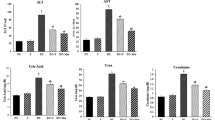Abstract
This study investigates blackberry juice’s effects on glucose metabolism, oxidative stress, inflammation, and endoplasmic reticulum stress (ER) in streptozotocin (STZ)-induced diabetic rats. Fifty Wistar rats were distributed to five groups randomly of ten rats each: Normal control, diabetic control, 9 mL/kg body weight (b.wt) blackberry juice only, blackberry juice plus diabetes, and 500 mg/kg b.wt metformin plus diabetes. A single intraperitoneal injection of 50 mg/kg b.wt STZ induced diabetes in the rats. This animal study continued for 56 days after the confirmation of diabetes. The levels of liver function and renal function, as well as insulin, glucose-6-phosphatase, glucokinase, and malondialdehyde (MDA) levels, and the activities of catalase (CAT) and superoxide dismutase (SOD), were determined. Additionally, interleukin-6 (IL-6) levels, tumor necrosis factor-alpha (TNF-α), and activated transcription factor 4 (ATF4) expressions were examined in the liver homogenate of rats. Furthermore, the liver tissues were utilized for histopathological examination. The results showed that blackberry juice prevented drastic loss of body weight and reduced food consumption in diabetic rats. Additionally, the levels of blood glucose, total protein, aspartate aminotransferase (AST), albumin, alanine aminotransferase (ALT), uric acid, creatinine, and urea improved after the administration of blackberry juice in diabetic rats. Blackberry juice significantly increased glucose metabolism and antioxidant status while reducing ER stress and inflammation in diabetic rats. Moreover, blackberry juice improved glucose metabolism by increasing insulin levels and improving the dysregulated activities of glucose-metabolizing enzymes. The microstructure of liver tissues in diabetic rats was also improved with blackberry juice treatment. Therefore, blackberry juice has the potential to alleviate diabetes in rats and could be considered as a functional food for people with diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus is a chronic metabolic disorder characterized by high blood glucose levels resulting from insulin resistance or impaired insulin secretion, or both (Katsarou et al. 2017). Type 2 diabetes (T2D) is the most common form of diabetes, accounting for approximately 90% of all diabetes cases (American Diabetes Association 2020). The global prevalence of diabetes was 9.3% in 2019 and is expected to increase to 10.2% by 2030 and 10.9% by 2045 (Saeedi et al. 2019).
One of the key organelles involved in T2D is the endoplasmic reticulum (ER), where protein synthesis and folding occur, making it a crucial component of cellular function. The unfolded protein response (UPR) is a pro-survival process that is triggered by disruptions in protein production homeostasis and ER folding capacity. However, ER stress, which is often linked to apoptosis and inflammation, results from the persistent activation of the UPR by factors such as excessive lipid load, hyperglycemia, oxidative stress, and excessive Ca2 + release from ER storage (Ozcan et al. 2004). Pancreatic β-cells, among other specialized cells, are responsible for producing, folding, and processing insulin (Oyadomari et al. 2002).
Blood glucose levels are regulated by enzymes that catalyze reactions in the gluconeogenic and glycolytic pathways, such as phosphofructokinase, hexokinase, and glucose-6-phosphatase (Petersen et al. 2017). Effective normal control of blood glucose levels is critical for treating and preventing diabetes and its consequences.
Diabetes complications can be reduced through lifestyle changes, such as dietary changes (Gregg et al. 2014). Nutrition also plays a significant role in the development or reduction of metabolic defects, and can therefore be considered a non-pharmacological supplement to the treatment of acute illnesses (Sears and Ricordi 2012). Blackberries are rich in phenolic chemicals, including ellagitannins and anthocyanins (Kaume et al. 2012). The black mulberry, or Morus nigra L., is the healthiest type of mulberry due to its high concentration of flavonoids, anthocyanins, and other phenols (Ercisli and Orhan 2007). These compounds have various health benefits, including protection against cardiovascular disease, inflammation, diabetes, cancer, and age-associated neurodegenerative illnesses (Kaume et al. 2012). These health benefits are attributed to the compounds' ability to reduce oxidative stress, which is the leading cause of disease (Finkel 2011).
Berries can be considered a source of exogenous antioxidants that can be obtained through diet and may help treat diabetes. The aim of this study was to evaluate the ability of anthocyanin-rich blackberry juice to mitigate inflammation, glucose metabolism, antioxidant status, and ER stress in diabetic rats.
Methods and materials
Preparing blackberry fruit juice
Fresh blackberry fruits were obtained from a garden in Minia, Egypt, washed, and homogenized to prepare the juice. For the preparation of the juice, a commercial Philips blender from China was used to blend the fruits. After blending, the resulting juice was diluted with distilled water in a 1:1 ratio and filtered through a Büchner funnel to remove any solid pieces, resulting in a clear liquid. The dosage of blackberry juice used in the experiment was based on previous studies by Ismail et al. (2014) and Siriwoharn et al. (2004), which recommended a dose of 9 mL/kg body weight.
Determination of bioactive compounds in anthocyanin-rich blackberry fruit juice
The procedure involves weighing 5 g of dried blackberry juice, soaking it in 25 mL of ethanol for a day, filtering it through Whatman No.1 filter paper and the solvent evaporated in vacuo at 50 °C. The resulting sample was then analyzed using Bioactive Compounds, HLPC, and GC/MS technology.
The total phenolic content (TPC) was determined using the Folin-Ciocalteu test (Maurya and Singh 2010) and was expressed as the amount of gallic acid equivalent per g of dry material. The total flavonoid concentration was measured using the procedure described in Jia et al. (1999) and expressed as quercetin equivalent per g of dry material. The total concentration of anthocyanins were determined using the method described by Ranganna (1977). The results are presented in milligrams of cyanidin 3-glucoside per 100 g.
Identification of anthocyanins in blackberry by HPLC
The HPLC analysis was done following the method described by Drust and Wrolstad (2001). It was carried out using an Agilent HPLC-LC1620A series model from Santa Clara, CA, USA, which was equipped with a degasser, an automatic injector for the autosampler, a high-pressure pump, and a UV/Visible detector with the ability to detect at various wavelengths. The chromatograms were monitored at 521 nm.
GC/MS analysis
The chromatography–mass spectrometry (GC–MS) analysis of blackberry juice was performed following a published procedure (Soraya et al. 2022). A thermal scientific trace GC Ultra/ISQ single Quadrupole MS, TG-5MS fused silica capillary column was utilized for the GC/MS analysis at the National Research Center, Dokki, Giza (30 m, 0.251 mm, and 0.1 mm film thickness). For GC/MS detection at a constant flow rate of 1 ml/min, an electron ionization system with 70 eV ionization energy and helium gas as the carrier gas was used. The injector and MS transfer line were kept at a constant temperature of 280 °C. To investigate the quantification of all the found components, a percent relative peak area was utilized. To establish a preliminary identification, the retention durations and mass spectra of the compounds were compared to those of the National Institute of Standards and Technology (NIST), WILLY library data from the GC/MS instrument.
Biological experiment protocol
Fifty male albino Wistar rats, weighing approximately 150 ± 20 g and 2 months old, were obtained from the Animal House et al. Nahda University’s Faculty of Pharmacy in Egypt. The rats were housed in polypropylene cages with husk bedding and maintained under a 12-h light–dark cycle during the study. They were fed a commercial pelleted diet and had access to water and libitum. The experiment was carried out according to the Ethics Committee for the care and use of animals, microorganisms and living cell cultures in education and scientific research at Faculty of Agriculture, Minia University (MU/FA/006/12/22).
Experimental design
The rats were distributed into five groups comprising ten animals each.
Normal control group: Animals received distilled water (10 mL/kg b.wt) by an oral tube for 56 days).
Blackberry juice group: Animals received blackberry juice at a dosage of 9 mL /kg b.wt by an oral tube for 56 days (Ismail et al. 2014).
Diabetic group: Animals treated STZ/50 mg/kg b.wt (Samarghandian et al. 2017).
Diabetic group + blackberry juice: Animals were treated with STZ followed by blackberry juice (9 mL g/kg b.wt) by an oral tube for 56 days.
Diabetic group + metformin group: Animals were treated with STZ followed by metformin (500 mg/kg/b.wt) by an oral tube for 56 days (Dimo et al. 2007).
Rats were given STZ in the form of a single intraperitoneal (i.p.) injection (50 mg/kg b.wt) in citrate buffer (pH 4.5) to induce diabetes, rats in the normal control group were given an identical but non-diabetic dose of buffer (Furman 2015). Those with sustained fasting blood glucose levels > 250 mg/dL 3 days after STZ injection were diagnosed with diabetes. Blackberry fruit juice was first administered 48 h following the injection of STZ.
The study lasted 56 days. The animals’ daily food amounts were recorded, and weights were recorded every week during that period. Food consumption and feeding efficiency ratio (%) were calculated. Blood glucose levels were measured on weekly tail blood samples during fasting by glucose reagent strips (Accu chek®, Roche Diagnostics, Indianapolis, USA). At the end of the trial, the rats were fasted overnight, anesthetized with ketamine (40 mg/kg body weight), and injected intraperitoneally (i.p.) with xylazine (5 mg/kg body weight) before being euthanized by cervical dislocation. Samples of blood were taken from the retro-orbital plexus in tubes. The tubes do not contain anticoagulants to separate the blood sample by centrifugation at 3000 g for 15 min and stored at − 20 °C till used in analyzing the samples biochemically. Small hepatic samples were placed in a 10% formalin solution to conduct the histopathological test. Other liver tissue samples were homogenized and utilized to evaluate MDA and CAT concentrations.
Biochemical assays
The serum total protein levels, aspartate aminotransferase (AST), albumin, alanine aminotransferase (ALT), uric acid, creatinine, urea, and direct bilirubin were evaluated by a commercial enzymatic kit following the instructions of the manufacturing company. During the difference between total protein and albumin, globulin was calculated. Serum total lipids (TL), total cholesterol (TC), triglycerides (TG), as well as high-density lipoprotein cholesterol (HDL-C), were assayed with a commercially available assay kit from Bio- Diagnostic Co., Egypt. Serum low-density lipoprotein cholesterol (LDL-c) and very low-density lipoprotein cholesterol (VLDL-c) were estimated following Lee and Nieman (1996) and Castelli et al. (1977), respectively.
Antioxidant biomarkers
Homogenization of liver tissue samples was performed in phosphate buffer at ice cold temperatures (pH7.4), and centrifugation at 3000 × g for 15 min was employed to separate the supernatant from homogenates, which was then used for biochemical analysis. The assay of malondialdehyde (MDA), superoxide dismutase (SOD), catalase activity (CAT), and glutathione peroxidase (GPx) was done in homogenate liver according to Aebi (1984), Ohkawa et al. (1979), Nishikimi et al. (1972), and Rotruck et al. (1973), in liver homogenate, respectively.
Glucose metabolism analysis
Insulin level, glucose-6-phosphatase activity, glucokinase activity, and hepatic glycogen content in the liver homogenate were estimated using the methods of Harper (1959), Brandstrup et al. (1957), and Roe and Dailey (1966), respectively.
Inflammatory biomarkers
ELISA kit was used to measure the concentration of IL-6 and TNF-α in liver homogenate following the manufacturer’s instructions using a test reagent kit (CUSABIO Company, 7505 Fannin St. Ste 610–312, Houston, TX 77054, USA).
Gene expression analysis
Following the manufacturer’s protocol, TRIzol Reagent (15,596,026, Life Technologies, USA) was used to carry out the total purification of RNA from blood samples. Briefly, Invitrogen™ TRIzol™ Reagent is an available reagent for isolating high-quality total RNA (in addition to proteins and DNA) from the cells and tissues of bacteria, yeast, plant, animal, or human in an hour. It is a monophasic solution of guanidine isothiocyanate, phenol, as well as other proprietary elements that enable isolating various small- or large-sized RNA types. It enables sequential precipitation of proteins, DNA, and RNA, using one single sample (Chomczynski 1993). After sample homogenization with TRIzol™ Reagent, chloroform can be provided, and the homogenate can separate into a transparent upper aqueous layer (with RNA), an interphone, and a red lower organic layer (with DNA and proteins). RNA is precipitated from the aqueous layer with isopropanol. DNA is precipitated from the interphase/organic layer with ethanol. Protein is precipitated from the phenol-ethanol supernatant by isopropanol precipitation. After washing the precipitated RNAs, DNAs, or proteins to get rid of impurities, they can be resuspended. A reverse-transcription of 1 μg of total RNA into single-stranded complementary DNA was possible by employing QuantiTects Reverse Transcription Kit (Qiagen, USA) using a random primer hexamer in a two-step RT-PCR reaction, where any genomic DNA (gDNA) impurity was removed by gDNA Wipeout buffer. Total cDNA (30 ng) was used as a template for amplifying using a certain primers pair (Table 1) employed at a concentration of 300 nM. The samples were exposed to real-time PCR in duplicate, and then the duplicates’ mean values were employed to be analyzed subsequently. GADPH was used as a reference gene. Rotor-Gene Q gathered data automatically and conducted the analysis of the threshold cycle (Ct) value standardized to an average Ct value of the housekeeping genes (∆Ct). Moreover, the relative expression of each representative was estimated as 2−∆∆ct.
Histopathological examination
Liver specimens were fixed in the solution of formalin (10%) before being rinsed in water, dehydrated in ascending grades of alcohol, and then cleaned in Xylene. Paraffin slices (5 µm) were cut from the specimens and stained with hematoxylin–eosin (H&E) to conduct a normal histopathological trial following Bancroft and Gamble’s method (2008). The assay of histopathological abnormalities in the livers was done, and scores (0–3) were taken by calculating the lesions’ percentage in 5 randomly inspected microscopic fields per animal. The percentage grades were: 0% = lack of change, 1% = slight change, 2% = moderate, and 3% = significant change. Changes below 30% were considered minor, 30–50% were deemed reasonable, and > 50% were considered severe (Ahmed et al. 2019).
Statistical analyses
Statistical analyses were carried out by SPSS 21.0. For group comparison, the one-way analysis of variance (ANOVA) was run before Tukey’s post hoc test. Additionally, P value < 0.05 was set to denote statistical significance. The data expression took the form of means ± standard errors (SE).
Results
Total flavonoids, total polyphenol concentration, and anthocyanin profile
The blackberry was reported to have total anthocyanin content (TAC) of about 312.9 mg cyanidin 3-glucoside/100 g DW, total flavonoids (TFs) of about 36.7 ± 1.49 mg QE / g DW, and total polyphenol concentration (TPC) of about 28.80 ± 1.29 mg GAE/ g DW.
Figure 1 shows the chromatographic analysis of blackberry anthocyanins at a wavelength of 521 nm. The analysis revealed the presence of three types of anthocyanins in blackberries: delphinidin-3-O-glycosides, pelargonidin-3-O-glycosides, and cyanidin-3-O-glycosides (Figs. 1 and 2). The most prevalent anthocyanins were delphinidin-3-O-glycosides (10.12 μg/mg DW), as shown in Table 2.
GC/MS analysis of blackberry juice
The compounds present in the blackberry juice were identified by GC–MS analysis (Fig. 3). Table 3 lists the active compounds in the blackberry juice, together with their concentration, MW, molecular formula, and retention time (RT). In blackberry juice, 10 chemicals were discovered. The major compounds in the blackberry juice were as follows: 9-borabicyclo-9-borabicyclo-9-borabicyclo-9-bor [3.3.1] nonane,9-[(2-pyridyl) amino]- (22.77%), benzenediamine, n,à,à-trimethyl (5.44%), 3,4,5,6-tetrahydroxy-2-oxo-hexanoic acid (9.32%), 1-deoxy-d-mannitol (3.42%), 2-nonyloxirane (3.16%), 2,4-pyridinedicarboxylic acid, dimethyl ester (3.75%), oxacyclotetradecane-2,11-dione, 13-methyl (11.57%), 11-octadecenoic acid, methyl ester (14.78%), 6H-1,2,5-oxadiazolo[3,4-E]indole-6,8a-diol, 4,5,5a,7,8,8a-hexahydro-, 3-oxide (6.07%), and Z,Z-4,16-octadecadien-1-ol acetate (3.54%).
Figure 4 shows the mass spectra and structures of 9-borabicyclo [3.3.1] nonane,9-[(2-pyridyl) amino]- (22.77%), 11-octadecenoic acid, methyl ester (14.78%), oxacyclotetradecane-2,11-dione, 13-methyl (11.57%), and 3,4,5,6-tetrahydroxy-2-oxo-hexanoic acid (9.32%), respectively. The GC–MS analysis of blackberry juice yielded the following phytocomponents and their biological activity (Table 3).
Changes in body weight
Table 4 illustrates the final weight (g), gained weight of the body (g), daily gained weight of the body (g), daily feed consumption (g), and feeding efficiency ratio (%) of the normal control and diabetic rats. There was a significant reduction (p < 0.05) in the final weight, gained weight of the body, daily gained weight of the body, feeding efficiency ratio (%), and considerable elevation (p < 0.05) in daily feed intake in the diabetic control group compared to the normal control group.
When diabetic rats were given blackberry juice, these symptoms were normalized, showing that diabetic control is needed to stop muscle loss (Table 4). Blackberry juice and the medication had comparable results (p < 0.05) (Table 4).
Changes in blood glucose levels
Animals with diabetes had sharply elevated (p < 0.05) blood sugar levels in comparison with the normal control group (Table 5). However, after 56 days of treatment, glucose levels considerably dropped in all treated groups in comparison with rats with diabetes (Table 5).
Changes in liver functions
Table 6 displays the mean values of serum protein, albumin, ALT, and AST for the normal control and experimental groups. STZ-induced diabetic rats revealed a substantial increase (p < 0.05) in serum protein, albumin, ALT, and AST compared to the normal control ones.
There was a significant improvement of these parameters after ingesting blackberry juice and metformin (Table 6).
Changes in kidney functions
Table 7 summarizes the impact of blackberry juice on the biochemical markers related to the kidneys. The serum levels of creatinine, uric acid, and urea showed a significant increase in rats with diabetes (p < 0.05), indicating that these animals had impaired kidney function.
While there were reductions in the serum levels of creatinine, uric acid, and urea by 23%, 23%, and 29%, respectively, in STZ-diabetic rats treated with blackberries, the reductions were statistically significant (p < 0.05) (Table 7). Similarly, metformin reduced the increases in these measures due to diabetes by 34%, 29%, and 39%, respectively, in comparison with the group with diabetes (p < 0.05).
Impact of blackberry juice on glucose metabolism
Table 8 shows no significant differences between the blackberry juice group and the normal control in hepatic glycogen level, insulin level, glucokinase activity, and glucose-6-phosphatase activity after 56 days. The STZ-treated group had a significant increase (p > 0.05) in glucose-6-phosphatase activity by about 75.54%. In contrast, the insulin, glucokinase, and glycogen activity were significantly lowered (p > 0.05) by approximately 86.48%, 74.38%, and 72.51%, respectively, compared to the normal control group.
The activity of glucose-6-phosphatase appeared significantly (p < 0.05) lower in treatment with blackberry juice and metformin concurrently with STZ. Blackberry decreased the elevation of glucose-6-phosphatase by about 25.21% in rats treated with STZ. Metformin concurrently with STZ significantly (p > 0.05) decreased glucose-6-phosphatase activity by about 30.17% compared with the STZ group. Table 8 shows that diabetic rats given blackberry juice for 56 days had significantly (p < 0.05) higher levels of insulin level and glycogen level, and glucokinase activity than diabetic control rats. In contrast, no differences were found between the metformin and blackberry juice groups.
Impact of blackberry juice on the oxidative stress of rats with diabetes
Table 9 illustrates the lack of a significant difference between the blackberry-only treated and the normal control groups in MDA level, SOD, CAT, and GPX activities after 56 days. It shows some markers of the antioxidant status, such as GPX, CAT, and SOD, in the liver homogenate. Also, Table 9 illustrates the levels of lipid peroxidation that indirectly indicate free radical damage.
In the utilized STZ model, reducing the activities of CAT and SOD was clear in the liver tissue of the diabetic group in comparison with the normal control group. The group with diabetes lost 81.55% and 77.73% of their activities of CAT and SOD, respectively, in comparison with the normal control one. Also, the treated with STZ had a significant decrease (p < 0.05) in GPX activity in the liver (− 79.80) in comparison to the normal control group. In contrast, the group with diabetes had a high level of MDA, i.e., 341.93 3%, compared to the normal control group.
According to the activities of CAT, SOD, and GPX, blackberry juice improved the antioxidant capacity to moderate levels between the diabetic and the normal control groups. The blackberry juice intake increases the activity to almost that of the normal control group, denoting that the blackberry relieves oxidative stress in rats. Therefore, blackberry juice revealed a significance (p < 0.05) in the activities of CAT, SOD, and GPX in the livers (164.44%,331.58%, and 167.11%, respectively) compared with the diabetic group. At the same time, the blackberry juice manages to decrease the lipid peroxidation levels in the liver (− 28.78%) to intermediate stations between the diabetic and the normal control groups.
Blackberry juice’s impact on the inflammatory markers of rats with diabetes
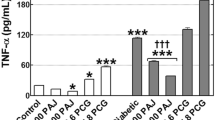
The findings in Table 10 showed no significant difference between the blackberry juice and the normal control groups in TNF-α and IL-6 activities after 56 days. Meanwhile, the treated with STZ increased significantly (p < 0.05) the IL-6 and TNF-α by about 9.3- and 4.8-fold compared with the control normal control group.
Blackberry juice significantly (p < 0.05) reduced increased hepatic TNF-α and IL-6 by about 65% and 69%, respectively, in rats with diabetes in comparison to only rats with diabetes (Table 10). Similarly, metformin significantly (p < 0.05) decreased the levels of hepatic TNF-α and IL-6 by about 84% and 87%, respectively, in rats with diabetes in comparison to only rats with diabetes (Table 10).
Blackberry juice modulates hepatic ER stress in diabetic rats
ATF4 expression was (p < 0.05) higher significantly in rats with diabetes in comparison with the normal control group (Fig. 5). In contrast, diabetic animals that received blackberry juice were significantly lower (p < 0.05) in the expression of ATF4 compared to diabetic animals. Blackberry juice and metformin had similar effects in suppressing ATF4 expression.
Histopathological examination of the liver
Microscopically, rats’ livers from the normal control group revealed the normal histoarchitecture of hepatic tissue (Fig. 6a and Table 11). Furthermore, the liver of rats from the blackberry juice group exhibited no histopathological lesions (Fig. 6b and Table 11). On the contrary, the diabetic control group rats’ livers displayed activating the Kupffer cells, necrosis of sporadic hepatocytes, hepatocellular vacuolar degeneration, portal edema, and thickening in the wall of the bile duct (Fig. 6c and Table 11).
Photomicrographs of the liver of different groups. a Photomicrographs of the livers of rats in the normal control group illustrating the normal histoarchitecture of hepatic tissue. b Photomicrographs of the livers of rats in the blackberry juice group displaying no histopathological lesions. c Photomicrographs of the livers of rats in the diabetic control group displaying the activation of the Kupffer cells and the necrosis of sporadic hepatocytes. d Photomicrographs of the livers of rats in the (diabetic + blackberry juice) group displaying slight Kupffer cells activation. e Photomicrographs of the livers of rats in the (diabetic + metformin) group 8 displaying the lack of histopathological alterations. (H&E × 400, scale bar 25 μm)
Meanwhile, the liver of rats from the diabetic + blackberry juice group described no changes except for slightly activating the Kupffer cells, sporadic hepatocytes necrosis, and slightly vacuolating some hepatocytes (Fig. 6d and Table 11). In contrast, the liver from the diabetic + metformin group displayed no histopathological alterations except for slight vacuolation of some hepatocytes and slight Kupffer cells activation (Fig. 6e and Table 11).
Discussion
When it comes to functional foods, researchers place more importance on the bioavailability of the nutrient rather than the amount of the nutrient itself in dietary supplements or foods (D’Archivio et al. 2010).
Diabetes metabolic control is influenced by body weight. In rats with diabetes, the final weight was significantly lower than that of the normal control group, likely due to the failure to use glucose for energy, increased use, and decreased protein storage, which depletes body proteins. These symptoms are indicative of type 2 diabetes in humans and animals, resulting from insulin deficiency (Swanston-Flatt et al. 1990). The reduced body weight in diabetic rats compared to normal rats suggests increased protein breakdown (Mishra et al. 2011). Jacobson (2010) found that STZ-induced diabetes leads to weight loss in rodent models because proteins are used for energy when carbohydrates are unavailable. Blackberry juice was found to increase body weight in diabetic rats, highlighting the importance of controlling diabetes to prevent muscle loss. Li et al. (2012) demonstrated that herbs in the diet can improve the feed conversion ratio and increase weight gain by boosting nutrient consumption and gut flora function. Berries contain active compounds such as flavonols, anthocyanins, and ellagitannins, which can improve protein assimilation and digestion (Yilmaz 2019).
The results showed that blood glucose levels increased significantly in infected diabetic rats compared to the normal control group. Compared to diabetic rats, blackberry juice reduced blood glucose levels by 62%. The reduction was statistically insignificant in comparison with the normal control or metformin-treated groups. Ragavan and Krishnakumari (2006) showed that blackberry fruit extract and its anthocyanin-rich fraction could considerably reduce blood glucose levels in rats with diabetes. Basu and Lyons (2012) concluded that various berries had an inhibitory action against α-glucosidase, α-amylase, and pancreatic lipase activities. Furthermore, He and Giusti (2010) reported a competitive attachment of the sodium-glucose co-transporter 1 (SGLT-1) with polyphenols, allowing the suggestion of a reduction in the uptake of glucose in the intestine, improving the hypoglycemic impact. Blackberry juice protected pancreatic beta cells from necrosis and apoptosis by decreasing lipid peroxide activity, enhancing catalase activity leading to a progressive recovery of insulin output, and improved management of hyperglycemia. Blackberry juice and its anthocyanin-rich fraction can have a significant impact on reducing the levels of blood glucose in rats with diabetes, possibly because of potent antioxidant chemicals (Zargar et al.2014).
Diabetic rats exhibited a significant increase in serum protein, albumin, ALT, and AST levels compared to the normal control group, indicating potential liver damage and dysfunction due to the metabolic changes associated with diabetes. Diabetes was also related to a significant rise in serum ALT and AST activity, as shown by Yazdi et al. (2019). Ghara et al. (2021) reported that STZ raised both blood glucose and liver enzyme activity (ALT and AST). Hepatocyte injury is detected by increased levels of ALT and AST in the blood (Mansourian et al. 2019). In the blackberry group, the serum levels of AST and ALT decreased by about 27 and 51%, respectively, compared with the diabetic group. This decrease may be due to its antioxidant properties, which protect against diabetes-related liver damage. Ismail et al. (2014) discovered that therapy with strawberry or blackberry alone decreased AST and ALT levels.
In rats with diabetes, impaired glucose metabolism accelerates protein catabolism, leading to an increase in blood urea levels. High blood sugar also causes kidney damage, resulting in elevated levels of creatinine and uric acid as demonstrated in the results of this study. Blackberries reduced serum creatinine, uric acid, and urea levels in diabetic rats. This finding is consistent with the previous report by Hassanalilou et al. (2017) that showed significantly lower serum levels of creatinine, uric acid, and urea in treated rats with diabetes compared with the normal control group after four weeks of administration of the blackberry extract. Abouzed et al. (2020) found that diets high in anthocyanins can reduce the incidence of diabetes and its complications. The present findings corroborate previous research on the putative biological activities of cyanidin 3-glucoside and cyanidin 3-rutinoside (Qin et al. 2018), demonstrating the potential utility of blackberry juice as a neuroprotectant in diabetic animals.
Diabetic rats showed increased glucose-6-phosphatase activity and decreased insulin levels, glucokinase activity, and hepatic glycogen levels compared to the normal control group. Defects in insulin production and action cause hyperglycemia and other metabolic abnormalities associated with T2D through uncontrolled hepatic gluconeogenesis and dyslipidemia due to the dysregulation of fatty acid, triglyceride, and lipoprotein metabolism (Muoio and Newgard 2008). Furthermore, the inability of the β-cells to secrete the amount of insulin required to maintain euglycemia is known to contribute to the development of T2D (Glaser 2007). The decreased insulin secretory capacity of β-cells observed in type 2 diabetes was associated with oxidative stress and inflammation in islet beta cells, causing beta cell death and loss of beta cell mass (Montane et al. 2014). Glucokinase catalyzes a rate-restricting step in the hepatic glucose consumption pathway by phosphorylating glucose to glucose-6-phosphate. Therefore, glucokinase is important in the regulation of blood glucose levels (Agius 2009). Additionally, glucose-6-phosphatase is an important regulator of the gluconeogenesis pathway and affects the development of hyperglycemia (Woerle et al. 2008). Altered enzymes of glucose metabolism were shown in T2D rats, as evidenced by decreased glucokinase activity and increased glucose-6-phosphatase activity (Patel and Goyal 2011). These changes lead to decreased glycogen levels in the liver and hyperglycemia (Ahmed et al. 2012). Our results showed that blackberry juice significantly improved insulin secretion in rats with diabetes, which can result from the effect of therapy on the secretory capacity of β-cells, as well as the improvement of oxidative stress and inflammation in cells of islets. This finding may also explain why the treatment modalities in this study had an antihyperglycemic effect. Adeva-Andany et al. (2016) reported that glucokinase activity raises the rate at which glucose can be used, while the final enzyme in the process of gluconeogenesis, the glucose-6-phosphatase enzyme, decreases the rate of new glucose production. Similarly, the increased glycogen level in the liver after the administration of blackberry juice to rats with diabetes can be associated with the increased availability of glucose-6-phosphate due to decreased Glucose-6-phosphatase activity and increased glucokinase activity. Abouzed et al. (2020) discovered that treating STZ- rats with diabetes using black mulberry fruit extract increased insulin levels compared to the normal control group, possibly due to anthocyanin content.
In diabetic rats, there was a significant decrease in CAT activity, SOD, and GPX levels compared to the normal control group, indicating lower antioxidant capacity. Conversely, the diabetic group had significantly higher levels of MDA, a marker of lipid peroxidation and oxidative stress. Oxidative stress in the diabetic group was connected to a lower antioxidant state that exacerbates the harmful impacts of free radicals (Picton 2001). Increased oxidative stress is a generally established component in the development of diabetes and its associated consequences. Increased oxidative stress may result from diabetes-related free radical generation or compromised antioxidant defenses (Maritim et al. 2003). In diabetes situations, the CAT activity and SOD values deteriorate due to unregulated production of hydrogen peroxide-induced by lipid peroxidation, protein glycation, and glucose autoxidation (Sefi et al. 2011). These findings agree with Abolfathi et al. (2012), who found that increased sensitivity to lipid peroxidation accompanied STZ-induced diabetes in rats. In diabetic rats, treatment with blackberry juice significantly reduced oxidative stress, as evidenced by increased CAT activity and levels of SOD and GPX, compared to the diabetic control group. Blackberry juice could inhibit lipid peroxidation, which is beneficial in maintaining the integrity of the transport layer fluid gradient and the actions of receptors and enzymes bound to the membrane. Maintaining the integrity of membrane lipid is crucial for preventing structural or functional problems linked to diabetes and its consequences, including atherosclerosis (Ma 2012).
Pro-inflammatory cytokines may affect the β-cell disease. It was reported that TNF-α and IL -6 were involved in β-cell dysfunction and death. At the same time, high IL-6 levels predicted developing T2D and significantly reduced insulin sensitivity in liver cells. IL-6 and TNF-α, two pro-inflammatory cytokines, affected the production of acute-phase proteins involved in developing T2D (Muhammad et al. 2016). As a result, the development of T2D is favored by increased inflammation. (Garg et al. 2012). The results showed that blackberry juice reduced hepatic TNF- and IL-6 levels in diabetic rats. These results agree with Ibitoye and Ajiboye (2018), who reported that blackberry juice was shown to reduce IL-6 and TNF-α in rats with metabolic syndrome caused by a high fructose diet.
Peripheral insulin resistance, hyperglycemia, inflammation, and gluconeogenesis in the liver increased the need for insulin production, increasing oxidative stress and ER stress in the beta cell (Papa 2012). TNF-α was shown to be directly related to ER stress and inflammation, forming a vicious cycle between the two. While suppressing NF-κB/TNF-α signaling prevented cell death in response to ER stress, it also prevented stress-induced cell death in ER. Both transcription and translation of insulin were reduced in ER-stressed β-cells (Walter and Ron 2011). ATF4 translation was increased under ER stress, despite the overall decrease in translation (Hamanaka et al. 2009). In this study, ATF4 was used as an indicator of ER stress. This study found that blackberry juice dramatically reduced the expression of ATF4 in diabetic rats, suggesting that ER stress decreased. Based on these results, insulin production and action improved. ATF4 was associated with regulating genes that protect cells from oxidative stress. Therefore, the improved antioxidant status in tissues, especially after treatment with blackberry juice, might have connections with the lower ER stress.
The liver, one of the essential endogenous organs, is severely impacted by diabetes. These findings corroborate Ghara et al. (2021), who found that STZ caused hepatocyte damage, including binucleated cells, and increased Kupffer cells, necrosis, and aberrant sinusoids. Liver damage in diabetic individuals is mainly caused by hyperglycemia-induced oxidative stress, which leads to abnormalities in glucose, protein, and lipid metabolisms (Mohamed et al. 2016). Blackberry fruit juice's antioxidant components, such as cyanidin 3-glucoside and cyanidin 3-rutinoside, have been shown to reduce oxidative stress in cells, resulting in expected structural and functional outcomes, as reported by Qin et al. (2018). Anthocyanin-rich diets were shown to reduce lipogenesis and ameliorate hepatic steatosis (Tsuda 2008). Cyanidin, a chemical found in the juice of blackberry (Pérez-Grijalva et al. 2018), was shown to drastically lower cellular lipid contents in steatotic hepatocytes loaded with lipids.
Conclusion
Finally, the paper on rats with diabetes gave significant findings regarding the consumption of blackberry juice as it could improve lipid profile and reduce elevated levels of total cholesterol, LDL, triglyceride, glucose metabolism, and oxidative stress modulating defense mechanism. Antioxidants reduce inflammation and endoplasmic reticulum (ER) stress. Thus, it gave exciting data for choosing blackberry to be included in the functional food group.
Limitation of study
The present study provides valuable insights into the potential therapeutic effects of blackberry juice on diabetic rats, but it is important to note its limitations. Firstly, the study was conducted on animal models and may not necessarily reflect the effects on humans. Additionally, the study only examined the effects of blackberry juice on a limited set of parameters related to diabetes and did not explore its effects on other important aspects such as lipid metabolism. Moreover, the study is limited by the lack of pancreatic tissue. Finally, the study did not investigate the potential side effects of blackberry juice consumption or its interactions with other medications. Therefore, further studies are necessary to confirm these findings and explore the full potential of blackberry juice as a therapeutic intervention for diabetes.
Data availability
The datasets utilized and analyzed during this investigation are available upon reasonable request from the corresponding author.
References
Abolfathi AA, Mohajeri D, Rezaie A, Nazeri M (2012) Protective effects of green tea extract against hepatic tissue injury in streptozotocin-induced diabetic rats. Evid-based Complement Altern Med 2012:740671. https://doi.org/10.1155/2012/740671
Abouzed TK, Sadek KM, Ghazy EW, Abdo W, Kassab MA, Hago, Assar DH (2020) Black mulberry fruit extract alleviates streptozotocin-induced diabetic nephropathy in rats: targeting TNF-α inflammatory pathway. J Pharm Pharmacol 72(11):1615–1628
Adeva-Andany MM, Pérez-Felpete N, Fernández-Fernández C, Donapetry-García C, Pazos-García C (2016) Liver glucose metabolism in humans. Biosci Rep 36(6):e00416. https://doi.org/10.1042/BSR20160385
Aebi H (1984) Catalase in vitro Methods Enzymol. Academic Press 105:121–126
Agius L (2009) Targeting hepatic glucokinase in type 2 diabetes: weighing the benefits and risks. Diabetes 58(1):18–20
Ahmed OM, Mahmoud AM, Abdel-Moneim A, Ashour MB (2012) Antidiabetic effects of hesperidin and naringin in type 2 diabetic rats. Diabetol Croat 41(2):53–67
Ahmed KA, Korany RMS, El Halawany HA, Ahmed KS (2019) Spirulina platensis alleviates arsenic-induced toxicity in male rats: biochemical, histopathological and immunohistochemical studies. Adv Anim Vet Sci 7(8):701–710
American Diabetes Association (2020) Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care 43(Suppl. 1):S14–S31. https://doi.org/10.2337/dc20-S002
Bancroft JD, Gamble M (2008) Theory and practice of histological techniques 7th ed., Churchill Livingstone London, Elsevier health sciences. UK,. 125–138 and 328–329.
Basu A, Lyons TJ (2012) Strawberries, blueberries, and cranberries in the metabolic syndrome: clinical perspectives. J Agric Food Chem 60(23):5687–5692
Brandstrup N, Kirk JE, Bruni C (1957) The hexokinase and phosphoglucoisomerase activities of aortic and pulmonary artery tissue in individuals of various ages. J Gerontol 12(2):166–171
Castelli WP, Doyle JT, Gordon T, Hames CG, Hjortland MC, Hulley SB, Zukel WJ (1977) HDL cholesterol and other lipids in coronary heart disease. The Cooperative Lipoprotein Phenotyping Study. Circulation 55(5):767–772
Chen Y, Gui D, Chen J, He D, Luo Y, Wang N (2014) Down-regulation of PERK-ATF4-CHOP pathway by Astragaloside IV is associated with the inhibition of endoplasmic reticulum stress-induced podocyte apoptosis in diabetic rats. Cell Physiol Biochem 33(6):1975–1987
Chomczynski P (1993) A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques 15(3):532–534
D’Archivio M, Filesi C, Varì R, Scazzocchio B, Masella R (2010) Bioavailability of the polyphenols: status and controversies. Int J Mol Sci 11(4):1321–1342
Dehkordi AH, Abbaszadeh A, Mir S, Hasanvand A (2019) Metformin and its antiinflammatory and anti-oxidative effects; new concepts. J Renal Inj Prev 8(1):54–61. https://doi.org/10.15171/jrip.2019.11
Dimo T, Rakotonirina SV, Tan PV, Azay J, Dongo E, Kamtchouing P, Gros G (2007) Effect of Sclerocarya birrea (Anacardia ceae) Stem bark methylene chloride/methanol extract on streptozotocin-diabetic rats. J Ethnopharmacol 110:434–438
Drust RW, Wrolstad RE (2001) Separation and characterization of Anthocyanins by HPLC. In: Wrolstad RE (ed) current Protocols in Foods Analytical Chemistry. John Wiley and Sons, New York, pp 1–13
Ercisli S, Orhan E (2007) Chemical composition of white (Morus alba), red (Morus rubra) and black (Morus nigra) mulberry fruits. Food Chem 103(4):1380–1384
Finkel T (2011) Signal transduction by reactive oxygen species. J Cell Biol 194(1):7–15
Furman BL (2015) Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol 70(1):5–47
Garg AD, Kaczmarek A, Krysko O, Vandenabeele P, Krysko DV, Agostinis P (2012) ER stress-induced inflammation: does it aid or impede disease progression? Trends Mol Med 18(10):589–598
Ghara R, Giri SK, Ciardi B, Mellema G, Zaroubi S (2021) Constraining the state of the intergalactic medium during the Epoch of Reionization using MWA 21-cm signal observations. Mon Not R Astron Soc 503(3):4551–4562
Glaser B (2007) Type 2 diabetes: hypoinsulinism, hyperinsulinism, or both? PLoS Med 4(4):e148
Gregg EW, Williams DE, Geiss L (2014) Changes in diabetes-related complications in the United States. New Engl J Med 371(3):286–287
Hamanaka RB, Bobrovnikova-Marjon E, Ji X, Liebhaber SA, Diehl JA (2009) PERK-dependent regulation of IAP translation during ER stress. Oncogene 28(6):910–920
Harper AE (1959) Hormonal factors affecting glucose 6-phosphatase activity. 2. Some effects of diet and of alloxan diabetes in the rat. Biochemical J 71(4):702
Hassanalilou T, Payahoo L, Shahabi P, Abbasi MM, Jafar-abadi MA, Bishak YK, Barzegar A (2017) The protective effects of Morus nigra L. leaves on the kidney function tests and histological structures in streptozotocin-induced diabetic rats. Biomed Res 28(14):6113–8
He J, Giusti MM (2010) Anthocyanins: natural colorants with health-promoting properties. Annu Rev Food Sci Technol 1(1):163–187
Ibitoye OB, Ajiboye TO (2018) Dietary phenolic acids reverse insulin resistance, hyperglycaemia, dyslipidaemia, inflammation and oxidative stress in high-fructose diet-induced metabolic syndrome rats. Arch Physiol Biochem 124(5):410–417
Ismail HAA, Hamza RZ, El-Shenawy NS (2014) potential protective effects of blackberry and quercetin on sodium fluoride-induced impaired hepato-renal biomarkers, sex hormones and hematotoxicity in male rats. J Appl Life Sci Int 1(1):1–16
Jacobson AJ (2010) Materials for solid oxide fuel cells. Chem Mater 22(3):660–674
Jia Z, Tang M, Wu J (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64:555–559
Jing-Jing L, Qi-Hua G, Min L, Hua-Ping Y, Li-Ming C, Cheng-Ping H (2013) Role of Ku70 and Bax in epigallocatechin-3-gallate-induced apoptosis of A549 cells in vivo. Oncol Lett 5(1):101–106
Katsarou A, Gudbjörnsdottir S, Rawshani A, Dabelea D, Bonifacio E, Anderson BJ, Lernmark Å (2017) Type 1 diabetes mellitus. Nat Rev Dis Primers 3(1):1–17
Kaume L, Howard LR, Devareddy L (2012) The blackberry fruit: a review on its composition and chemistry, metabolism and bioavailability and health benefits. J Agric Food Chem 60(23):5716–5727
Kothari V, Galdo JA, Mathews ST (2016) Hypoglycemic agents and potential anti-inflammatory activity. J Inflamm Res 9:27
Lee WY, Nieman TA (1996) Effect of organic solvent on tris (2, 2′-bipyridyl) ruthenium (III) chemiluminescent reactions in flowing streams. Anal Chim Acta 334(1–2):183–191
Li MH, Oberle DF, Lucas PM (2012) Effects of dietary fiber concentrations supplied by corn bran on feed intake, growth, and feed efficiency of channel catfish. North Am J Aquac 74(2):148–153
Ma Z (2012) The role of peroxidation of mitochondrial membrane phospholipids in pancreatic β-cell failure. Curr Diabetes Rev 8(1):69–75
Mansourian M, Mirzaei A, Azarmehr N, Vakilpour H, Kokhdan EP, Doustimotlagh AH (2019) Hepatoprotective and antioxidant activity of hydroalcoholic extract of Stachys pilifera. Benth on acetaminophen-induced liver toxicity in male rats. Heliyon 5(12):e03029
Maritim AC, Sanders A, Watkins Iii JB (2003) Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol 17(1):24–38
Maurya S, Singh D (2010) Quantitative analysis of total phenolic content in Adhatoda vasica Nees extracts. Int J Pharm Tech Res 2:2403–2406
Mishra SB, Verma A, Mukerjee A, Vijayakumar M (2011) Anti-hyperglycemic activity of leaves extract of Hyptis suaveolens L. Poit in streptozotocin induced diabetic rats. Asian Pac J Trop Med 4(9):689–693
Mohamed J, Nafizah AN, Zariyantey AH, Budin S (2016) Mechanisms of diabetes-induced liver damage: the role of oxidative stress and inflammation. Sultan Qaboos Univ Med J 16(2):e132
Montane J, Cadavez L, Novials A (2014) Stress and the inflammatory process: a major cause of pancreatic cell death in type 2 diabetes. Diabetes Metab Syndr Obes Targets Ther 7:25
Muhammad IF, Borné Y, Hedblad B, Nilsson PM, Persson M, Engström G (2016) Acute-phase proteins and incidence of diabetes: a population-based cohort study. Acta Diabetol 53(6):981–989
Muoio DM, Newgard CB (2008) Molecular and metabolic mechanisms of insulin resistance and β-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol 9(3):193–205
Nishikimi M, Rao NA, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46(2):849–854
Ohkawa H, Ohishi W, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Oyadomari S, Araki E, Mori M (2002) Endoplasmic reticulum stress-mediated apoptosis in pancreatic β-cells. Apoptosis 7(4):335–345
Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Hotamisligil GS (2004) Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306(5695):457–461
Papa FR (2012) Endoplasmic reticulum stress, pancreatic β-cell degeneration, and diabetes. Cold Spring Harb Perspect Med 2(9):a007666
Patel SS, Goyal RK (2011) Cardioprotective effects of gallic acid in diabetes-induced myocardial dysfunction in rats. Pharmacogn Res 3(4):239
Pérez-Grijalva B, Herrera-Sotero M, Mora-Escobedo R, Zebadúa-García JC, Silva-Hernández E, Oliart-Ros R, Guzmán-Gerónimo R (2018) Effect of microwaves and ultrasound on bioactive compounds and microbiological quality of blackberry juice. LWT 87:47–53
Petersen MC, Vatner DF, Shulman GI (2017) Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol 13(10):572–587
Picton HM (2001) Activation of follicle development: the primordial follicle. Theriogenology 55(6):1193–1210
Qin Y, Zhai Q, Li Y, Cao M, Xu Y, Zhao K, Wang T (2018) Cyanidin-3-O-glucoside ameliorates diabetic nephropathy through regulation of glutathione pool. Biomed Pharmacother 103:1223–1230
Ragavan B, Krishnakumari S (2006) Antidiabetic effect of T. arjuna bark extract in alloxan induced diabetic rats. Indian J Clin Biochem 21(2):123–128
Rajasekaran S, Sivagnanam K, Subramanian S (2005) Antioxidant effect of Aloe vera gel extract in streptozotocin-induced diabetes in rats. Pharmacol Rep 57(1):90–96
Ranganna S (1977) Manual of analysis of fruits and vegetable products. Tata MC. Graw – Hill Publishing Company Limited, New Delhi, India.
Roe JH, Dailey RE (1966) Determination of glycogen with the anthrone reagent. Anal Biochem 15:245–250
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra W (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179(4073):588–590
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, IDF Diabetes Atlas Committee (2019) Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract 157:107843
Samarghandian S, Azimi-Nezhad M, Farkhondeh T (2017) Catechin treatment ameliorates diabetes and its complications in streptozotocin-induced diabetic rats. Dose-Response 15:1–7
Sears B, Ricordi C (2012) Role of fatty acids and polyphenols in inflammatory gene transcription and their impact on obesity, metabolic syndrome and diabetes. Eur Rev Med Pharmacol Sci 16:1137–1154
Sefi M, Fetoui H, Lachkar N, Tahraoui A, Lyoussi B, Boudawara T, Zeghal N (2011) Centaurium erythrea (Gentianaceae) leaf extract alleviates streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. J Ethnopharmacol 135(2):243–250
Siriwoharn T, Wrolstad RE, Finn CE, Pereira CB (2004) Influence of cultivar, maturity, and sampling on blackberry (Rubus L. Hybrids) anthocyanins, polyphenolics, and antioxidant properties. J Agric Food Chem 52(26):8021–8030
Soraya S, Sukara E, Ernawati S (2022) Identification of chemical compounds in Ziziphus mauritiana Fruit juice by GC-MS and LC-MS/MS analysis. IJBPCS 4(2):11–19
Swanston-Flatt SK, Day C, Bailey CJ, Flatt PR (1990) Traditional plant treatments for diabetes. Studies in normal and streptozotocin diabetic mice. Diabetologia 33(8):462–464
Tsuda T (2008) Regulation of adipocyte function by anthocyanins, possibility of preventing the metabolic syndrome. J Agric Food Chem 56(3):642–646
Wacho P, Gildas A, Ulrich M, Modeste W, Benoit N, Albert K (2012) Hypoglycemic and hypolipidemic effects of Bersama engleriana leaves in nicotinamide/streptozotocin-induced type 2 diabetic rats. BMC Complement Altern Med 12(1):1–6
Walter P, Ron D (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334(6059):1081–1086
Woerle HJ, Albrecht M, Linke R, Zschau S, Neumann C, Nicolaus M, Schirra J (2008) Impaired hyperglycemia-induced delay in gastric emptying in patients with type 1 diabetes deficient for islet amyloid polypeptide. Diabetes Care 31(12):2325–2331
Yazdi MD, Wang Y, Di Q, Zanobetti A, Schwartz J (2019) Long-term exposure to PM2. 5 and ozone and hospital admissions of Medicare participants in the Southeast USA. Environ Int 130:104879
Yilmaz S (2019) Effects of dietary blackberry syrup supplement on growth performance, antioxidant, and immunological responses, and resistance of Nile tilapia, Oreochromis niloticus to Plesiomonas shigelloides. Fish Shellfish Immunol 84:1125–1133
Zargar BA, Masoodi MH, Ahmed B, Ganie SA (2014) Antihyperlipidemic and antioxidant potential of Paeonia emodi Royle against high-fat diet induced oxidative stress. Int Scholarly Res Notices 2014.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Sallam K. Tony and Hanaa S.S. Gazwi conceived the project. Sallam K. Tony, Mohamed SH. Hassan, Hamadi A. Ismail, Gamal F. Abd El-Naem, and Hanaa S.S. Gazwi designed and performed the experiments. Sallam K. Tony and Hanaa S.S. Gazwi analyzed the data. Sallam K. Tony, Mohamed SH. Hassan, Hamadi A. Ismail, Gamal F. Abd El-Naem, and Hanaa S.S. Gazwi wrote the manuscript. Mohamed SH. Hassan, Hamadi A. Ismail, Gamal F. Abd El-Naem, and Hanaa S.S. Gazwi provided critical discussion, editing, and final approval of the manuscript. All authors contributed to the preparation of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The research on animals was carried out according\s to the animal protection guidelines approved by the university authorities.
Consent for publication
Not applicable.
Consent to participate
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tony, S.K., Hassan, M.S., Ismail, H.A. et al. Effect of anthocyanin-rich blackberry juice on endoplasmic reticulum stress in streptozotocin-induced diabetic rats. Environ Sci Pollut Res 30, 79067–79081 (2023). https://doi.org/10.1007/s11356-023-27827-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-27827-z