Abstract
The fast-growing sector of insects for food and feed stimulates researchers and the industry to explore uncharted territories, such as insect breeding, to improve economically important insect fitness traits. The yellow mealworm, Tenebrio molitor L., is one of the most thoroughly studied insect species as food and feed. However, data on mating compatibility and the effect of cross-breeding between different strains on the performance and fitness of the hybrids are scarce. In the present study, we comparatively evaluated the mating compatibility between two T. molitor inbred strains (Greek and Italian) and their outbred strains, i.e., Italian (♀)-Greek (♂) and Greek (♀)-Italian (♂), as well as the performance of their hybrid offspring. Based on the results, there is good mating compatibility among adults of the strains tested. Offspring performance, quantified as larval survival and final larval weight, followed a similar pattern among the crossings examined. Even though differences were insignificant, the outbreeding of females of the Italian strain resulted in a higher cumulative number of eggs and hatching rate and higher offspring weight. The present study sheds light on the mating compatibility of different T. molitor strains and their hybrids' economically important life table characteristics to take the mass production of insects one step further.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insect production as an alternative protein source has been gaining upscaling attention during the last decade, as the development of feed sources derived from insects is consistent with the principles of the circular economy (Van Huis and Oonincx 2017). The recent European regulations consider insects as promising solutions for livestock producers who are seeking new protein-rich and nutritious products as feed (EC 2017, 2021b, EFSA et al.2021). Moreover, the European Commission aims the promotion of the organic sector; thus, two aspiring framework policies have introduced recently. One of the ambitions included in these framework policies is targeting the reduction of the farmland being used for organic farming by at least 25% by 2030. This goal would be of utmost importance for developing the insect sector in Europe, helping the transition to more sustainable food systems (EC 2020, 2021a). The yellow mealworm, Tenebrio molitor L. (Coleoptera: Tenebrionidae), is one of the most widely produced and commercialized insect species used as feed for farmed animals, in terms of novel protein sources (Rumbos and Athanassiou 2021; Van Huis 2013). The utilization of its larvae has started with the pet food industry (Martin et al. 1976), while during the last few years, it has been considered an essential alternative protein and lipid source for aquaculture (EC 2017), livestock (EC 2021b) as well as human consumption (EFSA et al. 2021).
Based on the above, the insect sector has to meet the needs of the fast-growing insect protein demands. In this context, diet optimization, as well as the improvement of rearing conditions, have attracted vast attention (Heckmann et al. 2018; Morales-Ramos et al. 2020; Rumbos et al. 2020). Another factor that has also been investigated and may contribute to the up-scaling of mass production of insects is the strain effect in terms of larval performance and adult fitness (Adamaki-Sotiraki et al. 2022a, b; Rumbos et al. 2021; Urs and Hopkins 1973b, a). Based on the available literature, an aspect that has not investigated thoroughly yet concerning insects as food and feed is the effect of cross-breeding of strains derived from different geographical areas, in respect of adult mating compatibility as well as hybrids’ fitness traits, but also on the performance of the hybrid populations that will produced after successful mating.
The idea of cross-breeding is not new for the traditional crops, livestock, and insects' sector (e.g., sericulture, apiculture, and biological pest control), as it has been operated for thousands of years and thus is currently a fine-tuned methodology (Eriksson and Picard 2021; Lippman and Zamir 2007; Notter et al. 2013). However, the main objective behind the idea of cross-breeding is to produce hybrids with superior phenotypes relative to their inbred parents regarding traits such as growth rate, reproductive success, and yield. The specific procedure is known as heterosis, or hybrid vigor (Lippman and Zamir 2007). For example, concerning sericulture, for many years now, commercial varieties of the silkworm have been produced by the single cross, double cross, or three-way cross (Sohn and Ramirez 1999). In this way, silkworm breeders managed to produce varieties with optimized economic traits such as a higher percentage of cocoon shell, raw silk, and greater egg production (Reddy et al. 2010; Sohn and Ramirez 1999). Additionally, in apiculture protocols focusing on the selective mating of queens, specific trait selection and selective breeding are well established, aiming to minimize the depletion of genetic variation (Büchler et al. 2013; Uzunov et al. 2017). Moreover, concerning the mass production of natural enemies, it is necessary to maintain separate laboratory strains and cross them systematically to increase the fitness traits of the F1 generation (Van Lenteren et al. 2004).
For T. molitor, only one work dating back to the 1930s is available on its genetics and the effect of cross-breeding on specific insect characteristics; however, this mainly focused on crosses with different eye color genotypes (Schuurman 2013). More recently published data reveal that genetic material affects economically important life table characteristics, which may contribute to the upscaling of the overall productivity of mass-rearing facilities. Indicatively, (Urs and Hopkins 1973b, a) evaluated two different T. molitor strains and reported that the faster-developing strain was capable of more efficient nutrient utilization. (Rumbos et al. 2021) reported that specific strains of T. molitor perform better concerning larval survival, weight, feed utilization, and development time. Similarly, (Adamaki-Sotiraki et al. 2022a) support that growth performance varies among different T. molitor strains reared under dry conditions. Moreover, (Berggreen et al. 2018) urge that there is a strain effect also on adults’ fitness-related traits, while (Adamaki-Sotiraki et al. 2022b) also reported that specific strains performed better in terms of adult survival and fecundity. It is a rationale that different strains express some, but not all, the desired characteristics. Cross-breeding of different strains could be utilized to produce hybrids with optimal traits derived from different parental strains. Cross-breeding has successfully applied though in other insect species. Indicatively, in the sector of sericulture, researchers utilized divergent parental strains of the silkworm Bombyx mori (L.) (Lepidoptera: Bombycidae) and produced hybrids with better economic traits and technological characteristics (Bhargava et al. 1995; Doddaswamy et al. 2009; Reddy et al. 2010).
The studies mentioned above bring to the fore the value of cross-breeding among strains toward optimization of economically important traits aiming to upscale the profit in large-scale production industries. Therefore, investigation and understanding the contribution and association of various performance and fitness traits are essential for any program aiming to improve insect-rearing practices. Based on the above, the present study aims to investigate the mating compatibility (in terms of adult survival, egg production, and larval hatching rate) among adults of different T. molitor strains, as well as the effect of cross-breeding on important fitness traits of F1 hybrid larvae. For this purpose, we have selected specific strains of T. molitor of different geographical origins that have shown desirable characteristics in earlier experimentation (Adamaki-Sotiraki et al. 2022a, b; Rumbos et al. 2021).
Materials and methods
Adult beetles and larvae of T. molitor derived from two different geographical areas, Greece and Italy, formed the materials for the present bioassays. The reason for the selection of the specific strains was the fact that they were reported to be among the strains that were previously shown to have superior performance in terms of adult fecundity, larval weight, and feed conversion efficiency (Adamaki-Sotiraki et al. 2022a; Rumbos et al. 2021). Rumbos et al (2021) have provided a detailed description of the traits of the strains used in the present study. The insects maintained at stable rearing conditions, i.e., continuous darkness, and 26 ± 0.5 °C, 50 ± 5% relative humidity, at the Laboratory of Entomology and Agricultural Zoology of the University of Thessaly. As insect feed, local wheat bran used, while agar provided as a moisture source.
Experimental design
Bioassay I
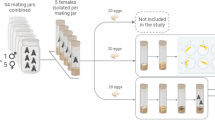
In this bioassay, the parameters that were evaluated were adult survival, egg production and hatching rate. At first, a stereoscope (Stereomicroscope Leica MZ12, Spach Optics Inc, NY, United States) was used to separate the female and male pupae based on the differences in abdominal appendages (Arena and Defagó 2020; Wang et al. 2013). After sex determination, female and male pupae were kept separately until adult emergence. Newly-emerged, virgin dark-colored adults (7–14 days old, to ensure sexual maturity) used in the experiment (Jehan et al. 2020). After that, four groups of five male and five female adult beetles were weighted and placed in the oviposition boxes for each one of the cross-breeding combinations between the two strains (two inbred strains and two outbred strains). In total, 24 oviposition boxes were utilized. As oviposition boxes, two cylindrical plastic vials (8.8 cm height, and 7.5 cm diameter) stuck inside one another. The bottom of the upper vial was removed and replaced by a circular plastic mesh (2 × 2 mm hole diameter) to permit egg oviposition and avoid egg cannibalism by the adults. The bottom vial was filled with white flour as an oviposition substrate, up to 5 mm above the mesh, to ensure that adult beetles have an adequate feed. Agar cubes (20 g/L, 1 × 1x1 cm) provided to beetles as a moisture source ad libitum (Deruytter et al. 2021). According to Gerber and Sabourin (1984) beetles mated and oviposited for seven days. After this period of time, a 600 μm opening sieve was utilized in order to sieve the flour and collect and count the laid eggs, whereas the alive and dead beetles in each vial were also counted to determine adult survival. Alive adults were then transferred to new vials. The exact process was repeated eight times with the same adults, for a total period of 56 days. The duration of the bioassay was 56 days as it has been reported that females may be fertile for up to two months (Berggreen et al. 2018). Collected eggs were transferred in new experimental units (vials) together with 1 g of wheat bran, which served as substrate for the newly-emerged larvae to feed. After a period of 8 days, the number of hatched larvae was estimated, by counting the larvae. For each treatment, six replicates were carried out.
Bioassay II
In this series of bioassays, the larval weight, survival and development time of the F1 generation larvae produced during the first and the eighth week of bioassay I were recorded. Larvae produced during the first week were separated into groups of 50 individuals at six replicates, while, due to lower availability, larvae produced during the eighth week divided into groups of 15 individuals at four replicates. Larvae produced during the first week were kept separately from the larvae produced during the eighth week. The bioassay was carried out in plastic cylindrical vials as above, and the newly-emerged larvae were provided with 4 g of local wheat bran and agar cubes (1 × 1x1 cm) as a moisture source. In total, 40 vials were utilized. Every two weeks, for each vial, larvae were isolated from the substrate utilizing sieves with openings ranging from 500 μm to 1 mm, depending on the age of the larvae, in order to sieve the feed, collect the larvae, and determine their total weight, and survival. The larval weight was recorded utilizing a precision balance (EQUINOX, Adam Equipment Inc Fox Hollow Road, Oxford, USA). The same procedure repeated up to one week after the appearance of the first pupa. The development time from newly-hatched larvae to first pupae appearance was also calculated. Feed and moisture sources provided ad libitum based on visual observations. Trials were performed in controlled rearing conditions, as previously described.
Data analysis
To determine the larval hatching rate at each evaluation point, the number of emerged larvae divided by the respective number of eggs. In order to calculate the cumulative hatching rate, the cumulative number of emerged larvae divided by the cumulative number of eggs for each tested strain and at each point of evaluation. Normal distribution tested by the Shapiro–Wilk test while homogeneity/homoscedasticity of variances tested using Levene's test checked for final adult survival, the cumulative number of eggs per adult, cumulative hatching rate, final larval weight and survival rate, as well as larval development time. Since the assumptions needed for parametric analysis met for the final adult survival, the cumulative number of eggs, cumulative hatchability, final larval weight, and survival rate, data were submitted to ANOVA to determine differences among strains, as well as differences between larvae produced from adults of different age for the same strain (P < 0.05). Concerning the adult survival, the number of eggs per adult, and the hatching rate of the larvae, a mixed effect modeling fitted to be performed due to the longitudinal nature of the data. As a consequence, a linear mixed effect model applied to investigate the interaction of different factors [strain and time (days)] on adult survival, on larval hatching rate and on the number of eggs per adult (Shek and Ma 2011). The linear mixed model used to compare the time-series data of all inbred and outbred strains in a holistic way and to supply a direct statistical comparison among inbred and outbred strains in terms of adult survival, larval hatching rate, and number of eggs per adult during the whole experimental period of time. The Kaplan–Meier method was applied to analyze the development time of larvae produced during the first and the eighth week after adults' mating, and afterwards, Mantel-Cox test used to detect contrasts between treatments.
Results
Bioassay I
For all inbred and outbred T. molitor strains, adult survival decreased over time. While no significant differences detected concerning the final survival (56 days), the linear mixed model analysis revealed that both strain (F = 7.5, P < 0.001) and time (F = 62.2, P < 0.001) significantly affected adult survival over time (Fig. 1; Table 1). The first sharp reduction in adult survival occurred 28 days after the onset of the bioassay for the inbred Italian strain (68% ± 17%) and the outbred Greek (♀)-Italian (♂) strain (72% ± 13%). For the same time interval, adult survival ranged between 80 and 88% for the outbred Italian (♀)-Greek (♂) strain and the inbred Greek strain, respectively. During the termination of the bioassay (56 days), the rate of adult survival ranged between 50% ± 16% and 57% ± 10% for all inbred and outbred strains. The highest survival rate recorded for the outbred Italian (♀)-Greek (♂) strain and the lowest for the outbred Greek (♀)-Italian (♂) strain.
Regarding the cumulative number of eggs per adult at the end of the bioassay, no significant differences were recorded among the different inbred and outbred strains tested (F = 0.49, P = 0.68, df = 3.23), while the overall egg production per adult consistently decreased over time (Fig. 2). The highest cumulative number of eggs was produced by the inbred Italian strain (154 ± 28 eggs per adult), while the lowest cumulative number of eggs produced by the inbred Greek strain (133 ± 32 eggs per adult). Considering the egg production at specific intervals over time, the highest number of eggs per adult was detected 7 days after the onset of the bioassay for all inbred and outbred strains. For instance, at this interval (7 days), the egg production ranged between 32 ± 6 and 48 ± 7 eggs per adult. On the other hand, the lowest number of eggs per adult recorded at the end of the bioassay for all inbred and outbred strains. Specifically, 6 ± 3 to 11 ± 8 eggs per adult produced at the end of the bioassay (56 days). Considering the overall data, the number of eggs per adult was significantly affected by time (F = 65.2, P < 0.001), but it was not affected by strain (F = 1.4, P = 0.23) (Table 1).
Oviposition every seven days of two inbred strains (Greek and Italian) and their outbred strains (Italian (♀)-Greek (♂) and Greek (♀)-Italian (♂)) of Tenebrio molitor over 56 days. The bars designate the number of eggs per adult at each time period. The dash lines reveal the fit curve for the cumulative number of eggs over time, and the dots symbolize the cumulative number of eggs at each time period. For dots, values depict means, while for bars, values depict means ± SE. Equations derived from the fit curve. The R.2 of the equation is also provided for each curve. For all inbred and outbred strains tested, n = 6
Moreover, no significant differences were detected in terms of cumulative hatching rate at the end of the bioassay among inbred and outbred strains (F = 2.35, P = 0.10, df = 3.23), with rates ranging between 64% ± 7% and 72% ± 4%, at the end of the bioassay (Fig. 3). However, noticeable fluctuations detected during the entire observation period for all inbred and outbred strains (Fig. 3). Hence, the outbred Greek (♀)-Italian (♂) strain hatching rate fluctuated between 60 and 70% during the whole bioassay period. Moreover, for the outbred Italian (♀)-Greek (♂) strain, hatching rate fluctuations were more apparent, reaching from 90 to 40% until the end of the bioassay. For the inbred strains hatching rate fluctuated between 70 and 40%. Based on the linear mixed model analysis, both factors, strain (F = 5.1, P = 0.002) and time (F = 159.1, P < 0.001), significantly affected the hatching rate (Table 1).
Hatching rate (%) of two inbred strains (Greek and Italian) and their outbred strains (Italian (♀)-Greek (♂) and Greek (♀)-Italian (♂)) of Tenebrio molitor over 56 days. The bars designate the larval hatching rate (%) at each time period. The dash lines reveal the fit curve for the cumulative hatching rate (%), and the dots symbolize the cumulative hatching rate at each time period. For dots, values depict means, while for bars, values depict means ± SE. Equations derived from the curve-fit. The R.2 of the equation is also provided for each curve. For all inbred and outbred strains, n = 6
Bioassay II
The larval survival rate was high until the end of the bioassay (> 87%) for all inbred and outbred strains for the larvae produced during the first week after parental mating (Fig. 4). In this context, the highest rate of larval survival was recorded for the inbred Greek strain (94% ± 3%), while the lowest rate of larval survival recorded for the outbred Italian (♀)-Greek (♂) strain (87% ± 2%). For the larvae produced during the eighth week after parental mating, the survival was also high (> 92%) for all inbred and outbred strains (Fig. 4). The highest survival rate observed for the outbred Greek (♀)-Italian (♂) strain (98% ± 3%). Regarding the survival rate for the larvae produced during the first week after parental mating, insignificant contrasts were detected among the tested strains. Similarly, the larvae produced during the eighth week after parental mating followed a similar pattern concerning their survival rate. In contrast, significant differences detected for the outbred strains; Italian (♀)-Greek (♂) strain (F = 8.1, P = 0.02, df = 1.9) as well as for Greek (♀)-Italian (♂) strain (F = 9.0, P = 0.02, df = 1.9) among larvae produced from parental adults of different age (i.e., during the first and the eighth week after adult’s mating).
Survival rate (%) of larvae of two inbred strains (Greek and Italian) and their outbred strains (Italian (♀)-Greek (♂) and Greek (♀)-Italian (♂)) of Tenebrio molitor produced during the first and the eighth week after adults’ mating (For the larvae produced on the first week after adults’ mating n = 6, while for the larvae produced on the eighth week after adults’ mating n = 4)
Concerning the final weight of larvae produced during the first week after parental mating, significant differences were recorded among the different strains tested (F = 13.13, P < 0.001, df = 3.23). The final average larval weight for all inbred and outbred strains ranged between 145 ± 17 and 182 ± 7 mg (Fig. 5). Among the strains tested, the lowest and the highest final larval weight recorded for the inbred Greek strain and the inbred Italian strain, respectively. The outbred strains provided similar results, gaining a final larval weight between 172 ± 7 and 173 ± 11 mg. During the eighth week after parental mating, the larvae produced gained a final larval weight between 181 ± 10 and 206 ± 11 mg (Fig. 5). The lowest final larval weight recorded for the outbred strain Greek (♀)-Italian (♂) and the inbred Greek strain (181 ± 10 and 186 ± 21 mg, respectively). The highest final weights calculated for the inbred Italian strain as well as for the outbred Italian (♀)-Greek (♂) strain (205 ± 21 mg). Nevertheless, no statistical differences were detected for the final larval weight of the larvae produced during the eighth week after parental mating among the different strains tested (F = 2.32, P = 0.13, df = 3.15). However, between larvae produced by adult parents of different ages (i.e., during the first and the eighth week after parental mating), significant differences were recorded for the inbred Italian (F = 6.2, P = 0.03, df = 1.9) and Greek (F = 11.1, P = 0.01, df = 1.9) strain, as well as for the outbred Italian (♀)-Greek (♂) strain (F = 35.9, P < 0.001, df = 1.9) while, for the outbred Greek (♀)-Italian (♂) no statistical differences recorded (F = 1.7, P = 0.22, df = 1.9). Interestingly, the highest larval weight recorded for the larvae produced during the eighth week after parental mating for all inbred and outbred strains tested.
The average weight of two inbred strains (Greek and Italian) and their outbred strains (Italian (♀)-Greek (♂) and Greek (♀)-Italian (♂)) of Tenebrio molitor produced during the first and the eighth week after adults’ mating (For the larvae produced on the first week after adults’ mating n = 6, while for the larvae produced on the eighth week after adults’ mating n = 4)
Concerning the larvae produced during the first week after parental mating, development time ranged between 74 ± 0.8 and 82 ± 0.9 days for all strains, while the respective development time for the larvae produced during the eighth week after parental mating ranged between 75 ± 1.8 and 79 ± 1.6 days (Fig. 6). The development time of larvae during the first week after parental mating they were significantly influenced by strain (Mantel-Cox χ2 = 20.4, P < 0.001, df = 3), while no significant differences recorded between inbred and outbred strains for larvae produced during the eighth week after parental mating.
Developmental time (days) of larvae of two inbred strains (Greek and Italian) and their outbred strains (Italian (♀)-Greek (♂) and Greek (♀)-Italian (♂)) of Tenebrio molitor produced during the first and the eighth week after adults’ mating (n = 6). Lowercase letters depict significant differences between larvae produced from adults of different strains for the same time interval, while the absence of letters depicts no significant differences. However, for each time interval means with the different lowercase letter, differ significantly. For each case, values designate means ± SEM (For the larvae produced during the first week after adults' mating, n = 6, while for the larvae produced during the eighth week after adults' mating, n = 4)
Discussion
Commercial insect producers and research institutions aim to reveal insects’ desired characteristics to improve the fast-growing sector of insect mass-rearing. A common way to improve fitness-related traits is cross-breeding among insect strains (Eriksson and Picard 2021). In the general context of artificial selection and selective breeding for T. molitor, a few studies consider improving important economic traits such as insects’ body size, weight and growth rate, fecundity, and feed conversion efficiency (Leclercq 1963; Morales-Ramos et al. 2019). Past and recent research reveals that the selection of specific strains may also be beneficial for the growth and development of T. molitor larvae, as well as offspring production and survival (Adamaki-Sotiraki et al. 2022a, b; Rumbos et al. 2021; Urs and Hopkins 1973b, a). Based on the aforementioned reported differences in larval fitness traits and adults' performance, it is evident that insect farming systems' production will be optimized by selecting the proper strain. However, our data indicate a gap in terms of the cross-breeding effect among different strains of T. molitor on adults' performance and their offspring's fitness characteristics, especially at the larval stage.
Our study is the first one to assess the cross-breeding of two strains of T. molitor. Our results indicate good mating compatibility among adults of the different strains tested. Good mating compatibility is evident from the high reproductive output and the hatching rates over time, both for inbred and outbred strains. In general, data on the evaluation of cross-breeding of different insect strains are scarce and usually oriented towards life table characteristics of the silkworm, B. mori, and the honeybee, Apis mellifera (L.) (Hymenoptera: Apidae), as well as specific biocontrol agents (Eriksson and Picard 2021). Several studies related to sericulture have shown that cross-breeding has been a valuable tool in creating new and productive silkworm strains (Doddaswamy et al. 2009; Neshagaran Hemmatabadi et al. 2016). For instance, Reddy et al (2010) reported that the outbred crosses of divergent geographic eco races of B. mori revealed high heterosis potential and good compatibility of parents for yield contributing traits; fecundity, shell weight, and silk ratio. Along the same lines, Doddaswamy et al (2009), while experimenting with inbred and outbred crosses of B. mori, reported that F1 hybrids of outbred crosses performed better in terms of specific economic traits, such as tightly formed shells of spun cocoons, uniform growth, and consistent crop performance, compared to inbred crosses. Similarly, Bhargava et al (1995) tested the cross-breeding of five B. mori races and reported differences for seven silk technological characters; the weight of cocoon and shell, cocoon shell ratio, percentage of raw silk, length of silk filament, silk reliability, as well as silk neatness. However, cross-breeding only sometimes results in improving all economically valuable traits. For instance, Sohn and Ramirez (1999) studied the single, three-way, and double cross hybrids from four parental lines of B. mori in terms of cocoon quality and reported the correlation between cocoon weight and the presence of silken shell of cocoon was negative. Although from another perspective, that of silk production, the studies mentioned above show that cross-breeding can be a precious tool in developing high-quality B. mori hybrids. Apart from being a primary producer of silk, this species recently approved in the EU as an ingredient of poultry and pig diets, as well as aquafeeds (EU Regulation 2021/1925 (EC 2021c). However, no studies have been published regarding the effect of cross-breeding on the growth parameters of B. mori as an edible insect. We did not detect any apparent heterosis effect for the outbred strains tested, as offspring performance, quantified as larval survival and final larval weight, followed a similar pattern among the crossings examined in our study. In one of the few studies on T. molitor crosses, the crossing of two strains with different lengths, i.e., a small and a large strain, resulted in individuals of the F1 generation with an intermediary size (Schuurman 2013). Therefore, it becomes evident that classical breeding, in which genes are rearranged randomly, does not always result in offspring with improved characteristics. However, the recent access to the entire T. molitor genome opens new opportunities for genetic breeding and substantially contributes to producing new T. molitor lines with desirable traits (Eleftheriou et al. 2021).
Focusing on adult survival, egg production, and hatching rate, the inbred Italian strain and the outbred Italian (♀)-Greek (♂) strain gave the best results, even though no significant differences recorded between the strains tested. Indicatively, the outbred Italian (♀)-Greek (♂) strain produced the highest cumulative number of eggs, while it came up with the highest cumulative hatching rate. In accordance with the conclusions of McNamara et al (2009), who stated that organisms might choose to invest more in reproduction while they are still young, adult fecundity in our study declined while adults were getting older for inbred and outbred strains. The highest number of eggs oviposited 7 days after the onset of the bioassay. According to Morales-Ramos et al (2012), the highest rates of reproduction (33 eggs per female per week) takes place early in life for T. molitor, i.e., within the first two to three weeks of adults’ life. However, Adamaki-Sotiraki et al (2022b) reported that there might be a strain effect on the exact time that beetles reach their highest reproductive output. Interestingly, in our study, the exact time might have been affected by female parents of the different strains. For instance, for the inbred Italian strain and the outbred Italian (♀)-Greek (♂) strain, the highest reproductive output occurred at 28 days, while for the inbred Greek strain and the outbred Greek (♀)-Italian (♂) strain at 35 days. Based on our data, fluctuations in egg production were not vigorously expressed in our study, and this may be due to the mesh that was utilized in order to separate the adults from their offspring. We hypothesize that adding this mesh will likely minimize cannibalism, which is very common in T. molitor (Berggreen et al. 2018; Gerber and Sabourin 1984; Halliday et al. 2015). However, in another study, Adamaki-Sotiraki et al (2022b) did not use a mesh to separate adults from their offspring and reported that the fluctuations in egg production were due to the proneness of adults of T. molitor to cannibalize their eggs. The female adult beetles used in our study were not labeled/colored. Consequently, whether female survival has affected the number of eggs produced could be argued with certainty. Nevertheless, based on the existing literature, the lifespan of T. molitor adults is normally not influenced by sex (Rho and Lee 2016).
The present study's data may also indicate that when female adults from the Italian strain are involved, better offspring, in terms of larval weight, are produced. For instance, larvae produced during the eighth week after mating for the inbred Italian strain and the outbred Italian (♀)-Greek (♂) strain cluster together, gaining the highest larval weight, while the inbred Greek strain and the outbred Greek (♀)-Italian (♂) strain gained a similar larval weight. Additionally, comparing weights recorded for larvae produced during the first week after parental mating with the weights of larvae produced during the eighth week after parental mating, it is apparent that older parents tend to produce larvae with higher weight. Although from another point of view, that of a stored-product insect, several studies have explored the effect of the parental age on the offspring fitness for T. molitor (Howe 1956; Ludwig and Fiore 1960; Tracey 1958). For instance, Ludwig and Fiore (1960) studied the correlation of parental age with the lifecycle of T. molitor larvae and reported that the increase in the growth rate of larvae was associated with an increase in parental age, while larvae from older parents also gained more weight. Similar results were also reported by Howe (1956), who found that larvae that originated from old T. molitor females developed faster than those that originated from young females. In addition, Tracey (1958) supported that larvae from older parents gain weight faster at an earlier age than larvae produced from younger parents, which is also evident in the results of our study. However, what should also pointed out is that, in our study, due to the lower availability of larvae, a lower larval density (15 instead of 50 individuals per vial) was used in the bioassay with larvae produced during the eighth week after adults’ mating compared to the bioassay with larvae produced during the first week. Consequently, the differences mentioned above could also be attributed to the different densities rather than other biological parameters. Finally, the compatibility of the different strains tested here can be considered high, considering the high larval survival rates recorded in the outbred strains.
Conclusions
The current study urges to take the strain effect on T. molitor fitness traits one step further, underlining the technique of cross-breeding of insect strains originating from different geographical areas and its utilization towards the production of new T. molitor lines with desirable characteristics. Our results indicate that strain-specific characteristics (i.e., larval weight) can be optimized by cross-breeding with an additional strain. In addition, even if, in some of the combinations examined here, the differences in larval weight were marginal, cross-breeding effects may be remarkable if translated at a commercial scale. Hence, this cross-breeding effort may contribute to the optimization of currently adopted rearing protocols, utilizing the divergent phenotypic characteristics of strains that provide specific traits to their offspring, heading off the generic mass-rearing procedure for T. molitor. Further research on phenotype-genotype relationships could be utilized to lead us to more optimal selective breeding approaches between different strains.
Data availability
Not applicable.
References
Adamaki-Sotiraki C, Rumbos C, Athanassiou C (2022a) Developmental plasticity among strains of the yellow mealworm, Tenebrio molitor L., under dry conditions. J Insects as Food Feed 8:281–288. https://doi.org/10.3920/JIFF2021.0087
Adamaki-Sotiraki C, Rumbos C, Athanassiou C (2022b) Strain effect on the adult performance of the yellow mealworm, Tenebrio molitor L. J Insects as Food Feed 8:1401–1410. https://doi.org/10.3920/JIFF2021.0207
Arena JS, Defagó MT (2020) A novel method for sexing live adult Alphitobius diaperinus. Entomol Exp Appl 168:416–419
Berggreen I, Offenberg J, Calis M, Heckmann L-H (2018) Impact of density, reproduction period and age on fecundity of the yellow mealworm Tenebrio molitor (Coleoptera: Tenebrionidae). J Insects as Food Feed 4:43–50. https://doi.org/10.3920/JIFF2017.0013
Bhargava SK, Majumdar MK, Datta RK (1995) Combining ability of seven silk technological characters in mulberry silkworm, Bombyx mori. Ital J Zool 62:359–362. https://doi.org/10.1080/11250009509356089
Büchler R, Andonov S, Bienefeld K, Costa C, Hatjina F, Kezic N, Kryger P, Spivak M, Uzunov A, Wilde J (2013) Standard methods for rearing and selection of Apis mellifera queens. J Apic Res 52:1–30. https://doi.org/10.3896/IBRA.1.52.1.07
Deruytter D, Coudron C, Claeys J (2021) The influence of wet feed distribution on the density, growth rate and growth variability of Tenebrio molitor. J Insects as Food Feed 7:141–149. https://doi.org/10.3920/JIFF2020.0049
Doddaswamy M, Subramanya G, Talebi E (2009) Studies on some economic traits and biological characters of regular and reciprocal cross between a multivoltine and bivoltine race of the silkworm Bombyx mori. J Entomol Nematol. 1:050–055 http://www.academicjournals.org/JEN
EC (2017) European Commission (EC), 2017. EU Commission Regulation 2017/893 of 24 May 2017 amending Annexes I and IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council and Annexes X, XIV and XV to Commission Regulation (EU) No 142/2011 as regards the provisions on processed animal protein. Official Journal of the European Union L 138: 92–116. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R0893&from=EN
EC (2020) European Commission (EC), 2020. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions: A Farm to Fork Strategy for a fair, healthy and environmentally-friendly food system COM/2020/381 final https://ec.europa.eu/food/system/files/2020-05/f2f_action-plan_2020_strategy-info_en.pdf
EC (2021a) European Comission (EC), 2021a. Communication from the commission to the european parliament, the council, the european economic and social committee and the committee of the regions on an action plan for the development of organic production https://ec.europa.eu/info/sites/default/files/food-farming-fisheries/farming/documents/com2021_141_act_organic-action-plan_en.pdf
EC (2021b) European Commission (EC), 2021b. Commission Regulation (EC) 2021b/1372 of 17 August 2021b amending Annex IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council as regards the prohibition to feed non-ruminant farmed animals, other than fur animals, with protein derived from animals. Official Journal of the European Union L 295:1–17 https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=OJ:L:2021:295:FULL&from=EN
EC (2021c): European Commission (EC), 2021c. Commission Regulation (EU) 2021/1925 of 5 November 2021 amending certain Annexes to Regulation (EU) No 142/2011 as regards the requirements for placing on the market of certain insect products and the adaptation of a containment method. Available at: https://eur-lex.europa.eu/legalcontent/EN/TXT/PDF/?uri=CELEX:32021R1925&from=EN
EFSA European Food Safety Authority (EFSA) NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens), Turck D, Castenmiller J, De Henauw S, Hirsch-Ernst KI, Kearney J, Maciuk A, Mangelsdorf I, McArdle HJ, Naska A, Pelaez C, Pentieva K, Siani A, Thies F, Tsabouri S, Vinceti M, Cubadda F, Frenzel T, Heinonen M, Marchelli R, Neuhäuser-Berthold M, Poulsen M, Maradona MP, Schlatter JR, Van Loveren H, Ververis E, Knutsen HK (2021) Safety of dried yellow mealworm (Tenebrio molitor larva) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J 19:6343. https://doi.org/10.2903/j.efsa.2021.6343
Eleftheriou E, Aury J-M, Vacherie B, Istace B, Belser C, Noel B, Moret Y, Rigaud T, Berro F, Gasparian S (2021) Chromosome-scale assembly of the yellow mealworm genome. Open Res Eur 1:94. https://doi.org/10.12688/openreseurope.13987.3
Eriksson T, Picard CJ (2021) Genetic and genomic selection in insects as food and feed. J Insects as Food Feed 7:661–682. https://doi.org/10.3920/JIFF2020.0097
Gerber G, Sabourin D (1984) Oviposition site selection in Tenebrio molitor (Coleoptera: Tenebrionidae). Can Entomol 116:27–39. https://doi.org/10.4039/Ent11627-1
Halliday WD, Thomas AS, Blouin-Demers G (2015) High temperature intensifies negative density dependence of fitness in red flour beetles. Eco Evo 5:1061–1067. https://doi.org/10.1002/ece3.1402
Heckmann LH, Andersen JL, Gianotten N, Calis M, Fischer CH, Calis H (2018) Sustainable mealworm production for feed and food. In: Halloran A, Flore R, Vantomme P, Roos N (eds) Edible Insects in Sustainable Food Systems. Springer, Cham. https://doi.org/10.1007/978-3-319-74011-9_19
Howe R (1956) The effect of temperature and humidity on the rate of development and mortality of Tribolium castaneum (Herbst) (Coleoptera, Tenebrionidae). Ann Appl Biol 44:356–368. https://doi.org/10.1111/j.1744-7348.1956.tb02128.x
Jehan C, Chogne M, Rigaud T, Moret Y (2020) Sex-specific patterns of senescence in artificial insect populations varying in sex-ratio to manipulate reproductive effort. BMC Evol Biol 20:1–13. https://doi.org/10.1186/s12862-020-1586-x
Leclercq J (1963) Artificial selection for weight and its consequences in Tenebrio molitor L. Nature 198:106–107. https://doi.org/10.1038/198106b0
Lippman ZB, Zamir D (2007) Heterosis: revisiting the magic. Trends Gen 23:60–66. https://doi.org/10.1016/j.tig.2006.12.006
Ludwig D, Fiore C (1960) Further studies on the relationship between parental age and the life cycle of the mealworm, Tenebrio molitor. Ann Entomol Soc Am 53:595–600. https://doi.org/10.1093/aesa/53.5.595
Martin R, Rivers J, Cowgill U (1976) Culturing mealworms as food for animals in captivity. Int Zoo Yearb 16:63–70. https://doi.org/10.1111/j.1748-1090.1976.tb00130.x
McNamara JM, Houston AI, Barta Z, Scheuerlein A, Fromhage L (2009) Deterioration, death and the evolution of reproductive restraint in late life. Proc R Soc b: Biol Sci 276:4061–4066. https://doi.org/10.1098/rspb.2009.0959
Morales-Ramos JA, Rojas MG, Kay S, Shapiro-Ilan DI, Tedders WL (2012) Impact of adult weight, density, and age on reproduction of Tenebrio molitor (Coleoptera: Tenebrionidae). Entomol Sci 47:208–220. https://doi.org/10.18474/0749-8004-47.3.208
Morales-Ramos JA, Kelstrup HC, Rojas MG, Emery V (2019) Body mass increase induced by eight years of artificial selection in the yellow mealworm (Coleoptera: Tenebrionidae) and life history trade-offs. J Insect Sci 19:4. https://doi.org/10.1093/jisesa/iey110
Morales-Ramos JA, Rojas MG, Dossey AT, Berhow M (2020) Self-selection of food ingredients and agricultural by-products by the house cricket, Acheta domesticus (Orthoptera: Gryllidae): A holistic approach to develop optimized diets. PLoS One. 15:e0227400. https://doi.org/10.1371/journal.pone.0227400
NeshagaranHemmatabadi R, Seidavi A, Gharahveysi S (2016) A review on correlation, heritability and selection in silkworm breeding. J Appl Anim Res 44:9–23. https://doi.org/10.1080/09712119.2014.987289
Notter DR, Scherf B, Hoffmann I (2013) Breeding of animals. Encyclopedia of Biodiversity, 2nd Edn. pp 636–649. https://doi.org/10.1016/B978-0-12-384719-5.00016-2
Reddy RM, Suryanarayana N, Rai S, Sinha MK, Hansda G, Ojha NG, Prasad BC (2010) The Impact of hybrid vigour on commercial traits of the Indian tropical tasar silkworm, Antheraea mylitta Drury (Lepidoptera: Saturniidae). Int J Entomol 20:61–67
Rho MS, Lee KP (2016) Balanced intake of protein and carbohydrate maximizes lifetime reproductive success in the mealworm beetle, Tenebrio molitor (Coleoptera: Tenebrionidae). J Insect Physio 91:93–99. https://doi.org/10.1016/j.jinsphys.2016.07.002
Rumbos CI, Athanassiou CG (2021) ‘Insects as food and feed: If you can’t beat them, eat them!’—To the magnificent seven and beyond. Oxford University Press US 21:9. https://doi.org/10.1093/jisesa/ieab019
Rumbos CI, Karapanagiotidis IT, Mente E, Psofakis P, Athanassiou CG (2020) Evaluation of various commodities for the development of the yellow mealworm, Tenebrio molitor. Sci Rep 10:11224. https://doi.org/10.1038/s41598-020-67363-1
Rumbos C, Adamaki-Sotiraki C, Gourgouta M, Karapanagiotidis I, Asimaki A, Mente E, Athanassiou C (2021) Strain matters: strain effect on the larval growth and performance of the yellow mealworm, Tenebrio molitor L. J Insects Food Feed 7:1195–1205. https://doi.org/10.3920/JIFF2021.0035
Schuurman J (2013) Contributions to the genetics of Tenebrio molitor L. Springer.https://doi.org/10.1007/BF02059645
Shek DT, Ma C (2011) Longitudinal data analyses using linear mixed models in SPSS: concepts, procedures and illustrations. Sci World J 11:42–76. https://doi.org/10.1100/tsw.2011.2
Sohn K-W, Ramirez L (1999) Comparison of the variation in cocoon quality among the single, three-way and double cross hybrids in the silkworm, Bombyx mori. Sericologia 39:15–31
Tracey KM (1958) Effects of parental age on the life cycle of the mealworm, Tenebrio molitor Linnaeus. Ann Entomol Soc Am 51:429–432. https://doi.org/10.1093/aesa/51.5.429
Urs K, Hopkins T (1973a) Effect of moisture on the lipid content and composition of two strains of Tenebrio molitor L. (Coleoptera, Tenebrionidae). J Stored Prod Res 8:299–305. https://doi.org/10.1016/0022-474X(73)90046-5
Urs K, Hopkins T (1973b) Effect of moisture on growth rate and development of two strains of Tenebrio molitor L. (Coleoptera, Tenebrionidae). J Stored Prod Res 8:291–297. https://doi.org/10.1016/0022-474X(73)90045-3
Uzunov A, Brascamp E, Büchler R (2017) The basic concept of honey bee breeding programs. Bee World 94:84–87. https://doi.org/10.1080/0005772X.2017.1345427
Van Huis A (2013) Potential of insects as food and feed in assuring food security. Ann Rev Entomol 58:563–583. https://doi.org/10.1146/annurev-ento-120811-153704
Van Huis A, Oonincx DG (2017) The environmental sustainability of insects as food and feed. A review. Agron Sustain Dev 37:1–14. https://doi.org/10.1007/s13593-017-0452-8
Van Lenteren J, Nicoli G, Heinz K, Parella M (2004) Quality control of mass-produced beneficial insects. Biocontrol in protected culture. Ball Publishing, Batavia, IL, pp 503–527
Wang Y, Zhang F, Ma J (2013) Sexing live pupae and adults of two wax blooming beetles, Colposcelis microderoides microderoides and Anatolica polita borealis. J Insect Sci 13:136. https://doi.org/10.1673/031.013.13601
Funding
Open access funding provided by HEAL-Link Greece. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript. The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
Christina Adamaki-Sotiraki, Christos I. Rumbos and Christos G. Athanassiou agreed with the content and all gave explicit consent to submit. Christina Adamaki-Sotiraki, Christos I. Rumbos and Christos G. Athanassiou obtained consent from the responsible authorities at the organization where the work has been carried out, before the work is submitted. Christina Adamaki-Sotiraki, Christos I. Rumbos and Christos G. Athanassiou made substantial contributions to the conception or design of the work and analysis of data, and revised the manuscript critically for important intellectual content. Moreover, Christina Adamaki-Sotiraki, Christos I. Rumbos and Christos G. Athanassiou approved the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethical approval
Research activities in the present study involved invertebrate animals, in particular insects. Insect mass rearing was performed taking into consideration insect welfare, treating insects as sentient beings and ensuring insect well-being. Particularly, trials were conducted with the following insect species: Tenebrio molitor (yellow mealworm, Coleoptera: Tenebrionidae). No ethical restrictions are applied for experimental and practical tests having as subject the above cited species. All the trials that involved insects were conducted by UTH.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Giovanni Benelli
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Adamaki-Sotiraki, C., Rumbos, C.I. & Athanassiou, C.G. Mating compatibility and offspring traits evaluation among different strains of Tenebrio molitor. Environ Sci Pollut Res 30, 97052–97062 (2023). https://doi.org/10.1007/s11356-023-29116-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-29116-1