Abstract
We assessed the association between long-term joint exposure to ambient air pollutants and the risk of laryngeal cancer and whether this risk was modified by genetic susceptibility. We used a multivariable Cox proportional hazards regression model to analyze data from UK Biobank to determine the relationship between long-term exposure to air pollutants–nitric oxide (NO), nitrogen dioxide (NO2), and 2.5-µm and 10-µm particulate matter (PM2.5 and PM10) and the risk of laryngeal cancer. In multivariable-adjusted models, in model 3 and compared with the participants with lower quintile scores for air pollution, the participants with the highest quintile scores for air pollution had a higher laryngeal cancer risk. The observed association was more pronounced among the participants who were female, were smokers, had a systolic blood pressure equal to or greater than 120 mmHg, and had diabetes. Compared with the participants with a low GRS and the lowest quintile score for air pollution exposure, those with an intermediate GRS and the highest quintile score for air pollution exposure had a higher risk of laryngeal cancer. Long-term exposure to NO2, NO, or PM2.5, individually or jointly, was associated with a risk of incident laryngeal cancer, especially in the participants with an intermediate GRS.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Laryngeal cancer is one of the most common and invasive malignant tumors of the head and neck, and has an annual incidence worldwide of approximately 2.4% (Christensen et al. 2013). The pathogenesis of laryngeal cancer has not been fully determined but is regarded as related to many factors, such as long-term excessive smoking, heavy drinking, viral infection, exposure to gaseous chemicals or severe air pollution, and/or long-term exposure to radionuclides (Edefonti et al. 2010).
This study focuses on the effect of air pollution on laryngeal cancer, given that air pollution—especially that due to the main atmospheric pollutants, such as nitrogen dioxide (NO2), nitric oxide (NO), and 2.5-µm and 10-µm particulate matters (PM2.5 and PM10)—is recognized as having adverse effects on human health (Khaniabadi et al. 2017). Exposure to air pollution is significantly related to an increased risk of lung cancer, bladder cancer, kidney cancer, and urinary tract cancer (Zare Sakhvidi et al. 2020); however, little is known about the effect of air pollution on the risk of laryngeal cancer in the general population. Genetic factors are also closely related to the pathogenesis of laryngeal cancer (Stanaway et al. 2018) and may interact with many environmental factors contributing to its pathogenesis (Schraufnagel et al. 2019). However, it remains unknown whether a genetic predisposition alters the association between combined exposure to various air pollutants and the risk of laryngeal cancer.
We used data from UK Biobank (UKB) to comprehensively investigate the association between PM2.5, PM10, NO2, and NO exposure, an air pollution score based on these pollutants, and the risk of laryngeal cancer among middle- and older-aged adults. Moreover, we explored whether the relationship between air pollution exposure and laryngeal cancer risk was affected by genetic susceptibility.
Methods
Study population and data sources
UKB is a large prospective cohort with more than 500,000 participants recruited during 2006–2010. At recruitment, the detailed information of each participant (such as their body measurements, health status, medical diagnosis, family history, and lifestyle behaviors) was collected using standard sociodemographic questionnaires. The UKB study was approved by the Northwest Research Ethics Committee (ref: 11/NW/0382), and all of its participants have provided written informed consent. The current study used UKB resources (application number 78619).
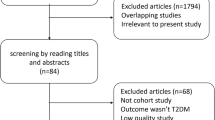
The initial sample of the current study was 502,490. We excluded participants with laryngeal cancer at baseline or who were missing data on exposure to any air pollutants. The final analytic sample comprised 418,914 participants. The detailed flow diagram used for participant inclusion is shown in supplementary Fig. 1.
Definition of the genetic risk score
Detailed information about genotyping, data imputation, and quality control in the UKB study has been described previously (Bycroft et al. 2018). We used a weighted method to calculate a genetic risk score (GRS) for laryngeal cancer using eight single-nucleotide polymorphisms (SNPs) that have been identified in genome-wide association studies (Supplementary Table 1) (Kampa and Castanas 2008). That is, the score of each SNP was determined by the number of risk alleles carried by an individual (0, l, or 2), and then the individual’s GRS for laryngeal cancer was calculated by the following weighted method: GRS = (β1 × SNP1 + β2 × SNP2 + ... + β8 × SNP8) × (8/sum of the β coefficients). We classified participants into tertiles based on their GRS: a low tertile (tertile 1), an intermediate tertile (tertile 2), and a high tertile (tertile 3).
Definition of outcome
Laryngeal cancer was defined according to code C32 in the International Statistical Classification of Diseases and Related Health Problems (10th Revision). Participants were followed from enrolment until the time of laryngeal cancer diagnosis or censoring, with the latter defined as the time of death, withdrawal from the study, or the end of follow-up, whichever came first.
Definition of the air pollution score
The annual average concentrations of NO2, NO, PM10, and PM2.5 were estimated using a land-use regression model developed from the European Study of Cohorts for Air Pollution Effects project and linked to participants’ residential addresses given at baseline (Cornean et al. 2019). The land-use regression model was calculated according to the data from European Study of Cohorts for Air Pollution Effects performed between January 26, 2010 and January 18, 2011. The spatial visualization mapping of geographic location information has been previously published [Obesity and the relation between joint exposure to ambient air pollutants and incident type 2 diabetes: a cohort study in UK Biobank] (Li et al. 2021).
We created an air pollution score to capture joint exposure to various air pollutants, which was calculated as follows: (β PM2.5 × PM2.5 + β PM10 × PM10 + β NO2 × NO2 + β NO × NO) × (4/sum of the β coefficients). The higher the air pollution score, the higher the degree of exposure to ambient air pollution. The β coefficient was obtained from the final model with each air pollutant considered (i.e., one at a time) as the independent variable.
Definition of covariates
The baseline questionnaire collected data on the participants’ age, sex (male/female), ethnicity (White/non-White), work (employed/unemployed), education level (0–7 years, 8–10 years, 11–15 years, or 16 or more years), income level (< 18,000; 18,000–52,000; 52,000–100,000; > 100,000 pounds, £), smoking status, and alcohol consumption status (current, previous, or never). In addition, body-mass index (BMI) was calculated by dividing the weight (in kg) by the square of the height (m2), using the height and weight data at baseline. Systolic blood pressure (SBP) was the average of two automatic or manual measurements at baseline, as measured by professionally trained nurses. Hypertension and diabetes were diagnosed based on participants’ self-reported and medical record data. Metabolic equivalent task (MET) was defined as minutes per week for all activities including walking, moderate, and vigorous activity.
Statistical analysis
Continuous variables are expressed as means ± standard deviations, and categorical variables are presented as percentages. Participants were stratified into quintiles based on air pollution exposure, with the first quintile as the reference.
We devised an air pollution score to assess joint exposure to NO2, NO, PM10, and PM2.5, which is determined by summing each pollutant concentration weighted by its regression coefficients with laryngeal cancer obtained from single-pollutant models. A multivariable Cox proportional risk model was used to estimate the risk ratio (hazard ratio (HR)) and 95% confidence interval (CI) for the relationship between each pollutant or air pollution score and laryngeal cancer. Model 1 was adjusted for age, sex, and ethnicity (White/non-White); model 2 was additionally adjusted for work (employed/unemployed), education (0–7 years, 8–10 years, 11–15 years, or 16 or more years), income (< 18,000; 18,000–52,000; 52,000–100,000; > 100,000), physical activity (continuous), alcohol-drinking status (never/past/current), and smoking status (never/past/current); model 3 was additionally adjusted for BMI (continuous), SBP (continuous), hypertension at baseline (yes/no), and diabetes at baseline (yes/no). Trends were estimated by regarding quintiles as continuous variables (i.e., 1–5), and restricted cubic splines were used to examine the relationship between each pollutant, air pollution scores, and the risk of laryngeal cancer (Licitra et al. 2003).
We also calculated the weighted GRS of laryngeal cancer to evaluate whether the genetic susceptibility of laryngeal cancer modified the association between the air pollution score and laryngeal cancer incidence. Thus, we tested the gene–air pollution interaction by adding variable cross-product terms of air pollution score with GRSs for laryngeal cancer into the models. Then, the presence of effect modification was examined with respect to sex (men vs. women), age (< 60 vs. ≥ 60 years), BMI (< 25 kg/m2 vs. ≥ 25 kg/m2), smoking status (no vs. yes), alcohol consumption status (no vs. yes), diabetes (no vs. yes), and SBP (< 120 mmHg vs. ≥ 120 mmHg). Sensitivity analyses were also conducted by excluding participants who had (Christensen et al. 2013) developed laryngeal cancer within the past 2 years, or (Edefonti et al. 2010) lived at their current address for fewer than 5 years.
All analyses were performed using SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA) and R software (version 3.5.1). All p-values for the tests were two sided, and P-values < 0.05 were considered as statistically significant.
Result
The baseline characteristics of the participants sorted according to the quintile of the air pollution score are shown in Table 1. The means ± standard deviations of air pollution score in the first to fifth quintiles were 44.7 ± 3.1, 52.1 ± 1.5, 56.6 ± 1.2, 61.1 ± 1.5, and 71.6 ± 8.2, respectively. Compared with participants in the lowest quintile for air pollution score, those in the highest quintile for air pollution score were younger, more likely to be male, non-White, current smokers, employed, have a lower education (0–7), have a low income (< 18,000), have hypertension, and have diabetes. The air pollutants were highly correlated with each other, and their Pearson correlation coefficients are shown in Supplementary Table 2.
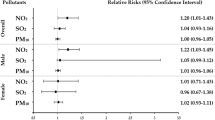
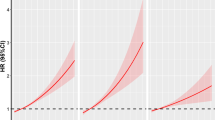
In the follow-up (a median follow-up period of 12.5 years), 1200 cases of laryngeal cancer occurred in 418,914 participants. The relationship between each air pollutant and the risk of laryngeal cancer is shown in Table 2. In model 3, we found that compared with the participants in the lowest quintile for exposure to NO2, NO, and PM2.5, the HRs of participants exposed to the highest quintile of the air pollutants NO2, NO, and PM2.5 were 1.7 (95% CI: 1.13–2.54), 2.13 (95% CI: 1.39–3.27), and 1.85 (95% CI: 1.20–2.85), respectively. A spline analysis showed a J-shaped relationship between exposure to NO2 and NO and the risk of laryngeal cancer (all p-nonlinearity < 0.001), but a linear association between exposure to PM2.5 and the risk of laryngeal cancer (p-linearity < 0.001) (Fig. 1). The associations between air pollution scores and the risk of developing laryngeal cancer are shown in Table 3. Compared with air pollution scores in the lower quintiles, air pollution scores in the highest quintile were found to be significantly associated with a higher risk of laryngeal cancer. In model 3 and compared with participants with an air pollution score in the lowest quintile, participants with an air pollution score in the highest quintile had a higher risk of laryngeal cancer [HR: 1.99 (95% CI: 1.30–3.03, P for trend = 0.001]. The spline analysis showed an S-shaped relationship between air pollution score and the risk of laryngeal cancer (p-nonlinearity < 0.001) (Fig. 1).
Associations of air pollutants (individually or jointly) with risk of laryngeal cancer. Cox proportional hazards models were used for analysis, including restricted cubic spline term for each air pollutant, with adjustment for age, sex, ethnicity (White/non-White), work (employed/unemployed), education (0–7 years, 8–10 years, 11–15 years, or 16– years), income (< 18,000/18,000–52,000/52,000–100,000/ > 100,000), physical activity (continuous), drinking status (never/past/current), smoking status (never/past/current), BMI (continuous), SBP (continuous), hypertension at baseline (yes/no), and diabetes at baseline (yes/no)
Stratified analyses revealed that sex, BMI, SBP, smoking status, drinking status, and diabetes significantly modified the association between air pollution score and the risk of laryngeal cancer (all p interactions < 0.01), with an overall HR of 0.85 (95% CI: 0.74–0.97), and individual HRs as follows: for participants who were female (HR = 3.73, 95% CI: 1.86–7.49), had an SBP equal to or greater than 120 mmHg (HR = 1.97, 95% CI: 1.61–2.41), were smokers (HR = 2.37, 95% CI: 1.64–3.42), or had diabetes (HR = 5.36, 95% CI: 2.10–13.69).
We also assessed the effect of the joint association between air pollution score and GRS for laryngeal cancer on the risk of laryngeal cancer (Table 4). Compared with participants with a low GRS and an air pollution score in the lowest quintile, those with an intermediate GRS and an air pollution score in the highest quintile had a higher risk of laryngeal cancer (HR = 2.64 (95% CI: 1.82–3.81); p-interaction = 0.001). Moreover, those with an intermediate GRS and NO2, NO, and PM2.5 scores in the highest quintile had the highest risk of laryngeal cancer.
We next performed a sensitivity analysis by excluding PM10 from the air pollution scores; after doing so, the association between air pollution scores for NO2, NO, and PM2.5 in the highest quintile and laryngeal cancer risk remained statistically significant (Supplementary Table 3). We also performed a sensitivity analysis by excluding participants who had developed laryngeal cancer within the past 2 years; after doing so, the association between air pollution scores in the highest quintile and laryngeal cancer risk remained statistically significant (Supplementary Table 4). Furthermore, we performed a sensitivity analysis by excluding participants who had lived in their place of residence for fewer than 5 years; after doing so, the association between air pollution scores in the highest quintile and the risk of laryngeal cancer remained statistically significant (Supplementary Table 5).
Discussion
In this large prospective cohort study, we found that an air pollution score indicating high individual or combined long-term exposure to various ambient air pollutants, namely, PM2.5, NO2, and NO, was significantly associated (after multivariable adjustment) with an increased risk of laryngeal cancer. Additionally, we identified significant interactions between air pollutants (PM2.5, PM10, NO2, and NO) and participants with an intermediate GRS; this interaction showed that such participants were more likely to develop laryngeal cancer than participants with a low GRS when exposed to high levels of air pollution.
To date, no studies have evaluated the association of different air pollutants with the risk of laryngeal cancer; instead, most studies have focused on the association of air pollutants with the risk of respiratory diseases, such as lung cancer. For example, a large meta-analysis by Yang et al. (Wei et al. 2014) of 21 cohort studies showed that for every 10 μg/m3 increase in the concentration of PM2.5, there was a 7.23% increase in the risk of lung cancer mortality or morbidity. Similarly, a cohort study of the effects of air pollution on nearly 313,000 people in Europe showed that long-term exposure to PM10 and PM2.5 was associated with an increased risk of lung cancer, particularly lung adenocarcinoma, with HRs of 1.22 for each 10 μg/m3 increase in PM10 and 1.18 for each 5 μg/m3 increase in PM2.5 (Shete et al. 2020).
In the current study, we found a significant joint association of air pollution and GRS with the risk of laryngeal cancer. That is, the risk of laryngeal cancer associated with air pollution scores in higher quintiles was enhanced by an intermediate GRS, suggesting that the interaction of these two factors led to a higher risk of laryngeal cancer than the sum of the risk associated with each factor alone. This shows that air pollution scores may more comprehensively reflect exposure to various air pollutants than single measure, and that people with an intermediate GRS for laryngeal cancer should be more concerned about air pollution than people with a low GRS for laryngeal cancer.
Moreover, we determined that PM2.5 was associated with a risk of laryngeal cancer. This is presumably due to changes in people’s employment patterns and lifestyle, and increasing atmospheric concentrations of PM2.5. Compared with coarse airborne PM (e.g., PM10), fine PM (e.g., PM2.5) is smaller, has a larger surface area, is more reactive, and more easily combines with toxic and harmful substances, such as heavy metal ions and microorganisms (Leikauf et al. 2020). Moreover, the low mass of fine PM particles means that they have a long residence time and long transport distance in the atmosphere, and so have a greater impact than coarse PM on human health and the quality of the air environment.
Furthermore, we found that the association between air pollution score and the risk of laryngeal cancer was significantly modified by sex, SBP, smoking status, and diabetes, consistent with the fact that lifestyle factors are recognized as the main factors affecting the occurrence of laryngeal cancer. For example, most scholars believe that smoking is the main risk factor for head and neck malignancies, such as laryngeal cancer (Eeftens et al. 2012). This may be because tobacco smoke contains many carcinogenic precursors (i.e., polycyclic aromatic hydrocarbons such as benzo[a]pyrene, heterocyclic amines, and nitroso compounds), which can be transformed into carcinogens in vivo. These carcinogens are electrophiles that can covalently bind to nucleophilic groups in intracellular macromolecules, such as DNA. In such cases, if DNA repair is inefficient or imperfect, dysregulation of gene expression can ensue, culminating in cellular carcinogenesis (Orsini et al. 2012).
The mechanisms by which other air pollutants increase the risk of laryngeal cancer are unclear, but several mechanisms have been proposed. PM-induced oxidative stress is considered one of the most probable mechanisms of toxicity and is thought to involve organic matter in PM being transformed into electrophilic reactive metabolites, which generate intracellular reactive oxygen species (ROS). Moreover, transition-metal ions (e.g., iron, copper, vanadium, and manganese ions) present in PM could induce production of ROS via the Fenton reaction (Desquilbet and Mariotti 2010). Increased intracellular ROS may disrupt the homeostasis of antioxidant functions and processes involving ROS in the cell, leading to increased oxidative stress and adverse effects on cellular and somatic health. This stems from ROS’ ability to directly damage cells by causing lipid peroxidation, oxidative protein modification, and DNA mutation (Yang et al. 2016), resulting in cytotoxicity and genotoxicity.
Air pollution and PM contain polyaromatic hydrocarbons (PAHs), and PAHs present on human cell-absorbed PM2.5 are activated by intracellular biochemical metabolism to produce ROS. A key metabolite of PAHs in the human body is benzo[a]pyrene diol epoxide (BPDE), which can covalently bind to the nucleophilic amino terminus of guanine in DNA to form BPDE–DNA adducts; this results in DNA damage, leading to genetic mutation and cell carcinogenesis, and is associated with tumor formation (Raaschou-Nielsen et al. 2013).
To the best of our knowledge, this was the first prospective study of various air pollutants to use an air pollution score to comprehensively examine the association between PM2.5, PM10, NO2, and NO and the risk of laryngeal cancer among middle- and older-aged adults using data from UKB. The strengths of our study were its large sample, prospective design, and the integration of scores for various air pollutants into an air pollution score. In addition, assessment of the contribution of genetic factors to the association between air pollution and laryngeal cancer allowed us to precisely determine the effects of air pollutants on groups of participants with different GRSs.
However, there are several limitations to this study. First, air pollution is a dynamic and complex mixture of substances containing many anthropogenic and natural pollutants with carcinogenic potential. Thus, UKB’s single measurement of air pollution at baseline did not take into account the changes in air pollution before and after registration. Second, the majority of UKB’s population is from Europe; thus, further investigation is needed to determine whether the observed associations can be applied to people from other regions and ethnic groups. Third, although we combined typical risk factors and potential confounders, residual confounding was unavoidable due to the design of the observational study. Last, the sources of air pollution data did not specify the length of outdoor exposure of the sample population; therefore, the analysis of air pollutants on the incidence of the risk of laryngeal cancer was limited.
In conclusion, we found that long-term exposure to various air pollutants, individually or jointly, was associated with an increased risk of laryngeal cancer, and that the association was more pronounced among participants who were female, were smokers, had an SBP equal to or greater than 120 mmHg, and/or had diabetes. Our findings highlight the importance of comprehensively assessing and minimizing exposures to various air pollutants and implementing good lifestyle management for preventing laryngeal cancer.
Data availability
The datasets generated during the current study are publically available.
References
Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K et al (2018) The UK Biobank resource with deep phenotyping and genomic data. Nature 562(7726):203–209
Christensen A, Kristensen E, Therkildsen MH, Specht L, Reibel J, Homøe P (2013) Ten-year retrospective study of head and neck carcinoma in situ: incidence, treatment, and clinical outcome. Oral Surg Oral Med Oral Pathol Oral Radiol 116(2):174–178. https://doi.org/10.1016/j.oooo.2013.03.002
Cornean CI, Cosgarea M, Cătană A, Mogoantă CA, Necula V, Maniu AA (2019) Do we know enough about the genetic involvement in laryngeal cancer susceptibility and prognostic outcome? Rom J Morphol Embryol 60(2):353–367
Desquilbet L, Mariotti F (2010) Dose-response analyses using restricted cubic spline functions in public health research. Stat Med 29(9):1037–1057. https://doi.org/10.1002/sim.3841
Edefonti V, Bravi F, Garavello W, La Vecchia C, Parpinel M, Franceschi S et al (2010) Nutrient-based dietary patterns and laryngeal cancer: evidence from an exploratory factor analysis. Cancer Epidemiol Biomarkers Prev 19(1):18–27. https://doi.org/10.1158/1055-9965.EPI-09-0900
Eeftens M, Beelen R, de Hoogh K, Bellander T, Cesaroni G, Cirach M et al (2012) Development of land use regression models for PM(2.5), PM(2.5) absorbance, PM(10) and PM(coarse) in 20 European study areas; results of the ESCAPE project. Environ Sci Technol 46(20):11195–205
Kampa M, Castanas E (2008) Human health effects of air pollution. Environ Pollut 151(2):362–367. https://doi.org/10.1016/j.envpol.2007.06.012
Khaniabadi YO, Goudarzi G, Daryanoosh SM, Borgini A, Tittarelli A, De Marco A (2017) Exposure to PM10, NO2, and O3 and impacts on human health. Environ Sci Pollut Res Int 24(3):2781–2789. https://doi.org/10.1007/s11356-016-8038-6
Leikauf GD, Kim SH, Jang AS (2020) Mechanisms of ultrafine particle-induced respiratory health effects. Exp Mol Med 52(3):329–337. https://doi.org/10.1038/s12276-020-0394-0
Licitra L, Bernier J, Grandi C, Locati L, Merlano M, Gatta G et al (2003) Cancer of the larynx. Crit Rev Oncol Hematol 47(1):65–80. https://doi.org/10.1016/S1040-8428(03)00017-9
Li X, Wang M, Song Y et al (2021) Obesity and the relation between joint exposure to ambient air pollutants and incident type 2 diabetes: a cohort study in UK Biobank. PLoS Medicine 18(8):e1003767. https://doi.org/10.1371/journal.pmed.1003767
Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D (2012) Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 175(1):66–73. https://doi.org/10.1093/aje/kwr265
Raaschou-Nielsen O, Andersen ZJ, Beelen R, Samoli E, Stafoggia M, Weinmayr G et al (2013) Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol 14(9):813–822. https://doi.org/10.1016/S1470-2045(13)70279-1
Schraufnagel DE, Balmes JR, De Matteis S, Hoffman B, Kim WJ, Perez-Padilla R et al (2019) Health benefits of air pollution reduction. Ann Am Thorac Soc 16(12):1478–1487. https://doi.org/10.1513/AnnalsATS.201907-538CME
Shete S, Liu H, Wang J, Yu R, Sturgis EM, Li G et al (2020) A genome-wide association study identifies two novel susceptible regions for squamous cell carcinoma of the head and neck. Cancer Res 80(12):2451–2460. https://doi.org/10.1158/0008-5472.CAN-19-2360
Stanaway JD, Afshin A, Gakidou E et al (2018) Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392(10159):1923–1994. https://doi.org/10.1016/S0140-6736(18)32225-6
Wei Q, Yu D, Liu M, Wang M, Zhao M, Liu M et al (2014) Genome-wide association study identifies three susceptibility loci for laryngeal squamous cell carcinoma in the Chinese population. Nat Genet 46(10):1110–1114. https://doi.org/10.1038/ng.3090
Yang WS, Zhao H, Wang X, Deng Q, Fan WY, Wang L (2016) An evidence-based assessment for the association between long-term exposure to outdoor air pollution and the risk of lung cancer. Eur J Cancer Prev 25(3):163–172. https://doi.org/10.1016/j.jhazmat.2010.12.036
ZareSakhvidi MJ, Lequy E, Goldberg M, Jacquemin B (2020) Air pollution exposure and bladder, kidney and urinary tract cancer risk: a systematic review. Environ Pollut 267:115328. https://doi.org/10.1016/j.envpol.2020.115328
Acknowledgements
We thank all authors for their contributions to the article.
Funding
This study was supported by the Zhejiang Provincial Natural Science Foundation of China (Nos. LQ21H130001 and LY19H160014), Ningbo Clinical Research Center for Otolaryngology Head and Neck Disease(No.2022L005), Ningbo Medical and Health Brand Discipline (No. PPXK2018-02); Zhejiang Provincial Medical and Health Science Research Foundation (No. 2021KY307), and Ningbo Public Science Research Foundation (No. 2021S171).
Author information
Authors and Affiliations
Contributions
Zhisen Shen: conceptualization; Yidian Chu: methodology; Chen Lin: data curation; Jiada Wang: writing—original draft preparation; Hongxia Deng: visualization and formal analysis; Zhisen Shen: writing—reviewing and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
The Institutional Review Board of Lihuili Hospital, China, reviewed and approved this study (2022).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, J., Lin, C., Chu, Y. et al. Association between long-term exposure to air pollution and the risk of incident laryngeal cancer: a longitudinal UK Biobank-based study. Environ Sci Pollut Res 30, 58295–58303 (2023). https://doi.org/10.1007/s11356-023-26519-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26519-y