factors such as microbial species and the structure of microbial cells have a significant impact on reaction mechanisms, kinetics, and performance, as do external factors such as pH, conductivity, and relative humidity (Murugesan et al. 2020a; Murugesan et al. 2020b).
Introducing CAP into the industry is of great importance, but a lot of challenges should be solved before achieving that. The ideal plasma system that can cover all the above-mentioned critical variables to implement this technology in the industry on a large scale has not been achieved yet. However, many developments based on pollutant degradation and consumed energy have been performed recently to achieve this. Many important perspectives should be considered for scaling up this technology such as maximization in the production of available reactive species, the large contact area between plasma and pollutants, and reaching the optimum treatment conditions with respect to energy and pollutant degradation efficiency. The sustainability of the treatment process indeed depends on the operation cost in terms of energy consumption, and recently a lot of efforts have been dedicated to decrease that cost, making plasma technology promising in water treatment. For example, Gerrity et al., using a pilot-scale cold plasma, reported that the electrical energy per order (EEO), defined as the amount of electrical energy in kWh needed to reduce the concentration of a contaminant by one order of magnitude in 1 m3 of polluted water, is less than 10 in the case of cold plasma treatment, while it is more than 100 in the case of biodegradation photocatalysis treatment. The energy supplied in any plasma system can be expressed by the applied voltage, power, and frequency, where cold plasma can be ignited with a wide range of frequency spectrum such as DC, AC (as in the case of the present study and many others) till nanosecond pulsed systems, which affects by its role the energy efficiency. For example, a significant enhancement in energy efficiency from 1 to 3 order of magnitude was observed when changing the ignition method from sinusoidal and/or microsecond pulsed high voltage to nanosecond pulsed systems. Accordingly, more efforts should be made to scale up plasma systems for water treatment, and more reference systems should be designed to help researchers around the world make a right comparison between systems and to bring plasma technology into water treatment plants (Aggelopoulos 2022; Gerrity et al. 2010).
Different studies have investigated the disinfection properties of CAP against both Gram-positive and Gram-negative bacteria. (Abreu et al. 2013; Younis et al. 2020). The reactive species can either interact with the bacterial outer membrane or quickly enter the cells and cause rupture of the bacterial cells (Abdel-Wahed et al. 2022). Additionally, there is a different sensitivity to CAP treatment between Gram-negative and Gram-positive bacteria. Typically, Gram-positive bacteria are more resistant to CAP compared to Gram-negative bacteria (Mai-Prochnow et al. 2014). CAP has also been proven to have the potential to treat bacteria in biofilms; however, more CAP exposure time is required for the complete removal of bacterial biofilm (Mai-Prochnow et al. 2014; Rao et al. 2020).
On the other hand, limited data on viral removal by CAP is available, and most studies have been conducted using dH2O. Norovirus, adenovirus, and hepatitis A viruses are common enteric viruses that have been treated with CAP (Abdel-Wahed et al. 2022; Aboubakr et al. 2016; Chen et al. 2020; Guo et al. 2018). CAP has successfully treated enteric viruses in aqueous solutions as well as in other liquid media. Also, CAP was applied to bacteriophages such as MS2, T4, and ɸX174 either through direct treatment or indirect exposure (Guo et al. 2018). All three types of bacteriophages were effectively inactivated using both methods. However, a shorter treatment time was sufficient to inactivate ɸX174 and MS2 compared to T4.
It is worth noting that there is no data available on the effect of CAP on Acanthamoeba. Whereas, others have tested the effect of CAP on protozoa using Cryptosporidium parvum as a model on cilantro-contaminated leaves (Craighead et al. 2020).
Here, we examined the effect of CAP on bacteria, viruses, and protozoa in different environmental water matrices. Additionally, the efficacy of CAP treatment was determined by the removal of total organic carbon (TOC) from the environmental samples. We used a high voltage AC power supply as a power source of our CAP with frequency in range of kHz as it will be discussed in detail in the manuscript, there are many CAP sources based on AC-driven sources with different configurations such as one atmosphere uniform glow discharge plasma (OAUGDP) (Park et al. 2012), the parallel plate reactor (Montie et al. 2000), the remote exposure reactor (Park et al. 2012), the two-rod configuration (Reece Roth et al. 2005), plasma jet with the powered electrode at the center of the tube (Perni et al. 2007), plasma jet with ring-shaped powered electrode (Deng et al. 2007), plasma jet with floating electrode inside a tube (Park et al. 2011), pin-to-pin electrodes (Sun et al. 2023), and pin-to-line (as in the case of our system configuration) (Abdel-Wahed et al. 2022; El-Kalliny et al. 2021; Park and Cha 2023).
Material and methods
The pin cold atmospheric plasma system
A plasma reactor with a high-voltage AC power supply capable of supplying an input voltage ranging from 0 to 18 V and an output voltage of up to 10 kV was used. The positive terminal of the power supply was connected to the pin electrode above the water surface, while the negative terminal was connected to the platinum metal electrode immersed in the water sample. The water sample of 10 mL was treated in a Petri dish (inner diameter of 5 cm, water thickness of 3.6 mm, and gap distance between the surface of the water and plasma of 1 mm). The Petri dish was placed in contact with ambient air. To measure the plasma voltage during treatment, the plasma electrodes were outfitted with a high-voltage probe (P5101) with a division ratio of 1/1000. Moreover, the plasma current was obtained from the measurements of the voltage across a high-voltage resistor 25 Ω, which also was used to protect the electric circuit from high short-circuit current, and then divided by 25 Ω to get the current value (Allam et al. 2016), see Fig. 1, and an oscilloscope (25 MHz) was used to observe the electrical characteristics; more details about the reactor setup can be found in Fig. 1.
Water samples
River Nile water samples were collected from the mainstream of the river in a one-liter wide-mouth sterile bottle. The final wastewater effluent sample was collected from the Zenin wastewater treatment plant (WWTP), Cairo. The collected samples were transferred in an icebox to the laboratory within 2 h. Some physicochemical parameters, including pH, electrical conductivity (EC), and total dissolved solids (TDS), were determined in the collected samples before and after CAP treatment according to APHA (2017). Cationic and anionic species were also measured by ion chromatography (Metrohm Ommnis Titrator). The degradation reaction of organic pollutants in wastewater and river Nile water was monitored by measuring TOC using the Analytik Jena, multi N/C 3100 TOC Analyzer. Moreover, 10 mL of each water type was used for CAP treatment after spiking with microbes under study.
Inactivation of viruses by CAP
Somatic coliphages
ɸX174 was exposed to plasma at different time intervals (0, 2, 5 and 10 min) in dH2O, river water and effluent of WWTP. ɸX174 was enumerated in water samples by a double agar layer plaque test according to ISO (2002). Briefly, Escherichia coli DSM 13127 grown on tryptone broth was used as a host. Coliphage was propagated in tryptone broth with a fresh (4 h-old) E. coli culture to generate a stock suspension. The quantity of infectious somatic coliphage was calculated as follows: 1 mL of exponentially growing host strain bacteria, 100 µL of treated or untreated water samples, and 3 mL of melting tryptone top agar were mixed and poured over modified tryptone bottom agar plates. Plaques were enumerated and expressed as PFU/mL within 3–5 h of incubation at 37 °C. Negative and positive controls were included in each test to ensure proper E. coli growth and coliphage detection.
Rotavirus
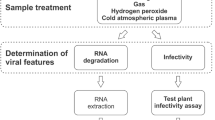
Rotavirus (RoV) SA11 was used as a model for RNA enteric viruses. Rotavirus was activated with 10 µg/mL trypsin before being propagated on MA-104 cell line monolayers using 2% FBS Dulbecco’s modified Eagle medium and incubated for 5 days at 37 °C in a 5% CO2 atmosphere. To eliminate cell debris, RoV-containing cell culture harvests of MA-104 cells was centrifuged for 5 min at 3000 g, and the supernatant was used as a stock suspension after filtration through a 0.22 µm membrane. RoV was subjected to different doses of plasma at different time intervals (0, 2, 5 and 10 min) in dH2O, river water and effluent of WWTP.
For quantitative measurement of RoV in the treated and untreated water samples, MA-104 cells were seeded into 24-well tissue culture plates (Nunclon, Roskilde, Denmark) and incubated further until they reached a confluent density of around 5.0 × 104 cells per well. The cells were then infected with 100 µL of a serial dilution of CAP-treated and non-treated water samples previously prepared in culture media without FBS. After 60 min of incubation, 900 µL of DMEM without FBS was added and cultured for 3–5 days at 37 °C under 5% CO2. Cells were microscopically examined, and the cytopathic effect was recorded. The viral concentration was indicated as TCID50/mL, which corresponded to 50% of wells exhibiting CPE at a given sample dilution. Integrated cell culture RT-qPCR (ICC-RT-qPCR) and RT-qPCR of RoV were used to compare the effect of CAP in dH2O on both viral nucleic acid and infectivity using the RoV primers and qPCR conditions as described in Hamza et al. (2009).
Inactivation of bacteria by CAP
E. coli ATCC 25922 was employed as a bacterial contamination model. In a falcon tube containing 50 mL of tryptic soya broth (Merck, Germany), the stock of E. coli strain was injected. The inoculated tube was incubated at 37 °C for 18–24 h before being centrifuged at 4000 rpm for 20 min. To remove any enrichment broth residue, the supernatant was removed, and the pellet was rinsed three times with sterile distilled water. The E. coli cells were then resuspended in 25 mL of sterile distilled water.
The prepared fresh E coli (~ 107 CFU/mL) was spiked in 10 mL of sterile distilled water (dH2O). The E. coli count was determined before and after treatment of environmental samples in a sterile petri dish. The samples were collected at zero-time exposure as an initial E. coli count, and after that at 2, 5, and 10 min. The E. coli density in the tested sterile distilled water was determined using the pour plate method on plate count agar (Merck, Germany) (APHA (2017). On the other hand, E. coli in the environmental water samples (river and effluent of WWTP samples) was determined on selective medium for E. coli namely HiCrome ECC agar (HiMedia, India) using spread plate method. All inoculated plates were incubated at 37 °C for 24 h. The E. coli colonies on agar plates were counted using the colony counter (Stuart, Germany). E. coli colonies on the surface of HiCrome ECC agar appeared in blue to purple color.
Inactivation of protozoa by CAP
Isolation of Acanthamoeba spp
Acanthamoeba used in the current research was isolated from the Egyptian aquatic environment and identified as Acanthamoeba T4. Briefly, using a stainless-steel filter holder attached to a suction pump, 1 L of river water sample was filtered via a nitrocellulose membrane (0.45-µm pore size and 47-mm diameter). Filtration was stopped just before dryness of the membrane (HPA 2014). After the filtration process, the membrane was inverted face-to-face on the surface of a non-nutrient agar plate seeded with heat-killed E. coli. The plates were incubated for 7 days and examined daily under the inverted microscope (Olympus CXK 41, Japan). Isolated and purified freshwater Acanthamoeba was identified based on trophozoite and cyst characteristics (Page 1988). Three sequential applications of freezing and thawing in liquid nitrogen were used to disrupt the wall of Acanthamoeba cysts, followed by a 2-min incubation in a water bath at 100 °C. The DNA of Acanthamoeba was then extracted using the QIAamp DNA Stool Mini Kit (Qiagen, Valencia, CA). PCR was used to amplify a 450–500 bp DNA fragment using general primers (JDP1 and JDP2) in order to identify Acanthamoeba spp. according to Schroeder et al. (2001). The positive PCR products were purified using the GeneJET PCR Purification Kit (Thermo Scientific, USA), according to the manufacturer’s instructions. Nucleotide sequences were analyzed and compared to the data on GenBank. The sequence of the isolated strain has been deposited in GenBank with accession number OR143778.
Exposure of Acanthamoeba to CAP
Acanthamoeba was subjected to different doses of plasma at different time intervals (5, 10, 15, and 30 min), followed by cultivation according to the standard methods (HPA 2014). In brief, 10 mL of each spiked water sample was concentrated by using a nitrocellulose membrane filter (0.45-µm pore size and 47-mm diameter), and cultivated as described above. Treatment experiments have been conducted in triplicate. In order to investigate the effect of CAP on Acanthamoeba DNA, the treated and non-treated Acanthamoeba cysts were disrupted by three consecutive cycles of freezing and thawing in liquid nitrogen, followed by incubation in a water bath at 100 °C for 2 min. Acanthamoeba DNA was then extracted using the QIAamp DNA Stool Mini Kit (Qiagen, Valencia, CA). PCR was performed to amplify 450–500 bp DNA fragment using JDP1 and JDP2 generic primers (Schroeder et al. 2001). SYBR Green PCR was used to compare the CT values of treated and no treated samples.
Results and discussion
The waveforms of plasma discharge voltage and current during the treatment can be seen in Fig. 2a. The plasma voltage was around 3.8 kV (peak-to-peak), and the plasma current was around 2 mA at plasma frequency of 13.27 kHz. The consumed power (P) can be obtained from the following equation:
where T is the periodic time of the discharge voltage, Vd(t) is the discharge voltage, and i(t) is the discharge current (Adhami Sayad Mahaleh et al. 2023; Ahmed et al. 2014). Then, the energy can be estimated through multiplying the consumed power by the periodic time. Following Eq. (1), it was found that the consumed power is 1.99 W, and the mean consumed energy per voltage period is 1.5 × 10−4 J. The optical emission spectra (OES) of plasma were measured above dH2O water using a spectral measurement system (SMS-500) with a wavelength accuracy of ± 0.25 nm (see Fig. 2b). Several lines can be observed in Fig. 2b, where OH can be seen between 306 and 317 nm with a maximum peak at 315 nm; N2 can be seen from 306 to 380 nm with peaks at 336, 357, and 380 nm.\({N}_{2}^{+}\) lines can also be detected between 391 and 470 nm. The atomic oxygen line at 777 nm is also observed. The OES of plasma above dH2O were compared with the OES above phenol (as a model compound of organic pollutants in water) in a previous work (Abdel-Wahed et al. 2022), where the spectra were higher in the case of dH2O than in the case of phenol, and this can be attributed to the difference in diffusivity and reactivity between the plasma-generated reactive species and the treated media (see Fig. 2c), or in other words: the phenol consumes the ROS and RNS much higher than the dH2O (Farhan Hanafi and Sapawe 2020). The electron density (ne) is one of the most important plasma parameters, and excited electrons are responsible for the induced chemistry in air and liquid as well. We used the Stark broadening method to estimate ne of our system, see Fig. 2d. ne can be estimated from the OES of plasma by choosing one of the spectral lines (we choose here the N2 336 nm line) and calculating the full width at half maximum (FWHM) of the Stark broadening λStark, and then substituting in the following equation:
The electron density was found (using Eq. (2_) to be approximately 5 × 1016 cm−3, where Fig. 2c shows Stark broadening and the Lorentzian fitting of the experimental data with \({\lambda }_{Stark}\approx 2.81 \mathrm{nm}.\) It is noteworthy that the electron temperature of this system was also estimated to be 6191 °K, in our previous work (Abdel-Wahed et al. 2022).
The performance of CAP was investigated in river water samples and the final effluents of the WWTP. Measuring TOC degradation in river water or wastewater is a useful tool for assessing the overall performance and efficiency of water or wastewater treatment facilities as well as an indication of water quality. It also allows for the evaluation of certain treatment methods or technologies in terms of their capacity to remove organic chemicals, process optimization and control, and regulatory compliance.
CAP treatment showed high efficiency for mineralization, as within 15 min there were 67 and 86% TOC removal for the Nile River water sample and final effluent of WWTP, respectively (Fig. 3a). This could be due to the bulk structure of humic acids (HA) in the river sample relative to small organic byproducts in the final effluent of the WWTP (Abdel-Wahed et al. 2021). This is also reflected in the slower rate of TOC degradation in the case of the river sample and low values for the rate constant (\({k}_{1}\)) (Table 1). Figure 3b shows non-linear regression for pseudo-first-order (Eq. (3)) and pseudo-second-order (Eq. (3)) kinetics. The non-linear equation forms for both orders are as follows (Perrin 2017):
where, \({C}_{o}\) (mg/L) is the concentration of the TOC at \({t}_{o}\) (0 min); \(C\) (mg/L) is the concentration of the TOC at \({t}_{t}\) (min); \({k}_{1}\) (1/min) and \({k}_{2}\) (L/mg.min) are the rate constants of pseudo-first-order (Eq. (3)) and pseudo-second-order (Eq. (4)), respectively. The TOC initial concentrations are 4.98 mg/L and 16.43 mg/L for the River Nile sample and final effluent of WWTP, respectively.
a The TOC percentage of removal by CAP treatment in the river and final effluent of WWTP samples. Checking of order kinetics for TOC removal of the river sample (b) and final effluent of the WWTP. Points and dashed lines represent the experimental values and curve fittings of the order kinetics of TOC removal, respectively
An assessment of the error function is typically needed to determine whether a model equation is appropriate for use with experimental results. Error functions were utilized to calculate the differences between theoretical and experimental data. Utilizing the coefficient of determination (\({R}^{2}\)), chi-square (\({\chi }^{2}\)) test, and sum of square error (SSE) statistical error functions, the best-fitting of TOC degradation kinetics was verified. These error functions show that pseudo-first-order kinetics is fitted to the experimental data for both two water samples (Table 1), as it shows the lowest SSE, \({\chi }^{2}\), and the closest value of \({R}^{2}\) to unity. The same behavior was observed by El-Kalliny et al. (2021) with the degradation of crystal violet dye in aqueous media by CAP treatment. To sum up, the final effluent of WWTP shows a faster degradation rate by CAP treatment compared with the river Nile sample, and the TOC degradation kinetics can be described as pseudo-first-order.
During CAP treatment, the pH of the aqueous solution changed. The pH of dH2O decreased, as shown in Fig. 4. The formation of nitrous and nitric acids in the plasma-treated fluids by the reaction of the electric charge with the nitrogen gas in the air was mostly responsible for the pH reduction (Lukes et al. 2012). However, the water matrix plays a role in the changing of the pH as well.
Also, Fig. 4 shows that temperature increases during CAP treatment are often limited. Temperature changes rely on a variety of parameters, such as plasma power, treatment duration, plasma-gas composition, water volume, and initial water temperature. Therefore, temperature monitoring and management are critical during CAP treatment to enable efficient treatment while preventing any unwanted thermal impacts on the treated water or its intended usage.
Table 2 presents the physicochemical properties of the two different water matrices before and after CAP treatment including the cationic and anionic species. CAP treatment leads to a significant increase in nitrites and nitrates in the treated water, and this is the main reason for decreasing the pH due to the formation of nitrous and nitric acids. However, the pH value slightly increased by CAP treatment for the WWTP final effluent. This can be attributed to the increase in the ammonia concentration which may be liberated by the CAP oxidation for N-containing organic compounds in the WW matrix. Generally, in both the Nile River and WWTP final effluent samples, the values of TDS increased by CAP treatment, and consequently the EC values increased. This is reflected in the increase of some cationic and anionic species in the treated water samples. This might be due to the concentration of the water samples through the non-equilibrium vaporization results from the bombardment of energetic ions from the solution surface when the plasma interacts with the water surface (Ekanayake et al. 2020). Accordingly, the CAP process is effective for the removal of organic pollutants, while it can cause an increase in some inorganic species in the final treated effluent.
The effect of CAP on viruses, bacteria, and protozoa was also investigated in river water and wastewater samples and compared to its performance in dH2O. In Fig. 5a, ~ 2log10 and 4log10 reductions of ɸX174 were obtained after 10 min of CAP exposure in river water and wastewater, respectively. Water matrices played a crucial role in the reduction of CAP efficacy because only 1 min was enough for the complete removal of ɸX174 from dH2O. Also, 5 min of CAP treatment was required for the complete removal of RoV from dH2O, whereas only ~ 2.0log10 was removed from river water and wastewater, at the same treatment time (Fig. 5b). The majority of viruses in surface water and wastewater are probably aggregated together or attached to organic materials, impairing the rates of viral inactivation (Kahler et al. 2016).
CAP can damage viral nucleic acids, leading to improper gene expression. The viral capsid proteins can get peroxidized and lose its infectivity when active species come into contact with it. Plasma can inactivate bacteriophages through a number of singlet oxygen-related mechanisms. Damage to viral DNA and proteins may result from viral DNA-DNA crosslinking or DNA–protein complexes caused by plasma exposure. Additionally, 1O2 was supposed to be the most efficient method for inactivating the bacteriophages T4 and feline calicivirus (FCV). Methionine molecules are altered, and histidine molecules are oxidized (Aboubakr et al. 2015). One way that 1O2 affects capsid proteins is by interfering with enzyme function when it reacts with certain amino acids (Guo et al. 2018). It can alter the nucleic acid and protein through the formation of cross-links between guanine and lysine as a result of the oxidation process.
Also, H2O2 has been shown to play a considerable role in the inactivation of enveloped viruses but only a minor effect on naked viruses (Sakudo et al. 2016). Considering that the current investigation employed naked viruses, it is anticipated that H2O2 will only have a slight effect on viral inactivation.
The inactivation of ɸX174 after brief contact with plasma might be attributable to viral capsid destruction or conformational change (Yasuda et al. 2010). It was reported that the role of plasma on bacteriophage lambda was predominantly caused by damage to the protein coat and, to a lesser extent, by damage to viral DNA. Whereas, if the virus is exposed to plasma for an extended period of time, both DNA and protein can be destroyed (Yasuda et al. 2010), and causing bacteriophage aggregations (Davies 2016). A comparable route of CAP inactivation was identified for bacteriophage MS2 and adenovirus (Zimmermann et al. 2011). The effect of CAP treatment on viral nucleic acid is presented in Table (3).
Data showed that lower action of RoV inactivation in the present study was attributed to the change in viral nucleic acid which was not increased with longer CAP exposure time, as indicated by direct RT-qPCR. RT-PCR analysis of a longer portion of the viral genome to assess genomic integrity can provide a stronger correlation with virus infectivity (Hamza et al. 2011). Also, damage to viral capsid proteins is possible, as estimated by ICC-RT-qPCR in which infectivity was lost, but minimal reduction (<0.5log10) by direct RT-qPCR at the same exposure time was observed (Table 3). This data is consistent with Aboubakr et al. (2018) and Yasuda et al. (2010), who demonstrated that during CAP treatment of bacteriophages and FCV, the damage of capsid proteins precedes the degradation of nucleic acids. In contrast, degradation of viral nucleic acid was the main effect of CAP treatment on FCV (Yamashiro et al. 2018), potato virus Y (Filipić et al. 2019) and adenovirus (Sakudo et al. 2016).
Similarly, E. coli counts were reduced by 3log10 and 4 log10 after 10 min of CAP treatment of river and wastewater samples, respectively. On the other hand, 4 log10 removal of E. coli was achieved in dH2O after 30 s of contact time (Fig. 5c). In another study, E. coli was reduced by 3log10 removal in wastewater effluent using CAP (Liao et al. 2021). According to Van Nguyen et al. (2019), CAP reduced the concentration of E. coli in river water samples by 99.8% for 15 kV and completely removed it for 18 kV. The given results support the conclusion that CAP has significant anti-bacterial properties due to the diffusion of RONS through the air gap and dissolving into the aqueous solution (Hefny and Tawfik 2022; Tawfik and Hefny 2021). CAP has ability to destroy the bacterial cell membranes of E. coli and damage the intracellular composition of bacterial cells such as bacterial nucleic acids and enzymes (Liao et al. 2021).
Peroxynitrite has been identified as one of the major reactive species generated by CAP and inactivate E. coli (Zhou et al. 2018). Su and Groves (2010) calculated the permeability coefficient for peroxynitrite to be 8.0 × 104 cm/s, which is comparable to that of H2O2 but about 400 times larger than that of superoxide. Because of its great permeability, peroxynitrite is particularly a potent oxidant for causing bacterial damage. It was shown that short exposure to CAP causes an instant loss of cell membrane integrity (Abdel-Wahed et al. 2022).
Using E. coli and total coliform, Nguyen et al. (2019) reported that CAP could be combined with conventional surface water pre-treatment methods for water disinfection. Interestingly, after treatment with CAP, all metrics of surface water met the acceptable criteria for drinking water quality.
The CAP system produces a variety of radicals of ROS and RNS with different levels depending on the type and composition of the water matrix. As a result, and for the sake of simplicity, radical scavengers can be used to estimate the contribution of the majority of generated radicals rather than tracking the concentration of these radicals. Distilled water is a good water matrix in terms of generalization to be a control for different water matrices. Figure 5d shows the role of the main reactive species O2·, 1O2, ·OH, and H2O2, produced in the CAP process into the chemical and microbial removal. In this Figure, instead of measuring TOC, the phenol was employed as a model water pollutant to eliminate any interference from the organic scavengers. Para-benzoquinone (p-BQ), sodium azide (SA), isopropyl alcohol (IPA), and catalase scavengers can trap effectively the reactive species O2·, 1O2, ·OH, and H2O2, respectively (Abdel-Wahed et al. 2022; Bekeschus et al. 2017; Santos et al. 2012). The experimental conditions of trapping are indicated in the Figure caption.
a, b Viral and c bacterial removal from river water and wastewater samples by CAP. The reactor diameter is 5.6 cm, and the experiments were conducted in a batch system. A two-way ANOVA with Tukey’s multiple comparisons test was conducted. Only comparisons with p ≤ 0.01 are displayed as asterisks. d Effect of scavengers on the removal efficiency of phenol, E. coli and ɸX174. All scavengers have a concentration of 100 mM except catalase (40 μg/10 mL). Data are an average of duplicate batch experiments, and the error bars indicate the SEM
After adding p-BQ, IPA, and SA, the percentage of phenol removed dropped, indicating that O2·, 1O2, and ·OH play a significant role in the degradation of phenol by CAP. The contribution of oxidizing species in the phenol degradation process occurs in the following order: O2· = 1O2 > ·OH. H2O2 had no effect, since adding catalase had no influence on the amount of phenol removed. Although, the virucidal impact (Fig. 5a, b) may result from the chemical reactions between the viral components and RONS like 1O2, O3, \({\mathrm{O}}_{2}^{\cdot -}\), and peroxynitrous acid, Fig. 5d shows that 1O2 has a substantial role in ɸX174 inactivation, as evidenced by the observed reduction in CAP performance to inactivate ɸX174 in the presence of SA; whereas, H2O2 has a significant a role in the reduction of E. coli in the treated samples.
Actually, there is a research gap on the effect of CAP on protozoa. Acanthamoeba was selected as a protozoan pathogen model to investigate the ability of CAP to disinfect its resistant cysts in different water matrices. This pathogen is characterized by a hard cyst wall, which can resist extreme environmental conditions and physical and chemical disinfectant doses such as chlorine, UV, and ozone (Dupuy et al. 2014; Hijnen et al. 2006; Thomas et al. 2004). Moreover, Acanthamoeba spp. is easy to cultivate using economic media (non-nutrient agar seeded with E. coli).
Data in Fig. 6 indicate that CAP has the potential to treat Acanthamoeba in water. However, it is clear that water matrices have a negative impact on the performance of CAP to remove protozoa; as in the case of dH2O, complete inactivation was obtained after 30-min treatment, while no removal of Acanthamoeba was observed after 30 min of exposure in river or wastewater samples (Fig. 6). Additionally, Acanthamoeba inactivated by CAP in dH2O showed negative PCR signals indicating degradation of Acanthamoeba nucleic acid.
The effect of CAP treatment on Acanthamoeba in dH2O. T0 shows cultivation of a spiked water sample without treatment. T30 represents the cultivation of a spiked sample after 30 min of CAP treatment showing only bacterial growth. Plates have been incubated for 1 week and examined daily for the presence of Acanthamoeba trophozoites or cysts
This means that the assay still needs more optimization in order to achieve complete removal of protozoa from complex water matrix. However, it is well established that Acanthamoeba is highly resistant to water disinfectants. Due to the limitations of the Acanthamoeba detection technique which involve the direct cultivation of the treated samples, Acanthamoeba might be reduced in environmental water samples by CAP treatment, but log10 reduction could not be measured after cultivation and cyst generation of Acanthamoeba. Therefore, the detection of Acanthamoeba in treated water samples was based on qualitative not quantitative method. The same experiments have been conducted using Nagleria clarki, which showed lower resistance to CAP compared to Acanthamoeba, represented by complete inactivation in 15 min treatment (data not shown).
The effect of CAP has been investigated before on another type of protozoa (Cryptosporidium parvum) oocysts (Craighead et al. 2020), revealing that oocysts were inactivated by 2.0 log10 after 3 min of treatment by CAP supporting the idea of the potential use of CAP to treat protozoa in water. This is a promising development in the field of water treatment, as protozoa can be harmful to human health if ingested and are more resistant to disinfectants compared to viruses and bacteria.
CAP technology may offer a more efficient and cost-effective solution compared to traditional water treatment methods. The difference between the microbial removal by CAP in dH2O and environmental water samples could be due to the presence of organic matter in environmental water which can directly interact with active species, reducing the biocidal activity of CAP. Therefore, it is important to consider the composition of the treated water and the potential impact of organic and inorganic materials on the effectiveness of microbial removal.
Barillas (2015) designed a prototype CAP reactor to be used a tertiary treatment of the industrial wastewater. One of the primary advantages of scaling up plasma devices for field applications is that the size of the plasma-liquid interface may be expanded allowing for the exposure of thin liquid layers to CAP which could enhance the efficacy of the treatment (Foster et al. 2018).
Conclusion
Water plasma treatment is an innovative approach to generating physiologically active solutions that may not be readily generated using chemical methods alone. As such, this method warrants additional research and development as a green stand-alone alternative or supplementary approach to traditional water treatment techniques. The plasma voltage and current waveforms were measured during the treatment, and the plasma power and the consumed energy were calculated as well. Moreover, the OES during plasma treatment of dH2O were measured and analyzed, and the electron density was estimated from the OES. CAP was examined for its effects on microbial and chemical pollutants in different types of water. Water type affected the efficacy of CAP treatment. For example, 1 min of exposure was enough to complete removal of ɸX174 in dH2O, whereas a 10-min application of CAP reduced ɸX174 by 2 log10 in river water and 4 log10 in wastewater. In the case of RoV, 5-min CAP treatment were required for its complete removal in dH2O and for ~ 2log10 in river water and wastewater. Similarly, E. coli reduced by 3log10 and 4log10 after 10-min treatment in river water or wastewater and 30 s achieved 4log10 E. coli reduction in dH2O.
The approach successfully reduced TOC from both surface and wastewater samples. TOC degradation kinetics may be represented by pseudo-first-order, and the final effluent of the WWTP exhibits a higher rate of CAP treatment degradation compared to the river Nile sample. According to the study, in the CAP process, singlet oxygen and superoxide radicals both have significant equal contributions to the phenol degradation. In addition, the CAP method removes organic contaminants effectively, but it also results in an increase in inorganic species in the final treated effluent. The data presented here support the potential combination of CAP with conventional pretreatment steps for water and wastewater purification. Indeed, comparisons of different studies of CAP are problematic due to the use of varied plasma system configurations. As a result, it is critical for researchers to develop a standardized process for evaluating and comparing CAP systems to assure accurate and consistent results. This will allow for the creation of more effective and efficient plasma-based technologies for a variety of applications. Further work is needed to fully understand the effectiveness and safety of this technology. In terms of cost, traditional procedures such as chlorination and UV irradiation have lower operational expenses than CAP. However, CAP is a newer technology that is still being optimized, and a cost-effective CAP system could accelerate the progress of the technology and become more commonly used.
Data availability
All the data generated or analyzed during this study are included in this published article.
References
Abdel-Wahed MS, Abdel-Karim A, Margha FH, Gad-Allah TA (2021) UV sensitive ZnO and TiO2-ZnO nanocrystalline transparent glass-ceramic materials for photocatalytic decontamination of surface water and textile industry wastewater. Environ Prog Sustain Energy 40:e13653
Abdel-Wahed MS, Hefny MM, Abd-Elmaksoud S, El-Liethy MA, Kamel MA, El-Kalliny AS, Hamza IA (2022) Removal of chemical and microbial water pollutants by cold plasma combined with Ag/TiO2-rGO nanoparticles. Sci Rep 12:9850
Abdo SM, El-Liethy MA, Doma HS, El Taweel GE, Ali GH (2022) Chlorine as an integrated approach for environmental health and hygiene: a case study on evaluation of the performance of waste stabilization ponds located at 11 governorates in Egypt. Emerg Contam 8:243–253
Aboubakr HA, Williams P, Gangal U, Youssef MM, El-Sohaimy SA, Bruggeman PJ, Goyal SM (2015) Virucidal effect of cold atmospheric gaseous plasma on feline calicivirus, a surrogate for human norovirus. Appl Environ Microbiol 81:3612–3622
Aboubakr HA, Gangal U, Youssef MM, Goyal SM, Bruggeman PJ (2016) Inactivation of virus in solution by cold atmospheric pressure plasma: identification of chemical inactivation pathways. J Phys D Appl Phys 49:204001
Aboubakr HA, Mor SK, Higgins L, Armien A, Youssef MM, Bruggeman PJ, Goyal SM (2018) Cold argon-oxygen plasma species oxidize and disintegrate capsid protein of feline calicivirus. PLoS ONE 13:e0194618
Abreu AC, Tavares RR, Borges A, Mergulhão F, Simões M (2013) Current and emergent strategies for disinfection of hospital environments. J Antimicrob Chemother 68:2718–2732
Adamovich I, Agarwal S, Ahedo E, Alves LL, Baalrud S, Babaeva N, Bogaerts A, Bourdon A, Bruggeman PJ, Canal C, Choi EH, Coulombe S, Donkó Z, Graves DB, Hamaguchi S, Hegemann D, Hori M, Kim HH, Kroesen GMW, Kushner MJ, Laricchiuta A, Li X, Magin TE, Mededovic Thagard S, Miller V, Murphy AB, Oehrlein GS, Puac N, Sankaran RM, Samukawa S, Shiratani M, Šimek M, Tarasenko N, Terashima K, Thomas E Jr, Trieschmann J, Tsikata S, Turner MM, van der Walt IJ, van de Sanden MCM, von Woedtke T (2022) The 2022 Plasma Roadmap: low temperature plasma science and technology. J Phys D Appl Phys 55:373001
AdhamiSayadMahaleh M, Narimisa M, Nikiforov A, Gromov M, Gorbanev Y, Bitar R, Morent R, De Geyter N (2023) Nitrogen oxidation in a multi-pin plasma system in the presence and absence of a plasma/liquid interface. Appl Sci 13:7619
Aggelopoulos CA (2022) Recent advances of cold plasma technology for water and soil remediation: a critical review. Chem Eng J 428:131657
Ahmed KM, Allam TM, El-sayed HA, Soliman HM, Ward SA, Saied EM (2014) Design, construction and characterization of AC atmospheric pressure air non-thermal plasma jet. J Fusion Energy 33:627–633
Allam TM, Ahmed KM, Abouelatta MA, Ward SA, Lashin AA, Soliman HM (2016) Development of a low-cost atmospheric non-thermal plasma jet and its characteristics in air and nitrogen. Eur Phys J Appl Phys 76:10803
APHA (2017) American Public Health Association, Standard methods for the examination of water and wastewater, 23rd edn. American Public Health Association, Washington, D.C
Arbuckle TE, Hrudey SE, Krasner SW, Nuckols JR, Richardson SD, Singer P, Mendola P, Dodds L, Weisel C, Ashley DL, Froese KL, Pegram RA, Schultz IR, Reif J, Bachand AM, Benoit FM, Lynberg M, Poole C, Waller K (2002) Assessing exposure in epidemiologic studies to disinfection by-products in drinking water: report from an international workshop. Environ Health Perspect 110(Suppl 1):53–60
Barillas L (2015) Design of a prototype of water purification by plasma technology as the foundation for an industrial wastewater plant. J Phys Conf Ser 591(1):012057. https://doi.org/10.1088/1742-6596/591/1/012057
Bekeschus S, Wende K, Hefny MM, Rödder K, Jablonowski H, Schmidt A, Woedtke Tv, Weltmann K-D, Benedikt J (2017) Oxygen atoms are critical in rendering THP-1 leukaemia cells susceptible to cold physical plasma-induced apoptosis. Sci Rep 7:1–12
Brany D, Dvorska D, Halasova E, Skovierova H (2020) Cold atmospheric plasma: a powerful tool for modern medicine. Int J Mol Sci 21:2932
Chen Z, Garcia G Jr, Arumugaswami V, Wirz RE (2020) Cold atmospheric plasma for SARS-CoV-2 inactivation. Phys Fluids (1994) 32:111702
Craighead S, Hertrich S, Boyd G, Sites J, Niemira BA, Kniel KE (2020) Cold atmospheric plasma jet inactivates Cryptosporidium parvum oocysts on cilantro. J Food Prot 83:794–800
Davies MJ (2016) Protein oxidation and peroxidation. Biochem J 473:805–825
Deng XT, Shi JJ, Kong MG (2007) Protein destruction by a helium atmospheric pressure glow discharge: capability and mechanisms. J Appl Phys 101:074701–0747019
Dupuy M, Berne F, Herbelin P, Binet M, Berthelot N, Rodier MH, Soreau S, Hechard Y (2014) Sensitivity of free-living amoeba trophozoites and cysts to water disinfectants. Int J Hyg Environ Health 217:335–339
Ekanayake UGM, Seo DH, Faershteyn K, O’Mullane AP, Shon H, MacLeod J, Golberg D, Ostrikov K (2020) Atmospheric-pressure plasma seawater desalination: clean energy, agriculture, and resource recovery nexus for a blue planet. Sustain Mater Technol 25:e00181
El-Kalliny AS, Abd-Elmaksoud S, El-Liethy MA, Abu Hashish HM, Abdel-Wahed MS, Hefny MM, Hamza IA (2021) Efficacy of cold atmospheric plasma treatment on chemical and microbial pollutants in water. ChemistrySelect 6:3409–3416
Farhan Hanafi M, Sapawe N (2020) A review on the water problem associate with organic pollutants derived from phenol, methyl orange, and remazol brilliant blue dyes. Mater Today: Proceed 31:A141–A150
Filipić A, Primc G, Zaplotnik R, Mehle N, Gutierrez-Aguirre I, Ravnikar M, Mozetič M, Žel J, Dobnik D (2019) Cold atmospheric plasma as a novel method for inactivation of potato virus Y in water samples. Food Environ Virol 11:220–228
Foster JE, Mujovic S, Groele J, Blankson IM (2018) Towards high throughput plasma based water purifiers: design considerations and the pathway towards practical application. J Phys D Appl Phys 51:293001
Fridman G, Friedman G, Gutsol A, Shekhter AB, Vasilets VN, Fridman A (2008) Applied plasma medicine. Plasma Process Polym 5:503–533
Gerrity D, Stanford BD, Trenholm RA, Snyder SA (2010) An evaluation of a pilot-scale nonthermal plasma advanced oxidation process for trace organic compound degradation. Water Res 44:493–504
Graumans MHF, Hoeben WFLM, Russel FGM, Scheepers PTJ (2020) Oxidative degradation of cyclophosphamide using thermal plasma activation and UV/H2O2 treatment in tap water. Environ Res 182:109046
Guo L, Xu R, Gou L, Liu Z, Zhao Y, Liu D, Zhang L, Chen H, Kong MG (2018) Mechanism of virus inactivation by cold atmospheric-pressure plasma and plasma-activated water. Appl Environ Microbiol 84:e00726-18
Hamza IA, Abd-Elmaksoud S (2023) Applicability of crAssphage as a performance indicator for viral reduction during activated sludge wastewater treatment. Environ Sci Pollut Res Int 30:50723–50731
Hamza IA, Jurzik L, Stang A, Sure K, Uberla K, Wilhelm M (2009) Detection of human viruses in rivers of a densly-populated area in Germany using a virus adsorption elution method optimized for PCR analyses. Water Res 43:2657–2668
Hamza IA, Jurzik L, Überla K, Wilhelm M (2011) Methods to detect infectious human enteric viruses in environmental water samples. Int J Hyg Environ Health 214(6):424–436. https://doi.org/10.1016/j.ijheh.2011.07.014
Hefny MM, Tawfik AM (2022) The fate of molecular species in water layers in the light of power-law time-dependent diffusion coefficient. Symmetry 14:1146
Hijnen WA, Beerendonk EF, Medema GJ (2006) Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: a review. Water Res 40:3–22
HPA (2014) Isolation and identification of Acanthamoeba species. Natl Stand Method W 17(2). http://www.hpa-standardmethods.org.uk/pdf_sops.asp
ISO (2002) Water quality. In: Detection and Enumeration of Bacteriophages - Part 2: Enumeration of Somatic Coliphages. International Organisation for Standardisation, Geneva ISO 10705-2
Kahler AM, Cromeans TL, Metcalfe MG, Humphrey CD, Hill VR (2016) Aggregation of Adenovirus 2 in source water and impacts on disinfection by chlorine. Food Environ Virol 8:148–155
Liao X, Liu D, Chen S, Ye X, Ding T (2021) Degradation of antibiotic resistance contaminants in wastewater by atmospheric cold plasma: kinetics and mechanisms. Environ Technol 42:58–71
Lukes P, Locke BR, Brisset J-L (2012) Aqueous-phase chemistry of electrical discharge plasma in water and in gas–liquid environments. Plasma Chem Catal Gases Liq 1:243–308
Mahmoud M, El-Liethy MA (2022) Three-dimensional, flow-through silver-magnetite nanocomposite–modified reactive electrochemical system with slow silver ions release for efficient bacterial disinfection of sewage. J Environ Chem Eng 10:106985
Mai-Prochnow A, Murphy AB, McLean KM, Kong MG, Ostrikov KK (2014) Atmospheric pressure plasmas: infection control and bacterial responses. Int J Antimicrob Agents 43:508–517
Montie TC, Kelly-Wintenberg K, Roth JR (2000) An overview of research using the one atmosphere uniform glow discharge plasma (OAUGDP) for sterilization of surfaces and materials. IEEE Trans Plasma Sci 28:41–50
Murugesan P, Moses JA, Anandharamakrishnan C (2020) Performance of an atmospheric plasma discharge reactor for inactivation of Enterocococcus faecalis and Escherichia coli in aqueous media. J Environ Chem Eng 8:103891
Murugesan P, Moses JA, Anandharamakrishnan C (2020) Water decontamination using non-thermal plasma: concepts, applications, and prospects. J Environ Chem Eng 8:104377
Nguyen DV, Ho PQ, Pham TV, Nguyen TV, Kim L (2019) Treatment of surface water using cold plasma for domestic water supply. Environ Eng Res 24:412–417
Nicol MJ, Brubaker TR, Honish BJ 2nd, Simmons AN, Kazemi A, Geissel MA, Whalen CT, Siedlecki CA, Bilén SG, Knecht SD, Kirimanjeswara GS (2020) Antibacterial effects of low-temperature plasma generated by atmospheric-pressure plasma jet are mediated by reactive oxygen species. Sci Rep 10:3066
Page FC (1988) A new key to freshwater and soil gymnamoebae: with instructions for culture. Freshw Biol Assoc, Ambleside, pp 3–170
Park JK, Nam SH, Kwon HC, Mohamed AA, Lee JK, Kim GC (2011) Feasibility of nonthermal atmospheric pressure plasma for intracoronal bleaching. Int Endod J 44:170–175
Park GY, Park SJ, Choi MY, Koo IG, Byun JH, Hong JW, Sim JY, Collins GJ, Lee JK (2012) Atmospheric-pressure plasma sources for biomedical applications. Plasma Sources Sci Technol 21:043001
Park J, Cha M S (2023) Successive multi-microdischarges occurring in pin-to-line geometry of dielectric barrier discharge. Plasma Chem Plasma Process. https://doi.org/10.1007/s11090-023-10357-4
Perni S, Shama G, Hobman JL, Lund PA, Kershaw CJ, Hidalgo-Arroyo GA, Penn CW, Deng XT, Walsh JL, Kong MG (2007) Probing bactericidal mechanisms induced by cold atmospheric plasmas with Escherichia coli mutants Applied Physics Letters 90(7). https://doi.org/10.1063/1.2458162
Perrin CL (2017) Linear or nonlinear least-squares analysis of kinetic data? J Chem Educ 94:669–672
Rao Y, Shang W, Yang Y, Zhou R, Rao X (2020) Fighting mixed-species microbial biofilms with cold atmospheric plasma. Front Microbiol 11:1000
Reece Roth J, Rahel J, Dai X, Sherman DM (2005) The physics and phenomenology of one atmosphere uniform glow discharge plasma (OAUGDP™) reactors for surface treatment applications. J Phys D Appl Phys 38:555
Sakudo A, Toyokawa Y, Imanishi Y (2016) Nitrogen gas plasma generated by a static induction thyristor as a pulsed power supply inactivates adenovirus. PLoS One 11:e0157922
Santos AL, Gomes NC, Henriques I, Almeida A, Correia A, Cunha  (2012) Contribution of reactive oxygen species to UV-B-induced damage in bacteria. J Photochem Photobiol, B 117:40–46
Schroeder JM, Booton GC, Hay J, Niszl IA, Seal DV, Markus MB, Fuerst PA, Byers TJ (2001) Use of subgenic 18S ribosomal DNA PCR and sequencing for genus and genotype identification of acanthamoebae from humans with keratitis and from sewage sludge. J Clin Microbiol 39:1903–1911
Shi Q, Chen Z, Liu H, Lu Y, Li K, Shi Y, Mao Y, Hu HY (2021) Efficient synergistic disinfection by ozone, ultraviolet irradiation and chlorine in secondary effluents. Sci Total Environ 758:143641
Su J, Groves JT (2010) Mechanisms of peroxynitrite interactions with heme proteins. Inorg Chem 49:6317–6329
Sun M-Y, Xu G-M, Zhang G-J (2023) Characteristics of micro-discharge process in saline solution with pin-to-pin electrodes driven by a low-voltage high-frequency AC power supply. J Appl Phys 133(9). https://doi.org/10.1063/5.0139438
Tawfik AM, Hefny MM (2021) Subdiffusive reaction model of molecular species in liquid layers: fractional reaction-telegraph approach. Fractal Fractional 5:51
Thomas V, Bouchez T, Nicolas V, Robert S, Loret JF, Lévi Y (2004) Amoebae in domestic water systems: resistance to disinfection treatments and implication in Legionella persistence. J Appl Microbiol 97:950–963
WHO (2022a) Drinking-water: fact sheet. https://www.who.int/news-room/fact-sheets/detail/drinking-water. Accessed 7 April 2023
WHO (2022b) Sanitation: Fact sheet. https://www.who.int/news-room/fact-sheets/detail/sanitation. Accessed 7 April 2023
Yamashiro R, Misawa T, Sakudo A (2018) Key role of singlet oxygen and peroxynitrite in viral RNA damage during virucidal effect of plasma torch on feline calicivirus. Sci Rep 8:17947
Yasuda H, Miura T, Kurita H, Takashima K, Mizuno A (2010) Biological evaluation of DNA damage in bacteriophages inactivated by atmospheric pressure cold plasma. Plasma Process Polym 7:301–308
Younis WO, Berekaa MM, Mohamed A (2020) Enhanced microbial decontamination using non-thermal low pressure argon plasma jet. Pak J Biol Sci: Pjbs 23:248–256
Zhou R, Zhou R, Prasad K, Fang Z, Speight R, Bazaka K, Ostrikov K (2018) Cold atmospheric plasma activated water as a prospective disinfectant: the crucial role of peroxynitrite. Green Chem 20:5276–5284
Zimmermann JL, Dumler K, Shimizu T, Morfill GE, Wolf A, Boxhammer V, Schlegel J, Gansbacher B, Anton M (2011) Effects of cold atmospheric plasmas on adenoviruses in solution. J Phys D Appl Phys 44:505201
Acknowledgements
We thank Professors Ahmad A-Herrawy, Tarek A. Gad-Allah, Mahmoud A Gad, and Kamal M. Ahmed for their valuable comments and suggestions on this manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This study was financially supported by the National Research Centre, Cairo, Egypt; project number: 13020304.
Author information
Authors and Affiliations
Contributions
I. A. H: acquired funding, conceptualization, formal analysis, investigation, and writing — review and editing. A. S. E: conceptualization, formal analysis, investigation, and review and editing, S. A: investigation, review, and editing. M. A. M: investigation, review, and editing. M. S. A: investigation, review, and editing. M. A. E: investigation, review, and editing. M. M. H: conceptualization, formal analysis, investigation, review, and editing. All the authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All the authors have read and agreed to the published version of the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Sami Rtimi
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hamza, I.A., El-Kalliny, A.S., Abd‑Elmaksoud, S. et al. Cold atmospheric plasma: a sustainable approach to inactivating viruses, bacteria, and protozoa with remediation of organic pollutants in river water and wastewater. Environ Sci Pollut Res 30, 116214–116226 (2023). https://doi.org/10.1007/s11356-023-30298-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-30298-x