Abstract
The projections for the production of insects as food and feed show an enormous increase for insect production in the near future, which will subsequently lead to the increase of the stored quantities of insect meals and related products. However, information on the susceptibility of insect meals to infestations by stored-product insects is rather limited. To this end, the objective of the present study was to evaluate the potential of major storage insect species to grow and reproduce on insect meals that are based on larvae of the lesser mealworm, Alphitobius diaperinus. The progeny production of thirteen stored-product insects on A. diaperinus meal, as well as their instantaneous rate of increase, as a measure of population growth, was recorded for each species. Based on the results, six out of the thirteen examined insect species (A. diaperinus, Tenebrio molitor, Trogoderma granarium, Lasioderma serricorne, Tribolium confusum, and Tribolium castaneum) were able to infest pure A. diaperinus meal, as they grew well and developed progeny on the insect meal substrate. Tribolium confusum, T. castaneum, and especially T. granarium gave the highest progeny production numbers in the A. diaperinus meal with the latter giving an instantaneous rate of increase of 0.067. Expecting the upcoming increase in the production of insect-based products globally, further research in this field is needed for improved production and storage facilities, detection and estimation methods, and technologies to minimize insect infestations without causing negative effects to farmed insects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The challenge to feed the increasing world’s population has led to excessive production and prolonged storage of food and feed with the preservation of their quantity and quality being at risk. Stored-product insects seem to be the major problem of all kinds of durable agricultural products during their storage with a wide range of insect species leading to considerable losses and qualitative degradations (Phillips and Throne 2010; Mason and Mcdonald 2012; Nayak and Daglish 2018). The enemies of stored products are already categorized according to their biology and food preferences. According to this, some of the major pests are primary colonizers and have the capacity to infest sound grain kernels, such as the species of genus Sitophilus (Coleoptera: Curculionidae), e.g., the granary weevil, Sitophilus granarius (L.), and the rice weevil, Sitophilus oryzae (L.), or of the genus Callosobruchus (Coleoptera: Bruchidae), such as the cowpea weevil, Callosobruchus maculatus (F.) (Rees 2007; Hagstrum et al. 2012; Athanassiou and Arthur 2018). Other species are classified as secondary colonizers, which are prone to infest processed commodities, or commodities that are already infested by primary colonizers, such as species of the genus Tribolium (Coleoptera: Tenebrionidae), e.g., the red flour beetle, Tribolium castaneum (Herbst), and the confused flour beetle, Tribolium confusum Jacquelin du Val (Nayak and Daglish 2018; Athanassiou and Arthur 2018; Campbell et al. 2022).
At the same time, the increasing global need for food and feed adequacy led to finding alternative nutritional sources with insects showing great promise towards this end (FAO 2013; Van Huis 2013; Kelemu et al. 2015; Yen 2015; Dossey et al. 2016; Rumbos and Athanassiou 2021). Although in its early steps, the massive production of insect meals and insect-based products has become a noticeable part of the production chain during the last few years due to the utilization of registered species for this purpose, such as the yellow mealworm, Tenebrio molitor L. (Coleoptera: Tenebrionidae), and the lesser mealworm, Alphitobius diaperinus Panzer (Coleoptera: Tenebrionidae) (Sánchez-Muros et al. 2014; Van Huis et al. 2015; Commission Regulation (EU) 2017/893; 2021/1372; Van Huis 2019; EFSA 2022). Furthermore, predictions show an enormously increasing insect production in the near future (Meticulous Market Research 2022).
The increase in the production of insect products demands a subsequent increase in the need for their storage. Thus, the edible insect market has to deal with all the problems the conventional agricultural products face during their storage including insect infestations that usually infest durable agricultural commodities (Rumbos et al. 2020a; Deruytter et al. 2021). Earlier work indicates that certain stored-product insect species can feed upon dead insect individuals (Hagstrum et al. 2012), but the degree that this infestation can be detrimental to insect meals is poorly understood. In an earlier study, Rumbos et al. (2020a) studied the vulnerability of insect-based substrates that are based on T. molitor to insect infestations and have shown that certain species can easily develop in such substrates including T. molitor itself. Hence, from a practical view, the increase of the storage capacity and duration of insect-based meals may concomitantly increase “post-harvest” infestations and losses by stored-product arthropods.
Despite these initial reports, the data available so far are focused only on a narrow range of stored-product insect species, while there is still inadequate information for the vast majority of the most common species that occur in durable agricultural commodities. The evaluation of a high number of stored-product insect species on insect meal substrates would be crucial for specifying their ability to infest stored products and food based on insects. This evaluation is also essential to draw the inferences necessary for control strategies that are species-specific, considering that some species that infest these substrates can be controlled easier than others (Deruytter et al. 2021). For instance, control of the Indian meal moth, Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae), can be done with the application of mating disruption, which avoids conventional chemical control but is not possible for most stored-product beetle species (Burks et al. 2011; Deruytter et al. 2021). A species-specific strategy for the control of insect infestations in facilities that produce insect meals is much more complicated in comparison with that in flour or feed mills, considering that certain control measures can also kill the “beneficial” insects (Rumbos et al. 2020a). In this context, we have evaluated the potential of thirteen common stored-product insect species to grow and reproduce on insect meals that are based on A. diaperinus larvae. In this effort, we have included species that range from primary to secondary colonizers, as well as species that can be classified either as stenophagous or polyphagous, to test their ability to constitute a risk in stored insect-based commodities.
Materials and methods
Insect rearing
All insect species used in the tests were reared at the Laboratory of Entomology and Agricultural Zoology, Department of Agriculture, Crop Production and Rural Environment, University of Thessaly. The thirteen species tested here were S. oryzae; S. granarius; C. maculatus; T. castaneum; T. confusum; T. molitor; A. diaperinus; the sawtoothed grain beetle, Oryzaephilus surinamensis (L.) (Coleoptera: Silvanidae); the khapra beetle, Trogoderma granarium Everts (Coleoptera: Dermestidae); the lesser grain borer, Rhyzopertha dominica (F.) (Coleoptera: Bostrychidae); the larger grain borer, Prostephanus truncatus (Horn) (Coleoptera: Bostrychidae); the cigarette beetle, Lasioderma serricorne (F.) (Coleoptera: Anobiidae), and the rusty grain beetle, Cryptolestes ferrugineus (Stephens) (Coleoptera: Laemophloeidae). Each species was reared on its preferred diet; namely, S. oryzae, S. granarius, T. granarium, and R. dominica were reared on soft wheat kernels, while T. castaneum and T. confusum were reared on white wheat flour (Loulis Mills, Organic category M soft wheat flour). Callosobruchus maculatus was reared on chickpeas (Tyrnavos Food S.A., Tyrnavos, Greece), O. surinamensis on oat flakes (Quaker Oats Company, Chicago, Illinois, USA), and P. truncatus on maize grains (bought from a local retailer). For L. serricorne, a mixture of maize flour and yeast (Angel Yeast Co. Ltd., Yichang, China) (18:1) was used, and for A. diaperinus, a mixture of wheat bran (bought from a local retailer) and egg layer hen pellets (3:1) (No 3–Compound Feed for Layers, Viozokat S.A., Katerini, Greece). Tenebrio molitor and C. ferrugineus were reared using wheat bran. Tenebrio molitor and A. diaperinus were weekly supplied with fresh potatoes and apples (bought from a local store), respectively, as a moisture source. Except for A. diaperinus and T. granarium which were kept at 30 °C and 55% relative humidity (r.h.) and 32 °C and 55% r.h., respectively, all the other species were kept at 26 °C and 55% r.h. All insect species were kept at continuous darkness. Only adults, < 1 month old, were used in the tests.
Insect meal preparation
For the A. diaperinus meal preparation, late-stage larvae were used after “harvesting” them through separation from the feeding substrate with sieving. The larvae were frozen at – 20 °C, chopped using a stainless-steel mill (Thermomix TM31-1, Vorwerk Elektrowerke GmbH & Co. KG, Wuppertal, Germany), dried for 72 h at 60 °C, and finally sieved with a 1-mm opening sieve. The insect meal produced was stored at − 20 °C until the initiation of the tests.
Experimental design
The population growth of each of the thirteen species was evaluated on pure A. diaperinus meal substrate (100% A. diaperinus meal). The substrates used as the control reference were those used for the rearing of each species described above. The whole experimental procedure was conducted in cylindrical plastic vials (Rotilabo®-sample tins with snap-on lid, 3.0 cm in diameter, 8.0 cm in height, Carl Roth GmbH & Co. Kg, Karlsruhe, Germany), on which an opening (1.5 cm in diameter) was created in the lid and covered with muslin gauze in order to ensure proper aeriation of the vial. Polytetrafluoroethylene preparation (Fluon, 60 wt% dispersion in water, Sigma-Aldrich Chemie GmbH, Steinheim, Germany) was applied on the upper and inner part of the vials to prevent insects from escaping the vials. Five grams of each of the mentioned substrates was placed in the vials, using different vials for each substrate. Finally, twenty mixed-sex adults of each of the thirteen insect species tested were placed into the vials, using different series of vials for each species. All vials were then placed at the conditions mentioned above, proper for each species, and were kept there for 65 days. In the case of T. molitor and A. diaperinus, a slice of potato and carrot, respectively, was placed inside the vials twice a week to cover the insect moisture needs. For all species, after 65 days, the vials were opened for the evaluation of the progeny production, through counting separately the total number of adults (alive or dead) and larvae. For O. surinamensis, L. serricorne, T. confusum, and T. castaneum, the number of pupae was also determined, whereas for A. diaperinus and T. molitor, the total larval weight was also recorded. For the latter species, the average individual larval weight was also calculated by dividing the total larval weight by the total number of the larvae. As a direct measure of population growth, for all species, the instantaneous rate of increase was calculated using the following equation: ri = ln(Nf/No)/ΔT, where Nf was the final number of individuals, No was the initial number of individuals, and ΔT was the change in time, i.e., the duration of the experiment. Positive values of ri suggest a growing population, ri = 0 shows a stable population, and negative ri values specify a population in decline (Stark and Banks 2003). There were three replicates for each treatment (three-vial replicates) with the whole procedure to be repeated two times (two series of vials) by preparing new vials each time (3 × 2 = 6 vials for each combination).
Statistical analysis
At first, the data were checked for normality using Shapiro–Wilk’s test (Zar 1999). For all species tested, the data for the different life stages that were evaluated, as well as the instantaneous rates of increase, were tested for normality and found that they were non-normally distributed. Therefore, data were compared with the Kruskal–Wallis H-test followed by multiple pairwise Mann–Whitney U tests using the Bonferroni correction (Zar 1999). All analyses were conducted using the IBM® SPSS® Statistics software, Version 25 (IBM Corporation, Armonk, NY, USA).
Results
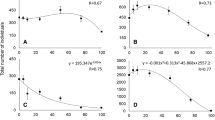
From all the insect species tested in the bioassays, S. oryzae, S. granarius, R. dominica, P. truncatus, O. surinamensis, C. maculatus, and C. ferrugineus did not manage to grow and develop on pure A. diaperinus meal (100% insect meal). Hence, for these species, there was no progeny production on A. diaperinus substrate, since the total number of individuals found at the termination of the tests was the twenty initially inserted adults, while in some cases, less than twenty adults were recorded (Tables 1, 2, 3, and 4). From the species tested, T. granarium and T. castaneum gave that highest progeny production in the control substrates among the species tested, which was 1570 and 445 individuals/vial, respectively, after 65 days (Tables 4 and 5) with T. granarium giving an instantaneous rate of increase of 0.067 (Table 4).
The rest of the examined species grew and developed on 100% A. diaperinus meal, giving a total progeny production ranging from 192 individuals (T. molitor) to 410 individuals (A. diaperinus) (Table 2) and instantaneous rates of increase ranging between 0.0317 and 0.0461 (Tables 2, 3, and 5). These numbers were found to be significantly higher than the control in most of the cases tested, with the exception of A. diaperinus and T. molitor, in which no significant differences were found, apart from their average individual larval weight (Table 5).
Discussion
Our results showed clearly that six out of the thirteen examined insect species in this study can indeed infest pure A. diaperinus meal. Particularly, A. diaperinus, T. molitor, T. granarium, L. serricorne, T. confusum, and T. castaneum grew well and produced progeny on the insect meal substrate. Moreover, in some of the cases tested, progeny production in the control vials was comparable with that for the vials that contained A. diaperinus meal, suggesting that the latter is not just a marginal rearing substrate for some species, but a good nutrient source that can support their development. The fact that, under certain circumstances, insect meals can be easily infested by stored-product insects has been recently examined by Rumbos et al. (2000a), using T. molitor meal. Our data indicate that A. diaperinus meal was equally susceptible to infestations by stored-product insects, if not even more susceptible than that of T. molitor. This fact is particularly important, as stored-product insects can pose a risk in insect farming that will require control measures.
Although some of the species tested here were not able to reproduce on A. diaperinus meal, we think that these species can still be a threat to insect farming, through their occurrence in the raw materials that are used as rearing substrates. In the case of both A. diaperinus and T. molitor, these raw materials are mostly based on amylaceous commodities, such as bran (Ribeiro et al. 2018; Rumbos et al. 2020b; c; 2021), that can be easily infested by a wide range of species, such as S. oryzae, S. granarius, R. dominica, and P. truncatus, which are primary colonizers in grain kernels (Rees 2007; Hagstrum et al. 2012; Athanassiou and Arthur 2018), or C. ferrugineus, which can easily develop in a wide range of amylaceous products (Hagstrum et al. 2012; 2013). On the other hand, species like C. maculatus are stenophagous and can infest only a certain range of commodities, which may be less important in insect farming. Nevertheless, the introduction of these species into a given insect production facility is likely to occur though the raw materials and can be rapidly increased at the first stages of the rearing, when the diet is introduced in the insect rearing. On the other hand, their importance can be decreased at the later stage of the rearing, when the insect diet becomes frass. In this context, any control effort should give emphasis to the disinfestation of the raw materials, before their introduction to the insect production lines. Finally, it should be noted that the primary colonizers tested here cannot develop easily in cracked kernels (Hagstrum et al. 2012), so chopping/milling of the grains that are to be used in insect farming may provide a certain degree of protection against these species.
Not surprisingly, species that are secondary colonizers and have a preference for processed amylaceous commodities (Hagstrum et al. 2012; 2013; Nayak and Daglish 2018; Athanassiou and Rumbos 2018) could develop easily in A. diaperinus meal. This observation stands in accordance with previous results reported by Rumbos et al. (2020a) for some of the species tested here. We think that this group of species poses the most considerable risk in terms of insect infestation, as they can infest cracked grain kernels during the feeding process to produce A. diaperinus meals, and, as such, their presence is even more important at that stage, as compared with their presence to the stored raw materials (e.g., grains). This fact also complicates the application of control measures, because, even if the raw materials are disinfested, they can establish high populations in the processing stage and infest A. diaperinus meals throughout the entire production line. For instance, fumigation with phosphine has been proved to be effective for stored-product insect control in storage and processing facilities (Nayak et al. 2020), but could not be used in certain parts of the production line in insect farming, due to the effect of phosphine in the “beneficial” insects.
From the species tested here, we found that T. confusum, T. castaneum, and, especially, T. granarium gave the highest progeny production numbers in the A. diaperinus meals. The two species of the genus Tribolium can feed on dead insects (Campbell 1989; Hill 2002), although to a lesser extent in comparison with other stored-product beetle species, but their population growth is highly enhanced by processed products, such as flour (Hagstrum and Subramanyam 2009; Hagstrum et al. 2013), which can partially explain the progeny production levels noted here. Moreover, apart from the progeny production capacity in absolute numbers, our results clearly demonstrate that the presence of A. diaperinus meal increased the speed of development of Tribolium spp., providing a faster larval growth and adult emergence. On the other hand, T. granarium is widely known as a “dirty feeder” and can feed upon dead insects as well (Athanassiou et al. 2019). In an earlier study, testing the competition capacity of T. granarium over other major stored-product beetle species, Kavallieratos et al. (2017) found that, at increased temperatures, T. granarium could outcompete S. oryzae and R. dominica, and after a certain period of time, the vials that initially contained all three species contained only T. granarium individuals.
Interestingly, we found that A. diaperinus can develop easily in A. diaperinus meals. While this may not be a serious problem in the mass rearing facilities of this species, the infestation of A. diaperinus in the final product during storage and transportation is likely to cause serious losses, as apart from the main infestation per se, this species can transmit a wide range of bacteria that may endanger human and animal health (Rumbos et al. 2019). Thus, any “escapees” from the production line may invade the final product, causing serious degradations, while remaining undetectable. Similarly, certain stored-product species can transmit pathogens that are not food-borne and can induce antibiotic resistance, as in the case of Tribolium spp. that can transfer certain species of Enterococci (Channaiah et al. 2010; Hubert et al. 2018; Parlapani et al. 2020). In the same way, the occurrence of larvae of T. granarium in the final commodity may cause serious allergenic reactions including skin and eye irritations (Athanassiou et al. 2019).
To conclude, the present study illustrated the ability of major stored-product insects to infest commodities of pure A. diaperinus meal in a way comparable with the infestations of the conventional agricultural stored products. The impact of such infestations may be visible in certain qualitative characteristics of the final product or even during the production chain through cross infestations. Expecting the upcoming increase in the production of insect-based products globally, research on this field should be more focused on improved production and storage facilities, detection and estimation methods, and technologies to minimize insect infestations, without detrimental effects on the insects that are to be produced in insect farming units.
Data availability
The datasets used in this study are available upon reasonable request from the corresponding author.
References
Athanassiou CG, Arthur FH (2018) Recent advances in stored product protection, Springer, Basel, Switzerland, pp 273. https://doi.org/10.1007/978-3-662-56125-6
Athanassiou CG, Rumbos CI (2018) Emerging pests in durable stored products. In: Athanassiou CG, Arthur FH (eds) Recent advances in stored product protection. Springer, Berlin, Heidelberg, Germany pp. 211–217 https://doi.org/10.1007/978-3-662-56125-6_10
Athanassiou CG, Phillips TW, Wakil W (2019) Biology and control of the khapra beetle, Trogoderma granarium, a major quarantine threat to global food security. Annu Rev Entomol 64:131–148. https://doi.org/10.1146/annurev-ento-011118-111804
Burks CS, McLaughlin JR, Miller JR, Brandl DG (2011) Mating disruption for control of Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae) in dried beans. J Stored Prod Res 47:216–221. https://doi.org/10.1016/j.jspr.2011.03.001
Campbell JM (1989) Canadian beetles (Coleoptera) injurious to crops, ornamentals, stored products and buildings. Agricultural Canada Publ 1826, Ottawa
Campbell FC, Athanassiou GC, Hagstrum DW, Zhu KY (2022) Tribolium castaneum: a model insect for fundamental and applied research. Annu Rev Entomol 67:347–365. https://doi.org/10.1146/annurev-ento-080921-075157
Channaiah LH, Subramanyam B, Zurek L (2010) Survival of Enterococcus faecalis OG1RF:pCF10 in poultry and cattle feed: vector competence of the red flour beetle, Tribolium castaneum (Herbst). J Food Prot 73:568–573. https://doi.org/10.4315/0362-028x-73.3.568
Deruytter D, Rumbos CI, Athanassiou CG (2021) Insect infestations in mealworm farming: the case of the pyralid moths. J Insects Food Feed 7:2352–4588. https://doi.org/10.3920/JIFF2021.0029
Dossey AT, Tatum JT, McGill WL (2016) Modern insect-based food industry: current status, insect processing technology, and recommendations moving forward. In: Dossey AT, Morales-Ramos JA, Guadalupe Rojas M (eds) Insects as sustainable food ingredients, Academic Press, pp 113–152. https://doi.org/10.1016/B978-0-12-802856-8.00005-3
EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA), Turck D, Bohn T, Castenmiller J, De Henauw S, Hirsch-Ernst KI, Maciuk A, Mangelsdorf I, McArdle HJ, Naska A, Pelaez C, Pentieva K, Siani A, Thies F, Tsabouri S, Vinceti M, Cubadda F, Frenzel T, Heinonen M, Marchelli R, Neuhäuser-Berthold M, Poulsen M, Maradona MP, Schlatter JR, Van Loveren H, Ververis E, Knutsen HK (2022) Safety of frozen and freeze-dried formulations of the lesser mealworm (Alphitobius diaperinus larva) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J 20(7):e07325. https://doi.org/10.2903/j.efsa.2022.7325
EU Commission Regulation 2017/893 of 24 May 2017 amending Annexes I and IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council and Annexes X, XIV and XV to Commission Regulation (EU) No 142/2011 as regards the provisions on processed animal protein. http://data.europa.eu/eli/reg/2017/893/oj. Accessed on 7 Feb2023
EU Commission Regulation 2021/1372 of 17 August 2021 amending Annex IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council as regards the prohibition to feed non-ruminant farmed animals, other than fur animals, with protein derived from animals. http://data.europa.eu/eli/reg/2021/1372/oj. Accessed on 7 Feb 2023
Food and Agriculture Organization (FAO) (2013) Edible insects: future prospects for food and feed security, by Van Huis A, van Itterbeeck J, Klunder H, Mertens E, Halloran A, Mui G, Vantomme P. FAO UN, Forestry Department, Rome, Italy. http://www.fao.org/docrep/018/i32-53e/i3253e.pdf. Accessed 15 Feb 2023
Hagstrum DW, Subramanyam Bh (2009) Stored-product insect resource. AACC International Inc, St Paul, MN, p 509
Hagstrum D, Phillips T, Cuperus G (2012) Stored product protection. Kansas State University, Manhattan, p 358
Hagstrum DW, Klejdysz T, Subramanyam B, Nawrot J (2013) Atlas of stored-product insects and mites. AACC International Inc., St. Paul, MN
Hill DS (2002) Pests of stored foodstuffs and their control. Kluwer Academic Publishers, Boston
Hubert J, Stejskal V, Athanassiou CG, Throne JE (2018) Health hazards associated with arthropod infestation of stored products. Annu Rev Entomol 63:553–573. https://doi.org/10.1146/annurev-ento-020117-043218
Kavallieratos NG, Athanassiou CG, Guedes RNC, Drempela JD, Boukouvala MC (2017) Invader competition with local competitors: displacement or coexistence among the invasive khapra beetle, Trogoderma granarium Everts (Coleoptera: Dermestidae), and two other major stored-grain beetles? Front Plant Sci 8:1837. https://doi.org/10.3389/fpls.2017.01837
Kelemu S, Niassy S, Torto B, Fiaboe K, Affognon H, Tonnang H, Maniania NK, Ekesi S (2015) African edible insects for food and feed: inventory, diversity, commonalities and contribution to food security. J Insect Food Feed 1:103–119. https://doi.org/10.3920/JIFF2014.0016
Mason LJ, McDonald M (2012) Biology, behaviour and ecology of stored grain and legume insects. In: Hagstrum DW, Phillips TW, Cuperus G (eds) Stored product protection, agricultural experiment station and cooperative extension service. Kansas State University, Manhattan, pp 7–20
Meticulous Market Research (2022) Edible insects market by product (whole insect, insect powder, insect meal, insect oil), insect type (crickets, black soldier fly, mealworms), application (animal feed, protein bar and shakes, bakery, confectionery, beverages), and geography - forecast to 2030. https://www.meticulousresearch.com/pressrelease/184/edible-insects-market-2030. Accessed on 7 Feb 2023
Nayak MK, Daglish GJ (2018) Importance of stored product insects In: Athanassiou CG, Arthur FH (eds) Recent advances in stored product protection, Springer, Basel, Switzerland, pp 1–18. https://doi.org/10.1007/978-3-662-56125-6_1
Nayak MK, Daglish GJ, Phillips TW, Ebert PR (2020) Resistance to the fumigant phosphine and its management in insect pests of stored products: a global perspective. Annu Rev Entomol 65:333–350. https://doi.org/10.1146/annurev-ento-011019-025047
Parlapani FF, Kyritsi M, Sakka M, Chatzinikolaou K, Donos S, Boziaris IS, Hadjichristodoulou C, Athanassiou CG (2020) Matrix-assisted laser desorption ionization–time of fight mass spectrometry reveals Enterococcus and Enterobacter spp. in major insect species involved in food security with resistance to common antibiotics. J Pest Sci 93:159–170. https://doi.org/10.1007/s10340-019-01125-5
Phillips TW, Throne JE (2010) Biorational approaches to managing stored-product insects. Annu Rev Entomol 55:375–397. https://doi.org/10.1146/annurev.ento.54.110807.090451
Rees D (2007) Insects of stored grain: a pocket reference, 2nd edn. CSIRO publishing, Colingwood, Australia, p 80
Ribeiro N, Abelho M, Costa R (2018) A review of the scientific literature for optimal conditions for mass rearing Tenebrio molitor (Coleoptera: Tenebrionidae). J Entomol Sci 53:434–454. https://doi.org/10.18474/JES17-67.1
Rumbos CI, Karapanagiotidis IT, Mente E, Athanassiou CG (2019) The lesser mealworm Alphitobius diaperinus: a noxious pest or a promising nutrient source? Rev Aquac 11:1418–1437. https://doi.org/10.1111/raq.12300
Rumbos CI, Rigopoulou M, Athanassiou CG (2020a) Are insect meals prone to insect infestation during storage? Development of major storage insects on substrates based on Tenebrio molitor larvae meal. J Pest Sci 93:1359–1367. https://doi.org/10.1007/s10340-020-01228-4
Rumbos CI, Karapanagiotidis IT, Mente E, Psofakis P, Athanassiou CG (2020b) Evaluation of various commodities for the development of the yellow mealworm. Tenebrio Molitor Sci Rep 10(1):11224. https://doi.org/10.1038/s41598-020-67363-1
Rumbos CI, Pantazis I, Athanassiou CG (2020c) Population growth of Alphitobius diaperinus (Coleoptera: Tenebrionidae) on various commodities. J Econ Entomol 113:1001–1007. https://doi.org/10.1093/jee/toz313
Rumbos CI, Athanassiou CG (2021) ‘Insects as food and feed: if you can’t beat them, eat them!’—to the magnificent seven and beyond. J Insect Sci 21:2. https://doi.org/10.1093/jisesa/ieab019
Rumbos CI, Bliamplias D, Gourgouta M, Michail V, Athanassiou CG (2021) Rearing Tenebrio molitor and Alphitobius diaperinus larvae on seed cleaning process byproducts. Insects 12:293. https://doi.org/10.3390/insects12040293
Sánchez-Muros MJ, Barroso FG, Manzano-Agugliaro F (2014) Insect meal as renewable source of food for animal feeding: a review. J Clean Prod 65:16–27. https://doi.org/10.1016/j.jclepro.2013.11.068
Stark JD, Banks JE (2003) Population-level effects of pesticides and other toxicants on arthropods. Annu Rev Entomol 48:505–519. https://doi.org/10.1146/annurev.ento.48.091801.112621
Van Huis A (2013) Potential of insects as food and feed in assuring food security. Annu Rev Entomol 58:563–583. https://doi.org/10.1146/annurev-ento-120811-153704
Van Huis A, Dicke M, van Loon JJA (2015) Insects to feed the world. J Insects Food Feed 1:3–5. https://doi.org/10.3920/JIFF2015.x002
Van Huis A (2019) Insects as food and feed, a new emerging agricultural sector: a review. J Insects Food Feed 6:27–44. https://doi.org/10.3920/JIFF2019.0017
Yen AL (2015) Insects as food and feed in the Asia Pacific region: current perspectives and future directions. J Insects Food Feed 1:33–55. https://doi.org/10.3920/JIFF2014.0017
Zar HJ (1999) Biostatistical analysis. Prentice Hall, Upper Saddle River
Funding
Open access funding provided by HEAL-Link Greece.
Author information
Authors and Affiliations
Contributions
Athanassiou Christos (AC) and Rumbos Christos (RC) contributed to the study conception and design. Rigopoulou Marianna (RM) conducted the experiments and analyzed the data together with RC and AC. All authors contributed to the writing of the manuscript with AC as the lead. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies involving human participants or animals.
Consent for publication
All authors agree and declare that the manuscript submitted to your journal has not been published elsewhere or submitted concurrently to other journals.
Competing interests
The authors declare no competing interests.
Disclaimer
The data and opinions expressed in this manuscript are solely our own, and we are solely responsible for their authenticity, validity, and originality. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the University of Thessaly.
Additional information
Responsible Editor: Giovanni Benelli
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
We declare that this manuscript is our original work and has not been copied from another source.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rigopoulou, M., Rumbos, C. & Athanassiou, C. Evaluation of the susceptibility of Alphitobius diaperinus meal to infestations by major stored-product beetle species. Environ Sci Pollut Res 30, 73628–73635 (2023). https://doi.org/10.1007/s11356-023-27602-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-27602-0