Abstract
Exposure to arsenic even at low levels can lead to adverse health outcomes, however, there is a paucity of research from South Africa in relation to human exposure to arsenic. We investigated long-term exposure of residents in Limpopo province, South Africa, in a cross-sectional study by analysing water, soil and blood arsenic concentrations from two arsenic-exposed (high and medium–low exposure) villages and one non-exposed (control) village. There were statistically significant differences in the distribution of arsenic in water, soil and blood amongst the three sites. The median drinking water arsenic concentration in the high-exposure village was 1.75 µg/L (range = 0.02 to 81.30 µg/L), 0.45 µg/L (range = 0.100 to 6.00 µg/L) in the medium- / low-exposure village and 0.15 µg/L (range = < limit of detection (LOD) to 29.30 µg/L) in the control site. The median soil arsenic concentration in the high-exposure village was 23.91 mg/kg (range = < LOD to 92.10 mg/kg) whilst arsenic concentrations were below the limit of detection in all soil samples collected from the medium-/low-exposure and control villages. In the high-exposure village, the median blood arsenic concentration was 1.6 µg/L (range = 0.7 to 4.2 µg/L); 0.90 µg/L (range = < LOD to 2.5 µg/L) in the medium-/low-exposure village and 0.6 µg/L (range = < LOD to 3.3 µg/L) in the control village. Significant percentages of drinking water, soil and blood samples from the exposed sites were above the internationally recommended guidelines (namely, 10 µg/L, 20 mg/kg and 1 µg/L, respectively). Majority of participants (86%) relied on borehole water for drinking and there was a significant positive correlation between arsenic in blood and borehole water (p-value = 0.031). There was also a statistically significant correlation between arsenic concentrations in participants’ blood and soil samples collected from gardens (p-value = 0.051). Univariate quantile regression found that blood arsenic concentrations increased by 0.034 µg/L (95% CI = 0.02–0.05) for each one unit increase in water arsenic concentrations (p < 0.001). After adjusting for age, water source and homegrown vegetable consumption in multivariate quantile regression, participants from the high-exposure site had significantly higher blood concentrations than those in the control site (coefficient: 1.00; 95% CI = 0.25–1.74; p-value = 0.009) demonstrating that blood arsenic is a good biomarker of arsenic exposure. Our findings also provide new evidence for South Africa on the association between drinking water and arsenic exposure, emphasising the need for the provision of potable water for human consumption in areas with high environmental arsenic concentrations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic exposure to arsenic is a significant environmental public health concern. The growing body of evidence suggests adverse health outcomes occur even at low levels of exposure (NRC 2014; Yang et al. 2021; Moon et al. 2017). Arsenic exposure affects several body organs leading to ill-health outcomes including skin lesions, neurological impairments, respiratory and cardiovascular disorders, and disruptions to immune and endocrine systems (Naujokas et al. 2013). Arsenic is classified as a Group 1 human carcinogen by the International Agency for Research on Cancer (IARC 2004) with exposure to elevated arsenic concentrations associated with increased risk of several human cancers (IARC 2012). Epidemiological studies suggest that major health risks from arsenic are due to chronic exposure to contaminated drinking water and soil. However, water is deemed the predominant source of arsenic. Several clinical and epidemiological studies conducted globally have assessed the health impacts of exposure to arsenic-contaminated drinking water (Chen et al. 2011; Ferreccio et al. 2013; Lamm et al. 2013; Liaw et al. 2008; Monrad et al. 2017). Arsenic-endemic areas, such as Bangladesh in India, the southwest coast of Taiwan, and parts of Chile, have reported elevated risks and prevalence of skin, bladder, liver, kidney and prostate cancer due to exposure to arsenic-contaminated drinking water (Steinmaus et al. 2013; Smith et al. 2000; Chen and Ahsan 2004; Chen 2014; Cheng et al. 2016; Kumar et al. 2021).
A predominant clinical manifestation of arsenic toxicity is black-foot disease. This is a peripheral vascular disease associated with long-term exposure to inorganic arsenic that is common in areas with elevated arsenic concentrations in groundwater used for human consumption (Nordberg et al. 2019). Increased risk and prevalence of cardiovascular diseases, such as myocardial infarction and diabetes, have also been associated with human consumption of arsenic-contaminated drinking water (D’Ippoliti et al. 2015; Chen et al. 2011; Tseng 2008; Del Razo et al. 2011). Neurologic examinations and assessments conducted on study participants exposed to high and even low concentrations of arsenic in drinking water in India (Mukherjee et al. 2003; Chakraborti et al. 2003), Taiwan (Tsai et al. 2003; Tseng et al. 2006), the United States of America (Wasserman et al. 2014) and Myanmar (Mochizuki et al. 2019) demonstrate varying types and severity of arsenic-typical neuropathy. Prolonged exposure to arsenic has also been found to reduce cognitive ability, especially in children (Nahar et al. 2014; Dong and Su 2009; Tolins et al. 2014). These studies provide evidence of the health risks of arsenic exposure from consuming ground water in several parts of the world. However, there is a scarcity of similar research in South Africa, where the majority of research is centred around quantifying environmental arsenic concentrations, with little focus on health effects. These include studies that have assessed arsenic contamination of soil (Kapwata et al. 2020; Kootbodien et al. 2012; Mathee et al. 2018) and water sources (Mudzielwana et al. 2020; Genthe et al. 2018; Munyangane et al. 2017) where results found that concentrations of arsenic were above international guidelines. An environmental study conducted in villages in Giyani, Limpopo province, found that 54% out of a total of 95 soil samples collected in one village had arsenic concentrations exceeded the Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health of 20 mg/kg (CEPA 2007) which considers lifetime cancer risk due to exposure. Unfortunately, there is no similar South African guideline to apply. The associated pollution index, which was calculated to categorise the level of arsenic contamination, determined that 57% of those samples were classified as ‘moderately to heavily’ and ‘extremely’ contaminated (Kapwata et al. 2020). Whilst a South African guideline limit for arsenic in residential soil of 47 mg/kg exists, it is based on soil contamination, not on evidence of adverse health outcomes. Therefore, Kapwata et al. (2020) applied the Canadian guideline, because it is based on adverse effects on humans and the environment. Other studies (Mudzielwana et al. 2020; Munyangane et al. 2017), have analysed arsenic concentrations in drinking water samples from boreholes in Giyani (including water samples from schools) and found concentrations that surpassed the limit of 10 µg/L recommended by both the South African and the World Health Organization (WHO) guidelines for drinking water quality (SANS 2015; WHO 2017).
Several South African studies have conducted arsenic health risk assessments and characterised the risks of personal or community exposure to arsenic; using metrics such as the hazard quotient (HQ), hazard index (HI) and chronic hazard index (CHI) in arsenic-exposed communities (Kamunda et al. 2016; Ngole-Jeme and Fantke 2017; Genthe et al. 2018). However, these studies did not involve the collection of biological and environmental samples to measure arsenic exposure and identify possible pathways of this exposure.
Therefore, there is a paucity of research in South Africa pertaining to human exposure to arsenic in communities that are potentially exposed to elevated concentrations of arsenic present in environmental media. One of the few such studies related to arsenic and human health found that arsenic concentrations in maternal blood of coastal residents at delivery were correlated with self-reported type of water source for drinking water (piped or borehole) (Röllin et al. 2017). Our study, one of the first in the country, aimed to establish if arsenic-exposed communities faced higher risks of adverse health effects due to elevated blood arsenic concentrations compared to people in non-exposed villages. Other considerations included whether the possible sources of this arsenic was from water and/or soil exposure by measuring arsenic concentrations in both. We investigated long-term exposure of residents living in areas known to have high environmental arsenic concentrations in Giyani, Limpopo province. A two-pronged approach was applied by (i) analysing the blood arsenic concentrations of individuals living in exposed and non-exposed areas; and (ii) evaluating arsenic concentrations in drinking water and soil samples from participants’ dwellings and gardens respectively, to identify potential sources of arsenic exposure.
Methods
Study area
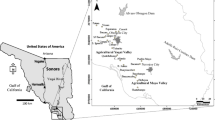
Greater Giyani is one of five local municipalities in the Mopani District Municipality in Limpopo province (Fig. 1). It is largely rural with an estimated total population of 244 217 consisting of approximately 91 villages and some semi-urban settlements (Giyani 2017). Poverty is rife in the area, with 42% of households reporting that they had no income or lived on ZAR800 per month, which is equivalent to ~ USD 51 (Giyani 2017). The study area was selected due to existing evidence of potentially high levels of arsenic in water and soil (Munyangane et al. 2017; Kapwata et al. 2020; Mudzielwana et al. 2020).
Study design and participant recruitment
A multi-disciplinary, cross-sectional study design with exposed and control sites was used to select study participants. This epidemiological design has been used in several arsenic exposure studies (National Research Council 1999; Roh et al. 2017). The two exposed villages were selected based on findings from previous studies which measured elevated soil and water concentrations of arsenic exceeding local and international guidelines (Munyangane et al. 2017; Kapwata et al. 2020; Mudzielwana et al. 2020). The exposed villages, Maswanganyi village and Muyexe village, were categorised as ‘high’ and ‘medium/low’ exposure sites, respectively. Tomu village, which was selected as a control site, had similar demographic characteristics to the exposed villages, but studies have not found elevated concentrations of arsenic in the soil and water environments there. Moreover, there was no evidence of activities or industry to indicate potential sources of arsenic.
Based on a sample size calculation, a minimum sample size of 124 participants, of which 62 were exposed and 62 were unexposed, was required to detect a difference of 20% or greater, assuming that the prevalence of abnormal arsenic concentrations in blood in unexposed individuals is 10%. Participants recruited for the study were adults between 18 and 65 years of age and had lived in the area for more than 10 years. This age range was selected as an inclusion criterion to provide information about the susceptibility to arsenic across different age groups and to reduce loss to follow-up (Chen et al. 2011; Zhang 2016). Households and eligible participants from each home were randomly selected. The purpose of our study was verbally explained to participants prior to obtaining informed signed consent. Questionnaires were administered at the participant’s residence, and blood samples were collected from consenting participants. Water and soil from their water sources and residential garden sites were also collected.
Questionnaire administration
Fieldworkers administered an electronic version of a structured questionnaire, which was developed using Redcap software (Harris et al. 2009). It is a secure web application used to conduct questionnaire surveys and to manage databases. The questionnaire requested information on socio-demographic information (i.e., age, level of education, employment status, employment history, sources of income and monthly household income), household characteristics (i.e., type and condition of dwelling, number of people living in the home, and fuel sources for cooking and heating), food sources (i.e., whether participants ate homegrown vegetables) and water sources (i.e., piped, borehole and river), current and past medical conditions (including chronic conditions) and behavioural practices (i.e., alcohol and tobacco use) that could be potential risk factors for arsenic exposure.
Blood samples
Blood serves as a biomarker of recent/short-term arsenic exposure (Arcega-Cabrera et al. 2018). However, with chronic and continuing exposure, steady-state concentrations are achieved thus blood has the potential to serve as a biomarker of past exposure (Arcega-Cabrera et al. 2018). Nurses registered and accredited with the Health Professions Council of South Africa and the South African Nursing Council collected venous, whole blood samples from study participants in 6 mL trace metal-free tubes. Samples were stored in portable coolers and delivered to the laboratory, daily.
For the measurement of arsenic in blood, samples were diluted 25-fold with a diluent (Ammonia 2.5 mL; Butanol 6 mL, 0.1% Triton-X 50 µL and Ethylenediaminetetraacetic acid (EDTA) 50 µg in 500 mL deionised water). The following internal standards were also added to the diluent: Indium (25 µL), Germanium (25 µL), Scandium (25 µL), Rhodium (250 µL) and Iridium (250 µL). The instrument used for the analyses, namely an Agilent Inductively Coupled Plasma Mass Spectrometry (ICP-MS) 7 900, was calibrated with calibration standards prepared in the diluent using a multi-element custom standard (i.e., SPECTRASCAN – SS 028 226). The specifications of the instrument include a standard low-flow, Peltier-cooled sample introduction system; an ultra-high matrix introduction (UHMI) that significantly increases matrix tolerance and improves plasma robustness; a plasma and shield torch system (STS) which ensures high sensitivity and effective interference removal in helium mode; a fast, frequency-matching plasma radio frequency generator; off-axis ion lens; hyperbolic quadrupole; an orthogonal detector system (ODS) and an efficient vacuum system The concentrations of the standards for arsenic ranged from 0.1 to 50 µg/L, whilst the Limit of Detection (LOD) for arsenic in blood is < 0.2 µg/L. There are no published guidelines for the permissible levels of arsenic in blood, however, clinical and epidemiological studies recommend a threshold of 1 µg/L (Kumar et al. 2020; NRC 1999; ATSDR 2002).
Water samples
The analytical technique used here has been used in previous studies to measure quantities of trace elements, including arsenic, in food and beverages consumed by both adults and children (Saghafi et al. 2021; Kiani et al. 2022; Karami et al. 2021).
Drinking water samples were collected from the main source used by the household, i.e., borehole, outdoor communal standpipe, indoor tap or outdoor storage tank. Samples were stored and transported at room temperature and analysed for arsenic concentration at an accredited laboratory. According to the United States Environmental Protection Agency (USEPA) guide for collecting and testing drinking water, samples do not need to be acid preserved if they are received by the laboratory within 14 days of sampling (USEPA 2016). Therefore, collected samples were delivered to the laboratory within the recommended times. An Inductively Coupled Plasma Mass Spectrometry Agilent 7 700 instrument was used for the analysis of arsenic concentration in each of the collected water samples. The specifications of the instrument include a low-flow Peltier cooled sample introduction system; electronic gas control to deliver precise control of all plasma and cell gases; a patented high matrix introduction (HMI) kit that virtually eliminates matrix suppression; a fast, frequency-matching plasma radio frequency generator; off-axis ion lens and efficient vacuum system (Agilent Technologies 2010). As part of quality control procedures, the methods used by the external laboratory, included the use of standard reference materials (SRMs) and quality control samples of known values. In addition, recoveries for the analysis were 94% for arsenic at 10 µg/L. The calibration range used was 0.01–500 µg/L and R2 value of the calibration was ≥ 0.995. The LOD and limit of quantification (LOQ) were 0.030 and 0.01 µg/L, respectively. Results were considered in the context of the WHO drinking water quality guideline—for arsenic in drinking water of 10 µg/L.
Soil samples
Soil samples were collected from the within the top 10 cm of exposed soil, in accordance with US EPA operating procedures (USEPA 2014). They were transported to the laboratory at room temperature then ground by hand and oven-dried at 40 °C over a 48-h period. The soil was sieved to retain particles less than 2 mm for further analysis. The concentrations of arsenic were measured using a portable Niton XL2 XRF fluorescence (XRF) spectrometer (Niton XRF 2015). The LOD was 5 mg/kg for arsenic. Results were considered in relation to the Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health of 20 mg/kg (CEPA 2007).
Statistical analysis
Descriptive analyses of arsenic concentrations in blood, soil and water samples from the participants were performed separately for each village. These included the high-exposure village (Maswanganyi); the medium-/low-exposure village (Muyexe); as well as the control village (Tomu). The Shapiro–Wilk test was used to assess normality of arsenic concentrations. The Spearman’s rank correlation was used to assess the relationship between arsenic concentrations in blood, water and soil results in paired samples (i.e., blood, soil and water results were merged for each individual). The Wilcoxon-rank sum test was used to test for statistically significant differences between the three villages for the blood, water and soil arsenic levels.
We commenced with univariate quantile regression analysis, assessing village (as a proxy for exposure level) as a predictor of blood arsenic levels. Multivariate quantile regression was then used to evaluate the associations between blood arsenic levels and the village category (high-exposure, medium-/low-exposure and the control), whilst adjusting for potential confounders and effect modifiers including gender, house type, employment status, tobacco use, consumption of homegrown vegetables and the use of piped or borehole water for consumption and domestic uses. A variable (i.e., potential confounder) was included in the adjusted analysis if it was significantly related to either arsenic concentration, or exposed/unexposed village; or if its inclusion changed the model coefficients for exposed/unexposed village by more than 10%. All analyses were performed using Stata 15 (StataCorp 2017), and p-values less than 0.05 were considered statistically significant.
Results
Descriptive statistics
The characteristics of study participants are presented in Table 1. These characteristics are also possible determinants of behaviours and exposures that affect arsenic concentration in blood (Rahbar et al. 2022; Mathee et al. 2022). Sixty-six participants were enrolled into the study, 43 from the two arsenic exposed villages known to have high- to medium- and low-environmental arsenic levels and 23 were in the unexposed control group living in a village known to not have high levels of arsenic. More than three-quarters of participants used borehole water for domestic purposes (86%) and to irrigate vegetable gardens for homegrown food (75%) (Table 1). Subsistence farming was a common practice, with 90% of participants reporting that they consumed vegetables grown in their own gardens. Almost half of the participants were unemployed (44%), and this could have contributed to households practicing subsistence farming and growing their own food to survive.
Arsenic concentrations in water, soil and blood
Figure 2 presents the means, medians and the inter quartile range of arsenic concentrations for the three villages. There was a statistically significant difference in the distribution of arsenic in water samples amongst the three villages (p-value = 0.0002). with significant differences observed for all pairwise comparisons. Median arsenic concentrations are reported because the means were affected by high outliers that were verified with the laboratory as true values.
Elevated arsenic concentrations in water were observed in the high-exposure village of Maswanganyi village (median = 1.75 µg/L; range = 0.02 to 81.30 µg/L), followed by Muyexe, the medium-/low-exposure village (median = 0.45 µg/L; range = 0.100 to 6.00 µg/L) and Tomu, the control village (median = 0.15 µg/L; range = < LOD to 29.30 µg/L). Thirty-nine percent of drinking water samples from the high-exposure village, had arsenic concentrations that were above the WHO drinking water quality guideline value for arsenic in drinking water (WHO 2017) (see supplementary Table S1).
There was a statistically significant difference in the distribution of arsenic concentrations in soil, amongst the villages (p-value = 0.0003). The median and range of arsenic concentrations in soil were 23.91 mg/kg and < LOD to 92.10 mg/kg, respectively. More than half (55%) of the soil samples collected from the high-exposure village, exceeded the recommended value of 20 mg/kg from the Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health (CEPA 2007). Arsenic concentrations in all soil samples collected from participants’ gardens in the medium-/low-exposure and control villages were below the LOD.
There was also a statistically significant difference in the distribution of arsenic in blood amongst participants living in the villages (p-value = 0.0005). In the high-exposure village, arsenic concentrations amongst participants ranged from 0.7 to 4.2 µg/L with a median of 1.6 µg/L; in the medium-/low-exposure village, concentrations ranged from < LOD to 2.5 µg/L with a median of 0.90 µg/L whilst in the control village, arsenic levels ranged from < LOD to 3.3 µg/L. Almost 80% of blood samples collected from participants in the high-exposure village had arsenic concentrations above the recommended threshold of 1 µg/L (ATSDR 2002) whilst 45% of blood samples from the medium-/low-exposure group exceeded this threshold (Table S1). Only 19% of blood arsenic concentrations from participants in the control village were above the level of concern.
Relations between environmental variables and blood arsenic concentrations
There were significant positive correlations between arsenic in blood and (Spearman’s rho = 0.391; p-value = 0.0015), specifically (Spearman’s rho = 0.292; p-value = 0.031). There was also a statistically significant positive association between arsenic concentrations in participants’ blood and soil collected from their gardens (Spearman’s rho = 0.290; p-value = 0.051).
The key exposure variable in our study was ‘village’ and it had three levels—high-exposure (Maswanganyi), medium-/low-exposure (Muyexe) and the control site (Tomu)—which was also the reference category in the regression. Univariate quantile regression analysis found that participant blood arsenic concentrations increased by 0.034 µg/L (95% CI = 0.02–0.05), for each one unit increase in matching water arsenic concentrations (p-value < 0.001) (Table S2a). However, participant blood and soil concentrations were not significantly associated (Table S2b). We also observed that individuals from the high-exposure village were associated with significantly higher blood arsenic levels, than individuals in the control site (coefficient = 1; 95% CI = 0.36–2.76; p-value = 0.007). However, individuals in the medium-/low-exposure site did not have significantly different blood arsenic concentrations, compared to individuals from the control village (coefficient = 0.3; 95% CI = − 0.39 to 0.99; p-value = 0.387).
For the multivariate regression analysis, we compiled a list of potential confounders for the relationship between exposure (i.e., ‘village’) and arsenic concentration in blood using existing literature for effects of borehole water, homegrown vegetables and age on arsenic in blood (Hall et al. 2006; Iyer et al. 2016; Rahbar et al. 2012; Bibi et al. 2015; Sekhar et al. 2003). Therefore, the variables included in the regression model were exposed/unexposed village, age, borehole water as main source of water, and consumption of homegrown vegetables (Table 2). After adjusting for age, water source and homegrown vegetable consumption, participants from the high-exposure village had significantly higher blood concentrations than those from the control village (Coefficient 1.00; 95% CI = 0.25–1.74; p-value = 0.009). These results concur with our initial hypothesis that the community of Maswanganyi likely experience higher arsenic exposure compared to residents of Tomu village which was the unexposed site.
Discussion
Arsenic contamination of water and soil has been investigated globally, given that arsenic is a known human carcinogen and has numerous adverse health effects. A limited amount of research has been undertaken in African countries, including South Africa, to assess arsenic exposure to contaminated water and/or soil, most of which have been desk-top human health risk assessment studies (Rosas-Castor et al. 2014; Affum et al. 2015; Wang et al. 2019; Bortey-Sam et al. 2015). Our study is considered the first amongst (South) African studies that analysed arsenic concentrations in water and soil, and took blood samples from individuals living in arsenic-exposed and non-exposed areas to quantitatively consider individual arsenic exposure.
Arsenic concentrations in water from our hypothesised high arsenic exposure site were above the WHO guideline value for arsenic in drinking water of 10 µg/L (WHO 2017). The WHO implemented this provisional guideline value taking into consideration the Joint Food and Agriculture Organization of the United Nations (FAO)/WHO Expert Committee on Food Additives (JECFA) evaluations of literature. They concluded that for certain regions of the world with concentrations of arsenic in drinking water that ranged between 50 to100 µg/L, evidence of adverse health effects existed. In other regions, where arsenic concentrations were between 10 and 50 µg/L, the possibility of adverse effects persisted, but would likely be at a low incidence (WHO 2018). Our study provided evidence of elevated arsenic levels in drinking water in the highly exposed village, with 39% of water samples exceeding the WHO recommendation and placing residents at risk of adverse health outcomes. For example, a large survey of Wisconsin residents who consumed groundwater with arsenic concentrations of 10 µg/L for 20 or more years had increased prevalence of chronic cardiac diseases and a high incidence of cardiac bypass surgery and depression (Zierold et al. 2004). Furthermore, a study using data from one of the largest populations at risk of arsenic exposure due to contaminated groundwater showed more than 24 000 adult deaths annually could be attributed to arsenic exposures of 10–50 µg/L (Flanagan et al. 2012).
The analysis of soil samples showed that arsenic concentrations in the control and medium–low exposure sites were below the LOD. However, high concentrations were found in soil samples from the high-exposure village. We also found a significant correlation between participant’s blood arsenic concentrations and matching soil arsenic concentrations; a relationship which is supported by other studies (Madrid et al. 2022; Li et al. 2021). Almost 90% of study participants reported that they consumed vegetables grown in the gardens from which soil samples were collected. Therefore, in addition to drinking water, the transfer of arsenic from soils to the edible parts of plants is another possible route of arsenic entry into the human body (Gebeyehu and Bayissa 2020).
As anticipated, the trend of blood arsenic concentrations showed that the highest levels were observed in the high-exposure site where almost 80% of blood samples exceeded the recommended threshold of 1 µg/L (ATSDR 2002). Similarly, 45% of samples from the medium-/low-exposure site exceeded the recommended threshold of 1 µg/L, whilst only 19% of samples from the control were above the threshold. Arsenic. Skin lesions were reported in about 23% of respondents with an arsenic blood range of 1.6–5.4 µg/L from a cohort study in Bangladesh exposed to high levels of arsenic from groundwater (Hall et al. 2006). The detection of arsenic in blood samples from non-exposed residents could have been caused by participants smoking tobacco products, arsenic is one of the chemical compounds found in cigarettes (WHO 2009). Although only a small percentage of respondents reported using tobacco products, the true prevalence of smoking could be higher as smoking status is often underreported due to stigma (Singh et al. 2022; Liber and Warner 2018).
Our univariate regression results reinforced the finding that the study participants from the high-exposure village had significantly higher blood arsenic concentrations than those participants from the control site. This was still true after adjusting for potential confounders such as age, water source and homegrown vegetable consumption in the multivariate regression. Therefore, our findings show that blood may be a useful biomarker for exposure to arsenic-contaminated water and soil. Previous studies also suggest blood arsenic concentrations are a reliable indicator of chronic, continuing exposure (Hall et al. 2006; McClintock et al. 2012).
A significant proportion of participants (86%) used borehole water. We found a significant correlation between participants’ blood arsenic concentrations and matching borehole water arsenic concentrations similar to other studies around the world (Katiyar and Singh 2014; Kumar et al. 2020; Rodrigues et al. 2016; Arikan et al. 2015; Arcega-Cabrera et al. 2017). Groundwater extracted via borehole or hand-dug wells may be contaminated with arsenic due to the hydrogeochemistry of the environment, for example arsenic present in coal in India (Patel et al. 2012), or from an anthropogenic source (Mudzielwana et al. 2020). Previous studies have found that arsenic contamination of drinking water sources can occur because of proximity to naturally-occurring arsenic found in certain types of bedrock and sediments. Whilst we did not ask participants their reasons for relying on borehole water as their main water source, which was identified as a shortcoming of our research, several reasons may be surmised. Water supply as a basic service is oftentimes unreliable in rural areas in Limpopo with frequent disruptions in supply (Nguyen et al. 2021; Luvhimbi et al. 2022; Majuru et al. 2012). Another consideration may be that surface water from rivers or dams may be deemed less suitable for human consumption; in terms of quality compared to groundwater (Madilonga et al. 2021). Using groundwater when it is more readily available than other sources, such as piped water supply, is thus a necessity but also introduces an issue of environmental injustice. There is no easy, low-cost solution at household or community level to remove arsenic from water. Therefore, it is imperative that appropriate policy implementation actions are taken to ensure safe drinking water is made available to all individuals and villages at risk of arsenic exposure via water, in particular from groundwater sources.
Study limitations
Several study limitations were considered. The timing of water sampling for the study was likely affected by season, since concentrations of chemicals in water may be diluted during the rainy season compared to the dry season, and additional samples, taken during different seasons may be required to supplement these findings. The minimum sample size of individuals required for the study was not met due to reluctance from potential participants to provide a blood sample due to traditional and cultural beliefs. There was also community unrest during the time of fieldwork caused by leadership contestations, and therefore, it was unsafe to proceed with data collection in some areas. Whilst participants were asked what their main water source was, multiple water sources could have been used in addition to their self-reported main source. Future studies should consider asking for the main water sources as well as secondary water sources.
Conclusions
Arsenic is naturally and anthropogenically present at high levels in groundwater in several countries around the world. Contaminated borehole water used for drinking and irrigation of food crops poses a public health threat. We assessed arsenic concentrations in study participants’ main water source, soil from their garden and participants’ blood samples. To the best of our knowledge, this is one of the first studies to analyse arsenic in blood at the individual level in Limpopo province, South Africa. We found significant associations between arsenic concentrations in borehole water and blood arsenic concentrations. This was particularly concerning because most of our study participants relied on borehole water as their main source of drinking water. In rural settings, such as the villages in our study, lack of access to piped water leads to a reliance on borehole water. Service provision of potable water for consumption, food preparation and irrigation of food crops in areas with arsenic-contaminated water needs to be improved to protect public health. Soil arsenic concentrations was correlated with blood arsenic concentrations, indicating possible exposure to arsenic through ingestion of homegrown produce. Further work is required to investigate arsenic concentrations in these plants and vegetables. We also found that study participants from the high-exposure village had significantly higher blood arsenic concentrations than those from the control site, thus demonstrating that blood arsenic is potentially a good biomarker of human arsenic exposure.
Data availability
The datasets generated during this study are available from the authors upon request.
Change history
30 January 2024
A Correction to this paper has been published: https://doi.org/10.1007/s11356-024-32103-9
References
Affum AO, Osae SD, Nyarko BJB, Afful S, Fianko JR, Akiti TT, Adomako D, Acquaah SO, Dorleku M, Antoh E (2015) Total coliforms, arsenic and cadmium exposure through drinking water in the Western Region of Ghana: application of multivariate statistical technique to groundwater quality. Environ Monit Assess 187:1–23
Agilent Technologies (2010) 'Agilent 7700 Series ICP-MS Brochure. Available at https://www.agilent.com/Library/brochures/5990-4025EN.pdf. Accessed on 8 February 2023
Arcega-Cabrera F, Fargher LF, Oceguera-Vargas I, Noreña-Barroso E, Yánez-Estrada L, Alvarado J, González L, Moo-Puc R, Pérez-Herrera N, Quesadas-Rojas M, Pérez-Medina S (2017) Water consumption as source of arsenic, chromium, and mercury in children living in rural Yucatan, Mexico: blood and urine levels. Bull Environ Contam Toxicol 99:452–459
Arcega-Cabrera F, Fargher L, Quesadas-Rojas M, Moo-Puc R, Oceguera-Vargas I, Noreña-Barroso E, Yáñez-Estrada L, Alvarado J, González L, Pérez-Herrera N (2018) Environmental exposure of children to toxic trace elements (Hg, Cr, As) in an urban area of Yucatan, Mexico: water, blood, and urine levels. Bull Environ Contam Toxicol 100:620–626
Arikan I, Namdar ND, Kahraman C, Dagci M, Ece E (2015) Assessment of arsenic levels in body samples and chronic exposure in people using water with a high concentration of arsenic: a field study in Kutahya. Asian Pac J Cancer Prev 16:3183–3188
ATSDR (2002). Agency for toxic substances and disease registry, United States Department of Health and Human Services. Available at https://www.atsdr.cdc.gov/HEC/CSEM/arsenic/docs/arsenic.pdf. Accessed 29 Jul 2020
Bibi M, Hashmi MZ, Malik RN (2015) ’Human exposure to arsenic in groundwater from Lahore district, Pakistan. Environ Toxicol Pharmacol 39:42–52
Bortey-Sam N, Nakayama SMM, Ikenaka Y, Akoto O, Baidoo E, Mizukawa H, Ishizuka M (2015) ’Health risk assessment of heavy metals and metalloid in drinking water from communities near gold mines in Tarkwa, Ghana. Environ Monit Assess 187:1–12
CEPA (2007) CEPA Canadian soil quality guidelines for the protection of environmental and human health. National Guidelines and Standards Office, Quebec
Chakraborti D, Mukherjee SC, Pati S, Sengupta MK, Rahman MM, Chowdhury UK, Lodh D, Chanda CR, Chakraborti AK, Basu GK (2003) Arsenic groundwater contamination in Middle Ganga Plain, Bihar, India: a future danger? Environ Health Perspect 111:1194–1201
Chen C-J (2014) Health hazards and mitigation of chronic poisoning from arsenic in drinking water: Taiwan experiences. Rev Environ Health 29:13–19
Chen Yu, Ahsan H (2004) Cancer burden from arsenic in drinking water in Bangladesh. Am J Public Health 94:741–744
Chen Yu, Graziano JH, Parvez F, Liu M, Slavkovich V, Kalra T, Argos M, Islam T, Ahmed A, Rakibuz-Zaman M (2011) Arsenic exposure from drinking water and mortality from cardiovascular disease in Bangladesh: prospective cohort study. BMJ 342:d2431
Cheng P-S, Weng S-F, Chiang C-H, Lai F-J (2016) Relationship between arsenic-containing drinking water and skin cancers in the arseniasis endemic areas in Taiwan. J Dermatol 43:181–186
D’Ippoliti D, Santelli E, De Sario M, Scortichini M, Davoli M, Michelozzi P (2015) Arsenic in drinking water and mortality for cancer and chronic diseases in central Italy, 1990–2010. PLoS ONE 10:e0138182
Dong Ju, Su SY (2009) The association between arsenic and children’s intelligence: a meta-analysis. Biol Trace Elem Res 129:88–93
Ferreccio C, Smith AH, Durán V, Barlaro T, Benítez H, Valdés R, Aguirre JJ, Moore LE, Acevedo J, Vásquez MI (2013) Case-control study of arsenic in drinking water and kidney cancer in uniquely exposed Northern Chile. Am J Epidemiol 178:813–818
Flanagan SV, Johnston RB, Zheng Y (2012) Arsenic in tube well water in Bangladesh: health and economic impacts and implications for arsenic mitigation. Bull World Health Organ 90:839–846
Gebeyehu HR, Bayissa LD (2020) Levels of heavy metals in soil and vegetables and associated health risks in Mojo area, Ethiopia. PLoS ONE 15:e0227883
Genthe B, Kapwata T, Le Roux W, Chamier J, Wright CY (2018) The reach of human health risks associated with metals/metalloids in water and vegetables along a contaminated river catchment: South Africa and Mozambique. Chemosphere 199:1–9
Giyani Greater (2017). Greater Giyani local municipality led strategy Available at http://www.greatergiyani.gov.za/docs/led/GREATER%20GIYANI%20LM%20LEDFinal.pdf. Accessed 12 Apr 2020
Hall M, Chen Yu, Ahsan H, Slavkovich V, Van Geen A, Parvez F, Graziano J (2006) Blood arsenic as a biomarker of arsenic exposure: results from a prospective study. Toxicology 225:225–233
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381
IARC (2004) ’Arsenic in drinking-water. Int Agency Res Cancer (IARC)—Summ Eval 84(2004):39 Available at http://www.inchem.org/documents/iarc/vol84/84-01-arsenic.html Accessed on 6 April 2020
IARC (2012) The International Agency for Research on Cancer: Working Group on the evaluation of carcinogenic risks to humans: arsenic, metals, fibres and dusts. Available from: https://www.ncbi.nlm.nih.gov/books/NBK304375/. Accessed 29 May 2020
Iyer S, Sengupta C, Velumani A (2016) Blood arsenic: Pan-India prevalence. Clin Chim Acta 455:99–101
Kamunda C, Mathuthu M, Madhuku M (2016) ’Health risk assessment of heavy metals in soils from Witwatersrand Gold Mining Basin, South Africa. Int J Environ Res Public Health 13:663
Kapwata T, Mathee A, Sweijd N, Minakawa N, Mogotsi M, Kunene Z, Wright CY (2020) Spatial assessment of heavy metals contamination in household garden soils in rural Limpopo Province, South Africa. Environ Geochem Health 42:4181–4191
Karami H, Shariatifar N, Khaniki GJ, Nazmara S, Arabameri M, Alimohammadi M (2021) Measuring quantities of trace elements and probabilistic health risk assessment in fruit juices (traditional and commercial) marketed in Iran. Int J Environ Anal Chem 1–15
Katiyar S, Singh D (2014) Prevalence of arsenic exposure in population of Ballia district from drinking water and its correlation with blood arsenic level. J Environ Biol 35:589
Kiani A, Arabameri M, Moazzen M, Shariatifar N, Aeenehvand S, Khaniki GJ, Abdel-Wahhab M, Shahsavari S (2022) Probabilistic health risk assessment of trace elements in baby food and milk powder using ICP-OES method. Biol Trace Elem Res 200:2486–2497
Kootbodien T, Mathee A, Naicker N, Moodley N (2012) ’Heavy metal contamination in a school vegetable garden in Johannesburg. SAMJ S Afr Med J 102:226–27
Kumar A, Ali M, Ranjit Kumar Md, Rahman AS, Chayal NK, Sagar V, Kumari R, Parween S, Kumar R (2020) ’High arsenic concentration in blood samples of people of village Gyaspur Mahaji, Patna Bihar drinking arsenic-contaminated water. Exposure Health 12:131–140
Kumar A, Ali M, Kumar R, Kumar M, Sagar P, Pandey RK, Akhouri V, Kumar V, Anand G, Niraj PK (2021) Arsenic exposure in Indo Gangetic plains of Bihar causing increased cancer risk. Sci Rep 11:1–16
Lamm SH, Robbins SA, Zhou C, Jun Lu, Chen R, Feinleib M (2013) Bladder/lung cancer mortality in Blackfoot-disease (BFD)-endemic area villages with low (< 150 μg/L) well water arsenic levels–an exploration of the dose–response Poisson analysis. Regul Toxicol Pharmacol 65:147–156
Li S, Wang J, Niu L, Wu Y, Teng T, and Xu J (2021) Analysis of arsenic exposure and its influencing factors in industrial areas. Chin J Endemiol 18–23
Liaw J, Marshall G, Yuan Y, Ferreccio C, Steinmaus C, Smith AH (2008) Increased childhood liver cancer mortality and arsenic in drinking water in northern Chile. Cancer Epidemiol Prev Biomarkers 17:1982–1987
Liber AC, Warner KE (2018) Has underreporting of cigarette consumption changed over time? Estimates derived from US national health surveillance systems between 1965 and 2015. Am J Epidemiol 187:113–119
Luvhimbi N, Tshitangano TG, Mabunda JT, Olaniyi FC, Edokpayi JN (2022) ’Water quality assessment and evaluation of human health risk of drinking water from source to point of use at Thulamela municipality, Limpopo Province. Sci Rep 12:1–17
Madilonga RT, Edokpayi JN, Volenzo ET, Durowoju OS, Odiyo JO (2021) ’Water quality assessment and evaluation of human health risk in Mutangwi River, Limpopo Province, South Africa. Int J Environ Res Public Health 18:6765
Madrid E, Gonzalez-Miranda I, Muñoz S, Rejas C, Cardemil F, Martinez F, Cortes JP, Berasaluce M, Párraga M (2022) Arsenic concentration in topsoil of central Chile is associated with aberrant methylation of P53 gene in human blood cells: a cross-sectional study. Environ Sci Pollut Res Int 29:48250–48259
Majuru B, Jagals P, Hunter PR (2012) Assessing rural small community water supply in Limpopo, South Africa: water service benchmarks and reliability. Sci Total Environ 435:479–486
Mathee A, Kootbodien T, Kapwata T, Naicker N (2018) Concentrations of arsenic and lead in residential garden soil from four Johannesburg neighborhoods. Environ Res 167:524–527
Mathee A, Haman T, Nkosi V, Naicker N, Street R (2022) Elevated soil and blood lead levels with increasing residential proximity to a mine tailings facility in Soweto, South Africa. Sci Total Environ 851:158158
McClintock TR, Chen Yu, Bundschuh J, Oliver JT, Navoni J, Olmos V, Lepori EV, Ahsan H, Parvez F (2012) Arsenic exposure in Latin America: biomarkers, risk assessments and related health effects. Sci Total Environ 429:76–91
Mochizuki H, Phyu KP, Aung MN, Zin PW, Yano Y, Myint MZ, Thit WM, Yamamoto Y, Hishikawa Y, Thant KZ (2019) Peripheral neuropathy induced by drinking water contaminated with low-dose arsenic in Myanmar. Environ Health Prev Med 24:1–10
Monrad M, Ersbøll AK, Sørensen M, Baastrup R, Hansen B, Gammelmark A, Tjønneland A, Overvad K, Raaschou-Nielsen O (2017) Low-level arsenic in drinking water and risk of incident myocardial infarction: a cohort study. Environ Res 154:318–324
Moon KA, Oberoi S, Aaron Barchowsky Yu, Chen EG, Nachman KE, Rahman M, Sohel N, D’Ippoliti D, Wade TJ (2017) A dose-response meta-analysis of chronic arsenic exposure and incident cardiovascular disease. Int J Epidemiol 46:1924–1939
Mudzielwana R, Gitari MW, Akinyemi SA, Talabi AO, Ndungu P (2020) Hydrogeochemical characteristics of arsenic rich groundwater in Greater Giyani Municipality, Limpopo Province, South Africa. Groundw Sustain Dev 10:100336
Mukherjee SC, Rahman MM, Chowdhury UK, Sengupta MK, Lodh D, Chanda CR, Saha KC, Chakraborti D (2003) ’Neuropathy in arsenic toxicity from groundwater arsenic contamination in West Bengal, India. J Environ Sci Health Part A 38:165–183
Munyangane P, Mouri H, Kramers J (2017) Assessment of some potential harmful trace elements (PHTEs) in the borehole water of Greater Giyani, Limpopo Province, South Africa: possible implications for human health. Environ Geochem Health 39:1201–1219
Nahar MN, Inaoka T, Fujimura M (2014) A consecutive study on arsenic exposure and intelligence quotient (IQ) of children in Bangladesh. Environ Health Prev Med 19:194–199
NRC (1999) National Research Council (US) Subcommittee on Arsenic in Drinking Water. Arsenic in Drinking Water. Washington (DC): National Academies Press (US); 1999. 6, Biomarkers of Arsenic Exposure. Available from: https://www.ncbi.nlm.nih.gov/books/NBK230898/.
Naujokas MF, Anderson B, Habibul Ahsan H, Aposhian V, Graziano JH, Thompson C, Suk WA (2013) The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect 121:295–302
Ngole-Jeme VM, Fantke P (2017) Ecological and human health risks associated with abandoned gold mine tailings contaminated soil. PLoS ONE 12:e0172517
Nguyen KH, Operario DJ, Nyathi ME, Hill CL, Smith JA, Guerrant RL, Samie A, Dillingham RA, Bessong PO, Rogawski ET, McQuade. (2021) ’Seasonality of drinking water sources and the impact of drinking water source on enteric infections among children in Limpopo, South Africa. Int J Hygiene Environ Health 231:113640
Niton XRF (2015) Thermo Scientific Niton XL2 100G XRF Analyzer, Availabe at https://www.sirioanalitix.com/sites/default/files/XL2.pdf. Accessed on 24 May 2019
Nordberg, GF, Fowler BA, Nordberg GF and Fowler BA (2019) “Examples of risk assessments of human metal exposures and the need for mode of action (MOA), toxicokinetic-toxicodynamic (TKTD) modeling, and adverse outcome pathways (AOPs)”, Risk Assessment for Human Metal Exposures, Elsevier, pp. 227–310
NRC (2014) NRC (National Research Council): Critical Aspects of EPA’s IRIS Assessment of Inorganic Arsenic: Interim Report. National Academies Press, Washington
NRC (1999). National Research Council (US) Subcommittee on Arsenic in Drinking Water, Arsenic in drinking water, Biomarkers of Arsenic Exposure, National Academies Press US: Washington, p 6. Available from: https://www.ncbi.nlm.nih.gov/books/NBK230898/
Patel KS, Ambade B, Jaiswal NK, Sharma R, Patel RK, Blazhev B, Lautent M, Bhattacharya P (2012) “Arsenic and other heavy metal contamination in central India.” In Understanding the geological and medical interface of arsenic—as 2012, In: Proceedings of the 4th international congress on arsenic in the environment, pp. 22–27
Rahbar MH, Samms-Vaughan M, Ardjomand-Hessabi M, Loveland KA, Dickerson AS, Chen Z, Bressler J, Shakespeare-Pellington S, Grove ML, Bloom K, Wirth J, Pearson DA, Boerwinkle E (2012) The role of drinking water sources, consumption of vegetables and seafood in relation to blood arsenic concentrations of Jamaican children with and without Autism Spectrum Disorders. Sci Total Environ 433:362–370
Rahbar MH, Samms-Vaughan M, Zhao Y, Saroukhani S, Zaman SF, Bressler J, Hessabi M, Grove ML, Shakspeare-Pellington S, Loveland KA (2022) Additive and interactive associations of environmental and sociodemographic factors with the genotypes of three glutathione S-transferase genes in relation to the blood arsenic concentrations of children in Jamaica. Int J Environ Res Public Health 19:466
Razo D, Luz M, García-Vargas GG, Valenzuela OL, Castellanos EH, Sánchez-Peña LC, Currier JM, Drobná Z, Loomis D, Stýblo M (2011) Exposure to arsenic in drinking water is associated with increased prevalence of diabetes: a cross-sectional study in the Zimapán and Lagunera regions in Mexico. Environ Health 10:1–11
Rodrigues EG, Bellinger DC, Linda Valeri Md, Hasan OSI, Quamruzzaman Q, Golam M, Kile ML, Christiani DC, Wright RO, Mazumdar M (2016) Neurodevelopmental outcomes among 2- to 3-year-old children in Bangladesh with elevated blood lead and exposure to arsenic and manganese in drinking water. Environ Health 15:44
Roh T, Lynch CF, Weyer P, Wang K, Kelly KM, Ludewig G (2017) Low-level arsenic exposure from drinking water is associated with prostate cancer in Iowa. Environ Res 159:338–343
Röllin HB, Channa K, Olutola BG, Odland JØ (2017) Evaluation of in utero exposure to arsenic in South Africa. Sci Total Environ 575:338–346
Rosas-Castor JM, Guzmán-Mar JL, Hernández-Ramírez A, Garza-GonzálezHinojosa-Reyes MTL (2014) Arsenic accumulation in maize crop (Zea mays): a review. Sci Total Environ 488:176–187
Saghafi M, Shariatifar N, Alizadeh Sani M, Dogaheh MA, Khaniki GJ, Arabameri M (2021). Analysis and probabilistic health risk assessment of some trace elements contamination and sulphur dioxide residual in raisins. Int J Environ Anal Chem 1–15
SANS (2015). South African National Standard of Drinking Water Available at: https://store.sabs.co.za/pdfpreview.php?hash=1cc8f679f228941e595094a46f19ef8b46434bcb&preview=yes. Accessed 29 May 2020
Sekhar KC, Chary NS, Kamala CT, Venkateswara Rao J, Balaram V, Anjaneyulu Y (2003) Risk assessment and pathway study of arsenic in industrially contaminated sites of Hyderabad: a case study. Environ Int 29:601–611
Singh PK, Jain P, Singh N, Singh L, Kumar C, YadavSubramanianSingh ASVS (2022) Social desirability and under-reporting of smokeless tobacco use among reproductive age women: evidence from National Family Health Survey. SSM-Popul Health 19:101257
Smith AH, Lingas EO, Rahman M (2000) Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bull World Health Organ 78:1093–1103
StataCorp (2017) StataCorp 2001. Statistical Software: Release 15.0. Stata Corporation, College Station
Steinmaus CM, Ferreccio C, Romo JA, Yuan Y, Cortes S, Marshall G, Moore LE, Balmes JR, Liaw J, Golden T (2013) Drinking water arsenic in northern Chile: high cancer risks 40 years after exposure cessation. Cancer Epidemiol Prev Biomarkers 22:623–630
Tolins M, Ruchirawat M, Landrigan P (2014) The developmental neurotoxicity of arsenic: cognitive and behavioral consequences of early life exposure. Ann Glob Health 80:303–314
Tsai S-Y, Chou H-Y, The H-W, Chen C-M, Chen C-J (2003) The effects of chronic arsenic exposure from drinking water on the neurobehavioral development in adolescence. Neurotoxicology 24:747–753
Tseng C-H (2008) Cardiovascular disease in arsenic-exposed subjects living in the arseniasis-hyperendemic areas in Taiwan. Atherosclerosis 199:12–18
Tseng HP, Wang YH, Wu MM, The HW, Chiou HY, Chen CJ (2006) Association between chronic exposure to arsenic and slow nerve conduction velocity among adolescents in Taiwan. J Health Popul Nutr 24:182–189
USEPA (2014) United States Environmental Protection Agency (USEPA): Soil Sampling Operating Procedure. Available at https://www.epa.gov/sites/production/files/2015-06/documents/Soil-Sampling.pdf. Accessed 9 Apr 2019
USEPA (2016) United States Environmental Protection Agency (USEPA): Quick guide to drinking water sample collection, second edition. Available at https://www.epa.gov/sites/production/files/2015-11/documents/drinking_water_sample_collection.pdf. Accessed 13 May 2020
Wang S, Wenyong Wu, Liu F (2019) Assessment of the human health risks of heavy metals in nine typical areas. Environ Sci Pollut Res 26:12311–12323
Wasserman GA, Liu X, LoIacono NJ, Kline J, Factor-Litvak P, van Geen A, Mey JL, Levy D, Abramson R, Schwartz A (2014) A cross-sectional study of well water arsenic and child IQ in Maine schoolchildren. Environ Health 13:1–10
WHO (2009) 'WHO Study Group on Tobacco Product Regulation. Report on the scientific basis of tobacco product regulation: third report of a WHO Study Group', World Health Organization technical report series, p 1
WHO (2017) 'World Health Organization Guidelines for drinking-water quality: first addendum to the fourth edition. Available at https://www.who.int/publications/i/item/9789241549950. Accessed on 15 February 2022
WHO (2018) 'World Health Organization (WHO) Arsenic Fact Sheet. Available at https://www.who.int/news-room/fact-sheets/detail/arsenic. Accessed on 5 July 2022
Yang K, Chen C, Brockman J, Shikany JM, He Ka (2021) Low-and moderate-levels of arsenic exposure in young adulthood and incidence of chronic kidney disease: findings from the CARDIA Trace Element Study. J Trace Elem Med Biol 63:126657
Zhang Z (2016) All-source exposures to arsenic for residents living near historic gold mine areas in northwest Romania
Zierold KM, Knobeloch L, Anderson H (2004) Prevalence of chronic diseases in adults exposed to arsenic-contaminated drinking water. Am J Public Health 94:1936–1937
Funding
Open access funding provided by South African Medical Research Council. All authors receive research funding from the SAMRC.
Author information
Authors and Affiliations
Contributions
Conceptualization: all authors; data collection: TK; formal analysis: TK, TR; writing—original draft: TK; writing—review and editing: all authors.
Corresponding author
Ethics declarations
Ethical approval
Research ethics clearance for the study was granted by the South African Medical Research Council Ethics Committee (Certificate No: EC022-7/2020).
Consent to participate
Informed consent to participate in the study was obtained from all recruited individuals.
Consent for publication
Consent to publish anonymized data/results was obtained from all participants included in the study.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The correct presentation of affiliation 4 is modified in the original published proof.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kapwata, T., Wright, C.Y., Reddy, T. et al. Relations between personal exposure to elevated concentrations of arsenic in water and soil and blood arsenic levels amongst people living in rural areas in Limpopo, South Africa. Environ Sci Pollut Res 30, 65204–65216 (2023). https://doi.org/10.1007/s11356-023-26813-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26813-9