Abstract
Recently, low-cost graphitic carbon nitride (g-C3N4) revealed high photocatalytic activities and provided solutions to environmental pollution. In this study, we synthesized brown mesoporous 2D g-C3N4 by calcination dicyandiamide with pluronic P123. This is followed by loading of Ag NPs on the prepared 2D g-C3N4 by photodeposition process. After that, a ternary composite 2% Ag/ 2D g-C3N4 / g-C3N4 QDs heterojunction photocatalyst has been successfully prepared. The prepared nanomaterials were comprehensively characterized by various analysis techniques such as XRD, UV–Vis., BET, XPS, SEM, TEM. This new system exhibited a large surface area with porous structure and a wide absorption of visible light. The results verified that Ag NPs decoration enhanced the charge separation of photo-generated carriers of g-C3N4 2D and g-C3N4 QDs, promote significant enhancement in the photocatalytic activity for reduction of p-nitrophenol with a rate constant (k) value of 0.49729 / min in 6 min. This rate is about two-fold higher than that observed for pure g-C3N4 2D and g-C3N4 QDs as well as shows an improvement compared to 2% Ag/ g-C3N4 2D and g-C3N4 2D/ g-C3N4 QDs. The results open the door to design highly efficient 2D/0D nanocomposite photocatalysts for a wide variety of environmental applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sustainably securing water supplies is increasingly recognized as a challenge for people, industry, and the environment. The challenge is likely due to environmental and global changes, industrial, domestic, and agricultural activities. Synthetic organic dye pollution is one of the major pollutants in the industrial sector. In this context, nitroaromatic compounds have been considered one of the most harmful environmental organic pollutants (Ju and Parales 2010; Spain 1995). P-nitrophenol (PNP) is one of such nitro-compound, which is widely used in industrial and agricultural processes, particularly in drug, dye, and pesticide manufacturing (Feng et al. 2013; Rocha et al. 2018; Yang et al. 2010) and found in industrial effluents because they are very soluble and stable. PNP is extremely harmful to human health; due to its toxic effects leading to a cancer (Yu et al. 2017). Therefore, numerous strategies have been developed for removing these organic pollutants from industrialized sewage, involving physisorption, chemical precipitation, membrane filtration, photocatalytic degradation, biological remedies, and catalytic decomposition, advanced oxidation processes (AOPs) (Chen et al. 2023; Peng et al. 2023; Tang et al. 2023) and enzymatic degradation (Guo et al. 2023). Heterogeneous photocatalysis is a promising approach that harnesses solar light to effectively oxidize contaminants under reasonable conditions (El-Hosainy et al. 2022; El-Sheshtawy et al. 2020; Ghubish et al. 2022; Shoueir et al. 2019; Dileepkumar et al. 2022; Prabagar et al. 2022).
Graphitic carbon nitride (g-C3N4) is one of the promising semiconductor photocatalyst materials with a layered structure that has a lot of covalent and hydrogen bonds and functions as an organic conjugated semiconductor photocatalyst. G-C3N4 has gained great attraction as a semiconductor in various applications due to its high stability, cost-effectiveness, excellent performance for solar energy use and a small band gap (2.82 eV) (Chen et al. 2023; Torad et al. 2021; Wang et al. 2009b; Dileepkumar et al. 2022; Prabagar et al. 2022), which can effectively satisfy the thermodynamic needs of different photo-redox processes (Wang et al. 2009a; Zhang et al. 2010). Nevertheless, the practical utilization of unmodified g-C3N4 is hindered by its low specific surface area, limited responsiveness to visible light (wavelengths less than 460 nm), and rapid recombination of photogenerated electron–hole pairs (Chen et al. 2023).
So, there are many efforts have been devoted to improve the g-C3N4 photocatalytic efficacy, including morphological controlled doping (Yu et al. 2015), metal deposition (Obregón et al. 2019), non-metal doping (Liu et al. 2010; Zhang and Antonietti 2010), construction of heterojunction or Z-scheme and S-scheme composites (She et al. 2017), introducing vacant defects (Niu et al. 2012) or designing porous structure (Li et al. 2014b). It has been demonstrated by Obregón et al. (2019); Wu et al. (2019); Yan et al. (2019) that the efficiency of photocatalytic materials can be enhanced by increasing the 2D surface area of g-C3N4, enlarging the number of active sites, and boosting mesoporosity in the photocatalysts. Furthermore, the photocatalytic efficiency of g-C3N4 can be enhanced by mixing with Quantum dots (QDs). Recently, Research has verified that merging g-C3N4 with QDs not only conquers the restrictions of each individual component but also synergistically combines their advantages (Chen et al. 2023).
Furthermore, the g-C3N4 2D photocatalytic efficiency can be maximized via forming co-catalysts like Au or Ag or Pd metals on its surface. These metals can accumulate electrons on these surfaces after light irradiation at the Fermi level and then release them to degrade pollutants quickly (Chiu and Hsu 2017; Choi et al. 2017; Li et al. 2008). All these modifications leads to use g-C3N4 in different applications such as pollutants degradation (Yan et al. 2010), cleansing (Huang et al. 2014), water splitting (Wang et al. 2009b; Zhang et al. 2012), CO2 reduction (Huang et al. 2015; Wang et al. 2015) and sensitive detection (Jiang et al. 2016; Tian et al. 2013a, 2013b, 2013c). Besides, many efforts have been widely employed to enhance the photocatalytic performance of g-C3N4 2D for potential applications, (including wastewater treatment, CO2 conversion, and PNP reduction) (El-Sheshtawy et al. 2019; Yang et al. 2013, 2017).
Herein, for the first time, we have combined 2D g-C3N4 with 0D g-C3N4 QDs and Ag as a cocatalyst to not only conquer the restrictions of each individual component but also synergistically combine their advantages. Thus, we have developed a new nanoporous brown photocatalyst (2% Ag/ g-C3N4 2D/ g-C3N4 QDs) using a facile approach for completely fast reduction of PNP pollutants when exposed to visible light. The spectral, structural features, morphological properties and the photocatalytic efficiency of this nanocomposite were determined. The novel configuration of this photocatalyst demonstrated an extensive surface area characterized by a porous structure and a remarkable capability for absorbing a broad spectrum of visible light. The mechanisms of the charge separation/transfer and exceptional photocatalytic degradation of PNP were also proposed.
Materials and methods
Dicyandiamide (DCDA), Pluronic (P123), and sodium citrate were purchased from Sigma-Aldrich, while silver nitrate (AgNO3) was supplied by Merck. Urea and Ethanol were obtained from Acros Organics-Fisher Scientific. Methanol was acquired from Chem-Lab Belgium. All materials were used without further purification.
Manufacture of brown g-C3N4 mesoporous (g-C3N4 2D)
The brownish g-C3N4 2D mesoporous was synthesized by mixing 7 gm of DCDA with 0.9 gm of P123 in an alumina crucible. Then, the mixture was transferred inside a muffle oven following ramping procedures. The temperature increased to 250°C by 2 °C/min from room temperature for 2 h. After that, it was raised to 350 °C for 1 h with a temperature rate of 1.5 °C per min, and further, increased to 550 °C for 1 h with a temperature rate of 3 °C per min for about 4 h then it was cooled to ambient condition. The produced g-C3N4 2D was calcinated at 450°C by 2 °C/min for 4 h.
Fabrication of Ag/ g-C3N4 2D by photodeposition method
Ag/g-C3N4 2D was synthesized via a simple photodeposition process. Typically, various concentrations of Ag (0.5, 1, and 2 wt%) were added to g-C3N4 2D (0.1 gm) dissolved in water/methanol (8:2 ml) and treated with visible light under stirring for 4 h. Then Ag/ g-C3N4 2D was separated via centrifugation and washed by water. The Ag/ g-C3N4 2D was used for further characterization after being dried at 80 oC for 24 h.
Synthesis of g-C3N4 QDs
G-C3N4 QDs can be prepared by this method (solid state method) without adding any solvent as follow: 1.68 mmol of urea (0.1015 gm) and 0.187 mmol of sodium citrate (0.055 gm) were blended using an agate mortar, in the presence of a few drops of ethanol to get a homogenous paste, followed by calcination at 80 °C. The produced powder was transferred into an autoclave and heated to 180°C over 1 h. The powder produced was dissolved in 5 ml water. The product was separated via centrifugation at 12000 rpm for 15 min. The supernatant was purified by dialyzing against ultra-pure for 24 h to obtain g-C3N4 QDs.
Ternary brownish nanocomposite photocatalyst Ag/ g-C3N4 2D / g-C3N4 QDs preparation
The ternary nanocomposite was synthesized by mixing 1ml of g-C3N4 QDs with 10 mg of Ag/ g-C3N4 2D sample. The mixture was kept under stirring for 24 h. Then after drying at 60°C, the produced sample was denoted as Ag/ g-C3N4 2D/ g-C3N4 QDs nanocomposite powder. For comparison, the nanocomposite without Ag was prepared by mixing 10 mg of g-C3N4 2D with 1ml of g-C3N4 QDs to confirm the g-C3N4 2D/ g-C3N4 QDs junction formation.
Characterization
X-ray diffraction (XRD) was acquired using Rigaku Smart Lab X-ray diffractometer (40 kV/ 30 mA) with speed 0.5°/min using Cu K α radiation source with λ = 1.54 Å. Transmission electron microscope (TEM model. JEOL-JEM-2100F) was used to examine the morphological nanostructure. FE-SEM was employed to view topography details on the surface of the samples model: Quattro S FEG-SEM – Thermo Fisher, NL. Energy dispersive X-ray spectroscopy (EDX) was performed for elemental distribution.
The absorption and the diffuse reflection spectra (UV–Vis DRS) of the nanostructure powder were investigated by UV spectrophotometer (JASCO V-770) using BaSO4 as a standard sample, and the band gap was followed the Kubelka–Munk equation. The photoluminescence spectra were measured using JASCO FP-8600 spectrofluorometer.
The chemical surface structure of the samples was recorded using X-ray Photoelectron Spectroscopy (XPS, Thermo-Scientific Kα) technique with X-ray source–Al Kα micro-focused monochromator – varying spot size (30–400 m in 5 m steps), ion gun – energy range 100–4000 eV, vacuum system – 2 × 220 l/s turbo molecular pumps for entry and analysis chambers – auto-firing, filament TSP was used. Zeta potential measurements were determined by dynamic light scattering operated by Brookhaven, USA. The BET surface area analyzer (Nova 2000 series, Quantachrome Instruments, UK) was used for determining the powder's surface area, pore volume, and pore size distribution.
Results and discussion
Characterization of Ternary Ag/ g-C3N4 2D / g-C3N4 QDs
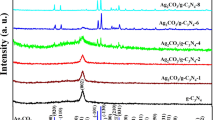
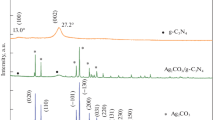
Scheme 1 illustrates the synthesis process of 2 wt% Ag/ g-C3N4 2D/ g-C3N4 QDs nanocomposite via multiple stages. As indicated in Fig. 1 a, the XRD patterns of g-C3N4 display a strong diffraction peak at 2θ = 27.3° with a great intensity that can be attributed to the (002) interlayer stacking plane of aromatic conjugated C-N ring. The lower peak at 2θ = 13.3° is related to the (100) plane, which characteristics of the H-bonds responsible for preserving long-range atomic order in interlayers of tri-s-triazine rings (He et al. 2013; Xiu et al. 2014). The g-C3N4 peaks intensity decreases upon addition of 2% Ag, which reflects the distribution of Ag NPs on the surface of 2D g-C3N4. The intensity of these peaks is further reduced by adding g-C3N4 QDs to the surface of 2% Ag/ g-C3N4 2D. The observed weak crystallinity of 2% Ag/ g-C3N4 2D/ g-C3N4 QDs indicated that both Ag NPs and g-C3N4 QDs were well-dispersed into the g-C3N4 nanosheets. No diffraction peaks were observed for Ag NPs in the XRD pattern due to their small amount embedded in g-C3N4 2D.
Figure 1 shows the FTIR spectra of the samples. g-C3N4 2D exhibits fork-like peaks at 3200 cm−1 and 3420 cm−1 corresponding to the stretching vibrations of amine N–H and -NH2 groups, respectively (Vidyasagar et al. 2018). The observed peak at 1650 cm−1 may be attributed to the C-N stretching bonds, while the obtained peaks at 1543 cm−1, 1460 cm−1, 1328 cm−1 and 1248 cm−1 may be attributed to the heterocyclic C–N and C = N stretching vibrations (Xu et al. 2013a). Moreover, the presence of the tri-s-triazine rings in g-C3N4 is confirmed by the strong and sharp peak observed at 810 cm−1 (Li et al. 2014a; Zhang et al. 2013). However, the short peak placed at 1024 cm−1 is characterized by the C − N–C groups (Bojdys et al. 2008). After decorating the g-C3N4 surface with Ag NPs, the main specific peaks featured to g-C3N4 can be distinctively located in the 2wt% Ag/ g-C3N4 2D composites, signifying that the overall g-C3N4 shape did not change, which agrees with XRD analysis. Furthermore, we observed that introducing Ag NPs into g-C3N4 2D/ g-C3N4 QDs does not promote a change in the position of the characteristic peak of tri-s-triazine in g-C3N4 2D (810 cm−1) suggesting that the configurational properties of the g-C3N4 2D were not altered after the addition of Ag particles (Li et al. 2017). However, after adding 2wt% Ag NPs to g-C3N4 2D a new peak appeared at 417 cm−1 due to (Ag–OH) which suggests the binding of Ag NPs to the mesoporous g-C3N4 2D surface. More importantly, upon addition of g-C3N4 QDs to the 2% Ag/ g-C3N4 2D sample, the N–H/-NH2 peak broadening at 3420 cm−1 directly relates to the hydroxyl (OH) groups involved in the 2% Ag/ g-C3N4 2D/ g-C3N4QDs sample which originate from physically adsorbed water. The results indicate that Ag NPs were loaded on the surface of g-C3N4 2D/ g-C3N4 QDs.
Figure 2 (a, b) shows the optical UV–Vis absorption spectra of the samples, it was observed that g-C3N4 2D exhibited an absorption band at ~ 550 nm, g-C3N4 QDs exhibited broad absorption peaks at ~ 400 and ~ 550 nm. While, the onset absorption bands of Ag/ g-C3N4 2D/ g-C3N4 QDs samples appeared at ~ 560 nm. On the other hand, the energy gap (Eg) was determined using the Kubelka–Munk formula (Li et al. 2015). Figure 2 b, shows the calculated Eg values for g-C3N4 2D, g-C3N4 QDs, 2% Ag/ g-C3N4, g-C3N4 QDs/ g-C3N4 2D, 0.5% Ag/ g-C3N4 2D/ g-C3N4 QDs, 1% Ag/ g-C3N4 2D/ g-C3N4 QDs and 2% Ag/ g-C3N4 2D/ g-C3N4 QDs were 2.82, 3.10, 2.80, 2.70, 2.71, 2.73 and 2.79 eV, respectively. All samples showed a brown color as shown in Fig. 2 c. The small change in the band gaps after the addition of Ag NPs confirms their embedding on the surface of g-C3N4 2D/ g-C3N4 QDs.
The surface charge and the stability of the 2% Ag/g-C3N4 2D /g-C3N4 QDs suspension were clarified by measuring their ζ potential at different pH values. As displayed in Fig. 2 d, the ζ potential of the 2% Ag/ g-C3N4 2D / g-C3N4 QDs at pH values; 2.2, 3.2, 6.8, 10.4 and 11.2 were 6.11 mV, 8.4 mV, -22.5 mV, -35.6 mV and -42.6 mV, respectively. The results reveal that the ζ potential value of 2% Ag/ g-C3N4 2D / g-C3N4 QDs increases gradually with rising pH and its isoelectric point (IEP = pH at ζ potential equal zero) is 4.2 (Torad et al. 2021; Zhu et al. 2015). The ζ potential outcomes also revealed that 2% Ag/g-C3N4 2D/ g-C3N4 QDs possess high negative charges above the IEP and have positive charges at pH values lower than this point. The surfaces with greatly negative charges provide a high electrostatic repulsion between the nanosheets resulting in better stability and homogeneity in aqueous solutions as recently (Milaneze et al. 2016) it was reported that the NPs exhibit ζ potential values (> + 25) mV or (< − 25 mV) display enough electrostatic repulsion to keep their solution stability. Hence, this can be highly beneficial for studying their optical and sensing characteristics. Thus, the examined nanocomposite provided high stability in the alkaline media.
The specific surface area, pore volume and size distribution were studied for g-C3N4 2D and 2% Ag/ g-C3N4 2D/ g-C3N4 QDs samples using the N2 adsorption–desorption isotherm. As shown in Fig. 3 a, the samples exhibited type IV isotherm that is accompanied by H3 hysteresis loops, suggesting their presence as the predominant mesopores structure of the obtained nanocomposite. Due to the significant amount of (CO2 and NH3) gases emitted from the precursor materials during calcination, mesoporosity has developed in the samples under investigation. The adsorbent amount of N2 molecules on the surface of 2% Ag/ g-C3N4 2D/ g-C3N4 QDs has been decreased as compared to g-C3N4 2D as displayed in Fig. 3 a. This is due to some Ag NPs having been adsorbed in the surface of g-C3N4 2D. So, the surface area of the 2% Ag/ g-C3N4 2D/ g-C3N4 QDs has been decreased from 150 m2g−1 to 140 m2g−1 upon the addition of Ag NPs and g-C3N4 QDs. As shown in Fig. 3 b, the average diameter of g-C3N4 2D pore size was determined from the distribution curves using the BJH model to be about 4 nm. This highly porous structure of g-C3N4 2D comes from the introducing holding sequences at intermediate temperatures around the surfactant decomposition (P123) during the calcination step to avoid volatilization (Wang et al. 2010). However, we observed broad and higher porosity for 2%Ag/ g-C3N4 2D/ g-C3N4 QDs with pore size distribution of ~ 13 nm suggesting that heterojunction occurred between the g-C3N4 2D and g-C3N4 QDs.
To elucidate the chemical oxidation state and the structure of 2% Ag/ g-C3N4 2D/ g-C3N4 QDs sample, we performed XPS measurements. Figure 4 a gives the distribution of Ag, N, C, and O species in the nanocomposite. As seen in Fig. 4 b, the observed two peaks in the C 1 s XPS spectra at 284.7 eV and 288 eV are characterized to sp2 C–C bonds and sp2 N–C = N bonds of the graphitic domains and the s-triazine units, respectively (Xu et al. 2013b). The observed peaks in the N 1 s spectrum at 398.6 eV and 400 eV is corresponding to sp2 (C–N = C) in the s-triazine unit and the sp3—located in N–(C)3, respectively as shown in Fig. 4 c (Xu et al. 2013c; Zhang et al. 2013). Furthermore, as shown in Fig. 4 d, we observed low amount of oxygen species probably due to two prominent peaks (in the O 1 s spectra) within 531.4 eV and 533.2 eV to chemisorbed oxygen species on the surface (Veerakumar et al. 2018). Moreover, Fig. 3 e showed the characteristic peaks for Ag 3d species. It displayed formation of two characteristic peaks at 367.8 eV and 373.8 eV, belonging to the metallic (Ag0) 3d 3/2 and (Ag0) 3d5/2, respectively, which is consistent with the literature value of Ag NPs (An et al. 2012).
The surface morphology, structural analysis, and size distribution of the g-C3N4 2D, g-C3N4 QDs, and 2%Ag/ g-C3N4 2D/ g-C3N4 QDs were performed by FE-SEM and HR-TEM analysis as shown in Fig. 5 (a-k). FE-SEM images of g-C3N4 2D show distributed multiple lamellar structures with numerous irregular worm-like holes; reflecting the pore structures within these nanosheets as revealed in Fig. 5 a. In comparison to the naked g-C3N4 2D, the 2% Ag/ g-C3N4 2D and heterojunction ternary 2% Ag/ g-C3N4 2D/ g-C3N4 QDs mesoporous nanocatalyst, exhibited a decrease in the porosity as shown in Fig. 5 (b, c). These results revealed that the Ag NPs are loaded on the surface and/or in between the g-C3N4 2D/ g-C3N4 QDs heterojunction.
FE-SEM images of (a) g-C3N4 2D, (b) 2% Ag/ g-C3N4 2D and (c) 2% Ag/ g-C3N4 2D/ g-C3N4 QDs samples, HRTEM images (d) g-C3N4 2D, (e) g-C3N4 QDs and (f) 2% Ag/ g-C3N42D/ g-C3N4QDs samples and (g) high angle annular dark field-scanning transmission electron microscopy (HAADF-STEM) image, and corresponding elemental mapping images of 2% Ag/ g-C3N4 2D/ g-C3N4 QDs sample (H–K)
Furthermore, the HR-TEM images of g-C3N4 2D also showed laminar multilayer morphology characteristic for the graphitic nature, consistent with the earlier observation (Kang et al. 2016), and the observed porous nanosheets are thinner with lateral thickness below 50 nm as displayed in Fig. 5 d (Veerakumar et al. 2018; Vidyasagar et al. 2018). The HR-TEM images of the as-synthesized g-C3N4 QDs monodisperse (Fig. 5 e)), showed the appearance of distinct clusters that were individually distributed without aggregation and exhibit sizes ranging from 5 to 15 nm, and its SAED image (inset Fig. 5 e) showed the crystal interplanar distance of 0.25 nm. Contrariwise, the images of HR-TEM (Fig. 5 f) of the 2% Ag/ g-C3N4 2D/ g-C3N4 QDs show Ag NPs well-tightly distributed around the g-C3N4 matrix's surface. The observed average size of Ag NPs is in the range 10–15 nm, suggesting a successful fabrication of Ag NPs on sheet matrix. The inset of Fig. 5 f shows the crystalline structure of Ag NPs within (d = 0.22 nm) and the SEAD displays a concentric rings because of the g-C3N4 QDs and g-C3N4 2D heterojunction (Li et al. 2017; Yu et al. 2020). The results suggest that Ag NPs are successfully adsorbed on the surface of g-C3N4 2D/ g-C3N4 QDs.
Figure 5 (g-k) shows the characteristics elemental mappings and distribution of 2% Ag/ g-C3N4 2D/ g-C3N4 QDs, using EDX-TEM scanning that shows the content of C (26.54%), N (61%0.76), O (10.22%), and Ag (1.48) mass %. Analysis results showed that Ag particles are successfully deposited on the g-C3N4 2D matrix which reflects their dense and uniform distribution throughout the whole composite surface.
Catalytic and photocatalytic ability of 2%Ag/ g-C3N4 2D/ g-C3N4 QDs nanocatalyst
The hydrogenation reaction of PNP in the presence of a reducing agent is used as a representative reaction to study both the catalytic and photocatalytic reduction action of the g-C3N4 2D, g-C3N4 QDs, and the three different ternary nanocomposites of Ag/ g-C3N4 2D/ g-C3N4 QDs nanocatalyst. Figure 6 shows the UV–Vis absorption spectra of PNP upon addition of a reducing agent (NaBH4).
There is a spectral shift on the absorption peak from 317 to 400 nm consistent with the formation of nitrophenolate ion. As shown in Fig. 6, we have studied the effect of adding ternary photocatalyst to PNP, and results showed a decrease in the absorption intensity of the 400 nm absorption peak which completely disappeared after 60 min and a novel peak appeared at 300 nm due to the produced p-aminophenol (PAP). In this system, we observed a change in the solution color from yellowish to colorless (Fig. 6 c).
The kinetic behavior of the reduction of PNP to PAP follows the empirical pseudo/first order kinetic equation: ln ([C]/[C]o) = kt, where C0 and C are the concentrations of PNP at zero and t time, respectively. The plotting of Ln(C/C0) versus time is linear and the rate constant (k) can be obtained from the slope.
As demonstrated in Fig. 7 (a-d) and Table 1, it is evident that both g-C3N4 2D and g-C3N4 QDs display a slow kinetic rate and then poor catalytic activity, whereas after photodeposition of Ag NPs into the g-C3N4 2D surface, the Ag NPs initiate the reduction process and there is a significant increase in the reaction rate with increasing the content of Ag NPs in the ternary catalyst followed by the degradation of PNP. The complete decomposition takes 60 min in the dark after the adsorption of 2% Ag NPs on the g-C3N4 2D /g-C3N4 QDs surface. After exposure to the PNP solution which contains the catalyst for solar light, the degradation of the PNP to the PAP is dramatically enhanced. The reduction rate is greatly accelerated by exposing visible light on (2% Ag/ g-C3N4 2D /g-C3N4 QDs)/ PNP/ NaBH4 structure.
As shown in Fig. 6 and Table 1, the reduction of PAP with 2%Ag/ g-C3N4 2D /g-C3N4 QDs photocatalyst under visible light only takes 6 min, whereas doing it in darkness with the same catalyst takes 60 min. As a result, the catalytic effect of light accelerates the reduction rate constant by 10 times that in the dark. As shown in Table 1, these data suggest that the designed photocatalyst enhances the conversion of PNP to the corresponding PAP compared to that of previously reported work (Table 2) (Jiang et al. 2021; Qu et al. 2022).
Mechanism for catalytic reaction
The catalytic performance of 2%Ag/ g-C3N4 2D/ g-C3N4 QDs nanomaterial is increasing the degradation of PNP both in dark and during exposure to light. In the case of dark, the electron transfer mechanism is responsible for the catalytic reduction (Zhao et al. 2015). Initially, PNP and the reducing agent (NaBH4) were both adsorbed on the porous g-C3N4 nanocatalyst. This is followed by the oxidation of NaBH4, which leads to the production of borate ions (BH4−) and the release of free electrons essential for PNP breakdown (Zhao et al. 2015).
Due to the formation of active hydrogen atoms on the surface of the Ag NPs, which react with the PNP and transform it into PAP as shown in Fig. 8 a, the degradation rate was significantly enhanced after addition of Ag NPs on the surface of g-C3N4 2D followed by addition of g-C3N4 QDs.
Mechanism for photocatalytic reduction of PNP
The reduction of PNP to PAP using 2% Ag/ g-C3N4 2D/ g-C3N4 QDs as photocatalyst proceeds via a charge separation mechanism (Hong et al. 2016). The CB and VB potentials were determined using the following equations (Zhang et al. 2015):
While ECB, Ee, and EVB represent the conduction band potential, released electrons energy compared to standard hydrogen potential (4.5 eV) and the valence band potential (Morrison and Morrison 1980) respectively, the electronegativity of semiconductors is χ can be determined using (Yuan et al. 2014):
where a, b and c represent the number of individual atoms.
The determined conduction band and valence band for g-C3N4 2D were -1.18 eV and 1.64 eV, respectively, and for the g-C3N4 QDs were -1.32 eV and 1.78 eV, respectively. Electron/hole species formed when visible light is used to irradiate a 2% Ag/g-C3N4 2D/g-C3N4 QDs sample. These photo-produced electrons in the g-C3N4 QDs CB levels shifted to g-C3N4 2D CB levels since the g-C3N4 QDs CB levels are higher than g-C3N4 2D CB levels in keeping with the Type I mechanism of electron/hole separation as shown in Fig. 8 b. Moreover, the photogenerated holes inside the g-C3N4 QDs valence band are shifted to the g-C3N4 2D due to the higher potential energy of the g-C3N4 QDs VB levels. As a result, the g-C3N4 2D conduction band drives the reduction process, and its VB regulates the oxidation process. When the CB of the photocatalyst is sufficiently negative (E = -1.18 eV compared to hydrogen electrode) to reduce the PNP to the corresponding PAP (E =—0.76 eV). Moreover, the g-C3N4 2D accumulated electrons in the CB are moving on the Ag NPs surface which performs as electron storage and is responsible for the complete PNP reduction to PAP as shown in Fig. 8 b. As well as the g-C3N4 2D holes within the valence band are responsible for some species of PNP oxidizing CO2 (2 µmole, measured using GC) as presented in Fig. 8 b. To confirm the importance of g-C3N4 QDs in this photocatalytic system, only 2% Ag/g-C3N42D is used as a photocatalyst for the reduction of PNP to PAP. Surprisingly, the results showed that the PNP was completely reduced to PAP within 20 min. Thus, we can conclude that the addition of g-C3N4 QDs (a photosensitizer) to the 2% Ag/ g-C3N4 2D system enhances the reduction of PNP to PAP within 6 min as shown in Fig. 7 b. Likewise, different amounts of Ag NPs were loaded into the g-C3N4 2D to prove the importance of Ag NPs. As can be seen from Fig. 7 (b, d) and Table 1, the observed data showed that increasing the amount of Ag NPs from 0.5% to 2% increases the photocatalytic reduction of PNP to PAP. Thus, we concluded that 2% Ag/ g-C3N4 2D/ g-C3N4 QDs photocatalyst is the optimum sample for enriching the PNP reduction ability of the corresponding PAP in a relatively short time (6 min).
To address the role of 2% Ag/ g-C3N4 2D/ g-C3N4 QDs in accelerating the photocatalytic ability for complete reduction of PNP compared to other samples. Photoluminescence (PL) study is performed as displayed in Fig. 9. The PL analysis helps determine in inspecting the efficiency of separation activity of charge carriers within this photocatalyst and investigating the electron/hole pair lifetime. All fabricated nanocomposites display a peak at 453 nm, indicating the trap energy state-defect emission. Furthermore, according to the obtained data, the most intense emitted portion is observed for g-C3N4 QDs sample and its intensity decreased after creating a heterojunction with g-C3N4 2D as well as reduced by adding more Ag NPs quantity which considers the main active sites in g-C3N4 2D/ g-C3N4 QDs nanocatalyst. This illustrates the importance of Ag NPs in reducing the recombination rate of the electron/hole pair in the 2% Ag/ g-C3N4 2D/ g-C3N4 QDs photocatalyst, resulting in superior photocatalytic efficiency as previously mentioned. The PL data were determined to give more proof of the photocatalytic efficacy improvement of the fabricated photocatalysts.
On the other hand, to confirm the durability and stability of the produced 2% Ag/ g-C3N4 2D/ g-C3N4 QDs nanocomposite in practical applications, we investigated its recyclability through four successive cycles of 4-NP photoreduction under illumination (refer to Fig. S1). Following each run, the solid catalyst was separated from the mixture via centrifugation, subsequently dried for employment in the subsequent cycle. The outcome illustrated a marginal reduction in the photocatalytic reduction efficacy, decreasing from 100 to 98% after four times. Furthermore, XRD analysis was executed on the reutilized sample, as depicted in Fig. S2. The XRD findings demonstrated an unaltered XRD pattern, affirming the impeccable reusability and stability of the synthesized 2% Ag/ g-C3N4 2D/ g-C3N4 QDs nanocomposite. Additionally, the FTIR examination (Fig. S3), for the same sample revealed typical spectra before and after usage of the % Ag/ g-C3N4 2D/ g-C3N4 QDs nanocomposite, indicating its stability.
Conclusion
In summary, this research presents novel nano-heterostructure brown g-C3N4 based materials for enhancing the photocatalytic reactivity, while ternary mesoporous Ag/ g-C3N4 2D/ g-C3N4 QDs photocatalysts are easily synthesized with different Ag NPs percentages via the photodeposition method. The characterization of the prepared photocatalyst components by various morphological and spectroscopic techniques proved that the Ag/ g-C3N4 2D/ g-C3N4 QDs photocatalysts have a mesoporous property with a wide absorption range in the visible light region. As well as, it evidenced that Ag NPs well distrusted on the surface g-C3N4 matrix. Moreover, the data illustrate and prove the considerable photocatalytic improvement of the ternary nanocomposite (2% Ag/ g-C3N4 2D/ g-C3N4 QDs) via electron/hole pairs separation. The fabricated nanocatalysts were tested to evaluate their ability to remove PNP with or without visible light. The ternary photocatalysts show significantly enhanced photocatalytic properties for the reduction of PNP in a short period of time (6 min) when subjected to visible light. According to the recorded findings, the fabricated ternary nanocomposite could be one of the potential solar light-induced nano-heterostructure photocatalysts for different environmental applications.
Data availability
All data are available from the corresponding author on request.
References
An C, Wang J, Jiang W, Zhang M, Ming X, Wang S, Zhang QJN (2012) Strongly visible-light responsive plasmonic shaped AgX: Ag (X= Cl, Br) nanoparticles for reduction of CO2 to methanol. Nanoscale 4:5646–5650
Bojdys M, Müller JO, Antonietti M, Thomas A (2008) Ionothermal synthesis of crystalline, condensed, graphitic carbon nitride. Chem Eur J 14:8177–8182
Chen Y, Cheng M, Lai C, Wei Z, Zhang G, Li L, Tang C, Du L, Wang G, Liu H (2023) The Collision between g-C3N4 and QDs in the Fields of Energy and Environment: Synergistic Effects for Efficient Photocatalysis. Small 19:2205902
Chiu YH, Hsu Y-J (2017) Au@ Cu7S4 yolk@ shell nanocrystal-decorated TiO2 nanowires as an all-day-active photocatalyst for environmental purification. Nano Energy 31:286–295
Choi Y, Koo MS, Bokare AD, Kim D-h, Bahnemann DW, Choi W (2017) Sequential process combination of photocatalytic oxidation and dark reduction for the removal of organic pollutants and Cr (VI) using Ag/TiO2. Environ Sci Techno 51:3973–3981
Dileepkumar VG, Balaji KR, Vishwanatha R, Basavaraja BM, Ashoka S, Al-Akraa IM, Santosh MS, Rtimi S (2022) CoSe2 grafted on 2D gC3N4: A promising material for wastewater treatment, electrocatalysis and energy storage. Chem Eng J 446:137023
El-Hosainy H, Alsaggaf WT, Zaki Z, Mahmoud MHH (2022) Facile synthesis of S-scheme NiS@ ZrO2 nano-heterostructure for superior degradation of antibiotic under visible light irradiation. Opt Mater 133:113011
El-Sheshtawy HS, El-Hosainy HM, Shoueir KR, El-Mehasseb IM, El-Kemary M (2019) Facile immobilization of Ag nanoparticles on g-C3N4/V2O5 surface for enhancement of post-illumination, catalytic, and photocatalytic activity removal of organic and inorganic pollutants. Appl Surf Sci 467:268–276
El-Sheshtawy HS, Shoueir KR, El-Kemary M (2020) Activated H2O2 on Ag/SiO2–SrWO4 surface for enhanced dark and visible-light removal of methylene blue and p-nitrophenol. J Alloys Compd 842:155848
Feng J, Su L, Ma Y, Ren C, Guo Q, Chen X (2013) CuFe2O4 magnetic nanoparticles: A simple and efficient catalyst for the reduction of nitrophenol. Chem Eng J 221:16–24
Ghubish Z, Kamal R, Mahmoud HR, Saif M, Hafez H, El-Kemary M (2022) Photocatalytic activation of Ag-doped SrSnO3 nanorods under visible light for reduction of p-nitrophenol and methylene blue mineralization. J Mater Sci 33:24322–24339
Guo C, Cheng M, Zhang G, Xiong W, Zhou C, Song B, Du L, Li L, Tang C, Wanga G, Liua H (2023) Degradation of organic contaminants by peroxymonosulfate activated with zeolitic imidazolate framework-based catalysts: performances, mechanisms and stability. Environ Sci Nano 10:1528–1552
He Y, Cai J, Li T, Wu Y, Lin H, Zhao L, Luo M (2013) Efficient degradation of RhB over GdVO4/g-C3N4 composites under visible-light irradiation. Chem Eng J 215:721–730
Hong Y, Jiang Y, Li C, Fan W, Yan X, Yan M, Shi W (2016) In-situ synthesis of direct solid-state Z-scheme V2O5/g-C3N4 heterojunctions with enhanced visible light efficiency in photocatalytic degradation of pollutants. Appl Catal b: Environ 180:663–673
Huang J, Ho W, Wang X (2014) Metal-free disinfection effects induced by graphitic carbon nitride polymers under visible light illumination. ChemComm 50:4338–4340
Huang Q, Yu J, Cao S, Cui C, Cheng B (2015) Efficient photocatalytic reduction of CO2 by amine-functionalized g-C3N4. Appl Surf Sci 358:350–355
Jiang T, Jiang G, Huang Q, Zhou H (2016) High-sensitive detection of dopamine using graphitic carbon nitride by electrochemical method. Mater Res Bull 74:271–277
Jiang J, Wang X, Yue C, Liu S, Lin Y, Xie T, Dong S (2021) Efficient photoactivation of peroxymonosulfate by Z-scheme nitrogen-defect-rich NiCo2O4/g-C3N4 for rapid emerging pollutants degradation. J Hazard Mater 414:125528
Ju K-S, Parales RE (2010) Nitroaromatic compounds, from synthesis to biodegradation. Microbiol Mol Biol Rev 74:250–272
Kang Y, Yang Y, Yin LC, Kang X, Wang L, Liu G, Cheng HM (2016) Selective breaking of hydrogen bonds of layered carbon nitride for visible light photocatalysis. Adv Mater 28:6471–6477
Li Q, Li YW, Wu P, Xie R, Shang JK (2008) Palladium oxide nanoparticles on nitrogen-doped titanium oxide: accelerated photocatalytic disinfection and post-illumination catalytic “memory.” Adv Mater 20:3717–3723
Li H, Liu J, Hou W, Du N, Zhang R, Tao X (2014a) Synthesis and characterization of g-C3N4/Bi2MoO6 heterojunctions with enhanced visible light photocatalytic activity. Appl Catal b: Environ 160:89–97
Li X, Ward AJ, Masters AF, Maschmeyer T (2014b) Solar Hydrogen from an Aqueous, Noble-Metal-Free Hybrid System in a Continuous-Flow Sampling Reaction System. Chem Eur J 20:7345–7350
Li WT, Zheng YF, Yin HY, Song XC (2015) Heterojunction BiOI/Bi2MoO6 nanocomposite with much enhanced photocatalytic activity. J Nanopart Res 17:1–8
Li H, Jing Y, Ma X, Liu T, Yang L, Liu B, Yin S, Wei Y, Wang Y (2017) Construction of a well-dispersed Ag/graphene-like gC3N4 photocatalyst and enhanced visible light photocatalytic activity. RSC Adv 7:8688–8693
Liu G, Niu P, Sun C, Smith SC, Chen Z, Lu GQ, Cheng H-M (2010) Unique electronic structure induced high photoreactivity of sulfur-doped graphitic C3N4. J Am Chem Soc 132:11642–11648
Milaneze BA, Oliveira JP, Augusto I, Keijok WJ, Côrrea AS, Ferreira DM, Nunes OC, Gonçalves Rd, Kitagawa RR, Celante VG (2016) Facile synthesis of monodisperse gold nanocrystals using Virola oleifera. Nanoscale Res Lett 11:1–9
Morrison SR, Morrison S (1980) Electrochemistry at semiconductor and oxidized metal electrodes. Angewandte Chemie 94:85–86
Niu P, Liu G, Cheng H-M (2012) Nitrogen vacancy-promoted photocatalytic activity of graphitic carbon nitride. J Phys Chem C 116:11013–11018
Obregón S, Vázquez A, Ruíz-Gómez M, Rodríguez-González V (2019) SBA-15 assisted preparation of mesoporous g-C3N4 for photocatalytic H2 production and Au3+ fluorescence sensing. Appl Surf Sci 488:205–212
Peng H, Xiong W, Yang Z, Tong J, Jia M, Xiang Y, Sun S, Xu Z (2023) Fe3O4-supported N-doped carbon channels in wood carbon form etching and carbonization: Boosting performance for persulfate activating. Chem Eng J 457:141317
Prabagar JS, Vinod D, Sneha Y, Anilkumar KM, Rtimi S, Wantala K, Shivaraju HP (2022) Novel gC3N4/MgZnAl-MMO derived from LDH for solar-based photocatalytic ammonia production using atmospheric nitrogen. Environ Sci Pollut Res Int 30:90383–90396
Qu X, Chen C, Lin J, Qiang W, Zhang L, Sun D (2022) Engineered defect-rich TiO2/g-C3N4 heterojunction: A visible light-driven photocatalyst for efficient degradation of phenolic wastewater. Chemosphere 286:131696
Rocha M, Costa P, Sousa CA, Pereira C, Rodríguez-Borges JE, Freire C (2018) l-serine-functionalized montmorillonite decorated with Au nanoparticles: A new highly efficient catalyst for the reduction of 4-nitrophenol. J Catal 361:143–155
She X, Wu J, Xu H, Zhong J, Wang Y, Song Y, Nie K, Liu Y, Yang Y, Rodrigues M (2017) High efficiency photocatalytic water splitting using 2D α-Fe2O3/g-C3N4 Z-scheme catalysts. Adv Energy Mater 7:1700025
Shoueir K, Kandil S, El-hosainy H, El-Kemary M (2019) Tailoring the surface reactivity of plasmonic Au@ TiO2 photocatalyst bio-based chitosan fiber towards cleaner of harmful water pollutants under visible-light irradiation. J Clean Prod 230:383–393
Spain JC (1995) Biodegradation of nitroaromatic compounds. Annu Rev Microbiol 49:523–555
Tang C, Cheng M, Lai C, Li L, Wei Z, Ma D, Du L, Wang G, Yang L, Tang L (2023) Multiple path-dominated activation of peroxymonosulfate by MOF-derived Fe2O3/Mn3O4 for catalytic degradation of tetracycline. J Environ Chem Eng 19:110395
Tian J, Liu Q, Asiri AM, Al-Youbi AO, Sun X (2013a) Ultrathin graphitic carbon nitride nanosheet: a highly efficient fluorosensor for rapid, ultrasensitive detection of Cu2+. J Anal Chem 85:5595–5599
Tian J, Liu Q, Asiri AM, Qusti AH, Al-Youbi AO, Sun X (2013b) Ultrathin graphitic carbon nitride nanosheets: a novel peroxidase mimetic, Fe doping-mediated catalytic performance enhancement and application to rapid, highly sensitive optical detection of glucose. Nanoscale 5:11604–11609
Tian J, Liu Q, Ge C, Xing Z, Asiri AM, Al-Youbi AO, Sun X (2013c) Ultrathin graphitic carbon nitride nanosheets: a low-cost, green, and highly efficient electrocatalyst toward the reduction of hydrogen peroxide and its glucose biosensing application. Nanoscale 5:8921–8924
Torad NL, El-Hosainy H, Esmat M, El-Kelany KE, Tahawy R, Na J, Ide Y, Fukata N, Chaikittisilp W, Hill JP, Zhang X, El-Kemary M, Yamauchi Y, (2021) Phenyl-modified carbon nitride quantum nanoflakes for ultra-highly selective sensing of formic acid: A combined experimental by QCM and density functional theory study. ACS Appl Mater Interfaces 13:48595–48610
Veerakumar P, Rajkumar C, Chen S-M, Thirumalraj B, Lin K-C (2018) Ultrathin 2D graphitic carbon nitride nanosheets decorated with silver nanoparticles for electrochemical sensing of quercetin. J Electroanal Chem 826:207–216
Vidyasagar D, Ghugal SG, Kulkarni A, Shende AG, Umare SS, Sasikala R (2018) Microwave assisted in situ decoration of a gC3N4 surface with CdCO3 nanoparticles for visible light driven photocatalysis. New J Chem 42:6322–6331
Wang X, Maeda K, Chen X, Takanabe K, Domen K, Hou Y, Fu X, Antonietti M (2009a) Polymer semiconductors for artificial photosynthesis: hydrogen evolution by mesoporous graphitic carbon nitride with visible light. J Am Chem Soc 131:1680–1681
Wang X, Maeda K, Thomas A, Takanabe K, Xin G, Carlsson JM, Domen K, Antonietti M (2009b) A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat Mater 8:76–80
Wang Y, Wang X, Antonietti M, Zhang Y (2010) Facile One-Pot Synthesis of Nanoporous Carbon Nitride Solids by Using Soft Templates. Chemsuschem 3:435–439
Wang K, Li Q, Liu B, Cheng B, Ho W, Yu J (2015) Sulfur-doped g-C3N4 with enhanced photocatalytic CO2-reduction performance. Appl Catal b: Environ 176:44–52
Wu X, Cheng J, Li X, Li Y, Lv K (2019) Enhanced visible photocatalytic oxidation of NO by repeated calcination of g-C3N4. Appl Surf Sci 465:1037–1046
Xiu Z, Bo H, Wu Y, Hao X (2014) Graphite-like C3N4 modified Ag3PO4 nanoparticles with highly enhanced photocatalytic activities under visible light irradiation. Appl Surf Sci 289:394–399
Xu H, Yan J, Xu Y, Song Y, Li H, Xia J, Huang C, Wan H (2013a) Novel visible-light-driven AgX/graphite-like C3N4 (X= Br, I) hybrid materials with synergistic photocatalytic activity. Appl Catal b: Environ 129:182–193
Xu J, Wang Y, Zhu Y (2013b) Nanoporous graphitic carbon nitride with enhanced photocatalytic performance. Langmuir 29:10566–10572
Xu J, Zhang L, Shi R, Zhu Y (2013c) Chemical exfoliation of graphitic carbon nitride for efficient heterogeneous photocatalysis. J Mater Chem A 1:14766–14772
Yan S, Li Z, Zou Z (2010) Photodegradation of rhodamine B and methyl orange over boron-doped g-C3N4 under visible light irradiation. Langmuir 26:3894–3901
Yan Q, Zhao C, Zhang L, Hou Y, Wang S, Dong P, Lin F, Wang Y (2019) Facile two-step synthesis of porous carbon nitride with enhanced photocatalytic activity using a soft template. ACS Sustain Chem Eng 7:3866–3874
Yang L, Luo S, Li Y, Xiao Y, Kang Q, Cai Q (2010) High efficient photocatalytic degradation of p-nitrophenol on a unique Cu2O/TiO2 pn heterojunction network catalyst. Environ Sci Technol 44:7641–7646
Yang Y, Guo Y, Liu F, Yuan X, Guo Y, Zhang S, Guo W, Huo M (2013) Preparation and enhanced visible-light photocatalytic activity of silver deposited graphitic carbon nitride plasmonic photocatalyst. Appl Catal b: Environ 142:828–837
Yang Z, Xu X, Liang X, Lei C, Cui Y, Wu W, Yang Y, Zhang Z, Lei Z (2017) Construction of heterostructured MIL-125/Ag/g-C3N4 nanocomposite as an efficient bifunctional visible light photocatalyst for the organic oxidation and reduction reactions. Appl Catal b: Environ 205:42–54
Yu B, Liu Y, Jiang G, Liu D, Yu W, Chen H, Li L, Huang Q (2017) Preparation of electrospun Ag/g-C3N4 loaded composite carbon nanofibers for catalytic applications. Mater Res Express 4:015603
Yu B, Meng F, Khan MW, Qin R, Liu X (2020) Facile synthesis of AgNPs modified TiO2@ g-C3N4 heterojunction composites with enhanced photocatalytic activity under simulated sunlight. Mater Res Bull 121:110641
Yu Q, Li X, Zhang L, Wang X, Tao Y, Wang X (2015) Synthesize of PANI/gC3N4 Composites by Interfacial Polymerization Method and Study of the Visible-light Driven Photocatalytic Performance of the Composites. J Polym Mater 32:411–422
Yuan Q, Chen L, Xiong M, He J, Luo S-L, Au C-T, Yin S-F (2014) Cu2O/BiVO4 heterostructures: synthesis and application in simultaneous photocatalytic oxidation of organic dyes and reduction of Cr (VI) under visible light. Chem Eng J 255:394–402
Zhang Y, Antonietti M (2010) Photocurrent generation by polymeric carbon nitride solids: an initial step towards a novel photovoltaic system. Chem Asian J 5:1307–1311
Zhang J, Chen X, Takanabe K, Maeda K, Domen K, Epping JD, Fu X, Antonietti M, Wang X (2010) Synthesis of a carbon nitride structure for visible-light catalysis by copolymerization. Angew Chem Int Ed 49:441–444
Zhang G, Zhang J, Zhang M, Wang X (2012) Polycondensation of thiourea into carbon nitride semiconductors as visible light photocatalysts. J Mater Chem A 22:8083–8091
Zhang J, Wang Y, Jin J, Zhang J, Lin Z, Huang F, Yu J (2013) Efficient visible-light photocatalytic hydrogen evolution and enhanced photostability of core/shell CdS/g-C3N4 nanowires. ACS Appl Mater Interfaces 5:10317–10324
Zhang L, Wang X, Nong Q, Lin H, Teng B, Zhang Y, Zhao L, Wu T, He Y (2015) Enhanced visible-light photoactivity of g-C3N4 via Zn2SnO4 modification. Appl Surf Sci 329:143–149
Zhao X, Li Q, Ma X, Xiong Z, Quan F, Xia Y (2015) Alginate fibers embedded with silver nanoparticles as efficient catalysts for reduction of 4-nitrophenol. RSC Adv 5:49534–49540
Zhu B, Xia P, Ho W, Yu J (2015) Isoelectric point and adsorption activity of porous g-C3N4. Appl Surf Sci 344:188–195
Acknowledgements
The authors acknowledge Dr. Moatz Rashad, Institute of Nanoscience & Nanotechnology, Kafrelsheikh University for his support in TEM and SEM measurements.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research was sponsored by the STDF—Science, Technology & Innovation Funding Authority, Egypt for providing financial support to this study, Project (46050).
Author information
Authors and Affiliations
Contributions
Sandy Maged: Investigation, Formal analysis, Visualization, Writing – original draft. Ola M. El-Borady: Supervision, Methodology, Validation, Writing – review & editing. Hamza El-Hosainy: Conceptualization, Methodology, Validation, Writing – review & editing. Maged El-Kemary: Supervision, Conceptualization, Methodology, Validation, Writing – review & editing. Project administration and Funding acquisition. The final manuscript was read and approved by all authors.
Corresponding author
Ethics declarations
Ethical approval
All authors have approved the manuscript and agreed to submit it as an original research article for possible publication in this journal, the corresponding author would like to undertake that the manuscript is not under consideration by another journal.
Consent to participate
Not applicable.
Consent to publish
The corresponding author on the behalf of all authors declares that the submitted manuscript can be published as an original research article in regular issue of the journal in case of acceptance.
Competing interests
The authors declare that they have no known competing financial interests associated with this publication, and there has been no significant financial support for this work that could have influenced its outcome.
Additional information
Responsible Editor: Sami Rtimi
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maged, S., El-Borady, O.M., El-Hosainy, H. et al. Efficient photocatalytic reduction of p-nitrophenol under visible light irradiation based on Ag NPs loaded brown 2D g-C3N4 / g-C3N4 QDs nanocomposite. Environ Sci Pollut Res 30, 117909–117922 (2023). https://doi.org/10.1007/s11356-023-30010-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-30010-z