Abstract
Goethite was modified by chitosan (CS) or poly(acrylic acid) (PAA) to improve its adsorptive abilities toward components of agrochemicals, i.e., copper ions (Cu), phosphate ions (P), and diuron. The pristine goethite effectively bound Cu (7.68 mg/g, 63.71%) and P (6.31 mg/g, 50.46%) only in their mixed systems. In the one adsorbate solutions, the adsorption levels accounted for 3.82 mg/g (30.57%) for Cu, 3.22 mg/g (25.74%) for P, and 0.15 mg/g (12.15%) for diuron. Goethite modification with CS or PAA did not yield spectacular results in adsorption. The maximum increase in adsorbed amount was noted for Cu ions (8.28%) after PAA modification as well as for P (6.02%) and diuron (24.04%) after CS modification. Both goethite modifications contributed to clear reduction in desorption of pollutants (even by 20.26% for Cu after PAA coating), which was mainly dictated by electrostatic attractive forces and hydrogen bonds formation occurring between macromolecules and impurities. The only exception in this phenomenon was Cu desorption from CS-modified solid—the polymer made it higher (to 95.00%). The Cu adsorption on PAA-modified goethite enhanced solid aggregation and thus facilitated metal cation separation from aqueous media. Consequently, the goethite modification with PAA was considered more promising for environmental remediation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Expansively growing human population is a major challenge for agriculture. It contributes to the increasing demand for food and thus application of more and more plant protection products and fertilizers improving crop quality and quantity (Kopittke et al. 2019). Excessive use of agrochemicals leads to accumulation of various harmful substances in natural ecosystems. The group of compounds most frequently detected in water-soil environment includes, among others, phosphates (P), copper (Cu), and diuron.
Phosphate fertilizers are applied regularly in agriculture to avoid physiological disorders in plant growth (Xu et al. 2019). This usually leads to eutrophication in neighboring water reservoirs manifested by algal overgrowth and oxygen depletion (Awual 2019a). The P concentration equal to 100 mg/L is high enough to induce this phenomenon (Kumar et al. 2019; Nazari-Sharabian et al. 2018). The phosphates use in various industries has also became larger and larger in recent years. As a result, they are present in industrial sewage and can cause additional environmental pollution due to illegal waste discharges or insufficient treatment (Ganesh et al. 2012). In waters, the mean detected P concentrations are 552.63 ng/L in Nanjing (China), 226.6 ng/L in Shihwa Lake (Korea), and 265.3 ng/L in Sydney (Australia) (Qiao et al. 2022). In the USA, the P level in soils typical of the mid-Atlantic region is 12.5 mg/kg (Maguire et al. 2005), whereas soils from poultry production areas contains even 568 mg/kg of these compounds (Hooda et al. 2001). Cu is classified as a micronutrient essential for proper plant growth. However, at higher concentrations this is a toxic element having negative effect on organisms (Ju et al. 2019). Anthropogenic activities including application of copper-based fertilizers, mining, and metal ore processing contribute to excessive accumulation Cu in the environment (Rehman et al. 2019). The Cu toxicity occurs when its concentration exceeds 50 mg/L in sandy soils or 150 mg/L in silty-clay or clay soils (Droz et al. 2021). The maximum Cu amounts detected in surface waters were 9.0-261.0 μg/L, while in groundwater, 64.0-2,783.0 μg/L (ATSDR 2022). Diuron (3-(3,4-dichlorophenyl)-1,1-dimethylurea) is applied to control unwanted weeds. Due to its high persistence in the environment, it is considered a serious threat for soils and waters (Egea et al. 2017). Diuron is metabolized by soil bacteria and fungi to several toxic metabolites, among which DCA (3,4-dichloroaniline) has the highest nephrotoxicity and hepatotoxicity (Mohammed et al. 2020). UE has set the maximum permissible residue level of diuron in the soil to 0.01 mg/kg for fruits, vegetables, cereals, and sugar crops; 0.02 mg/kg for nuts and oil crops; and 0.05 mg/kg for herbs, tea, coffee, herbal infusions, spices, and animal products (Szewczuk-Karpisz et al. 2021a). So far, its average concentration detected in Australia was 397 μg/kg in (Stork et al. 2008), in turn in the USA, 380 μg/kg (Field et al. 2003).
The harmful effects of phosphates, copper ions, and diuron can be reduced by their adsorption on the solid surfaces. As a consequence, their leaching into neighboring waters and bioavailability can be reduced significantly. Nowadays, many researchers are looking for environmentally friendly, biodegradable, and low-cost adsorbents that are able to bind large amounts of pollutants and can be applied for environmental remediation. For Cu detection and removal from wastewater, several composite materials based on silica were developed and described. There are mesoporous silica with 5-tert-butyl-2-hydroxybenzaldehyde thiosemicarbazone (THTB) ligand (Awual 2015), mesoporous silica with Schiff base ligand containing nano-composite adsorbent (NCA) (Awual et al. 2015b), mesoporous silica monoliths with (3-(3-(methoxycarbonyl)benzylidene) hydrazinyl)benzoic acid ligand (Awual et al. 2013), mesoporous silica monolith with 3-(((5-ethoxybenzenethiol)imino)methyl)-salicylic acid (EBMS) ligand (Awual et al. 2015c), mesoporous silica monolith with N,N-bis(salicylidene)1,2-bis(2-aminophenylthio)ethane ligand (Awual et al. 2014a), mesoporous silica with sulfur donor containing organic ligand of ammonium (4-chlro-2-mercaptophenyl)carbamodithioate (ACMPC) (Awual et al. 2016), mesoporous silica with N,N-disalicylidene-4,5-dimethyl-phenylenedene (DDPD) ligand (Awual 2017), reusable ligand anchoring stable composite (Awual 2019b), porous silica with 2-methyl-8-quinolinol ligand (Awual et al. 2019), mesoporous silica with 4-tert-Octyl-4-((phenyl)diazenyl)phenol (TPDP) ligand (Salman et al. 2023a), and porous silica with 4-dodecyl-6-((4-(hexyloxy)phenyl)diazenyl)benzene-1,3-diol (DPDB) ligand (Kubra et al. 2021). Composite material for simultaneous removal of Cu and lead(II) ions, that is, 6-((2-(2-hydroxy-1-naphthoyl)hydrazono)methyl)benzoic acid (HMBA) embedded onto mesoporous silica monoliths, was also investigated (Awual et al. 2014b). Similar materials were successively applied for thulium(III) (Kubra et al. 2023), lutetium(III) (Hasan et al. 2023a, 2023b), palladium(II) (Awual and Yaita 2013), and cadmium(II) (Hasan et al. 2023a, 2023b) capturing. Silica-based composites with immobilized organic ligands are very promising as well as show high sensitivity and sorption capacity toward metal ions.
The second group of composites used to remove metals and other pollutants are those prepared on the basis of soil minerals and macromolecular compounds. So far, the effect of macromolecular compound on adsorption capacity of minerals or mineral composites toward toxic substances has been tested several times. Szewczuk-Karpisz et al. (2021b) examined impact of poly(acrylic acid) on Cu adsorption on the carbon-mineral composites with metallic elements. The same team determined the impact of bacterial exopolysaccharide on accumulation of Cu, chromium (Cr), and carboxin on montmorillonite (Szewczuk-Karpisz et al. 2022). Fijałkowska et al. (2021) described adsorption mechanism of lead (Pb) on kaolinite and montmorillonite modified with cationic and anionic polyacrylamide (PAM). Medykowska et al. (2022) examined the removal of diclofenac and heavy metal ions (Pb, zinc (Zn)) using zeolitic materials in the systems containing also PAA 2000 or PAA 240 000. The impact of CS coating on mineral adsorptive abilities has rarely been investigated. Most researchers applied chitosan as a substrate to synthesize new materials (Da Silva Alves et al. 2021). Sutirman et al. (2018) performed the chemical and physical modification of CS in order to improve removal of metal ions from aqueous solutions. Chen et al. (2020) obtained chitosan-carboxylmethyl starch composites and examined their adsorption capactity relative to Cu. Gu et al. (2019) prepared chitosan-lignosulfonate composite for removal of dyes and metals from wastewater. Salman et al. (2023b) investigated CS-coated cotton fiber composite for dye removal. Many researchers used CS for hydrogel synthesis, e.g., magnetic chitosan/poly(vinyl alcohol) beads (Zhu et al. 2012), methacrylamide/chitosan crylogels (Kundakci 2020), and polyacrylamide-g-chitosan gel (Da Silva et al. 2020). Goethite coated with PAA or CS has not been reported yet.
Goethite (α-FeOOH) is one of the most stable iron oxyhydroxides in nature, present in almost all soil types (Liu et al. 2013), as well as a waste product of hydrometallurgical processes, e.g., zinc production. In the second case, goethite may be an environmental problem and limit light penetration and photosynthesis when discharged into waters (Szewczuk-Karpisz and Wiśniewska 2014). Goethite application for adsorption purposes creates an opportunity to manage it and reduce potential threat to ecosystems (Szewczuk-Karpisz et al. 2019). Since the specific surface area of this mineral varies in a large range and may be as low as 10 m2/g (Liu et al. 2013), it seems necessary to modify it and improve its sorption capacity.
Therefore, in this work, an attempt was made to coat goethite with two polymeric substances of different properties and origins, i.e., CS and PAA, to explore new potential soil conditioners or adsorbents for contaminated surface or groundwater treatment. The adsorption capacity of the goethite with and without macromolecular compounds was examined toward Cu, P, and diuron, in the systems containing one or two of them at the same time. The mechanism of Cu/P/diuron adsorption on goethite, coated or uncoated with CS or PAA, was explored based on the study including isotherms and kinetics, adsorption data modeling, potentiometric titration, and Fourier transform infrared spectroscopy (FTIR). The strength of pollutants binding was estimated during the desorption study. Zeta potential and turbidimetric measurements were used to determine the stability of the goethite suspension with and without adsorbed polymers/pollutants. It was predicted that goethite modification with CS or PAA will improve its adsorption abilities toward various substances due to introduction of additional functional groups together with polymers. The presence of the polymer may also stimulate the aggregation of goethite with adsorbed impurities, which is desirable during the purification of aqueous systems. It is worth emphasizing that the presented composites were prepared using only safe, environmentally friendly substrates which made them suitable for environmental remediation. CS is obtained by chemical or enzymatic deacetylation of chitin (the main component of the cuticle of crustaceans) (Saheed et al. 2021), whereas PAA is a non-toxic polymer produced by the radical polymerization of acrylic acid (Szewczuk-Karpisz et al. 2021b).
The presented issue can be considered highly innovative and important from the point of view of water and soil protection. Simultaneous adsorption of copper ions, phosphate ions, and diuron on the goethite has not been described in the literature. The impact of the goethite modification of PAA or CS on its adsorption capacity toward selected impurities and aggregation also remains unknown. This paper is part of the search for new means to remediate degraded ecosystems. It also describes how waste goethite is managed in order to enable closed-loop operation.
Experimental
Materials
Goethite (CAS 20344-49-4) was applied as an adsorbent in the experiments. Chitosan (CAS 9012-76-4) of medium molecular weight and poly(acrylic acid) (CAS 9003-01-4) were used as macromolecular compounds for goethite coating. CS average molecular weight (Mw), determined by size exclusion chromatography (SEC-MALS), was equal to 411 kDa (Matusiak et al. 2022). Conductometric titration with HCl indicated that the deacetylation degree (DD) of the applied CS was 75%. The average molecular weight of PAA was 2000 Da. Both polymers were delivered by Sigma Aldrich and were not further cleaned. Copper ions, phosphate ions, and diuron (C9H10Cl2N2O, CAS 330-54-1) were used as pollutants. Copper(II) chloride dihydrate, CuCl2 × 2H2O (CAS 10125-13-0) was applied as a source of copper ions and monosodium phosphate, (CAS 7558-80-7) as a source of phosphate ions. The formulas and structures of adsorbent and adsorbates are summarized in Table 1. Before the experiments, the goethite was washed out using demineralised water to a conductivity of < 2 μS/cm.
To adjust pH value of the examined systems, hydrochloric acid (HCl, CAS 7647-01-0) and sodium hydroxide (NaOH, CAS 1310-73-2) were used. In the determination of polymer concentration sulfuric acid (H2SO4, 98%, CAS 7664-93-9) and hyamine 1622 (0.004 mol/dm3, CAS 121-54-0) were applied. Sodium chloride (NaCl, CAS 7647-14-5) was used as a supporting electrolyte. All the chemicals were delivered by Sigma Aldrich.
The concentrations of stock solutions of copper ions and phosphate ions as well as CS and PAA were 1000 mg/L. In turn, the concentration of the stock solution of diuron, due to its limited solubility in water, was 10 mg/L.
Methods
Goethite characterization
The goethite sample was characterized using Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM-EDS), nitrogen adsorption/desorption, energy dispersive X-ray analyses (EDX), and potentiometric titration.
Determination of goethite surface groups was performed using a Nicolet 6700 Fourier-transform infrared (FTIR) spectrometer (Thermo Scientific) equipped with a Smart Orbit diamond-attenuated reflectance attachment. The spectra were recorded in the range of 4000–400 cm-1, at 4 cm-1 intervals, from 128 scans, and were corrected with a linear baseline using the OMNIC v.8.2 software (Thermo Scientific).
The morphology of the goethite was observed using a scanning electron microscopy (SEM) (Phenom ProX, PiK Instruments) equipped with an energy dispersive spectrometer (EDS). The applied acceleration voltage was 10 kV. The analyses were carried out in a low vacuum mode without coating the sample with conductive layer.
Based on nitrogen adsorption/desorption isotherms, measured using an analyzer 3Flex (Micromeritics, USA), the specific surface area (SBET) and porosity parameters (total pore volume—Vp, pore diameter—D) of goethite were determined. Specific surface area was calculated using the Brunauer-Emmet-Teller (BET) equation as well as the capacity of monolayer formed on the mineral surface. The porosity parameters were estimated using nitrogen desorption isotherm. Before the measurement, the sample was dried and out-gassed (105 °C, 12 h).
Energy dispersive X-ray analyses (EDX-7200, Shimadzu) were used to determine elemental composition of goethite.
Potentiometric titration was applied to estimate the point of zero charge (pHpzc) of the mineral. The titration was performed using an automatic burette (Titrino 702 SM, Metrohm) and 0.1 M sodium hydroxide (NaOH) as the titrant, at pH values of 3-10. The surface charge density (σ0) was calculated using the method developed by Janusz (1994).
Goethite modification
Goethite modification was performed by its coating with polymers via adsorption. At the beginning, the samples (10 mL) containing 0.04 g of the goethite, the supporting electrolyte (0.01 mol/L NaCl) and the polymer (50 or 100 mg/L of CS/PAA) were prepared. After the pH adjustment to the value of 5, the adsorption was conducted for 24 h. This time ensured the achievement of equilibrium state in the examined systems. After process completion, the samples were centrifuged to separate modified goethite from the solution. The amounts of adsorbed polymers were determined based on the difference in their concentration in the solution before and after the adsorption, using the following formula (Ościk 1969):
where Cads—the adsorbed amount of the polymer (Cads = C0 − Ceq) [mg/L], V—the system volume [L], m—the weight of the goethite [g].
In turn, the efficiency, E, of the polymer adsorption was calculated using the equation (Szewczuk-Karpisz et al. 2022):
where CA—the concentration of polymer adsorbed on the goethite [mg/L], C0—the initial polymer concentration in the sample [mg/L].
The concentration of CS was determined using the method developed by Albalasmeh et al. (2013), in turn the concentration of PAA—by the method with hyamine 1622 (Kang et al. 2013). During the adsorption on solid surfaces, macromolecular compounds behave differently than ions and small molecules. They assume specific conformations including structures like “loops” and “tails” and one polymer chain can interact with several active sites (Fijałkowska et al. 2019). Therefore, the adsorbed amounts of CS/PAA on goethite are presented only as a histogram.
Study on pollutant adsorption in the single systems
To examine kinetics of the pollutant adsorption on the goethite, a series of samples was prepared. They consisted of 0.04 g of the mineral and 10 mL of the solution containing 0.01 mol/L NaCl as a supporting electrolyte and the selected pollutant (copper ions/phosphate ions/diuron). The applied concentration of copper ions/phosphate ions was 100 mg/L, while that of diuron, 5 mg/L. After the pH adjustment to the value of 5, the adsorption was started and conducted for 5, 15, 30, 60, 120, 180, 300 min using a rotator (Multi RS-60, Biosan, 30 rpm). When the process was complete, the solid with adsorbed impurities was separated from the solution by centrifugation (3000 rpm, 10 min, centrifuge SBS-LZ-4000/20-6, Steinberg Systems) and using syringe filters (0.22 μm, AlfaTec Technology). The concentration of phosphate in the obtained clear solutions was determined by ion chromatography (ICS-1100, Dionex), the concentration of copper ions—by an atomic absorption spectrometer working in the technique with a graphite cuvette (contrAA 900, Analytic Jena), whereas the concentration of diuron—by high pressure liquid chromatography (Dionex Ultimate 3000 equipped with a diode array detector).
To prepare adsorption isotherms, the samples were prepared as mentioned above. The applied concentration of ions was in the range of 10-200 mg/L, whereas that of diuron was in the range of 1-9 mg/L. After the samples preparation, their pH value was adjusted to 5 using 0.1 mol/L HCl or 0.1 mol/L NaOH and a pH meter (CX-505, Elmetron), and the adsorption was started. The process was conducted under conditions of continuous mixing (rotator Multi RS-60, Biosan, 30 rpm), for 24 h. This time was chosen based on the kinetics study results (it ensured equilibrium state in the examined systems). The concentration of pollutants in the obtained clear solutions was analyzed in the same way as during the study on adsorption kinetics.
As in the case of polymer adsorption, the adsorbed amount of copper ions/phosphate ions/diuron on the goethite was calculated based on the difference in their concentrations in the solution before and after the adsorption process using Eq. (1). The efficiency of pollutant adsorption was determined using Eq. (2).
Study on the pollutant adsorption in the mixed systems
During the adsorption study in the mixed systems, the samples were prepared using 0.04 g of the goethite as well as 10 mL of the solution containing 0.01 mol/L NaCl as a supporting electrolyte and 50 mg/L of two different substances, either two pollutants or one pollutant and one polymer. In this way, it was possible to measure the mutual influence of impurities on their adsorption on the goethite surface, as well as the influence of CS/PAA on the adsorption of impurities. The adsorption process and the determination of copper ions/phosphate ions/diuron concentration were performed in the same way as described in the section above.
Study on pollutant desorption
The goethite with adsorbed impurities, obtained in the adsorption study with or without polymers, was dispersed in an aqueous solution at pH 5 (10 mL, the pH value of desorbing solution was adjusted using 0.1 mol/L HCl). Then the desorption process was conducted for 1 h under continuous shaking (rotator Multi RS-60, Biosan, 30 rpm). After that, the solid was separated from the solution using centrifuge (3000 rpm, 10 min, centrifuge SBS-LZ-4000/20-6, Steinberg Systems) and syringe filter (0.22 μm, AlfaTec Technology), and the concentration of pollutants was analyzed in the same way as during “adsorption study.” The desorption degrees (%) of copper ions/phosphate ions/diuron from pristine and modified goethite were calculated using the equation:
where Cdes is the desorbed concentration of the ion or herbicide [mg/L].
Adsorption data modeling
Experimental adsorption isotherms were modeled using Langmuir, Freundlich and Redlich-Peterson models. The kinetics data was fitted to the pseudo I-order (PFO) and pseudo II-order (PSO) equations. The Langmuir (Eq. 4) and Freundlich (Eq. 5) models are described as (Foo and Hameed 2010):
where KF [mg/g(mg/L)-1/nF]—the Freundlich parameter, KL [L/mg]—the Langmuir parameter, qe [mg/g]—the equilibrium adsorption capacity, Ce [mg/L]—the equilibrium liquid phase concentration; qm [mg/g]—the maximum adsorption capacity in Langmuir model, n—the Freundlich constant related to adsorption intensity.
The Redlich-Peterson isotherm model (Eq. 6) is defined as (Kumara et al. 2014):
where KRP—the Redlich–Peterson adsorption capacity constant [L/mg], aRP—the Redlich–Peterson isotherm constant [(L/mg)β], β—the exponent.
In turn, the pseudo I-order (Eq. 7) and pseudo II-order (Eq. 8) equations are expressed as (Ho and McKay 1998, 2002):
where qe—the adsorbed amount at equilibrium [mg/g]; qt—the adsorbed amount after time t [mg/g]; and k1 [1/min] and k2 [g/mg·min]—the rate constants.
Zeta potential study
The zeta potential values of the goethite with and without polymers/pollutants were calculated based on electrophoretic mobility values measured using ZetaPlus (Brookhaven Instruments Corporation). At first, 0.001 g of goethite was added to 100 mL of sodium chloride solution (0.01 mol/L). The pH of the solution was adjusted to the value of 5 by adding hydrochloric acid. After 10 min of sonication, the suspension was divided into several parts, to which a certain volume of impurities and polymers was added to obtain their final concentration of 50 mg/L for copper ions/phosphate ions/polymers and 5 mg/L for diuron. Such prepared suspensions were stirred on the magnetic stirrer (HI 190M, Hanna Instruments) for 30 min to allow adsorption to occur. In the systems with electrostatic repulsion between adsorbate and adsorbent, this time was extended to 20 h to ensure that equilibrium was established. If the pH of the suspensions changed after adding polymer or pollutant, it was restored using several drops of 0.1 mol/L HCl or 0.1 mol/L NaOH. The pH of the systems was measured using a pH-meter (827 pH lab, Metrohm). After the suspension stirring for the selected time, the electrophoretic mobility of the goethite was measured.
Stability study
The stability of the goethite suspensions, with and without pollutants/polymers, was measured using a UV-Vis spectrophotometer (Jasco V-530) at a wavelength of 500 nm. The samples were prepared by adding 0.01 g of the goethite the solution of supporting electrolyte (0.01 mol/L NaCl). After 10 min of sonication, the selected substance (polymer, pollutant, or polymer + pollutant) was added and the pH value of the samples was adjusted to 5. The concentration of copper ions/phosphate ions and CS/PAA in the examined systems was 50 mg/L, whereas that of diuron, 5 mg/L. One stability measurement lasts 1 h, during which the absorbance was measured every 10 s.
Statistical analysis
All the adsorption/desorption measurements were made in triplicate, in turn zeta potential measurements were repeated 10 times. The measurement uncertainty was determined by calculation of standard deviation (Statistica software and Microsoft Office Excel). The measurement error did not exceed 5%.
Results and discussion
Goethite characteristics
Goethite is an iron oxyhydroxide of the formula α-FeOOH, mainly composed of iron hydroxide (80-90%) and water (10-20%). In its crystals, oxygen and hydroxyl anions are packed hexagonally in arrays, whereas all iron atoms are coordinated octahedrally (Table 1) (The Editors of Encyclopaedia Britannica 1998). The EDX analyses indicated that the applied goethite was composed of the following: iron (Fe)—about 32% and oxygen (O)—about 67%, as well as trace amounts of sulfur (S), zinc (Zn), copper (Cu), manganese (Mn), and strontium (Sr).
Textural parameters of the goethite were rather poor. The N2 adsorption/desorption experiment indicated that its SBET parameter equaled 11 m2/g, and the Vp was 0.034 cm3/g (Table 2). The obtained nitrogen adsorption/desorption isotherms represented type IV with the H3 hysteresis loop (Fig. 1a). Such an isotherm type describes a multilayer adsorption with capillary condensation on mesoporous materials. The H3-type hysteresis loop is present in aggregate, parallel-corrugated pellets with slit-like pores. The calculated pore size distribution (Fig. 1a) indicated mesoporous character of the goethite. The DPSD value was 4.7 nm, whereas D4V/A was 12.2 nm. The obtained SBET parameter corresponded with that of synthetic goethite prepared by Ulatowska (2022).
The SEM images showed that the goethite particles were needle-like and elongated (Fig. 1b).
Potentiometric titration results showed that the point of zero charge (pHpzc) of the goethite was equal to 8.2 (Szewczuk-Karpisz et al. 2019). This means that at pH = 8.2, the surface charge of the goethite is equal to 0 and then neutral groups, ≡Fe2OH, are dominant on its surface. At pH < 8.2, the mineral is positively charged because ≡FeOH1/2+ and ≡Fe3OH1/2+ groups prevail. At pH > 8.2, the goethite surface charge is negative due to the domination of ≡FeO1/2- and ≡Fe3O1/2- moieties on the surface. The above specific groups are created on the goethite surface because, according to Pauling’s rules, oxygens are singly, doubly, and triply coordinated and all iron atoms are octahedrally coordinated in its structure (Tadanier and Eick 2002).
Adsorption mechanism of copper ions/phosphate ions on the pristine goethite
At pH 5, at which adsorption study was performed, copper ions occurred as Cu2+, whereas phosphate ions were mainly in the form of H2PO4- (Kabata-Pendias and Pendias 1999; Varlot et al. 1999). The goethite surface was positively charged (Szewczuk-Karpisz et al. 2019).
In Fig. 2a, the time effect on the Cu and P adsorption on goethite is presented. At the beginning, there was a rapid increase in adsorbed amount associated with the ions bonding on external surface of mineral crystals. Then, after 100 min for Cu and 300 min for P, the kinetic curves increased more slowly due to the adsorbate diffusion into internal sites. Experimental kinetic data were best fitted to the pseudo II-order equation (R2 was in the range of 0.959–0.999, Table 3). This means that Cu and P adsorption on goethite could be described as chemisorption. Cu ions can form inner- and outer-sphere complexes on the goethite surface. In the first case, the metal ions interact with the surface directly based on covalent or ionic bonds (specific adsorption), whereas in the second one, at least one water molecule is located between the system components and the adsorption process is of coulombic character (non-specific adsorption) (Perelomov et al. 2013; Grossi and Sparks 1994). Outer-sphere surface complexation prevails at low pH, while, the inner-sphere one, at high pH values (Liu et al. 2013). Phosphate ions adsorption on the goethite is mainly based on the surface complexation (Fe-O-P-O-Fe type) and formation of hydrogen bridges. The replacement of hydroxyl groups by adsorbing anions also occurs in the system (Liu et al. 2013). Due to the fact that goethite is positively charged at pH 5, there is electrostatic attraction between the solid particles and the H2PO4- ions (Ler and Stanforth 2003). Surface complexation reactions occurring between goethite active sites and ions can be described as follows (Jaiswal et al. 2013; Katz 2017):
Adsorption isotherms and kinetics of copper ions, phosphate ions (a) and diuron (b) on goethite with the fitting to Redlich-Peterson and pseudo second-order (PSO) models as well as comparison of adsorbed amounts of copper ions (c), phosphate ions (d), and diuron (e) in the systems containing one or two pollutants simultaneously
where Me2+—the metal ions involved in inner-sphere reaction, Cat2+—the metal ions involved in outer-sphere reaction, OxAn2-—the anions involved in inner-sphere reaction, An-—the anions involved in outer-sphere reaction.
The experimental isotherms of Cu and P adsorption, presented in Fig. 2b, were best fitted to the Redlich-Peterson model (R2 was in the range of 0.997–0.999, Table 3). The β parameter for both ions was close to unity, which meant that their adsorption was localized (ions did not move on the surface and one ion interacted with one active site) (Majd et al. 2022). Ulatowska (2022) reported that arsenic(V) adsorption on synthetic goethite was best described by Dubinin-Radushkevich and pseudo second-order models. Jaiswal et al. (2013) indicated that Cu and Cd adsorption on synthetic goethite was best fitted to Freundlich and pseudo second-order equations. Similarly, cobalt (Co) and nickel (Ni) adsorption on natural iron oxide and synthetic goethite was best described by Freundlich and pseudo second-order models (Nangah et al. 2019).
The EDX analyses (Fig. 3) confirmed that copper and phosphate ions were adsorbed on the goethite surface. There are additional peaks in the spectra registered for goethite after ions adsorption, not visible in the spectrum for the goethite before the process. There are peak around 9 keV corresponding to Cu (Fig. 3b) and peak around 2 keV corresponding to P (Fig. 3c).
After ions adsorption, the changes occurred also in the FTIR spectrum of goethite (Fig. 4a). The spectrum of pristine goethite consisted of the following bands at: 3112 cm-1 (the typical OH stretching band of oxyhydroxides), 906 cm-1 and 794 cm-1 (which can be attributed to the FeOOH bending), 604 cm-1 (the Fe–O stretching). After the Cu and P adsorption, the 3112 cm-1 bands shifted to 3095 cm-1 and 3087 cm-1, respectively. Moreover, the intensity of the bands at 904, 794, and 604 cm-1 was decreased. All these changes were probably dictated by the interactions of copper and phosphate ions with Fe–O–H and Fe–O groups (Salimi et al. 2018).
It is also worth mentioning that the adsorption capacity of the goethite was slightly higher toward Cu than toward P. For initial ions concentration of 50 mg/L, the adsorbed amount was 3.82 mg/g and 3.22 mg/g for copper and phosphate ions, respectively (Fig. 2). The higher adsorption capacity can be explained by different size of ions. Ionic radius of Cu2+ is 0.73 Å, whereas that of H2PO4-, 2.13 Å (Kadim and Gamaj 2020). Smaller ions can be bounded in larger amount on unit surface area of the adsorbent.
The measured adsorbed amounts of Cu and P on the pristine goethite were considered rather low. For the initial ions concentration of 200 mg/L, the obtained adsorbed amounts were 9.78 mg/g (19.36%) and 3.50 mg/g (7.00%), respectively. A comparative study indicated that a few other mineral adsorbents had similar, also unsatisfactory, adsorptive abilities. What is more, the adsorption capacity of synthetic goethite was much higher than that of natural one, applied in this study (Table 4). This means that the modification of goethite improving its adsorptive abilities is highly needed.
Adsorption mechanism of diuron on the pristine goethite
The pKa value of diuron is 13.55, so at pH 5, it has a slight positive charge (Wong et al. 2016). Under these conditions, the amount of the positive species (DH+) is about 10% (Deng et al. 2012).
Figure 2b presents the kinetics of diuron adsorption on the goethite surface. A rapid increase in pesticide adsorbed amount was observed up to 120 min. Then, the kinetics curve was close to the equilibrium. Experimental data were best fitted to the pseudo II-order equation with R2 equal to 0.955 (Table 3), which indicated chemical character of the tested process. The adsorption of organic molecules on goethite involves mainly electrostatic effects, ligand exchange, and hydrogen bonding (Liu et al. 2013). The donor-acceptor interactions between hydroxyl groups of goethite and amino/carbonyl moieties of diuron are dominant (Szewczuk-Karpisz et al. 2021a). The experimental isotherms were best described by Redlich-Petersen model (R2 = 0.999, Table 3). The β parameter was close to 0, which meant that the adsorption of diuron met the assumptions of Freundlich model (the pesticide molecules show some mobility in the adsorption layer, and they occupied centers of the highest adsorption energy at first).
The adsorption of diuron was visible in the FTIR spectrum of goethite (Fig. 4b). The band at 3112 cm-1 was shifted to 3093 cm-1, whereas the bands at 904 cm-1, 794 cm-1, and 604 cm-1 were intensified. All these changes were associated with the interactions of above mentioned groups of diuron and goethite. The band at 1646 cm-1, that appeared in the spectrum, was attributed to the bending vibration of –OH or N=C groups present in the pesticide structure (Correa-Navarro et al. 2020).
Minerals are usually characterized by low adsorption capacity toward pesticides due to high hydrophobicity of adsorbate (Hundal et al. 2001). The largest adsorbed amount of diuron, noted for its initial concentration of 9 mg/L, was 0.25 mg/g (11.15%). Kaolinite, montmorillonite, and fly ash bound fewer molecules of diuron (Table 4). The solid modification with polymeric compounds should enhance its interactions with hydrophobic substances.
Adsorption of copper ions/phosphate ions/diuron on the goethite in the mixed systems
In the systems containing two types of adsorbates (two ions or ion + pesticide), adsorption capacity of pristine goethite was different than that in the systems with only one adsorbate (Fig. 2). The addition of 50 mg/L of phosphate ions to 50 mg/L of copper ones increased the Cu adsorbed amount from 3.82 mg/g (30.57%) to 7.96 mg/g (63.71%). At pH 5, a certain amount of HPO42- ions was present in the system. They could form specific bridges between positive goethite surface and copper cations and, as a result, weaken electrostatic repulsion between positively charged system compounds. Enhanced adsorption of Cu, Zn, Cd, and Pb on goethite was also observed in the presence of sulfate (Swelund et al. 2009). The addition of diuron had different effect on heavy metal adsorption, that is, it limited the tested process. After the addition of 50 mg/L of diuron to 50 mg/L of copper ions, the Cu adsorbed amount changed from 3.82 mg/g (30.57%) to 2.36 mg/g (18.86%). This was probably dictated by the competition between positive diuron molecules and copper cations for goethite active sites.
Regarding the phosphate ions adsorption, the addition of 50 mg/L of Cu to 50 mg/L of phosphate ions strengthened the binding of the latter. The phosphate adsorption increased from 3.22 mg/g (25.74%) to 6.31 mg/g (50.46%). The HPO42- ions, a small amount of which was present in the system together with H2PO4-, were probably responsible for the formation of the second, third, and next layers of pollutants on goethite. One HPO42- ion interacted with one adsorbed Cu ion and another Cu ion from the solution, which contributed to larger metal adsorption. The addition of 50 mg/L of diuron to 50 mg/L of phosphate ions also increased the phosphate binding from 3.22 mg/g (25.74%) to 3.78 mg/g (30.21%). Between these pollutants hydrogen bonds were created, which contributed to adsorption of additional P amounts.
The addition of ions, copper, or phosphate (50 mg/L), to 5 mg/L of diuron increased the adsorbed amount of this pesticide from 0.15 mg/g (12.15%) to 0.318 mg/g (25.47%) and 0.558 mg/g (44.65%), respectively. Bivalent cations can form cation-herbicide or cation-herbicide-cation complexes, which can precipitate and thus contribute to higher adsorption of herbicide on the solid surface. Metal ions interact with two chlorine atoms attached to an aromatic ring of diuron, i.e., the site of high electronegativity capable of attracting cations (Das Chagas et al. 2019). On the other hand, phosphate ions are attracted to protonated region of diuron molecules by electrostatic forces. In this case, HPO42- species could also act as specific bridges between positive goethite particles and positive diuron molecules, which increased the herbicide adsorption.
Modification of the goethite with polymeric substances
Goethite modification with macromolecular compounds was performed based on the adsorption process. Figure 5a shows the adsorbed amounts of PAA and CS on the mineral for the polymer initial concentrations of 50 or 100 mg/L. Both polyelectrolytes were adsorbed on the goethite surface efficiently. Larger adsorbed amounts were noted for PAA due to attractive electrostatic conditions. They were equal to 11.25 mg/g (90.00%) and 21.46 mg/g (85.83%) for 50 mg/L and 100 mg/L initial concentrations, respectively. On the other hand, the CS-adsorbed amounts were 4.95 mg/g (39.63%) and 15.82 mg/g (63.27%) for the same initial concentrations. The PAA adsorption on goethite was based on electrostatic attraction and hydrogen bonding between positively charged solid particles and negative PAA macromolecules. In turn, for chitosan attachment to the mineral, the interactions between goethite hydroxyl groups and CS amino groups were responsible (Munagapati et al. 2017). Between positive CS chains and positive solid particles there was also electrostatic repulsion, which hindered their mutual contact.
The FTIR spectra of the goethite with and without PAA and CS as well as of the applied polymers are presented in Fig. 6. The bands in the PAA spectrum (Fig. 6a) are as follows: 1687 cm-1 (can be assigned to C=O stretching), 1421 cm-1 (C-O stretching coupled with O-H in-plane bending), 1139 cm-1 (the C-O stretching). Due to the PAA adsorption, in the spectrum of goethite + PAA, the additional bands around 1664 cm-1 and 1137 cm-1 were visible. Moreover, the bands near 906 cm-1, 794 cm-1, and 604 cm-1 were significantly decreased and shifted to 896 cm-1, 792 cm-1, and 601 cm-1, respectively. This was caused by the poly(acrylic acid) interaction with Fe–O–H and Fe–O groups of the goethite (Dong et al. 1998; Najim et al. 2012).
The spectrum of CS (Fig. 6b) consisted of the following bands: 2830 cm-1 (attributed to –NH and –OH groups stretching), 1342 cm-1 (CH3 deformations), 1122 cm-1 (asymmetric stretching of C-O-C), 1035 cm-1, and 1010 cm-1 (C-O stretching). The spectrum of the goethite modified with chitosan showed significant decreased bands at 906 cm-1, 794 cm-1, and 604 cm-1 (compared to the spectrum of the pristine goethite). Moreover, they were shifted to the regions of: 894 cm-1, 790 cm-1, and 590 cm-1, respectively (Queiroz et al. 2015; Lustriane et al. 2018). This was the evidence of the interactions occurring between the goethite hydroxyl groups and chitosan amino moieties (Munagapati et al. 2017).
Based on these results, it can be stated that, due to the goethite modification with chitosan, additional groups (amino and hydroxyl ones) were introduced to the solid surface. In turn, the mineral modification with poly(acrylic acid) contributed to both change in surface charge sign of the goethite and introduction of carboxylic groups.
To confirm goethite coating with the selected polymers, the zeta potential values of the mineral with and without PAA/CS were determined (Fig. 7). In the system without polymeric substances, the goethite isoelectric point (pHiep) was about 7.4. In the PAA presence (50 mg/L), electrokinetic potential was negative in almost entire pH range, and the pHiep value was shifted toward the value of 3.1. On the other hand, after the chitosan addition (50 mg/L), zeta potential of the mineral became positive over entire tested pH range, and the pHiep was close to 9. Such significant changes in the slipping plane charge, observed in the presence of PAA or CS, was the evidence for goethite coating with selected polymers.
Modification of the goethite with polymeric substances in the presence of pollutants
The pollutants presence influenced the adsorptive modification of the goethite (Fig. 5a). Copper ions and diuron (with the concentration of 50 mg/L) slightly increased the adsorbed amount of PAA on goethite. For initial polymer concentration of 50 mg/L, it increased from 11.25 mg/g (90.00%) to 12.00 mg/g (96.00%) and 11.37 mg/g (91.00%) with Cu and diuron, respectively. On the other hand, for initial PAA concentration of 100 mg/L, its adsorption changed from 21.46 mg/g (85.83%) to 22.71 mg/g (90.83%) and 23.79 mg/g (95.17%) with Cu and diuron, respectively. Positively charged ions and molecules were involved in the formation of second polymer adsorption layer. They simultaneously interacted with polymer chains adsorbed and those from the solution and create intermolecular complexes. PAA can also form intramolecular complexes with divalent ions (when one ion interacts with two carboxylic groups of one polymer chain). These complexes had more coiled structure than PAA molecules and, as a result, their larger amount can be adsorbed on the goethite surface (Fijałkowska et al. 2019). The phosphate ions presence affected PAA adsorption in the opposite way. A clear decrease in the adsorption rate from 11.25 mg/g (90.00%) to 2.17 mg/g (17.3%) (for initial PAA concentration of 50 mg/L) and from 21.46 mg/g (85.83%) to 12.50 mg/g (50.00%) (for initial PAA concentration of 100 mg/L) was observed. This was dictated by the competition between negatively charged phosphate ions and negatively charged polyelectrolyte molecules for active sites on the goethite surface.
All applied pollutants reduced the chitosan adsorption on goethite. In the Cu presence (50 mg/L), the CS-adsorbed amount decreased from 4.95 mg/g (39.64%) to 3.32 mg/g (26.55%) (when initial concentration was 50 mg/L) as well as from 15.82 mg/g (63.27%) to 11.86 mg/g (47.45%) (when initial concentration was 100 mg/L). Similarly with phosphate ions, a decrease to 2.11 mg/g (26.54%) and 7.55 mg/g (30.18%) was noted for the initial CS concentrations of 50 and 100 mg/L, respectively. Diuron reduced CS-adsorbed amount to the values of 4.09 mg/g (32.73%) and 10.52 mg/g (42.09%) when initial polymer concentration was 50 mg/L and 100 mg/L, respectively. All molecules and ions of pollutants, definitely smaller in size than the polymer macromolecules, reached the surface as first and block some active sites of the adsorbent.
Adsorption mechanism of copper ions/phosphate ions/diuron on the modified goethite
The goethite modification with PAA affected the adsorption of all pollutants (Fig. 5b). There was a slight increase in the Cu adsorbed amount from 3.82 (30.57%) to 4.39 mg/g (35.14%) and 4.86 mg/g (38.85%), after PAA addition of 50 mg/g and 100 mg/L, respectively. A certain amount of Cu cations is attracted electrostatically by the PAA macromolecules due to their negative charge. The Cu-PAA complexes formed in the solution, could also be adsorbed on the solid surface. As a consequence, the additional amount of metal ions was accumulated in the adsorption layer (Szewczuk-Karpisz et al. 2021a). In the case of phosphate ions, the increase in their adsorbed amount was also clear after PAA coating. Then, their adsorbed amount changed from 3.22 mg/g (25.74%) to 3.97 mg/g (31.76%) and 3.56 mg/g (29.48%) for 50 and 100 mg/L PAA. Probably, under these conditions, ions interacted with PAA by hydrogen bonding, which contributed to higher adsorption of phosphate. In the contrast, in the presence of 100 mg/L of PAA, the phosphate adsorbed amount decreased to the value of 3.09 mg/g (24.79%). Such a high concentration of the polymer reflected in a very strong electrostatic repulsion between PAA and phosphate ions. As a result, they repelled each other and the ions adsorption was reduced. For diuron adsorption, the impact of the goethite modification with PAA was positive, but significant increase was noted only for lower PAA concentration. The diuron adsorption changed from 0.15 mg/g (12.15%) to 0.42 mg/g (33.77%) and to 0.18 mg/g (14.58%) for 50 mg/L and 100 mg/L of PAA, respectively. This was associated with electrostatic attraction between positively charged diuron molecules and dissociated carboxylic groups of the polymer.
The goethite modification with chitosan, regardless of the applied concentration of the polymer, caused an dramatic decrease in the Cu adsorption (Fig. 5c). The Cu adsorbed amount was reduced from 3.82 mg/g (30.57%) to 1.42 mg/g (11.43%) and 0.21 mg/g (1.71%) for the CS concentrations of 50 mg/L and 100 mg/L, respectively. This phenomenon was caused by strong competition between positively charged chitosan and metal ions. The pKa parameter of chitosan is in the range of 6.39-6.51 (Wang et al. 2006), which means that at pH 5 most amino groups within chitosan chains are protonated. On the goethite covered with CS, P, and diuron were better adsorbed. The phosphate adsorption increased from 3.22 mg/g (25.74%) to 4.22 mg/g (33.72%) (for 50 mg/L of CS) and 4.02 mg/g (32.16%) (for 100 mg/L of CS). The adsorbed amount of herbicide changed from 0.15 mg/g (12.15%) to 0.35 mg/g (27.73%) and 0.45 mg/g (36.19%) for 50 mg/L and 100 mg/L of CS, respectively. Phosphate ions interacted with protonated amino groups (-NH3+) by electrostatic attractive forces (Kim et al. 2022). On the other hand, diuron could form hydrogen bonds with hydroxyl groups of CS as well as amino groups (-NH2), small amounts of which were also present in chitosan macromolecules at pH 5 (Pavinatto et al. 2005).
Binding strength of copper ions/phosphate ions/diuron on the pristine and modified goethite
Desorption degrees of the pollutants measured in the examined systems are summarized in Table 5. The results obtained for pristine goethite indicated that Cu was desorbed in the highest amount from the solid surface (26.14%). This was associated with acidic character of the applied desorbing solution. Acids, such as sulfuric acid, nitric acid, and hydrochloric acid, are very efficient desorbing agents (Abdo Allozy and Khairil Juhanni 2020). They usually cause protonation of solid surface and enhanced electrostatic repulsion between adsorbent and adsorbate (Isaac and Siddiqui 2022). Desorbed amounts of phosphate ions and diuron were significantly lower (8.01% and 9.89%, respectively). During phosphate desorption, the hydroxyl groups could replace adsorbed anions (Zhang et al. 2015). In the mixed systems, i.e., containing two types of pollutants, the desorption degrees were different. In most solutions, they were lower than those observed in the systems with one impurity. Copper ions were desorbed in the amount of 21.57% and 21.21% in the presence of phosphate ions and diuron, respectively. Diuron desorption was also smaller with other pollutants—1.76% with Cu and 5.70% with phosphate ions. In the case of phosphate ions, the presence of metal ions and herbicide increased their desorption to the values of 18.39% and 9.43%, respectively. This meant that the binding of phosphate ions to the surface was weaker under these conditions.
In the systems containing modified goethite, desorption degrees were generally lower than those measured for pristine goethite, with the exception of copper ions. The CS polymer layer enhanced desorption of Cu (91.67–95%), but reduced that of phosphate anions (4.84–7.46%) and diuron (0.76–4.52%). The PAA modification decreased desorption of all tested impurities. Then, desorption degrees for copper ions were in the range of 5.88–12.19%, those of phosphate ions, 5.61–6.62%, whereas those of diuron, 1.41–3.91%. Reducing desorption of pollutants is highly desirable during the remediation of polluted ecosystems.
Zeta potential values of the goethite with pollutant or pollutant/polymer
Adsorption of pollutants affected goethite zeta potential significantly (Table 6). Cu contributed to increase in absolute values of positive zeta potential, diuron decreased absolute values of positive zeta potential, whereas adsorption of phosphate ions led to the negative zeta potential values. In the case of Cu, the non-specifically adsorbed ions, located in the outer Helmholtz layer were responsible for increase of absolute zeta potential values (Birdi 2003). In the system containing diuron, the reduction in absolute values of positive electrokinetic potential was associated with the presence of herbicide fragments containing chlorides (of high electronegativity) in the slipping plane area. The negative zeta potential of goethite observed after the addition of phosphate ions was mainly dictated by the adsorption of HPO42-. Then, one negative charge interacted with the solid surface, whereas the second one was located in the slipping plane area.
In the presence of both polymer and pollutant, zeta potential of goethite was mainly determined by the adsorbed macromolecular compound. In the systems with PAA, zeta potential was negative, whereas in those with CS, positive. The polymer chains formed structures like “loops” and “tails” on the goethite surface, which contained dissociated carboxylic groups (in the case of PAA) or protonated amino groups (CS). Exactly these moieties were located in the slipping plane area and affected its charge. The increase in absolute values of negative zeta potential was also dictated with the offset of slipping plane as a result of the adsorption of poly(acrylic acid) (Fijałkowska et al. 2019; Wiśniewska et al. 2015).
Stability mechanism of the goethite suspension with and without pollutants/polymers
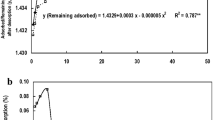
At pH 5, the aqueous suspension of pristine goethite had relatively high stability—the absorbance remained constant during the measurement (Fig. 8). Positively charged particles were surrounded by chloride ions coming from the supporting electrolyte, which limited their mutual contact (electrostatic stabilization) (Szewczuk-Karpisz et al. 2015).
Most of the goethite suspensions containing polymers and/or pollutants were also relatively stable. A clear reduction in the system stability, which is highly desirable during wastewater treatment, was noted only for the suspensions containing goethite/phosphate ions or PAA-modified goethite/copper ions. Then, the absorbance gradually decreased over time. Phosphate anions, added to the system, neutralized positive charge of the positive particles and, as a consequence, the electrostatic repulsive forces were weakened. On the other hand, copper ions acted as bridges between adsorbed PAA macromolecules, which facilitated the goethite aggregation.
Conclusions
Goethite was modified in order to improve its adsorption capacity toward pollutants as well as its subsequent use as an adsorbent in environmental remediation. In this way, an attempt was made to manage waste mineral.
Due to low specific surface area, pristine goethite was an effective adsorbent of Cu and phosphate ions only in the systems containing both pollutants at the same time. Goethite modification with macromolecular compounds introduced additional functional groups to the solid and affected its surface charge. The PAA coating slightly increased Cu adsorption due to the formation of Cu-PAA complexes. It also enhanced the binding of phosphates based on the hydrogen bonds creation. The CS modification made the adsorption of phosphates and diuron higher. P ions interacted with protonated amino groups (-NH3+) of CS by electrostatic attractive forces, whereas diuron could form hydrogen bonds with amino (-NH2) and hydroxyl groups of this polymer. In the systems with modified goethite, the pollutant desorption was generally lower, which meant that polymers contributed to strong immobilization of impurities. The only exception was the CS effect on Cu desorption—its intensification was observed. The presence of macromolecular compound did not destabilize the system due to relatively high absolute values of zeta potential of the mineral. A clear aggregation was noted only in the system containing pristine goethite and phosphates, which was caused by the neutralization of positive solid charge by anions, and in the system containing Cu and PAA-modified goethite, where divalent ions created “bridges” between adsorbed polymer chains. Taking all these results into account, the goethite modification with PAA was considered more promising for environmental remediation.
References
Abdo Allozy HG, Khairil Juhanni AK (2020) Removal of copper ions from aqueous solutions using poly(vinylbenzyl chloride). Malaysian J Anal Sci 24(6):978–991
Agency for Toxic Substances and Disease Registry (ATSDR) (2022) Toxicological profile for copper (draft for public comment). U.S. Department of Health and Human Services, Public Health Service
Akpomie KG, Dawodu FA (2015) Treatment of an automobile effluent from heavy metals contamination by an eco-friendly montmorillonite. J Adv Res 6:1003–1013
Albalasmeh AA, Berhe AA, Ghezzehei TA (2013) A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohydr Polym 97:253–261
Álvarez-Ayuso E, Garcia-Sánchez A, Querol X (2003) Purification of metal electroplating waste waters using zeolites. Water Res 37:4855–4862
Arkaban H, Barani M, Akbarizadeh MR, Chauhan NPS, Jadoun S, Soltani MD, Zarrintaj P (2022) Polyacrylic acid nanoplatforms: antimicrobial, tissue engineering, and cancer theranostic applications. Polymers 14(6):1259
Awual MR, Yaita T (2013) Rapid sensing and recovery of palladium(II) using N,N-bis(salicylidene)1,2-bis(2-aminophenylthio)ethane modified sensor ensemble adsorbent. Sens Actuators B Chem 185:332–341
Awual MR, Yaita T, El-Safty SA, Shiwaku H, Suzuki S, Okamoto Y (2013) Copper(II) ions capturing from water using ligand modified a new type mesoporous adsorbent. Chem Eng J 221:322–330
Awual MR, Ismael M, Khaleque MA, Yaita T (2014a) Ultra-trace copper(II) detection and removal from wastewater using novel meso-adsorbent. J Ind Eng Chem 20(4):2332–2340
Awual MR, Rahman IMM, Yaita T, Khaleque MA, Ferdows M (2014b) pH dependent Cu(II) and Pb(II) ions detection and removal from aqueous media by an efficient mesoporous adsorbent. Chem Eng J 236:100–109
Awual MR (2015) A novel facial composite adsorbent for enhanced copper(II) detection and removal from wastewater. Chem Eng J 266:368–375
Awual MR, Eldesoky GE, Yaita T, Naushad M, Shiwaku H, AlOthman ZA, Suzuki S (2015b) Schiff based ligand containing nano-composite adsorbent for optical copper(II) ions removal from aqueous solutions. Chem Eng J 279:639–647
Awual MR, Ismael M, Yaita T, El-Safty SA, Siwaku H, Okamoto Y, Suzuki S (2015c) Trace copper(II) ions detection and removal from water using novel ligand modified composite adsorbent. Chem Eng J 222:67–76
Awual MR, Hasan MM, Khaleque MA, Skeikh MC (2016) Treatment of copper(II) containing wastewater by a newly developed ligand based facial conjugate materials. Chem Eng J 288:368–376
Awual MR (2017) New type mesoporous conjugate material for selective optical copper(II) ions monitoring & removal from polluted waters. Chem Eng J 307:85–94
Awual MR (2019a) Efficient phosphate removal from water for controlling eutrophication using novel composite adsorbent. J Clean Prod 228:1311–1319
Awual MR (2019b) Novel ligand functionalized composite material for efficient copper(II) capturing from wastewater sample. Composites B: Eng 172:387–396
Awual MR, Hasan MM, Rahman MM, Asiri AM (2019) Novel composite material for selective copper(II) detection and removal from aqueous media. J Mol Liq 283:772–780
Bhattacharyya KG, Gupta SS (2006) Kaolinite, montmorillonite, and their modified derivatives as adsorbents for removal of Cu(II) from aqueous solution. Sep Purif Technol 50:388–397
Birdi KS (2003) Handbook of Surface and Colloid Chemistry, Second edition. CRS Press, Boca Raton, New York.
Boujelben N, Bouzid J, Elouear Z, Feki M, Jamoussi F, Montiel A (2008) Phosphorus removal from aqueous solution using iron coated natural and engineered sorbents. J Hazard Mater 151:103–110
Correa-Navarro YM, Giraldo L, Moreno-Piraján JC (2020) Biochar from fique bagasse for remotion of caffeine and diclofenac from aqueous solution. Molecules 25:1849
Chen L, Hao H, Zhang W, Shao Z (2020) Adsorption mechanism of copper ions in aqueous solution by chitosan-carboxylmethyl starch composites. J Appl Polym Sci 137(18):48636
Chen J, Yan L-G, Yu H-Q, Li S, Qin L-L, Liu G-Q, Li Y-F, Du B (2016) Efficient removal of phosphate by facile prepared magnetic diatomite and illite clay from aqueous solution. Chem Eng J 287:162–172
Das Chagas PSF, dr Freitas Souza M, Domborski JLD, dr Oliveira Junior RS, de Sousa Nunes GH, Pereira GAM, Silva TS, de Jesus Passos ABR, dos Santos JB, Silva DV (2019) Multivariate analysis reveals significant diuron-related changes in the soil composition of different Brazilian regions. Sci Rep 9:7900
Da Silva Alves DC, Healy C, de Ameida Pinto LA, Sant’Anna Cadaval TR, Breslin CB (2021) Recent developments in chitosan-based adsorbents for the removal of pollutants from aqueous environments. Molecules 26:594
Da Silva RC, de Aguiar SB, da Cunha PLR, de Paula RCM, Feitosa JPA (2020) Effect of microwave on the synthesis of polyacrylamide-g-chitosan gel for azo dye removal. React Funct Polym 148:104491
Deng J, Shao Y, Gao N, Deng Y, Tan C, Zhou S, Hu X (2012) Multiwalled carbon nanotubes as adsorbents for removal of herbicide diuron from aqueous solution. Chem Eng J 193-194:339–347
Dong J, Ozaki Y, Nakashima K (1998) FTIR studies of conformational energies of poly( acrylic acid) in cast films. J Polym Sci B: Polym Phys 35:507–515
Droz B, Payraudeau S, Martín JAR, Tóth G, Panagos P, Montanarella L, Borrelli P, Imfeld G (2021) Copper content and export in european vineyard soils influenced by climate and soil properties. Environ Sci Technol 55(11):7327–7334
Egea TC, da Silva R, Boscolo M, Rigonato J, Monteiro DA, Grünig D, da Silva H, van der Wielen F, Helmus R, Parsons JR, Gomes E (2017) Diuron degradation by bacteria from soil of sugarcane crops. Heliyon 3(12):e00471
Fernández-Cori RA, Morales-Gomero JC, Huayhuas-Chipana BC, Taboada-Sotomayor MDP, Ruíz-Montoya JG (2015) Nanostructured sensors for determination of 3-(3,4-dichlorophenyl)-1,1-dimethylurea based in molecularly imprinted polymers (mips) deposited in screen printed carbon nanotubes. ECS Trans 66(37):33–41
Field JA, Reed RL, Sawyer TE, Griffith SM, Wigington JRPJ (2003) Diuron occurrence and distribution in soil and surface and ground water associated with grass seed production. J Environ Qual 2:171–179
Fijałkowska G, Szewczuk-Karpisz K, Wiśniewska M (2019) Anionic polyacrylamide as a substance strengthening the Pb(II) immobilization on the kaolinite surface. Int J Environ Sci Technol 17:1101–1112
Fijałkowska G, Wiśniewska M, Szewczuk-Karpisz K, Jędruchniewicz K, Oleszczuk P (2021) Comparison of lead(II) ions accumulation and bioavailability on the montmorillonite and kaolinite surface in the presence of polyacrylamide soil flocculant. Chemosphere 276(1):130088
Foo KY, Hameed BH (2010) Insight into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10
Ganesh S, Khan F, Ahmed MK, Velavendan P, Pandey NK, Kamachi Mudali U (2012) Spectrophotometric determination of trace amounts of phosphate in water and soil. Water Sci Technol 66(12):2653–2658
Grossi PR, Sparks DL (1994) Rapid kinetics of Cu(II) adsorption/desorption on goethite. Environ Sci Technol 28:1422–1429
Gu F, Geng J, Li M, Chang J, Cui Y (2019) Synthesis of chitosan-lignosulfonate composite as an adsorbent for dyes and metal ions removal from wastewater. ACS Omega 4:21421–21430
Hasan MM, Kubra KT, Hasan MN, Awual ME, Salman MS, Sheikh MC, Rehan AI, Rasee AI, Waliullah RM, Islam MS, Khndaker S, Islam A, Hossain MS, Alsukaibi AKD, Alshammari HM, Awual MR (2023a) Sustainable ligand-modified based composite material for selective and effective cadmium(II) capturing from wastewater. J Mol Liq 371:121125
Hasan MN, Salman S, Hasan MM, Kubra KT, Sheikh MC, Rehan AI, Rasee AI, Awual ME, Waliullah RM, Hossain MS, Islam A, Khandaker S, Alsukaibi AKD, Alshammari HM, Awual MR (2023b) Assessing sustainable lutetium(III) ions adsorption and recovery using novel composite hybrid nanomaterials. J Mol Struct 1276:134795
Ho YS, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70:115–124
Ho YS, McKay G (2002) Application of kinetics models to the sorption of copper(II) onto peat. Adsorption Sci Technol 20:797–815
Hooda PS, Truesdale VW, Edwards AC, Withers PJA, Aitken MN, Miller A, Rendell AR (2001) Manuring and fertilization effects on phosphorus accumulation in soils and potential environmental implications. Adv Environ Res 5:13–21
Hundal LS, Thompson ML, Laird DA, Carmo AM (2001) Sorption of phenanthrene by reference smectites. Environ Sci Technol 25:3456–3461
Icenhower J, Qafoku NP, Zachara JM, Martin WJ (2010) The biogeochemistry of technetium: a review of the behavior of an artificial element in the natural environment. Am J Sci 310(8):721–752
Isaac R, Siddiqui S (2022) Adsorption of divalent copper from aqueous solution by magnesium chloride co-doped Cicerarietinum husk biochar: Isotherm, kinetics, thermodynamic studies and response surface methodology. Biores Technol Rep 18:101004
Janusz W (1994) Electrical double layer at the metal oxide/electrolyte interface in interfacial forces and fields: theory and applications. in M. Decker (Ed.), Surfactant Sci., vol. 85, chapter 4, New York
Jaiswal A, Banerjee S, Mani R, Chattopadhyaya MC (2013) Synthesis, characterization and application of goethite mineral as an adsorbent. J Environ Chem Eng 1:281–189
Ju W, Liu L, Fang L, Cui Y, Duan C, Wu H (2019) Impact of co-inoculation with plant-growth-promoting rhizobacteria and rhizobium on the biochemical responses of alfalfa-soil system in copper contaminated soil. Ecotoxol Environ Saf 167:218–226
Kabata-Pendias A, Pendias H (1999) Biogeochemistry of trace elements. PWN, Warsaw (in Polish).
Kadim MJ, Gamaj MI (2020) Estimation of the diffusion coefficient and hydrodynamic radius (Stokes radius) for inorganic ions in solution depending on molar conductivity as elektro-analytical technique – A review. J Chem Rev 2(3):182–188
Kang J, Sowers TD, Duckworth OW, Amoozegar A, Heitman JL, McLaughlin RA (2013) Turbidimetric determination of anionic polyacrylamide in low carbon soil extracts. J Environ Qual 42:1902–1907
Katz LE (2017) Adsorption equilibrium and kinetics at goethite-water and related interfaces. Raport. Grant: DE-FG02-04ER15496. University of Texas at Austin.
Kim T, Shin J, An B (2022) Adsorption characteristics of Cu(II) and phosphate in chitosan beads under single and mixed conditions. Polymers 15(2):421
Kopittke PM, Menzies NW, Wang P, McKenna BA, Lombi E (2019) Soil and the intensification of agriculture for global food security. Environ Int 132:105078
Kubra KT, Salman MS, Hasan MN, Islam A, Hasan MM, Awual MR (2021) Utilizing an alternative composite material for effective copper(II) ion capturing from wastewater. J Mol Liq 336:116325
Kubra KT, Hasan MM, Hasan MN, Salman MS, Khaleque MA, Sheikh MC, Rehan AI, Rasee AI, Waliullah RM, Awual ME, Hossain MS, Alsukaibi AKD, Alshammari HM, Awual MR (2023) The heavy lanthanide of thulium(III) separation and recovery using specific ligand-based facial composite adsorbent. Colloids Surf. A: Physicochem. Eng. Asp. 667:131415
Kumar PS, Korving L, van Loosdrecht MCM, Witkamp G-J (2019) Adsorption as a technology to achieve ultra-low concentrations of phosphate: research gaps and economic analysis. Water Res 4:100029
Kumara NTRN, Hamdan N, Petra MI, Tennakoon KU, Ekanayake P (2014) Equilibrium isotherm studies of adsorption of pigments extracted from Kuduk-kuduk (Melastoma malabathricum L.) pulp into TiO2 nanoparticles. J Chem ID:468975.
Kundakci S (2020) Synthesis of methacrylamide/chitosan polymeric cryogels and swelling/dye sorption properties. Polym Sci Ser A 62:481–493
Ler A, Stanforth R (2003) Evidence for surface precipitation of phosphate on goethite. Environ Sci Technol 37(12):2694–2700
Li P, Wang Y, Peng Z, She FH, Kong L (2011) Development of chitosan nanoparticles as drug delivery systems for 5-fluorouracil and leucovorin blends. Carbohydr Polym 85(3):698–704
Liu H, Chen T, Frost RL (2013) An overview of the role of goethite surfaces in the environment. Chemosphere 103:1–11
Lustriane C, Dwivany FM, Suendo V, Reza M (2018) Effect of chitosan and chitosan-nanoparticles on post harvest quality of banana fruits. Plant Biotechnol J 45:36–44
Maguire RO, Chardon WJ, Simard RR (2005) Assessing potential environmental impacts of soil phosphorus by soil testing, phosphorus: agriculture and the environment (eds. J. Thomas Sims, A.N. Sharpley and D.T. Westermann), Agronomy Monographs, Wiley.
Majd MM, Kordzadeh-Krmani V, Ghalandari V, Askari A, Sillanpaa M (2022) Adsorption isotherm models: a comprehensive and systematic review (2010-2020). Sci Tot Environ 812:151334
Matusiak J, Grządka E, Maciołek U, Godek E, Guzman E (2022) The journey of tunning chitosan properties in colloidal systems: interactions with surfactants in the bulk and on the alumina surface. Chem Eng J 450(2):138145
Medykowska M, Wiśniewska M, Szewczuk-Karpisz K, Panek R (2022) Management of hazardous fly-ash waste in adsorptive removal of diclofenac by the use of synthetic zeolitic materials. Environ Sci Pollut Res https://doi.org/10.1007/s11356-022-24619-9
Mohammed AM, Huovinen M, Vähäkangas KH (2020) Toxicity of diuron metabolites in human cells. Environ Toxicol Pharmacol 78:103409
Moharami S, Jalali M (2013) Removal of phosphorus from aqueous solution by Iranian natural adsorbents. Chem Eng J 223:328–339
Munagapati VS, Yarramuthi V, Kim D-S (2017) Methyl orange removal from aqueous solution using goethite, chitosan beads and goethite impregnated with chitosan beads. J Mol Liq 240:329–339
Musso TB, Parolo ME, Pettinari G, Francisca FM (2014) Cu(II) and Zn(II) adsorption capacity of three different clay liner materials. J Env Manage 146:50–58
Najim T, Yasin S, Dawood BA (2012) Synthesis of peppermint stem-g-poly(acrylic acid) and its application for nickel ions removal. Iraq J Polym 16(2):34–46
Nangah CR, Marlain TG, Nsami NJ, Tubwoh CP, Foba-Tendo J, Mbadcam KJ (2019) Synthesized goethite and natural iron oxide as effective absorbents for simultaneous removal of Co(II) and Ni(II) ions from water. J Encapsulation Adsorpt Sci 9(3):127–147
Nazari-Sharabian M, Ahmad S, Karakouzian M (2018) Climate change and eutrophication: a short review. Eng Technol Appl Sci Res 8(6):3668–3672
Olu-Owolabi BI, Popoola DB, Unuabonah EI (2010) Removal of Cu2+ and Cd2+ from aqueous solution by bentonite clay modified with binary mixture of goethite and humic acid. Water Air Soil Pollut 211:459–474
Ościk J (1969) Adsorption (in Polish). UMCS, Lublin
Queiroz MF, Melo KRT, Sabry DA, Sassaki GL, Rocha HAO (2015) does the use of chitosan contribute to oxalate kidney stone formation? Marin Drug 13:141–158
Qiao Y, Liu D, Feng C, Liu N, Wang J, Yan Z, Bai Y (2022) Ecological risk assessment for tris(2-chloroethyl) phosphate to freshwater organisms. Front Environ Sci 10:963918
Pavinatto FJ, dos Santos Jr DS, Oliveira ON Jr (2005) Interaction between cholesterol and chitosan in Langmuir monolayers. Polimeros 15(2). https://doi.org/10.1590/S0104-14282005000200006
Perelomov L, Cozzolino V, Pinga M, Violante A (2013) Adsorption of Cu and Pb on goethite in the presence of low-molecular mass aliphatic acids. Geomicrobiol J 28(7):582–589
Polati S, Angioi S, Gianotti V, Gosetti F, Gennaro MC (2006) Sorption of pesticides on kaolinite and montmorillonite as a function of hydrophilicity. J Environ Sci Health B 41(4):333–344
Quirantes M, Nogales R, Romero E (2017) Sorption potential of different biomass fly ashes for the removal of diuron and 3,4-dichloroaniline from water. J Hazard Mater 331:300–308
Rehman M, Liu L, Wang Q, Saleem MH, Bashir S, Ullah S, Peng U (2019) Copper environmental toxicology, recent advances, and future outlook: a review. Environ Sci Pollut Res 26:18003–18016
Rodrigues LA, da Silva MLCP (2009) An investigation of phosphate adsorption from aqueous solution onto hydrous niobium oxide prepared by co-precipitation method. Colloids Surf A 334(1-3):191–196
Sakadevan K, Bavor HJ (1998) Phosphate adsorption characteristics of soils, slags and zeolite to be used as substrates in constructed wetland systems. Water Res 32(2):393–399
Salimi F, Rahimi H, Karami C (2018) Removal of methylene blue from water solution by modified nanogoethite by Cu. Desalin Water Treat 137:334–344
Salman MS, Hasan MN, Hasan MM, Kubra KT, Sheikh MC, Rehan AI, Waliullah RM, Rasee AI, Awual ME, Hossain MS, Alsukaibi AKD, Alshammari HM, Awual MR (2023a) Improving copper(II) ion detection and adsorption from wastewater by the ligand-functionalized composite adsorbent. J Mol Liq 1282:135259
Salman MS, Sheikh MC, Hasan MM, Hasan MN, Kubra KT, Rehan AI, Awual ME, Rasee AI, Aliullah RM, Hossain MS, Khaleque MA, Alsukaibi AKD, Alshammari HM, Awual MR (2023b) Chitosan-coated cotton fiber composite for efficient toxic dye encapsulation from aqueous media. Appl Surf Sci 622:157008
Saheed IO, Oh WD, Suah FBM (2021) Chitosan modifications for adsorption of pollutants – A review. J Hazard Mater 408:124889
Stork PR, Bennett FR, Bell MJ (2008) The environmental fate of diuron undera conventional production regime in asugarcane farm during the plant cane phase. Pest Manage Sci 64:954–963
Sutirman ZA, Sanagi MM, Karim KJA, Naim AA, Ibrahim WAW (2018) Chitosan-based adsorbents for the removal of metal ions from aquoeus solutions. Malaysian J Anal Sci 22(5):839–850
Swelund PJ, Webster JG, Miskelly GM (2009) Goethite adsorption of Cu(II), Pb(II), Cd(II), and Zn(II) in the presence of sulfate: properties of the ternary complex. Geochim Cosmochim Acta 73(6):1548–1562
Szewczuk-Karpisz K, Bajda T, Tomczyk A, Kuśmierz M, Komaniecka I (2022) Immobilization mechanism of Cd2+/HCrO4-/CrO42- ions and carboxin on montmorillonite modified with Rhizobium leguminosarum bv. trifolii exopolysaccharide. J Hazard Mater 428:128228
Szewczuk-Karpisz K, Krasucka P, Boguta P, Skic K, Sokołowska Z, Fijałkowska G, Wiśniewska M (2019) Anionic polyacrylamide efficiency in goethite removal from aqueous solutions: goethite suspension destabilization by PAM. Int J Environ Sci Technol 16:3145–3154
Szewczuk-Karpisz K, Tomczyk A, Celińska M, Sokołowska Z, Kuśmierz M (2021a) Carboxin and diuron adsorption mechanism on sunflower husks biochar and goethite in the single/mixed pesticide solutions. Materials 14:2584
Szewczuk-Karpisz K, Wiśniewska M (2014) Adsorption properties of the albumin-chromium(III) oxide system – effect of solution pH and ionic strength. Soft Mater 12(3):268–276
Szewczuk-Karpisz K, Wiśniewska M, Medykowska M, Galaburda MV, Bogatyrov VM, Oranska OI, Błachnio M, Oleszczuk P (2021b) Simultaneous adsorption of Cu(II) ions and poly(acrylic acid) on the hybrid carbon-mineral nanocomposites with metallic elements. J Hazard Mater 412:125138
Szewczuk-Karpisz K, Wiśniewska M, Myśliwiec D (2015) Albumin adsorption influence on the stability of the mesoporous zirconia suspension. J Ind Eng Chem 32:113–119
Tadanier CJ, Eick MJ (2002) Formulating the charge-distribution multisite surface complexation model using FITEQL. Soil Sci. Soc Am J 66(5):1505–1517
The Editors of Encyclopaedia Britannica (1998) Goethite mineral. Encyclopaedia Britannica, London
Ulatowska J (2022) Adsorption behaviour of As(III) onto synthetic iron-based minerals: a comparative study of akaganeite, goethite and magnetite. Physicochem Probl Miner Process 58(2):144818
Varlot K, Martin JM, Grossiord C, Vargiolu R, Vacher B, Inoue K (1999) A dual-analysis approach in tribochemistry: application to ZDDP/Calcium Borate additive interactions. Tribol Lett 6(3):181–189
Wang QZ, Chen XG, Liu N, Wang SX, Liu CS, Meng XH, Liu CG (2006) Protonation constans of chitosan with different molecular weight and degree of deacetylation. Carbohydr Polym 65(2):194–201
Wiśniewska M, Szewczuk-Karpisz K, Ostolska I, Urban T, Terpiłowski K, Zarko V, Gunko V (2015) Effect of polyvinyl alcohol adsorption on the mixed alumina-silica-titania suspension stability. J Ind Eng Chem 23:265–272
Wong A, de Oliveira FM, Tarley CRT, Sotomayor MDPT (2016) Study on the cross-linked molecularly imprinted poly(methacrylic acid) and poly(acrylic acid) towards selective adsorption of diuron. React Funct Polym 100:26–36
Xu J, Koopal LK, Wang M, Xiong J, Hou J, Li Y, Tsn W (2019) Phosphate speciation on Al-substituted goethite: ATR-FTIR/2D-COS and CD-MUSIC modeling. Environ Sci Nano 6:3625–3637
Zhang L, Gao Y, Xu Y, Liu J (2015) Different performances and mechanisms of phosphate adsorption onto metal oxides and metal hydroxides: a comparative study. Chem Technol Biotechnol 91(5):1232–1239
Zhu H-Y, Fu Y-Q, Jiang R, Yao J, Xiao L, Zheng G-M (2012) Novel magnetic chitosan/poly(vinyl alcohol) hydrogel beads: preparation, characterization and application for adsorption of dye from aqueous solution. Bioresour Technol 105:24–30
Funding
The research was partially financed by National Science Centre, Poland (2021/41/B/NZ9/03059). The authors are thankful to Croatian Science Foundation for the support through project IPS-2020-01-6126.
Author information
Authors and Affiliations
Contributions
Katarzyna Szewczuk-Karpisz: conceptualization, investigation, writing—original draft preparation, writing—reviewing and editing, methodology, supervision, project administration; Sylwia Kukowska: investigation, writing—original draft preparation, methodology; Katarzyna Grygorczuk-Płaneta: writing—original draft preparation, investigation; Bartosz Kondracki: investigation, visualization; Katarina Jerin: investigation, writing—reviewing and editing, methodology; Davor Kovačević: writing—reviewing and editing, resources, supervision, project administration.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Guilherme L. Dotto
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Szewczuk-Karpisz, K., Kukowska, S., Grygorczuk-Płaneta, K. et al. Scavenging of copper(II) ions, phosphate(V) ions, and diuron from aqueous media by goethite modified with chitosan or poly(acrylic acid). Environ Sci Pollut Res 30, 79980–80000 (2023). https://doi.org/10.1007/s11356-023-27783-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-27783-8