Abstract
Ferrous oxalate dihydrate (FOD) can be used as a photo-Fenton catalyst with remarkable photo-Fenton catalytic and photocatalytic performances on organic pollutant degradation. Various reduction processes were compared in the current study to synthesize FODs from ferric oxalate solution utilizing the iron source in alumina waste red mud (RM), including natural light exposure (NL-FOD), UV light irradiation (UV-FOD), and hydroxylamine hydrochloride hydrothermal method (HA-FOD). The FODs were characterized and employed as photo-Fenton catalysts for methylene blue (MB) degradation, and the effects of HA-FOD dosage, H2O2 dosage, MB concentration, and the initial pH were investigated. The results show that HA-FOD has submicron sizes and lower impurity contents with more rapid degradation rates and higher degradation efficiencies compared with the other two FOD products. When using 0.1 g/L of each obtained FOD, 50 mg/L of MB can be rapidly degraded by HA-FOD by 97.64% within 10 min with 20 mg/L of H2O2 at pH of 5.0, while NL-FOD and UV-FOD achieve 95.52% in 30 min and 96.72% in 15 min at the same conditions, respectively. Meanwhile, HA-FOD exhibits strong cyclic stability after two recycling experiments. Scavenger experiments reveal that the predominant reactive oxygen species responsible for MB degradation are hydroxyl radicals. These findings demonstrate that submicron FOD catalyst can be synthesized using hydroxylamine hydrochloride hydrothermal process from ferric oxalate solution with high photo-Fenton degradation efficiency and reduced reaction time for wastewater treatment. The study also provides a new pathway of efficient utilization for RM.
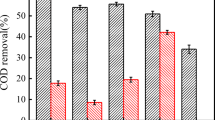
Graphical abstract






Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Abbreviations
- AOPs:
-
Advanced oxidation processes
- ROSs:
-
Reactive oxygen species
- FOD:
-
Ferrous oxalate dihydrate
- RM:
-
Red mud
- NL-FOD:
-
Ferrous oxalate dihydrate obtained under natural light irradiation
- UV-FOD:
-
Ferrous oxalate dihydrate obtained under UV irradiation
- MB:
-
Methylene blue
- HA:
-
Hydroxylamine hydrochloride
- HA-FOD:
-
Ferrous oxalate dihydrate obtained by HA hydrothermal reduction
References
Agrawal S, Dhawan N (2021) Evaluation of red mud as a polymetallic source - a review. Miner Eng 171:107084. https://doi.org/10.1016/j.mineng.2021.107084
Angermann A, Töpfer J (2008) Synthesis of magnetite nanoparticles by thermal decomposition of ferrous oxalate dihydrate. J Mater Sci 43:5123–5130. https://doi.org/10.1007/s10853-008-2738-3
Baba Y, Yatagai T, Harada T, Kawase Y (2015) Hydroxyl radical generation in the photo-Fenton process: effects of carboxylic acids on iron redox cycling. Chem Eng J 277:229–241. https://doi.org/10.1016/j.cej.2015.04.103
Bento NI, Santos PSC, de Souza TE, Oliveira LCA, Castro CS (2016) Composites based on PET and red mud residues as catalyst for organic removal from water. J Hazard Mater 314:304–311. https://doi.org/10.1016/j.jhazmat.2016.04.066
Bi W, Dong W (2021) The degradation of oxytetracycline with ferrous oxalate under different light irradiation. Environ Technol 42:1084–1091. https://doi.org/10.1080/09593330.2019.1652698
Brillas EF (2022) Fenton, photo-Fenton, electro-Fenton, and their combined treatments for the removal of insecticides from waters and soils A review. Sep Purif Technol 284:120290. https://doi.org/10.1016/j.seppur.2021.120290
Chen N, Zhao Y, Li M, Wang X, Peng X, Sun H, Zhang L (2022) FeC2O4•2H2O enables sustainable conversion of hydrogen peroxide to hydroxyl radical for promoted mineralization and detoxification of sulfadimidine. J Hazard Mater 436:129049. https://doi.org/10.1016/j.jhazmat.2022.129049
Dhal JP, Mishra BG, Hota G (2015) Ferrous oxalate, maghemite and hematite nanorods as efficient adsorbents for decontamination of Congo red dye from aqueous system. Int J Environ Sci Technol 12:1845–1856. https://doi.org/10.1007/s13762-014-0535-x
Dias IN, Souza BS, Pereira JHOS, Moreira FC, Dezotti M, Boaventura RAR, Vilar VJP (2014) Enhancement of the photo-Fenton reaction at near neutral pH through the use of ferrioxalate complexes: a case study on trimethoprim and sulfamethoxazole antibiotics removal from aqueous solutions. Chem Eng J 247:302–313. https://doi.org/10.1016/j.cej.2014.03.020
Fan X, Zhang L, Li M, Wang M, Zhou X, Cheng R, Zhou Y, Shi J (2016) α-Ferrous oxalate dihydrate: a simple coordination polymer featuring photocatalytic and photo-initiated Fenton oxidations. Sci China Mater 59:574–580. https://doi.org/10.1007/s40843-016-5064-y
Ferroudj N, Talbot D, Michel A, Davidson A, Abramson S (2017) Increasing the efficiency of magnetic heterogeneous Fenton catalysts with a simple halogen visible lamp. J Photochem Photobiol A 338:85–95. https://doi.org/10.1016/j.jphotochem.2017.01.029
Gan L, Li B, Guo M, Weng X, Wang T, Chen Z (2018) Mechanism for removing 2,4-dichlorophenol via adsorption and Fenton-like oxidation using iron-based nanoparticles. Chemosphere 206:168–174. https://doi.org/10.1016/j.chemosphere.2018.04.162
Gu H, Hargreaves JSJ, Jiang J-Q, Rico JL (2017) Potential routes to obtain value-added iron-containing compounds from red mud. J Sustain Metall 3:561–569. https://doi.org/10.1007/s40831-016-0112-2
Guo X, Jia J, Xu Y, Meng Q, Zha F, Tang X, Tian H (2021) FeS2-Fe1-xS heterostructure as a high-efficient Fenton-like catalyst for ultrafast degradation of orange II. Appl Surf Sci 556:149786. https://doi.org/10.1016/j.apsusc.2021.149786
Hajjaji W, Pullar RC, Labrincha JA, Rocha F (2016) Aqueous Acid Orange 7 dye removal by clay and red mud mixes. Appl Clay Sci 126:197–206. https://doi.org/10.1016/j.clay.2016.03.016
He J, Yang X, Men B, Wang D (2016) Interfacial mechanisms of heterogenous Fenton reactions catalyzed by iron-based materials: a review. J Environ Sci 39:97–109. https://doi.org/10.1016/j.jes.2015.12.003
Hu L, Wang P, Xiong S, Chen S, Yin X, Wang L, Wang H (2019) The attractive efficiency contributed by the in-situ reactivation of ferrous oxalate in heterogeneous Fenton process. Appl Surf Sci 467–468:185–192. https://doi.org/10.1016/j.apsusc.2018.10.151
Huang Q, Cai X, Chen M, Yang Q, Fan S, Zhang Y, Hu H, Gan T, Huang Z (2022) A stepwise processing strategy for treating manganese residue and the remediation of hexavalent chromium in water and soil by manganese residue-derived (Fe, Mn)C2O4. Chem Eng J 436:135258. https://doi.org/10.1016/j.cej.2022.135258
Karimi S, Shokri A, Aghel B (2020) Remediation of spent caustic in the wastewater of oil refinery by photo-Fenton process. Arch Hyg Sci 9:179–188. https://doi.org/10.29252/archhygsci.9.3.179
Khairul MA, Zanganeh J, Moghtaderi B (2019) The composition, recycling and utilisation of Bayer red mud. Resour Conserv Recycl 141:483–498. https://doi.org/10.1016/j.resconrec.2018.11.006
Kim EJ, Baek K (2019) Selective recovery of ferrous oxalate and removal of arsenic and other metals from soil-washing wastewater using a reduction reaction. J Clean Prod 221:635–643. https://doi.org/10.1016/j.jclepro.2019.03.014
Kim SR, Kim S, Kim EJ (2020) Photoreaction characteristics of ferrous oxalate recovered from wastewater. Chemosphere 249:126201. https://doi.org/10.1016/j.chemosphere.2020.126201
Lai C, Shi X, Li L, Cheng M, Liu X, Liu S, Li B, Yi H, Qin L, Zhang M, An N (2021) Enhancing iron redox cycling for promoting heterogeneous Fenton performance: a review. Sci Total Environ 775:145850. https://doi.org/10.1016/j.scitotenv.2021.145850
Li K, Liang Y, Yang J, Yang G, Xu R, Xie X (2018) α-Ferrous oxalate dihydrate: an Fe-based one-dimensional metal organic framework with extraordinary photocatalytic and Fenton activities. Catal Sci Technol 8:6057–6061. https://doi.org/10.1039/c8cy01779d
Li H, Chai W, Cao Y, Yang S (2022a) Flotation enhancement of low-grade bauxite using oxalic acid as surface pretreatment agent. Appl Surf Sci 577:151964. https://doi.org/10.1016/j.apsusc.2021.151964
Li W, Li Z, Wang N, Gu H (2022b) Selective extraction of rare earth elements from red mud using oxalic and sulfuric acids. J Environ Chem Eng 10:108650. https://doi.org/10.1016/j.jece.2022.108650
Li Z, Gu H, Hong B, Wang N, Chen M (2022c) An innovative process for dealkalization of red mud using leachate from Mn-containing waste. J Environ Chem Eng 10:107222. https://doi.org/10.1016/j.jece.2022.107222
Lin R, Li Y, Yong T, Cao W, Wu J, Shen Y (2022) Synergistic effects of oxidation, coagulation and adsorption in the integrated Fenton-based process for wastewater treatment: a review. J Environ Manage 306:114460. https://doi.org/10.1016/j.jenvman.2022.114460
Liu Z-J, Liu W, Wang Y, Guo M-L (2016) Preparation of β-ferrous oxalate dihydrate layered nanosheets by mechanochemical method and its visible-light-driven photocatalytic performance. Mater Lett 178:83–86. https://doi.org/10.1016/j.matlet.2016.04.201
Liu S, Yu B, Wang S, Shen Y, Cong H (2020) Preparation, surface functionalization and application of Fe3O4 magnetic nanoparticles. Adv Colloid Interface Sci 281:102165. https://doi.org/10.1016/j.cis.2020.102165
Liu X, Han Y, He F, Gao P, Yuan S (2021) Characteristic, hazard and iron recovery technology of red mud - a critical review. J Hazard Mater 420:126542. https://doi.org/10.1016/j.jhazmat.2021.126542
Liu Y, Wang X, Sun Q, Yuan M, Sun Z, Xia S, Zhao J (2022) Enhanced visible light photo-Fenton-like degradation of tetracyclines by expanded perlite supported FeMo3Ox/g-C3N4 floating Z-scheme catalyst. J Hazard Mater 424:127387. https://doi.org/10.1016/j.jhazmat.2021.127387
Ma S, Gu H, Mei Z, Yang Y, Wang N (2020) Conversion synthesis of manganese sulfate residue into iron hydroxide adsorbent for Cu(II) removal from aqueous solution. Environ Sci Pollut Res 27:23871–23879. https://doi.org/10.1007/s11356-020-08819-9
Miklos DB, Remy C, Jekel M, Linden KG, Drewes JE, Hübner U (2018) Evaluation of advanced oxidation processes for water and wastewater treatment - a critical review. Water Res 139:118–131. https://doi.org/10.1016/j.watres.2018.03.042
Mohadesi M, Aghel B, Razmegir MH (2021) COD reduction in petrochemical wastewater using the solar photo-Fenton process. J Chem Petro Eng 55:69–81. https://doi.org/10.22059/jchpe.2020.310442.1330
Mohamed HH, Besisa DHA (2022) Eco-friendly and solar light-active Ti-Fe2O3 ellipsoidal capsules’ nanostructure for removal of herbicides and organic dyes. Environ Sci Pollut Res 2022:1–11. https://doi.org/10.1007/s11356-022-23119-0
Nie X, Li G, Li S, Luo Y, Luo W, Wan Q, An T (2022) Highly efficient adsorption and catalytic degradation of ciprofloxacin by a novel heterogeneous Fenton catalyst of hexapod-like pyrite nanosheets mineral clusters. Appl Catal B 300:120734. https://doi.org/10.1016/j.apcatb.2021.120734
Niveditha SV, Gandhimathi R (2020) Flyash augmented Fe3O4 as a heterogeneous catalyst for degradation of stabilized landfill leachate in Fenton process. Chemosphere 242:125189. https://doi.org/10.1016/j.chemosphere.2019.125189
Oturan MA, Aaron J-J (2014) Advanced oxidation processes in water/wastewater treatment: principles and applications. A review. Crit Rev Environ Sci Technol 44:2577–2641. https://doi.org/10.1080/10643389.2013.829765
Peng S, Zhang W, He J, Yang X, Wang D, Zeng G (2016) Enhancement of Fenton oxidation for removing organic matter from hypersaline solution by accelerating ferric system with hydroxylamine hydrochloride and benzoquinone. J Environ Sci 41:16–23. https://doi.org/10.1016/j.jes.2015.05.006
Pliego G, Zazo JA, Garcia-Muñoz P, Munoz M, Casas JA, Rodriguez JJ (2015) Trends in the intensification of the Fenton process for wastewater treatment: an overview. Crit Rev Environ Sci Technol 45:2611–2692. https://doi.org/10.1080/10643389.2015.1025646
Rahimi M, Aghel B, Sadeghi M, Ahmadi M (2014) Using Y-shaped microreactor for continuous decolorization of an Azo dye. Desalin Water Treat 52:5513–5519. https://doi.org/10.1080/19443994.2013.807471
Ribeiro JP, Nunes MI (2021) Recent trends and developments in Fenton processes for industrial wastewater treatment - a critical review. Environ Res 197:110957–110973. https://doi.org/10.1016/j.envres.2021.110957
Samal S (2021) Utilization of red mud as a source for metal ions - a review. Materials (basel) 14:2211. https://doi.org/10.3390/ma14092211
Samsami S, Mohamadizaniani M, Sarrafzadeh M-H, Rene ER, Firoozbahr M (2020) Recent advances in the treatment of dye-containing wastewater from textile industries: overview and perspectives. Process Saf Environ Prot 143:138–163. https://doi.org/10.1016/j.psep.2020.05.034
Sukhatskiy Y, Sozanskyi M, Shepida M, Znak Z, Gogate PR (2022) Decolorization of an aqueous solution of methylene blue using a combination of ultrasound and peroxate process. Sep Purif Technol 288:120651. https://doi.org/10.1016/j.seppur.2022.120651
Tanvar H, Mishra B (2021) Hydrometallurgical recycling of red mud to produce materials for industrial applications: alkali separation, iron leaching and extraction. Metall Mater Trans B 52:3543–3557. https://doi.org/10.1007/s11663-021-02285-5
Tkaczyk A, Mitrowska K, Posyniak A (2020) Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: a review. Sci Total Environ 717:137222. https://doi.org/10.1016/j.scitotenv.2020.137222
Vorontsov AV (2019) Advancing Fenton and photo-Fenton water treatment through the catalyst design. J Hazard Mater 372:103–112. https://doi.org/10.1016/j.jhazmat.2018.04.033
Wang J, Tang J (2021) Fe-based Fenton-like catalysts for water treatment: preparation, characterization and modification. Chemosphere 276:130177. https://doi.org/10.1016/j.chemosphere.2021.130177
Wang F, Wu Y, Gao Y, Li H, Chen Z (2016) Effect of humic acid, oxalate and phosphate on Fenton-like oxidation of microcystin-LR by nanoscale zero-valent iron. Sep Purif Technol 170:337–343. https://doi.org/10.1016/j.seppur.2016.06.046
Wang G, Zhou A, Xu Q (2019a) α-Ferrous oxalate with different micro scale: synthesis and catalytic degradation effect to rhodamine B. Solid State Sci 91:54–60. https://doi.org/10.1016/j.solidstatesciences.2019.03.004
Wang N, Chen J, Wang J, Feng J, Yan W (2019b) Removal of methylene blue by polyaniline/TiO2 hydrate: adsorption kinetic, isotherm and mechanism studies. Powder Technol 347:93–102. https://doi.org/10.1016/j.powtec.2019.02.049
Wang Z, Qiu W, Pang S, Jiang J (2019c) Effect of chelators on the production and nature of the reactive intermediates formed in Fe(II) activated peroxydisulfate and hydrogen peroxide processes. Water Res 164:114957. https://doi.org/10.1016/j.watres.2019.114957
Wang J, Kang D, Shen B, Sun H (2020) Wu C (2020) Enhanced hydrogen production from catalytic biomass gasification with in-situ CO2 capture. Environ Pollut 267:115487. https://doi.org/10.1016/j.envpol.2020.115487
Wu S, Deng S, Ma Z, Liu Y, Yang Y, Jiang Y (2022) Ferrous oxalate covered ZVI through ball-milling for enhanced catalytic oxidation of organic contaminants with persulfate. Chemosphere 287:132421. https://doi.org/10.1016/j.chemosphere.2021.132421
Xue S, Wu Y, Li Y, Kong X, Zhu F, Hartley W, Li X, Ye Y (2019) Industrial wastes applications for alkalinity regulation in bauxite residue: a comprehensive review. J Cent South Univ 26:268–288. https://doi.org/10.1007/s11771-019-4000-3
Yoo SH, Jang D, Joh H-I, Lee S (2017) Iron oxide/porous carbon as a heterogeneous Fenton catalyst for fast decomposition of hydrogen peroxide and efficient removal of methylene blue. J Mater Chem A 5:748–755. https://doi.org/10.1039/C6TA07457J
Yu Z-L, Shi Z-X, Chen Y-M, Niu Y-J, Wang Y-X, Wan P-Y (2012) Red-mud treatment using oxalic acid by UV irradiation assistance. Trans Nonferr Metal Soc China 22:456–460. https://doi.org/10.1016/S1003-6326(11)61198-9
Zeng Q, Huang Y, Huang L, Hu L, Xiong D, Zhong H, He Z (2020) Efficient removal of hexavalent chromium in a wide pH range by composite of SiO2 supported nano ferrous oxalate. Chem Eng J 383:123209. https://doi.org/10.1016/j.cej.2019.123209
Zeng Q, Wang S, Hu L, Zhong H, He Z, Sun W, Xiong D (2021) Oxalic acid modified copper tailings as an efficient adsorbent with super high capacities for the removal of Pb2+. Chemosphere 263:127833. https://doi.org/10.1016/j.chemosphere.2020.127833
Zhang Y, Zhou M (2019) A critical review of the application of chelating agents to enable Fenton and Fenton-like reactions at high pH values. J Hazard Mater 362:436–450. https://doi.org/10.1016/j.jhazmat.2018.09.035
Zhang M, Dong H, Zhao L, Wang D, Meng D (2019) A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci Total Environ 670:110–121. https://doi.org/10.1016/j.scitotenv.2019.03.180
Zhu X, Li J, Xie B, Feng D, Li Y (2020) Accelerating effects of biochar for pyrite-catalyzed Fenton-like oxidation of herbicide 2,4-D. Chem Eng J 391:123605. https://doi.org/10.1016/j.cej.2019.123605
Acknowledgements
All authors wish to thank Prof. Wan’s Group for FTIR determination.
Funding
The current research was funded by the National Natural Science Foundation of China (U1812402), the Youth Innovation Promotion Association, CAS (2021400), and Guizhou Outstanding Young Scientific and Technological Talents Project (2021–5641).
Author information
Authors and Affiliations
Contributions
Yuxin Yang: Investigation, resources, data curation, writing—original draft. Ning Wang: Conceptualization, resources, supervision. Hannian Gu: Writing—review and editing, conceptualization, validation, resources, methodology, supervision, funding acquisition.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The authors are willing to permit the Journal to publish the article.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ricardo A. Torres-Palma
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. Iron source in waste red mud was reused to synthesize different FOD products.
2. Through HA reduction from ferrioxalate solution to obtain HA-FOD.
3. HA-FOD was synthesized with submicron size and less impurities.
4. HA-FOD presents high efficiency in photocatalysis and photo-Fenton process.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Y., Wang, N. & Gu, H. Synthesis of submicron ferrous oxalate from red mud with high Fenton catalytic performance on degradation of methylene blue. Environ Sci Pollut Res 30, 85210–85222 (2023). https://doi.org/10.1007/s11356-023-28308-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-28308-z