Abstract
The identification of the degradation products in objects of cultural significance, including musical instruments (e.g., a piano), is a key issue for the preservation and valorisation processes of cultural heritage. The aim of this study is to characterize the degradation products of lead weights from an important Steinway & sons piano using a multi-analytical approach that includes ionic chromatography (IC), X-ray diffraction (XRD) and Fourier transform-infrared (FTIR) spectroscopy analyses. These techniques allowed us to identify hydrocerussite as the main degradation product on the superficial layer of lead weights, followed by lead acetate and formate. Moreover, accelerated corrosion experiments in closed environments were performed under acetic and formic acid atmospheres to evaluate the development of lead acetate and formate over time. Exposure of lead weights to formic and acetic acid vapours leads to the prevalent formation of basic lead formate, which promotes the formation of hydrocerussite. These results can help to limit the degradation of these piano components and consequently preserve the sound of the piano itself.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Musical instruments constitute a specific type of cultural heritage and require special care from experts of various disciplines, such as conservators, restorers and scientists (Vaiedelich and Fritz 2017). To study the degradation phenomena of the materials that make up the cultural object turns out to be essential for preserving its unique characteristics. To limit their extent, it is necessary to consider what environmental conditions favour these phenomena. Also, particularly important is the relationship existing between the constituent materials and the sounds developed, especially in the case of violins, that are among the most investigated musical instruments (Fichera et al. 2018; Invernizzi et al. 2020). Regarding the piano, an instrument of choice in classical music (Bokiau et al. 2017), in addition to the preservation of its constituent wood, other elements are crucial in the restoration operations: these are the so called lead weights (LW) that constitute a fundamental part of piano keys, since they enable its sound (Costa and Urban 2005; Watkinson 2010). Moreover, the nature of the materials of which the piano is made and the environmental conditions to which it is exposed must also be considered (Butlin 1990; Chiavari et al. 2008; Fichera et al. 2018).
Microclimatic conditions and indoor pollutants are often responsible for some degradation phenomena of works of art (Slezakova et al. 2011; Kontozova-Deutsch et al. 2011b; Chiantore and Poli 2021; Motta et al. 2022; Pironti et al. 2022). In addition, building materials can act as sources of indoor pollutants affecting the work of art itself. Air pollutants that are typically monitored at cultural heritage sites, such as museum environments and religious sites (churches, sanctuaries), include sulphur, nitrogen and carbon oxides, but also ozone, ammonia and particulate matter (Krupińska et al. 2012; Griesser et al. 2016; Pironti et al. 2023). In fact, high concentrations of these compounds are responsible for the degradation phenomena of the artifacts exposed to them. Carboxylic acids, such as acetic acid (AcOH) and formic acid (FOH), are considered dangerous for some works of art, even at low concentrations (μg/m3), so they pose a major problem for the conservation in museums (Grzywacz and Tennent 1994). Limestone, ceramics, bronze, lead and copper undergo irreversible damage due to the presence of these organic acids (Tétreault et al. 2003; Hodgkins et al. 2011), and, over time, this phenomenon may cause the complete deterioration of the artifacts containing these materials. The source of AcOH and FOH is well known and mostly includes wood and wooden products (Kontozova-Deutsch et al. 2008; Alapieti et al. 2020). As a consequence, several studies are reported in the literature concerning sampling and analytical methodologies for AcOH and FOH detection in the field of cultural heritage (Johnson et al. 1994; Kontozova-Deutsch et al. 2008, 2011a; Krata et al. 2009; Degano and La Nasa 2016; Smedemark et al. 2020; Kraševec et al. 2021; Michalski et al. 2021).
Concerning the atmospheric degradation of lead, generally lead oxide (PbO) is produced first; subsequently plumbonacrite (Pb5O(OH)2(CO3)2) and hydrocerussite (Pb3(OH)2(CO3)2) are formed by reaction with carbon dioxide (Niklasson et al. 2008; Inberg et al. 2018). AcOH and FOH are very reactive with lead, so the LW can be easily degraded due to the exposure to these acids emitted from the wood from which the piano is made (Graedel 1994; Lyon 2010; Deflorian and Fedel 2013; Eggert and Fischer 2021). Literature studies suggest that such acid compounds can accelerate the lead corrosion and degradation of lead artifacts by promoting the formation of cerussite (PbCO3), plumbonacrite and hydrocerussite (Graedel 1994; Tétreault et al. 1998).
Recently, we analysed for the first time the degradation compounds of one LW sample from a Steinway & sons piano (Faggiano et al. 2022). These preliminary results suggested that the lead of LW degraded into hydrocerussite. In this study, we extended the number of samples analysed in order to confirm this interesting finding and completely characterize these compounds using a multi-analytical approach. Furthermore, we decided to perform accelerated corrosion experiments under AcOH and FOH atmospheres to evaluate the development of the degradation products over time.
Materials and methods
Materials
All the chemicals used for the measurements (NaOH solution 49–51%, CH3CO2Na, HCO2Na, NaNO3, NaCl, Na2SO4, Acetic acid 96%, Formic acid, KBr, Mg(NO3)2) were purchased from Sigma-Aldrich (St. Louis, MO, USA). LW samples (6 not degraded and 4 degraded) from a Steinway & sons piano (manufactured in 1953 and made of Bavarian spruce wood) were provided by a private restorer.
Samples exposed to formic and acetic acid vapours
FOH and AcOH atmospheres were prepared by adding 1 mL of both acids to 50 mL of saturated solution of Mg(NO3)2 at the bottom of the 750 cm3 desiccator, as previously reported (Gibson and Watt 2010). The use of saturated solution of Mg(NO3)2 allows us to obtain a value of relative humidity (RH) of about 54%, which is typical of museum environments. The atmospheric concentrations of FOH and AcOH were determined experimentally using passive samplers after 1 week. Prior to acid vapours exposure, non-degraded samples were sonicated in distilled water and the IC analysis of the washings confirmed the absence of soluble anions on LW surface. Four non-degraded samples (one per exposure time) were placed in the mixed acids environment. As before the start weight, m0, after the specific time of exposure (1, 2, 3 and 4 weeks), the sample was removed, equilibrated in a desiccator at 25 °C for 2 h, and finally weighed. The weight increment (M) at the different exposure times was calculated using the following equation:
where mi is the mass (g) of LW after the specific time of exposure (i = 1, 2, 3 and 4 weeks) and m0 is the mass (g) of LW before the exposure to organic acid vapours. Similarly, the weight increment of formate (F) and acetate (A) was calculated using Eqs. 2 and 3.
where fi and ai are the mass (g) of formate and acetate ions, respectively, obtained from ion chromatography measurements, after the specific time of exposure (i = 1, 2, 3 and 4 weeks) and m0 is the mass (g) of LW before the exposure to organic acid vapours.
Passive sampling
Palmes tubes were purchased by Gradko Ltd., UK, and used to evaluate the atmospheric concentration of FOH and AcOH in the prepared mixed acids environment. Samplers were prepared by pipetting an exact volume (40 μL) of the sorbent solution (1 M of KOH and 10% v/v of glycerol) into two stainless steel mesh discs placed inside one cap of the tube and stored in the refrigerator before use (Gibson et al. 1997a, b; Malagodi et al. 2017). After sampling, 3 mL of distilled water was used to extract the trapped analytes on the stainless-steel mesh discs. The concentration of acetate and formate ions present in the extracts was determined by ion chromatography and used to obtain AcOH and FOH masses sampled. The values were converted into air concentrations, expressed as mg/m3, using the procedure reported in the supporting material.
Sample treatment and analytical determinations
To recover the degradation patina on LW, samples were placed into test tubes together with 6 mL of distilled water and put into an ultrasonic bath for 1 h. Subsequently, the obtained suspensions were divided into two parts: one was filtered on 0.45 μm filter and stored for ion chromatography analyses (isocratic hydroxide selective anion-exchange column and conductivity system detector (Ricciardi et al. 2022)); the other was slowly evaporated on a glass slide to perform directly X-ray diffraction measurements (Ricciardi et al. 2018), thereafter, the solid was removed from the glass slide and mixed with potassium bromide to prepare the disk for infrared spectroscopy analyses (Della Monica et al. 2019). In order to achieve proper separation of formate and acetate ions peaks, several experimental conditions were tested: sodium hydroxide eluent concentration from 1 to 20 mM, suppressor current from 20 to 60 mA and flow rate from 0.8 to 1.2 mL/min. The best results were achieved using a sodium hydroxide concentration of 5.0 ± 0.3 mM, a suppressor current of 50 mA and a flow rate of 1.0 mL/min. Under these optimized conditions, we obtain the following parameters for the two carboxylates:
-
Acetate: regression line y = 0.06286 x + 0.00330; R2 = 0.99905; LOD: 0.28 mg/L; LOQ: 0.86 mg/L;
-
Formate: regression line y = 0.16146 x − 0.02249; R2 = 0.99993; LOD: 0.12 mg/L; LOQ: 0.35 mg/L.
For all the instrumental details, see supporting materials.
Results and discussion
Analyses of degradation products on LW samples
Degraded LW samples from a Steinway & sons piano were characterized in terms of composition of the degraded layer using different analytic techniques: IC, XRD and FTIR. The last two were performed on the patina removed by sonication in water and dried on a glass slide in order to identify the overall deterioration products formed on LW.
X-ray diffraction spectra of degraded samples (Fig. 1) show the typical diffraction pattern of the trigonal (R\(\overline{3}\)m) structure of hydrocerussite, Pb3(OH)2(CO3)2, as identified by Miller indices. FTIR spectra (Fig. 2) confirmed the presence of hydrocerussite as main component of degraded patina on lead artifacts, with the following characteristic signals: O—H stretching vibrations band (3500 cm−1), ν3 asymmetric C—O stretching vibrations of CO32− anions (1400 cm−1), ν1 symmetric C—O stretching vibrations of CO32−anions (1045 cm−1), ν2 out-pf-plane bending vibrations of CO32−anions (835 and 805 cm−1) and ν4 in plane bending vibrations of CO32−anions (684 cm−1) (Brooker et al. 1983; Siidra et al. 2018).
The determination of water-soluble compounds on degraded samples was performed by anionic chromatography on the patina removed by sonication in water and filtration on a 0.45 μm filter. Amounts of soluble anions, in terms of milligrams of anion per grams of LW and weight percentage with respect to the weight of removed patina, were reported in Table 1.
Acetate results to be the most abundant anion (5.6 ± 0.2 %), followed by formate (1.99 ± 0.02 %), nitrate (0.28 ± 0.01) and chloride (0.17 ± 0.01). These results are in line with the ambient exposure of these LW to acid vapours. As a consequence, the release of VOCs, especially AcOH and FOH, from the wood of the piano plays an important role in the degradation of its LW. In more detail, the piano under study is made of Bavarian spruce, which, as softwood, releases elevated concentrations of AcOH and FOH, prompting the formation of lead acetate and formate on LW surface (Adamová et al. 2019). Among all the degradation compounds, we can clearly observe the signals of hydrocerussite from XRD and FTIR spectra, since this compound is the main component of degraded patina. If, on the one hand, the detection of a not negligible amount of acetate and formate confirms the exposure of lead to organic acids, it also pushes toward the search for the contribution of these acids in accelerating the degradation of these LW and promoting the formation of hydrocerussite.
Samples exposed to formic and acetic acid vapours
In order to evaluate the effect of both FOH and AcOH on LW deterioration, undegraded samples were exposed to FOH and AcOH vapours in controlled conditions: RH around 54%, close to that typical of museum environments, and FOH and AcOH concentration of 246 ± 25 mg/m3 and 115 ± 12 mg/m3, respectively. Anionic chromatography on the soluble part of the formed patina, allowed us to detect mainly formate and small amounts of acetate. For each exposure time (1, 2, 3, 4 weeks), the total weight increment (M) and that related to acetate (A) and formate (F) were calculated according to Eqs. 1, 2 and 3. Figure 3 shows the trend of calculated values as a function of exposure time.
The total weight increment (M) and the formate weight increment (F) follow the same trend with the increase of exposure time: a large rise in the first week, which then holds constant in the second week, then increases again in the third week and remains constant in the fourth week. On the contrary, acetate has a weight increment (A) that remains almost constant as exposure time changes. The higher amount of formate detected with respect to acetate (up to nearly 10 times higher) could be due to the fact that the action of AcOH on lead can be hindered in the presence of a comparable amount of FOH (Tétreault et al. 2003). The weight increment rate changes of the formic acid were probably due to an initial fast rate of formate formation on the bare lead and then possibly due to changes in the rate of formate formation associated with its conversion to hydrocerussite.
Although many studies have looked at lead corrosion triggered by exposure to AcOH and FOH alone (Niklasson et al. 2005, 2008; Gibson and Watt 2010; Malagodi et al. 2017), only one example involves the simultaneous presence of AcOH and FOH (Niklasson et al. 2007). In the latter case, a synergistic effect was observed due to the simultaneous presence of the two acids: corrosion is faster and more difficult to remove. Moreover, the predominant formation of a water-soluble crystalline phase (unassigned structure) containing both formate and acetate was observed (Niklasson et al. 2007).
In this study, to find the chemical nature of degradation compounds formed under controlled exposure to acid vapours, FTIR (Fig. 4) and XRD (Fig. 5) measurements were performed on the patina removed by sonication in water at different exposure times.
FTIR spectra show the typical signal of carboxylates: C=O stretching at 1590 cm−1 and 1628 cm−1, C-O stretching at 1346 cm−1 and 1385 cm−1. As exposure time increases from 1 week to 4 weeks, there is a reduction in the intensities of the bands at 1385 cm−1 and 1628 cm-1.
X-ray diffraction spectra of samples exposed to FOH and AcOH vapours (Fig. 5) show the diffraction pattern of both basic lead formate (*), Pb2O(HCOO)2 and hydrocerussite (H). Peaks with 2θ (hkl) 12.5° (110), 19.8° (111), 29.4° (221), 30.7° (002), 38° (330), 41.5° (241), 51.2° (350) and 65.6° (114) were assigned to the orthorhombic (Cccm) structure of basic lead formate (Mauck et al. 2010). At the same time, identified hydrocerussite peaks are at 2θ (hkl) 20.4° (101), 24.6° (104), 27.4° (015), 34° (009) and 47° (027).
As the exposure time increases, the intensity of basic lead formate peaks decreases slightly with a corresponding increase in the intensity of hydrocerussite peaks. Regarding the FTIR spectra (Fig. 4), it is likely that the hydrocerussite signals are masked by the more intense signals of lead carboxylates. No signals attributable to crystalline structures of lead acetates are observed. This may be due, in the first instance, to the lower measured amount of acetate compared to formate. In addition, a very intense signal at 17.2° is observed, which could be associated with crystalline forms containing both lead formate and acetate, as previously reported (Niklasson et al. 2007).
The results of our study confirm how exposure to AcOH and FOH vapours, under controlled humidity conditions, promotes lead corrosion and hydrocerussite formation over time, even in the case of piano LW. The effect of these acids on the atmospheric corrosion of lead suggests that it is important to monitor the concentration of both AcOH and FOH in theatres and museums.
Conclusions
In this paper, the degradation compounds on LW from a Steinway & Sons piano were characterized using a multi-analytical approach. XRD and FTIR measurements allowed to identify hydrocerussite as the main component of the degradation products, whereas acetate (5.6 ± 0.2%) and formate (1.99 ± 0.02 %) are the most abundant among soluble anions detectable by IC analyses. Accelerated corrosion experiments in closed environments highlight how exposure of LW to AcOH and FOH vapours leads to the prevalent formation of basic lead formate, which promotes the formation of hydrocerussite over time. These results proved that VOCs (AcOH and FOH) emitted from the wood of the piano may trigger the degradation of piano LW.
Data availability
Not applicable
References
Adamová T, Hradecký J, Prajer M (2019) VOC Emissions from spruce strands and hemp shive: In Search for a low emission raw material for bio-based construction materials. Materials 12:2026. https://doi.org/10.3390/ma12122026
Alapieti T, Mikkola R, Pasanen P, Salonen H (2020) The influence of wooden interior materials on indoor environment: a review. Eur J Wood Prod 78:617–634. https://doi.org/10.1007/s00107-020-01532-x
Bokiau B, Ceulemans A-E, Fisette P (2017) Historical and dynamical study of piano actions: a multibody modelling approach. J Cult Herit 27:S120–S130. https://doi.org/10.1016/j.culher.2016.04.010
Brooker MH, Sunder S, Taylor P, Lopata VJ (1983) Infrared and Raman spectra and X-ray diffraction studies of solid lead(II) carbonates. Can J Chem 61:494–502. https://doi.org/10.1139/v83-087
Butlin RN (1990) Effects of air pollutants on buildings and materials. Proc R Soc Edinburgh, Sec B: Biol Sci 97:255–272. https://doi.org/10.1017/S0269727000005376
Chiantore O, Poli T (2021) Indoor air quality in museum display cases: volatile emissions, materials contributions, impacts. Atmosphere 12:364. https://doi.org/10.3390/atmos12030364
Chiavari C, Martini C, Prandstraller D et al (2008) Atmospheric corrosion of historical organ pipes: the influence of environment and materials. Corros Sci 50:2444–2455. https://doi.org/10.1016/j.corsci.2008.06.045
Costa V, Urban F (2005) Lead and its alloys: metallurgy, deterioration and conservation. Stud Conserv 50:48–62. https://doi.org/10.1179/sic.2005.50.Supplement-1.48
Deflorian F, Fedel M (2013) Electrochemical analysis of the degradation of lead alloy organ-pipes due to acetic acid. J Cult Herit 14:254–260. https://doi.org/10.1016/j.culher.2012.06.002
Degano I, La Nasa J (2016) Trends in high performance liquid chromatography for cultural heritage. Top Curr Chem (Z) 374:20. https://doi.org/10.1007/s41061-016-0020-8
Della Monica F, Ricciardi M, Proto A et al (2019) Regioselective ring-opening of glycidol to monoalkyl glyceryl ethers promoted by an [OSSO]-FeIII triflate complex. ChemSusChem 12:3448–3452. https://doi.org/10.1002/cssc.201901329
Eggert G, Fischer A (2021) The formation of formates: a review of metal formates on heritage objects. Herit Sci 9:26. https://doi.org/10.1186/s40494-021-00499-z
Faggiano A, Ricciardi M, Pironti C et al (2022) Investigation on the deterioration of lead artifacts. A case study on piano keys and their degradation products. J Phys: Conf Ser 2204:012026. https://doi.org/10.1088/1742-6596/2204/1/012026
Fichera GV, Rovetta T, Fiocco G et al (2018) Elemental analysis as statistical preliminary study of historical musical instruments. Microchem J 137:309–317. https://doi.org/10.1016/j.microc.2017.11.004
Gibson LT, Cooksey BG, Littlejohn D, Tennent NH (1997a) Determination of experimental diffusion coefficients of acetic acid and formic acid vapours in air using a passive sampler. Anal Chim Acta 341:1–10. https://doi.org/10.1016/S0003-2670(96)00566-1
Gibson LT, Cooksey BG, Littlejohn D, Tennent NH (1997b) A diffusion tube sampler for the determination of acetic acid and formic acid vapours in museum cabinets. Anal Chim Acta 341:11–19. https://doi.org/10.1016/S0003-2670(96)00567-3
Gibson LT, Watt CM (2010) Acetic and formic acids emitted from wood samples and their effect on selected materials in museum environments. Corros Sci 52:172–178. https://doi.org/10.1016/j.corsci.2009.08.054
Graedel TE (1994) Chemical mechanisms for the atmospheric corrosion of lead. J Electrochem Soc 141:922. https://doi.org/10.1149/1.2054858
Griesser M, Kockelmann W, Hradil K, Traum R (2016) New insights into the manufacturing technique and corrosion of high leaded antique bronze coins. Microchem J 126:181–193. https://doi.org/10.1016/j.microc.2015.12.002
Grzywacz CM, Tennent NH (1994) Pollution monitoring in storage and display cabinets: carbonyl pollutant levels in relation to artifact deterioration. Stud Conserv 39:164–170. https://doi.org/10.1179/sic.1994.39.Supplement-2.164
Hodgkins RE, Grzywacz CM, Garrell RL (2011) An improved ion chromatography method for analysis of acetic and formic acid vapours
Inberg A, Ashkenazi D, Cohen M et al (2018) Corrosion products and microstructure of copper alloy coins from the Byzantine-period Ma’agan Mikhael B shipwreck, Israel. Microchem J 143:400–409. https://doi.org/10.1016/j.microc.2018.08.033
Invernizzi C, Fiocco G, Iwanicka M et al (2020) Non-invasive mobile technology to study the stratigraphy of ancient Cremonese violins: OCT, NMR-MOUSE, XRF and reflection FT-IR spectroscopy. Microchem J 155:104754. https://doi.org/10.1016/j.microc.2020.104754
Johnson BJ, Huang SC, Wong A, Yao L (1994) Collection and analysis of trace formic and acetic acid vapors in air using aminopropyl-modified surfaces. Microchem J 49:78–84. https://doi.org/10.1006/mchj.1994.1011
Kontozova-Deutsch V, Deutsch F, Bencs L et al (2011a) Optimization of the ion chromatographic quantification of airborne fluoride, acetate and formate in the Metropolitan Museum of Art, New York. Talanta 86:372–376. https://doi.org/10.1016/j.talanta.2011.09.030
Kontozova-Deutsch V, Deutsch F, Godoi RHM et al (2011b) Urban air pollutants and their micro effects on medieval stained glass windows. Microchem J 99:508–513. https://doi.org/10.1016/j.microc.2011.07.003
Kontozova-Deutsch V, Krata A, Deutsch F et al (2008) Efficient separation of acetate and formate by ion chromatography: application to air samples in a cultural heritage environment. Talanta 75:418–423. https://doi.org/10.1016/j.talanta.2007.11.025
Kraševec I, Menart E, Strlič M, Kralj Cigić I (2021) Validation of passive samplers for monitoring of acetic and formic acid in museum environments. Herit Sci 9:19. https://doi.org/10.1186/s40494-021-00495-3
Krata A, Kontozova-Deutsch V, Bencs L et al (2009) Single-run ion chromatographic separation of inorganic and low-molecular-mass organic anions under isocratic elution: application to environmental samples. Talanta 79:16–21. https://doi.org/10.1016/j.talanta.2009.02.044
Krupińska B, Worobiec A, Gatto Rotondo G et al (2012) Assessment of the air quality (NO2, SO2, O3 and particulate matter) in the Plantin-Moretus Museum/Print Room in Antwerp, Belgium, in different seasons of the year. Microchem J 102:49–53. https://doi.org/10.1016/j.microc.2011.11.008
Lyon SB (2010) 3.11 - Corrosion of lead and its alloys. In: Cottis B, Graham M, Lindsay R et al (eds) Shreir’s corrosion. Elsevier, Oxford, pp 2053–2067
Malagodi M, Milanese C, Licchelli M, et al (2017) Alteration processes of pigments exposed to acetic and formic acid vapors. In: 2017 IEEE International Conference on Environment and Electrical Engineering and 2017 IEEE Industrial and Commercial Power Systems Europe (EEEIC / I&CPS Europe). pp 1–6
Mauck CM, van den Heuvel TWP, Hull MM et al (2010) Synthesis and structures of Pb3O2(CH3COO)2·0.5H2O and Pb2O(HCOO)2: two corrosion products revisited. Inorg Chem 49:10736–10743. https://doi.org/10.1021/ic101904j
Michalski R, Pecyna-Utylska P, Kernert J (2021) Ion chromatography and related techniques in carboxylic acids analysis. Crit Rev Anal Chem 51:549–564. https://doi.org/10.1080/10408347.2020.1750340
Motta O, Pironti C, Ricciardi M et al (2022) Leonardo da Vinci’s “Last Supper”: a case study to evaluate the influence of visitors on the Museum preservation systems. Environ Sci Pollut Res 29:29391–29398. https://doi.org/10.1007/s11356-021-13741-9
Niklasson A, Johansson L-G, Svensson J-E (2008) The influence of relative humidity and temperature on the acetic acid vapour-induced atmospheric corrosion of lead. Corros Sci 50:3031–3037. https://doi.org/10.1016/j.corsci.2008.08.009
Niklasson A, Johansson L-G, Svensson J-E (2005) Influence of acetic acid vapor on the atmospheric corrosion of lead. J Electrochem Soc 152:B519. https://doi.org/10.1149/1.2084348
Niklasson A, Johansson L-G, Svensson J-E (2007) Atmospheric corrosion of lead: the influence of formic acid and acetic acid vapors. J Electrochem Soc 154:C618. https://doi.org/10.1149/1.2775173
Pironti C, Ricciardi M, Proto A et al (2022) New analytical approach to monitoring air quality in historical monuments through the isotopic ratio of CO2. Environ Sci Pollut Res 29:29385–29390. https://doi.org/10.1007/s11356-020-12215-8
Pironti C, Ricciardi M, Motta O et al (2023) Sulphurous air pollutants and exposure events of workers in thermal-mineral springs: a case study of Contursi Terme (Salerno, Italy). Environ Sci Pollut Res 30:3112–3120. https://doi.org/10.1007/s11356-022-22432-y
Ricciardi M, Falivene L, Tabanelli T et al (2018) Bio-glycidol conversion to solketal over acid heterogeneous catalysts: synthesis and theoretical approach. Catalysts 8:391. https://doi.org/10.3390/catal8090391
Ricciardi M, Pironti C, Motta O et al (2022) Investigations on historical monuments’ deterioration through chemical and isotopic analyses: an Italian case study. Environ Sci Pollut Res 29:29409–29418. https://doi.org/10.1007/s11356-021-15103-x
Siidra O, Nekrasova D, Depmeier W et al (2018) Hydrocerussite-related minerals and materials: structural principles, chemical variations and infrared spectroscopy. Acta Crystallogr B Struct Sci Cryst Eng Mater 74:182–195. https://doi.org/10.1107/S2052520618000768
Slezakova K, Castro D, Begonha A et al (2011) Air pollution from traffic emissions in Oporto, Portugal: health and environmental implications. Microchem J 99:51–59. https://doi.org/10.1016/j.microc.2011.03.010
Smedemark SH, Ryhl-Svendsen M, Schieweck A (2020) Quantification of formic acid and acetic acid emissions from heritage collections under indoor room conditions. Part I: laboratory and field measurements. Heritage. Science 8:58. https://doi.org/10.1186/s40494-020-00404-0
Tétreault J, Cano E, van Bommel M et al (2003) Corrosion of copper and lead by formaldehyde, formic and acetic acid vapours. Stud Conserv 48:237–250. https://doi.org/10.1179/sic.2003.48.4.237
Tétreault J, Sirois J, Stamatopoulou E (1998) Studies of lead corrosion in acetic acid environments. Stud Conserv 43:17–32. https://doi.org/10.1179/sic.1998.43.1.17
Vaiedelich S, Fritz C (2017) Perception of old musical instruments. J Cult Herit 27:S2–S7. https://doi.org/10.1016/j.culher.2017.02.014
Watkinson D (2010) 4.43 - Preservation of metallic cultural Heritage. In: Cottis B, Graham M, Lindsay R et al (eds) Shreir’s corrosion. Elsevier, Oxford, pp 3307–3340
Acknowledgements
Authors gratefully acknowledged Luigi Barone for providing LW samples.
Funding
Open access funding provided by Università degli Studi di Salerno within the CRUI-CARE Agreement. This research was supported by Consorzio Interuniversitario Nazionale per la Scienza e Tecnologia dei Materiali (INSTM).
Author information
Authors and Affiliations
Contributions
Conceptualization: MR, AP and OM. Data curation: MR, AFa and CP. Formal analysis: AFa, MR and CP. Funding acquisition: AP. Investigation: MR and AFa. Methodology: AFa, MR, CP, YM and AFi. Project administration and resource: AP. Software: AF and MR. Supervision and validation: MR and AP. Visualization: MR, AFa, AFi, AP and NM. Writing—original draft: MR. Writing—review and editing: MR, AFa, YM and OM.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Michel Sablier
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 24 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Faggiano, A., Pironti, C., Motta, O. et al. Insight on the deterioration of cultural objects: a multi-analytical approach to characterize degradation products of lead weights from a Steinway & sons piano. Environ Sci Pollut Res 30, 104633–104639 (2023). https://doi.org/10.1007/s11356-023-29790-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-29790-1


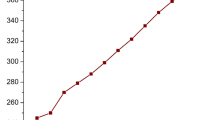




 ) and mass of formate F (
) and mass of formate F (
 ) with their standard errors
) with their standard errors
