Abstract
The present study, La0.7Sr0.3MO3 (M = Mn-, Co-, and Fe-), perovskite, has successfully been synthesized via co-precipitation and sol–gel auto-combustion. XRD, SEM, and EDX characterized the prepared samples. XRD and SEM showed that the as-prepared La0.7Sr0.3MnO3 and La0.7Sr0.3CoO3 have multiphase. La0.7Sr0.3FeO3, in comparison, is nanosized, has a single-phase perovskite, and has a rather homogenous particle size distribution. Additionally, EDX mapping analysis shows that all pieces are distributed uniformly. According to X-ray diffractometer results, all calcined powders contain 100% LSF, more than 15% perovskite phase of LSC, 47% LSM, and other secondary phases, such as cobalt oxide. Aِt room temperature and magnetic field of ± 20 kG, La0.7Sr0.3MnO3 exhibited weak ferromagnetic behavior in a low magnetic field, whereas diamagnetic behavior was seen in a high magnetic field. La0.7Sr0.3FeO3 samples behave as strong ferromagnetic. On the contrary, the photodegradation of La0.7Sr0.3MnO3 is 99% compared to 75% and 91% for other samples under UVC lights of wavelength = 254 nm. The degradation rate for La0.7Sr0.3MnO3 is 0.179 higher, about 3.25 and 2.23, than the other samples. A La0.7Sr0.3MnO3 nanocomposite performs as a photocatalyst to enhance the efficiency of methylene blue photodegradation. This study boosts good UVC photocatalysts with high efficiency for different kinds of dyes. Hence, the catalyst possessed high stability and efficiency for continuous wastewater treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Manganites as perovskite structures with the general formula RAMO3, R is the cation for rare earth like La3+, Nd3+, and Gd3+; A is an alkaline cation such as Sr2+, Ca2+, and Ba2+; M is a transition metal such as Mn-, Co-, and Fe-; and O−2 is oxygen anion. A-site is occupied at corners by rare earth elements or/and alkaline earth metal, B-site is occupied at the center with transition metals (3d, 4d, and 5d), and the oxygen atom is centered at the face (Smitha and Murugendrappa 2019). Due to its unique electrical and magnetic performance, such as ferromagnetic, colossal magnetoresistance (CMR), metal–insulator transitions (M-I), and paramagnetic transition (FM–PM), perovskite materials have been studied extensively during these last few years (Sfirloaga et al. 2019). The perovskite family has received considerable attention recently because of its interesting features for potential applications, such as data storage, spintronics, sensors, resistant switching elements, transducers, solid oxide fuel cells, moisture sensing actuators, and catalysts (Verma et al. 2019, Lijak et al. 2016, Tran et al. 2019, Abdel-Latif et al. 2011, Hilal et al. 2012).
Alkyl amine has been demonstrated as the ligand for all-inorganic perovskite nanocrystals with well-defined morphology with considerable attention due to their unique size/shape-dependent optical properties (Feiyan et al. 2020) (Hu et al. 2022) obtained CsPbBr3 NRs and showed superior optical performance, demonstrating promising application projections in the photoelectric field. But these practical applications are restricted by poor stability against water, heat, and polar solvents. To avoid such defects, Li et al. (2022) reported CsPbBr3@Cs4PbBr6/SiO2 with their double coating structure and found owing stability in a polar solvent (ethanol), water, and heat.
Structures of the perovskites are ideal cubic, but the combination of various ionic radii causes distortion in the structure. These distortions in the octahedral of MnO6 can lead to the transition from cubic crystal to orthorhombic, rhombohedral, tetragonal, or hexagonal crystalline phase (Jayakumar et al. 2020, Londoño-Calderón et al. 2018). Several theories have been developed to explain fascinating electrical, magnetic, and photocatalytic properties in several doped manganites, such as double exchange (DE) (Jadli et al. 2021) models between cations, grain boundaries (GBs), Jahn–Teller effect (Li et al. 2019a), and oxygen deficiency (Londoño-Calderón et al. 2018). Partial replacement, La- with divalent alkaline earth elements, Sr-, produces some changes at the A-site and generates mixed M3+/M4+ valence that causes the ferromagnetic ordering and conductivity of the doping. The average cation radius also influences the tolerance factor, affecting the properties of manganese oxide perovskite (Li et al. 2019b). Relative to the DE model, the metallic characteristics and ferromagnetism are derived from the transfer of roaming electrons, e.g., in the M4+ matrix. Manganites’ properties also depend on oxygen deficiency, which might majorly cause expanding the crystal lattice in perovskite ABO3 cells (Londoño-Calderón et al. 2018), vacancy, and stress. Currently, the role of B-sites (electron configuration and ionic radius) is also highly considered in manganites; this type of transitional metal causes a density change for the itinerant electrons leading to variations in Curie temperature, magnetization, and conductivity (Ulyanov et al. 2019).
In the present work, the choice of three specific cations, Mn3+, Co3+, and Fe3+, is based on their similar ionic radii due to their adjacent in the periodic table. Recently, various mixed ionic electronics with perovskite structures, including manganite, La1-xSrxMnO3, La1-xSrxCoO3, cobaltite, and La1-xSrxFeO3 orthoferrite, were proposed due to their low price, non-toxicity, capacity, and excellent electrochemical performance (Utami et al. 2019, Abdullah et al. 2020, Ehsani and Raouf 2018, Yang et al. 2019).
When La3+ ion is doped by Sr2+ ion, an amount of Mn3+ (3d4, t32g↑e1g↑,:S = 2) ion is replaced by Mn4+ (3d3, t32g↑e0g↑,:S = 3/2) ion that comes from the motion between two partially filled d shells of an, e.g., electron. Some factors affect perovskite’s magnetic and photocatalysis properties, such as synthesis route grain size, shape, and density on the particles. Zhou et al. (2018) state that the optimum ratio for the transport property of the system is expected when the ratio of Mn4+/ Mn3+ is 1:2; this ratio is attained at 33% of the Sr2+-doping amount. He reported that the number of manganese ions could also be changed by creating vacancies in oxygen (oxygen deficiency), which can cause modification in the exchange interactions. It also concludes that oxygen deficiency was observed in the vacuum and nitrogen samples. At the same time, the relative concentration of Mn4+/Mn3+ was reduced, weakening the FM-inflammation interaction between them, resulting in reduced magnetization values. Yousefi and Ranjbar reported that strontium atoms replace atoms of lanthanum in the LSM structure, which are coordinated by twelve oxygen atoms. A Sr2+-doping needs to be carefully chosen at between 10 and 30% because a few characteristics, like increasing the coefficient of thermal expansion, can be positively modified (Yousefi and Ranjbar 2017).
Shinde et al. (2011) found that the coercivity of manganite annealed at 900 °C is 26.94 G, attributed to increase grain size. But they explain decreasing coercivity to 10.8 G when the annealing temperature reaches 1200 °C to oxygen deficiency. In the view of the photocatalyst, Ghiasi and Malekzadeh (2014) reported that the photocatalysis activity of La0.7Sr0.3MnO3 nanoparticles in the case of acidic medium or K2S2O8 as an electron accepter was increased. The results, however, showed that the degradation percentage depends more on the particle size and the SSA of samples as a catalyst for photocatalytic activities (Esmaeili et al. 2019). Although ACoO3 was discovered in 1950, the cobaltite of perovskite was still attracted by the cobalt’s main aspect, which clearly distinguished the cobalt oxides of transition metal oxides, that is, Co3+/III and CoIV degree of freedom of spin state: the cobalt oxides could possibly be low, intermediate, or high spin state (S = 0, 1, 2 for Co3+/III and S = 1/2, 3/2, 5/2 for CoIV). ACoO3 cobaltite with 3D corner-sharing network CoO6 octahedra often changes from the low spin state (LS, t62g e0g, S = 0) to the intermediate spin state (IS, t52g e1g, S = 1) or high spin state (HS, t42g e2g, S = 2) (Shinde et al. 2012). Moreover, it is possible to achieve oxygen non-stoichiometry by changing preparation methods and the heat treatment temperature. Otherwise, Zhang et al. (2019) reported that Fe(III) compounds are always highly spin, resulting in complex multiples, i.e., a 30% Sr doping in the A-site has resulted in an increase in the oxygen vacancies and enhanced mobility of the oxygen lattice, from Fe3+ to Fe4+ at the B-site with the proportion of 61.91% and 38.09%, respectively.
While phase purity and particle size depend on preparation methods, several synthesis methods are used, such as solid-state reaction, co-precipitation, sol–gel, microemulsion, frozen-drying, flame hydrolysis, polymeric precursors, hydrothermal synthesis, and citrate-complexation (Sfirloaga et al. 2019). For example, a solid-state reaction method occurs at high temperatures (> 1000 °C), which results in non-porous, irregular powder products, uncontrolled particle size, and low surface area (Kaewpanha et al. 2019). Other interesting chemical approaches in low sintering temperature and short reaction time (Li et al. 2019b), such as the sol–gel process and co-precipitation, have been reported to prepare materials with improved control of grain size and uniform particle size distribution and morphology (Hannora and Hanna 2019, Ghozza et al. 2013, Shlapa et al. 2018). Therefore, by the co-precipitation method, we focus on preparing La0.7Sr0.3MnO3 and La0.7Sr0.3CoO3, but the sol–gel auto-combustion route to prepare La0.7Sr0.3FeO3 samples.
Organic and toxic pollutants pollute water sources in different dyeing factories (Sakamoto et al. 2019). Dyes are problematic because the chemical families that produce good dyes also cause human toxicity and are regarded as fatal poisons (Qu et al. 2014). The most serious problem in developing countries is environmental pollution from dye wastewater. Common methods of treatment dyes with a complex aromatic structure can be very difficult to degrade since products resulting from different reactions, such as hydrolysis and oxidation in aqueous media, produce toxicity and carcinogenic substances. These products should be removed with appropriate treatment methods for public health and safety (Gowthami et al. 2018). In recent years, enormous research and development have gained considerable importance as an environmental remediation process in photocatalysis because it can fully remove organic and inorganic toxins from water pollutants by using nanosized metal oxides as catalysts (Yerkinova et al. 2018; Kanakaraju and Wong 2018; Guillaume et al. 2018). Several metal oxide nanosized semiconductor nanoparticles have been used in different applications, including dye solution photodegradation and contaminated water. The predominant research in the photocatalysis field for wastewater purification has included semiconductors of wide bandgap and high surface-to-volume ratio material such as titanium dioxide (TiO2), zinc oxide (ZnO), tin oxide (SnO2), and zirconium oxide (ZrO2) (Anandan et al. 2020). This compound is called the hole-doped. That hole doping plays a role in transferring the electronic system to the Fermi level to ensure that conductivity and valence band behavior is observed. The coexistence of electrons and holes in manganites introduces them into optical systems as promising materials (Esmaeili et al. 2019). Chahar et al. (2021) observed that the ferrite’s degradation was enhanced by an increasing cobalt level with a maximum degradation efficiency (77%) for x = 0.5. The lowest degradation efficiency (~ 65%) for x = 0.0 per 1 h was achieved by visible light irradiation. However, due to their unique electronic properties and crystal structures, many perovskite oxides have visible light photocatalytic activity (Pena and Fierro 2001a, b). Doped alkaline rare earth transition metal perovskite-like structure oxides reduce the bandgap energy values because this feature increases the charge carrier separation (photogenerated electrons and holes) (Ismail et al. 2010). Intense studies were conducted on the materials because their electrical and optical properties can be tuned, indicating control of the rational design structure in ABO3 perovskite by cationic substitutions (Ismail et al. 2009; Shui et al. 2000). So, we can say that perovskite compounds are one of the promising structures that adapt the bandgap values to collect the visible-light absorption and the capacity of the band edge to meet the specific photocatalysis needs. Abdel-Latif et al. (2017) say that Nd0.6Sr0.4MnO3 has a small bandgap of 2 to 2.98 eV, which can be controlled by changing the annealing temperature of its rings. The researchers investigated the photocatalysis efficiency of the annealed Nd0.6Sr0.4MnO3 perovskite under visible light and found that annealed perovskite at 500 °C was a better photocatalyst than that of 800, 1000, and 1150 °C. However, little work has been used perovskite nanoparticles as a catalyst. Previous works have not comprehensively considered the synthesis route and transition metal spin role in the magnetic and photocatalysis process.
Experimental technique
Synthesis of La0.7Sr0.3MnO3, La0.7Sr0.3CoO3, and La0.7Sr0.3FeO3 nano-perovskites
Nano-perovskites La0.7Sr0.3MnO3 and La0.7Sr0.3CoO3 (hereafter will be called LSM and LSC, respectively) were prepared by a co-precipitation route. According to Eqs. 1 and 2, the stoichiometric quantity of lanthanum nitrate-hexahydrate (La(No3)3.6H2O), strontium chloride hexahydrate (SrCl2.6H2O), manganese sulfate (MnSO4), and cobalt chloride hexahydrate (CoCl2. 6H2O) was weighed by a digital balance and dissolved separately in 50 mL distilled water afterward; the solution was put on a hot-stirring plate at 600 rpm and 60 °C for 1 h until complete dissolution occurred. The solutions were mixed in one beaker and on magnetic stirring again; then, 100 mL of CTAB (cetyltrimethylammonium bromide) was added to prevent particle aggregation. To avoid the NOx gases, the solution moves to an isolated position and adds NH4OH carefully as a reaction medium to reach a pH = 10. Finally, this solution was heated and stirred under the same conditions until a precipitate was formed. The residue was washed several times with filtration paper and dried in the furnace at 80 °C overnight.
The ultimate products were sintered at 1000 °C for 10 h in the air to obtain the perovskite phase. La0.7Sr0.3FeO3 sample (hereafter will be called LSF) was prepared with a low cost and easily modified sol–gel/auto-combustion process based on an easy precursor. High-purity lanthanum nitrate (LaN3O9.6H2O), strontium nitrate (Sr(NO3)2), and iron nitrate (Fe(NO3)3.9H2O) as starting materials were taken in appropriate stoichiometric ratio. The precursor solution was prepared by fully dissolving the constituents in a beaker in distilled water (precursor/starter materials). As a chelating agent, a specific amount of citric acid (C6H8O7·H2O) is dissolved in distilled water in a separate beaker. The molar ratio of citric acid to La0.7Sr0.3FeO3 was 1:1. The dissolution was carried out by magnetic stirring at 600 rpm. The nitrates, citric acid, and NH4OH (to control pH at 9) were put together immediately into a third big beaker. The solution was mixed at 80 °C using a magnetic stirrer for 1 h until a clean and transparent solution was found, while some ethylene glycol was added. The homogeneous precursor solution was then dried at 120 °C for 24 h to remove the water content. The powder is put in the beaker on a hot plate at a temperature of 250 °C until instantly combustion occurs and continues for 30 s. The obtained powder was calcined at 1000 °C for 6 h. The obtained powder was pressed at 10 tons into pellets of a diameter of 13 mm and thickness of 2 mm with a stainless steel dye mold to calculate the density.
Devices and measurements
XRD and SEM measurements
The X-ray diffractometer Bruker D8 Advance checked the sample structure with a CuKα radiation (λ = 1.5406 Å) system. The data was collected in step-scanning mode within the 2θ range of 20–80° for LSM and LSC and 10–80° for LSF with a step size of 0.02°. An XRD diffraction pattern investigated the samples’ phase identification and structural analysis. The XRD peaks were indexed using the open crystallography database (COD) and X’Pert high score software program. The XRD pattern analysis used Fullprof software to characterize the crystalline structure. The surface morphology of the three annealed at 1000 °C LSMO and coated with gold for 4 min samples were observed using a scanning electron microscope (SEM, Vega 3 SBU Tescan, Czech Republic instrument operated at 30 kV) equipped with an energy dispersive spectrometer (EDX).
Magnetic measurement
Magnetization was measured at the room temperature of three samples using a vibrating VSM (Lake Shore 7410) magnetometer in the magnetic field range of ± 20 kG.
Photocatalytic activity
The photocatalytic activity of La0.7Sr0.3MO3 was inspected using MB dye (MB, C37H27N3Na2S3O9) under UVC- radiation for the model pollutant using a wooden photoreactor at room temperature, designed by I.S. Yahia and his group at NLEBA/ASU/Egypt; more details about the photoreactor are mentioned in Hussien et al. (2020a, b). The 5 mg catalyst and 100 mL dye solution (10 mg/L) were mixed using a magnetic stirrer for about 30 min to achieve the La0.7Sr0.3MO3 adsorption/MB balance between the photocatalyst and the MB. The suspension was collected by the irradiation process at 1 mL every 5 min from the mixture. The mixture was then irradiated with UVC lamps of 18 watts of wavelength = 254 nm. For analysis, a solution of 1 mL was obtained and filtered inside the quartz cuvettes for the absorbance measurements versus the incident wavelength in the range from 400 to 800 nm using a UV–Vis Double Beam Spectrophotometer. The reusability and stability of the synthesized samples were monitored repeatedly for 5 cycles.
Result and discussion
X-ray analysis of La0.7Sr0.3MO3, nano-perovskites
Figure 1a shows the XRD pattern for the La0.7Sr0.3MnO3 sample sintering at 1000 °C. The LMS sample reveals a little perovskite phase ratio of about 15%, belonging to ICSD# 00–053-0075 (Q) according to the X’pert high score program besides SrO1.962 and La4Sr3O9 as secondary phases. The compound is crystallized in an orthorhombic structure with the symmetry of the Pcmn space group. LSC sample sintering at 1000 °C shows a perovskite phase with a ratio of 47.5% belonging to ICSD# 00–36-1393 (Q), according to X’pert high score program having Rhombohedra structure system with space group R-3C (167) beside one secondary phase of LaH3O3 ICSD# 42–0343 (D). LSF sample is indexed as Rhombohedra with 100% perovskite phase structure with space group R-3C (167) ICSD# 01–082-1964. The variation in the perovskite forming ratio may be due to the difference in the synthesis route. It indicates that the sol–gel/auto-combustion method saves time and energy and is better in crystalline phase forming. The lattice parameters and volume of the unit cell are represented in Table 3.
Because of the multiphase, performing refinement for LSM and LSC samples is difficult. Rietveld refinement analysis was carried out for the LSF sample. The background is refined by using the pseudo-Voigt function with the 12-coefficients Fourier-cosine series and other parameters such as scale factor, sample displacement, isotropic thermal parameter Biso, and peak asymmetry shown in Fig. 1d. The inset in Fig. 1d is the structure of LSF perovskite. The compound is crystallized into an R-3C symmetry rhombohedral structure. The small value of the fitness indicator χ2 confirmed the perfect quality of the refining. The Wyckoff equivalent positions in the cell follow: (La, Sr) at Fig. 2a (0, 0, ¼), (Fe) at Fig. 2b (0, 0, 0), and O at 18e (x, 0, ¼). The lattice parameters, unit cell volume (V), value of goodness of fit χ2, and reliability factors (R%) are listed in Table 1. To check the structure stability, Goldschmidt tolerance factor, t, Eq. 1 is used and calculated using ionic radii from Shannon (1976) as La+3 = 1.36, Sr+2 = 1.44, Mn+3 = 0.645, Mn+4 = 0.56, Co+3 = 0.61, Co+4 = 0.53, Fe3+ = 0.645, Fe4+ = 0.585, and O−2 = 1.4 Å. The calculated values have proven little distortion perovskite structure and are between 0.97 and 0.99 as shown in Table 1 as
Figure 3a reveals the most intense peak crystallite size (CS) according to Scherer’s formula with Eq. 2 as well as William’s son and Hamilton (CSW-H) having formula with Eq. 3 by XPowder software computer’s program (Ghozza et al. 2020) vs. perovskite ratio. The behavior of CS and CSW-H increases with increasing perovskite ratio, contrary to particle size due to secondary phases. The crystallite size estimated using Scherer’s equation is found to be 32.3, 38.3, and 44.2 nm for compositions LSM, LSC, and LSF, respectively. At the same time, CSW-H has the same trend that decreases with increasing perovskite phase formed for compositions LSM, LSC, and LSF are 48.7, 50.8, and 51.6 nm, respectively. Figure 3b reveals the linear dependence of the crystallite size (CS), dislocation, and strain lattice on the perovskite ratio as
The calculated density of the La0.7Sr0.3MO3 uses normal relation ρcal. = m/V, where m is the sample’s mass in g and v = π2rd. The porosity of the pellets was calculated by Eq. 4 (da Conceição et al. 2011):
The whole structure parameters, measured and calculated, are represented in Table 1. The porosity is estimated to be 30%, 40%, and 22% for LSM, LSC, and LSF samples; these values are less than those obtained in other published (da Conceição et al. 2009) (see Table 2).
Microstructure and surface morphology
The surface morphology in the three samples has not changed dramatically (see Fig. 4a–f). The three samples were similar in mean grain sizes. These images indicate that particles in each sample are well-resolved and distinctive. For LSM specimens, grain agglomeration increased, intergranular gaps increased, and the stepped morphology increased, which agreed with the literature. These morphological aspects are due to whether the oxygen-deficient in the sample, the escape of Mn- cation from the sample, and the presence of some impurities such as Mg- and S- elements as contamination during the preparation process. In addition, the particles are irregular in form and quite uniform, particularly the Fe- sample, due to the different preparation methods that agree with previous work (Wiglusz et al. 2015; Xu et al. 2008). The diameters of the particles are determined using the ImageJ software program by Gaussian fit of the total partitions. With the largest ionic radius of rare earth ion doping, the average particle size increases, and the samples of LSM, LSC, and LSF showed 214.5, 190, and 174.2 nm, respectively. The variance between the calculated crystallite size by Scherrer’s and W–H formula may be due to the chosen peak. Some perovskite-formed peaks for calculating CS and CSW-H are 15%, 47%, and 100% for LSM, LSC, and LSF samples. But grain size, D, considers agglomerates of various crystallite size of different phases. The indicator of agglomeration n is 4.76, 3.91, and 3.26 for LSM, LSC, and LSF samples, respectively.
Additionally, the chemical constitution of the samples has been checked by EDX analysis in Fig. 4a–c to examine the possibility of cation non-stoichiometry along different preparation routes. The relative atomic percentage of sampled elements of La-, Sr-, M-, and O- has been shown to be roughly unsatisfactory in the measurement, confirming the presence of certain accumulations and the lack of cations sensitive to the synthesized method variations. The best explanation for that percentage discrepancy is that EDX can only give you the percentage composition of the selected region you map in your analysis, which may vary when you choose another position on your sample. However, the parameter of the specific surface area, SSA, is extremely determined by the following Eq. 5, since photoreaction occurs on the surface area (Esmaeili et al. 2019):
where \(\rho\) is density from XRD and D grain size. Indeed, the presence of more reactive sites is more likely because of the smaller particles. This means that the smaller the size, the bigger the SSA. The calculated SSA is 4.25, 4.72, and 5.82 for LSM, LSC, and LSF samples, respectively. Berger et al. (2009) obtained the same results.
Magnetic properties
Figure 5a–c display the M–H curves of LSM, LSC, and LSF perovskite samples under the applied magnetic field of ± 20 kG at room temperature. The corresponding saturation magnetizations (Ms), coercivity (Hc), retentivity (Mr), and squareness (S) are listed in Table 3. The M-H hysteresis loop of the LSM powder exhibited two distinct behaviors. Uncommon weak-ferromagnetic-like behaviors at the low applied magnetic field, ± 1000 G, with saturation magnetization and coercivity values, are 0.0359 emu/g and 133.78 G, respectively. Whereas increasing the applied magnetic field was nearly above ± 1500 G, the magnetization increased in the direction opposite to the externally applied magnetic field (anti-S shaped), demonstrating the intrinsic diamagnetic behavior of LSM. Such behavior is shown by Dong et al. (2020). The uncommon ferromagnetic performance of the polycrystalline LSM in the low magnetic field is likely to take place due to the existence of some potential point defects as vacancies that might form at the surface or near-surface region in the polycrystalline sample during synthesis (Handal et al. 2019); the existence of secondary phases beside perovskite phase confirms that opinion. Furthermore, these unusual behaviors are ascribed to particles’ surfaces which might exhibit uncompensated spins that were ferromagnetically ordered and are missing in the bulk counterpart (Handal et al. 2019). Similar notes were reported for nanosized oxides due to the origin of ferromagnetism in near-surface regions where the thermal treatment could significantly reduce their density (Qin et al. 2010). In addition, such observation could be attributed to the bulk (Jayakumar et al. 2020) LSM samples, where a particle size of 214.5 nm and a great aggregation indicator factor of 4.76 are represented in Table 2.
The saturation magnetization increases with a change in the transition ion from LSM (Ms = 0.0359 emu/g) to good agreement with Ms = 0.0552 emu/g (Jayakumar et al. 2020), LSC (Ms = 0.0800 emu/g) to LSF (Ms = 0.4301 emu/g). LSC samples exhibit paramagnetic behavior. LSF showed a clear hysteresis loop that indicates ferromagnetic behavior over the whole magnetic field at room temperature. Furthermore, the squareness ratio (SQR) of LSF is quite large compared to the two other samples. The large SQR value indicates that the homogeneity of the particle (Nguyen and LeMinh 2012) is predominant in the LSF sample and confirms the ferromagnetic behavior. Other authors (Handal et al. 2019) reported a peculiar ferromagnetic behavior for the perovskite. The ferromagnetic behavior was interpreted by the presence of F-centers created by oxygen vacancy—the F-center exchange coupling (FCE). With the FCE mechanism, a magnetic ion such as TM or RE is trapped in the form of oxygen vacancies which act as a coupling center. The bounding magnetic polaron (BMP) model is also used to explain the ferromagnetism of this type (Handal et al. 2019). The interplay of the connected carrier with the magnetic ions under its surface (donor or acceptor such as vacancies) results in the polar magnet bound (BMP) formation. Ferromagnetic interaction is caused when two polarons overlap. When the polarons overlap largely and this type can remain at high temperatures, strong ferromagnetic interactions are caused.
For the LSM sample, the weak ferromagnetic property in a low magnetic field can reasonably be explained in a slightly canted spin arrangement (Zhang and Jianhua 2010). In comparison, diamagnetic behavior was seen in a high magnetic field due to the presence of impurity phases, which may be prevented the magnetic domain rotation in the direction of the magnetic field from the low values of the coercive field. The magnetization versus magnetic field shows a small coercive field and an unsaturated magnetization, indicating that some LSM sample nanoparticles are blocked at room temperature (Rostamnejadi et al. 2009). Abdel-Khalek et al. (2009) explain that the decrease of coercive field can be attributed to the increase of the grain size for the polycrystalline ferromagnets, and grain boundaries are magnetic domain boundaries. Additionally, the coercive field decreases with increasing grain size as follows: Hc = Hc,o + (KM/D) where Hc,o reflect the coercivity due to other effects, such as internal stresses and impurities, KM is a constant, and D is the grain size.
Photocatalytic process
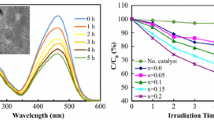
The photocatalytic absorbance of MB of the different catalysts (blank, LSM, LSC, and LSF) at room temperature in dark and UVC- light is shown in Fig. 6a–c, respectively; for three samples, the maximum absorbance reveals at 664 nm wavelength. The MB is adsorbed over the La0.7Sr0.3MO3, and an adsorption–desorption balance is reached after 30 min during the photocatalytic treatment (see Fig. 7); the kinetics and rate constants were analyzed and computed with the help of Eq. 6 (Hussien et al. 2020a, b):
where Ao is the initial absorbance, A is absorbance after time t, and k is rate constant; the plot of ln(A/Ao) against the time t is linear according to Eq. 6. Thus, the value of k can be obtained directly via its slope (see Fig. 7) to be 0.179, 0.055, and 0.08 for LSM, LSC, and LSF, respectively. These results agree well with another catalyst, as mentioned in Table 4. Figure 8 plots the k values for the studied three samples. The following equation calculated the efficiency of the photocatalytic degradation process of the studied samples (Hassan et al. 2022):
where Ao is the initial MB solution absorbance without any pre-degradation exposure and A is the solution MB absorbance after photo-irradiation for time t. MB absorption was estimated at 664 nm of peak wavelength, the maximum absorption for MB taken in the UVC- spectrum at first and every 5 min. A degradation of MB dye under UVC- light irradiation determined the photocatalytic activities of candidate materials. The degradation percentage of LSM, LSC, and LSF for 30 min is 99%, 78%, and 95.5%, respectively, as shown in Fig. 9.
As well known, OH• radicals degrade dye molecules into simpler compounds (CO2 and H2O). The impact of irradiation time on the percentage of dye degradation can be seen in Fig. 7b. From the curve, the amount of degraded OH• radicals increases with increasing irradiation time (Rahmayeni et al. 2020). The photo-degradation originated when the UVC- light irradiated the catalyst, so the photodegradation mechanism of dye solutions is based on the redox reactions for perovskite samples. The photon energy (hυ) absorbed is equal to/or greater than the perovskite energy gap. During the photoexcitation process, the valence band (VB) electron jumps to the conduction band (CB) and lets h+ on VB, which reacts with the adsorbed −OH ions or the H2O on the perovskite surface, producing OH•.
On the other hand, the electron reduces the amount of O2. On the other hand, O2• increases the number of other oxidative O2 (i.e., OH• and H2O2). It takes the oxidation portion and prevents e−/h+ pair recombination at VB. The protonation of superoxides produced peroxides, and the formation of OH• radicals was dissociated. Conversely, h+ is responsible for producing reactivity species OH• radicals using the VB hydroxyl ions. The radicals manufactured on the surface of radiated La0.7Sr0.3MO3 and OH• are very strong agents that attack and cause the dye molecules to become mineralized as simple molecules (CO2 and H2O) (Rahmayeni et al. 2020). It is clear from the photocatalytic process of the LSM sample, with a crystalline size of 48.7 nm, that the mixed structure La0.7Sr0.3MnO3 (15% orthorhombic” and 85.82% impurities) is a superior photocatalyst candidate than that of two other samples having the mixed structure (47% rhombohedral and 53% secondary phases). The observed photodegradation was 99% in the LSM sample, and the photodegradation rate of the sample LSM (0.179 min−1) is significantly about 3.25–2.23 times higher than that of the LSC and LSF samples, respectively, which is good agreement with the previous work (Abdel-Latif et al. 2017). These results quite agree with the obtained results by Ghiasi and Malekzadeh (2014) and Hussien and Yahia (2021). The superiority of the La0.7Sr0.3MnO3 can be attributed to an irregular crystallographic structure, with the Mn–O-polyhedral distortion of the secondary phases. This work showed that the high UVC- light uptake, lattice distortion, and narrow bandgap are key factors for the high photocatalytic activity of the obtained lanthanum strontium-doped manganite.
Furthermore, the degradation rate for La0.7Sr0.3MnO3 is 0.179 higher, about 3.25 and 2.23, than the other samples. A La0.7Sr0.3MnO3 nanocomposite performs as a photocatalyst to enhance the efficiency of methylene blue photodegradation owing to some structural, morphological, and electronic configuring reasons. The structural reason involved the presence of SrO1.962 and La4Sr3O9 as secondary phases indexed by XRD, besides Mg- and S- elements as contamination indexed by EDX analysis. As the orthorhombic phase’s Mn–O bond length is much smaller than the rhombohedral phase (Co–O and Fe–O), LSM perovskite nanoparticles may appreciably decrease effective bandgap energy with increasing orthorhombic phase (Tütüncü and Srivastava 2008). Arabi et al. established that LSM has 2 eV as a bandgap which is a main factor for enhancing photocatalytic activities (Arabi et al. 2019a, b). Morphological reason denotes to rod-like shape of LSM nanoparticles, as shown in the SEM image of Fig. 4a, that could accomplish maximum MB degradation in minimum time owing to the electron relay process (Shenoy et al. 2020; Anchan et al. 2019). Furthermore, the high degradation rate for La0.7Sr0.3MnO3 is more about 3.25 and 2.23, than the other samples ascribed to reduce optical bandgap energy, Eg, which increases the free electron–hole pair (Gratzel 2001), where M–O bond length and M–O–M bond angle depend on the type of transition metal cation (Mn-Co-Fe), which plays a critical role in modifying electron bandwidth (W) and hence the bandgap of perovskite (Z. Zhang et al. 2010). The empirical formula relating W with bond length and angle is W α cos θ/d3.5Mn-O, where θ is ½ [π–(Mn–O-Mn)] and dMn–O is the M–O bond length (P.G. Radaelli et al. 1997). The bandgap relates to W as follows: Eg = Δ-W, where Δ is the charge–transfer energy)M. Medardeet al. 1995). LSM perovskite nanoparticles may appreciably increase W’s value and decrease effective bandgap energy. In addition, the 3d conduction band edge of Mn4+ (Ecb = − 5.83 eV) is lower than Fe3+ (Ecb = − 4.78 eV) state. It brings holes in the d band, which may reduce the effective energy gap between the O 2p valence band and the Mn-3d conduction band, decreasing the optical bandgap width of LSM nanoparticles (Hasan et al. 2016). On the contrary, Co4+ in the LSC sample is unstable, where oxygen can be released, leading to oxygen vacancies and a decrease in catalytic activity (Pena and Fierro 2001a, b).
In general, the magnetic properties of perovskite NPs, such as saturation magnetization (MS) and coercivity (HC), are strongly affected by four main factors, including finite size effects, surface effects, magnetic anisotropy, and degree of crystallinity besides size and shape of nanoparticles (Nguyen et al. 2021). Ferromagnetic behavior was observed at room temperature due to nanosized particle formation (Hannora and Hanna 2019), while nano wire morphology of La0.7Sr0.3MnO3 sample with bandgap energy of 2 eV introduces 99% photodegradation under UVC- lights, which is confirmed by Arabi et al. (2019a, b).
Radical trapping study and photodegradation mechanism
The generation of reactive oxygen species (ROS) was detected with scavenger’s ascorbic acid (ASC), sodium chloride (NaCl), sodium nitrate (NaNO3), and isopropyl alcohol (IPA) for the best sample LSM to understand which type of oxidizing agent mostly promotes the degradation of MB dye under UVC- irradiation. It is well known that ASC, NaCl, NaNO3, and IPA are scavengers for superoxide anion (O2•−), holes (h+), electrons (e−), and hydroxyl radical (•OH) radicals, respectively (Hassan et al. 2022, Hussien and Yahis 2021). Figure 10 shows how the values of MB degradation percentage decrease with NaNO3 from 99 to 30.6% while using IPA degradation decreases to 14.5% after 30 min. This trend indicates that the main oxidizing agent participating in the degradation of MB is the •OH radical.
Recycle and stability
The reusability or recyclability of the LSM material is an essential factor to assess since it delivers a detailed insight into the proposed system’s real-time implementation. A cycling experiment was performed to analyze the chemical stability and effective reusability of LSM material. Here, four successive cycles of constant photocatalytic MB degradation were performed. Each cycle involved extracting, rinsing, centrifuging, and drying the LSM catalyst. The degradation percentage of the LSM catalyst after various cycles is reported in Fig. 10c. The LSM catalyst displayed acceptable activity after four cycles, but its photodegradation performance was slightly decreased. Particularly, even after the four cycles, there was only a 9% decrease in degradation, so the possible application of LSM photocatalyst.
Conclusion
A different route was prepared for La0.7Sr0.3MO3 perovskite compositions. The perovskite phase ratio is 100% by the sol–gel/auto combustion technique compared to 15% and 47% by co-precipitation. The crystallite size is between 32.3 and 44.2 nm. The particle size is between 214.5 and 174.2 nm. The sample porosity, P, and SSA are calculated to be 30, 40, and 22% and 4.25, 4.72, and 5.82 for LSM, LSC, and LSF, respectively. The FCE mechanism interprets a ferromagnetic behavior at room temperature in three samples. Among the prepared samples, the Fe- sample has shown the highest saturation magnetization of 0.4301 emu/g and better coercivity of 2905.4 G. The boost in the magnetic of La0.7Sr0.3FeO3 opens the door for its usage in a wide range of multifunctional devices. The photocatalytic decomposition of MB has also been investigated under the effect of UVC lights. The optimization process parameters were presented, such as the type and dye concentration. The percentage degradation of LSM, LSC, and LSF for 30 min is 99%, 78%, and 95.5%, respectively. The degradation rate k is 0.179, 0.055, and 0.08 min−1 for LSM, LSC, and LSF.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Abdel-Khalek EK, El-Meligy WM, Mohamed EA, Amer TZ, Sallam HA (2009) Study of the relationship between electrical and magnetic properties and Jahn–Teller distortion in R0.7Ca0.3Mn0.95Fe0.05O3 perovskites. J Phys: Condens Matter 21:026003. https://doi.org/10.1088/0953-8984/21/2/026003 (5pp)
Abdel-Latif A, Al-Hajry A, Hashem H, Ghozza MH, El-Sherbini TH (2011) The particle size effect on some physical properties of neodymium cobaltate-manganites for hydrogen storage use. AIP Conf Proc 1370:158–164
Abdel-Latif IA et al (2017) J Taiwan Inst Chem Eng 75:174–182
Abdullah AS, Alias SH, Darus M, Somalu MR, Baharuddin NA, N Osman (2020) Thermal decomposition, phase formation and microstructure analysis of surfactant assisted sol-gel derived La0.6Sr0.4CoO3-δ material. Earth Environ Sci 463:012095. https://doi.org/10.1088/1755-1315/463/1/012095
Anandan K, Rajesh K, Gayathri K, Vinoth Sharma S, Mohammed Hussain SG, Rajendran V (2020) Effects of rare earth, transition and post transition metal ions on structural and optical properties and photocatalytic activities of zirconia (ZrO2) nanoparticles synthesized via the facile precipitation process. Physica E 124:114342. https://doi.org/10.1016/j.physe.2020.114342
Anchan S, Pai S, Sridevi H, Varadavenkatesan T, Vinayagam R, Selvaraj R (2019) Biogenic synthesis of ferric oxide nanoparticles using the leaf extract of Peltophorum pterocarpum and their catalytic dye degradation potential. Biocatal Agric Biotechnol 20:101251
Arabi A, Ehsani MH, Fazli M (2019) Hydrothermal synthesis of La0.7Sr0.3MnO3 and its application in visible light photocatalytic activity. J Mater Sci: Mater Electron 30:19001–19008
Arabi A, Ehsani MH, Fazli M (2019) Hydrothermal synthesis of La0.7Sr03Mn0.3 and its application in visible light photocatalytic activity. J Mater Sci: Mater Electron 30:19001–19008. https://doi.org/10.1007/s10854-019-02257-1
Berger D et al (2009) Synthesis of La1-xSrxMO3 (M = Mn, Fe Co, Ni) nanopowders by alanine-combustion technique. J Eur Ceram Soc. https://doi.org/10.1016/j.jeurceramsoc.2009.07.032
da Conceição L, Ribeiro NFP, Souza MMVM (2011) Synthesis of La1-xSrxMnO3 powders by polymerizable complex method: evaluation of structural, morphological and electrical properties. Ceramics Int 37:2229–2236. https://doi.org/10.1016/j.ceramint.2011.03.069
da Conceição L, Silva CRB, Ribeiro NFP, Souza MMVM (2009) Influence of the synthesis method on the porosity, microstructure and electrical properties of La0.7Sr0.3MnO3 cathode materials. Mater Charact 60:1417-1423. https://doi.org/10.1016/j.matchar.2009.06.017
Deepika C, Taneja S, Bisht S, Kesarwani S, Thakur P, Thakur A, Sharma PB (2021) Photocatalytic activity of cobalt substituted zinc ferrite for the degradation of methylene blue dye under visible light irradiation. J Alloys Compd 851:156878. https://doi.org/10.1016/j.jallcom.2020.156878
Dong MC, Lee YH, Lee KW, Park WK, EuiLee C (2020) Room-temperature ferromagnetism and hydrogen shallow donors in rare-earth Eu-doped ZnO nanorods. Physica B 585:412117. https://doi.org/10.1016/j.physb.2020.412117
Ehsani MH, Raouf T (2018) Effect of Gd substitution on the critical scaling of the ferromagnetic transition of La0.6-xGdxSr0.4MnO3 (x=0, 0.05, 0.1) manganite. J Alloys Compd 769:649e659
Esmaeili S, Ehsani MH, Fazli M (2019) Structural, optical and photocatalytic properties of La0.7Ba0.3MnO3 nanoparticles prepared by microwave method. Chem Phys. https://doi.org/10.1016/j.chemphys.2019.110576
Feiyan Xu, Meng K, Cheng B, Wang S, Jingsan Xu, Jiaguo Yu (2020) Unique S-scheme heterojunctions in self assembled TiO2/CsPbBr 3 hybrids for CO2 Photoreduction. Nat Commun 11(1):4613
Ghiasi M, Malekzadeh A (2014) Solar photocatalytic degradation of methyl orange over La0.7Sr0.3MnO3 nano-perovskite. Sep Purif Technol 134:12–19. https://doi.org/10.1016/j.seppur.2014.07.022
Ghozza MH, Abdel-Latif IA, Allm SH (2013) Properties of 3d-4f oxides nanoparticles: synthesis, structural, electrical and magnetic. Scholars’ Press, Edition: 1st; Publisher: Scholars’ Press; Editor: Verlag Publisher
Ghozza MH, Yahia IS, El-Dek SI (2020) Role of B-site cation on the structure, magnetic and dielectric properties of nanosized La0.7Sr0.3Fe1-xMxO3 (M= Mn; Co and x= 0, 0.5) perovskites. Mater Res Express
Gowthami K, Suppuraj P, Thirunarayanan G, Krishnakumar B, Sobral AJFN, Swaminathan M, Muthuvel I (2018) Fe2V4O13 assisted hetero-fenton mineralization of methyl orange under UV-A light irradiation. Iran Chem Commun 6:97–108
Gratzel M (2001) Photoelectrochemical cells. Nature 414:338–344
Guillaume PLA, Chelaru AM, Visa M, Lassine O (2018) Titanium oxide-clay as adsorbent and photocatalysts for wastewater treatment. J Membr Sci Technol 8(176):1000176. https://doi.org/10.4172/2155-9589.1000176
Handal HT, Mousa HA, Mabrouk Yakout S, Sharmoukh W, Thangadurai (2019) Effect of Mn and Ni-doping on structure, photoluminescence and magnetic properties of perovskite-type BaSn0.99Gd0.01O3. J Magn Magn Mater. https://doi.org/10.1016/j.jmmm.2019.165946
Hannora AE, Hanna FF (2019) Preparation of nanostructured manganites SmMnO3 and Sm0.5Sr0.5MnO3 by mechanochemical synthesis method. J Mater Sci: Mater Electron 30:12456–12464. https://doi.org/10.1007/s10854-019-01605-5
Hasan M, Basith MA, Zubair MA, Hossain MDS, Rubayyat M, HakimMA, Islam MDF (2016) Saturation magnetization and band gap tuning in BiFeO3 nanoparticles via co-substitution of Gd and Mn. J Alloys Compd 687(5 December 2016):701–706. https://doi.org/10.1016/j.jallcom.2016.06.171
Hassan AE, Hussien MSA, Elsayed MH, Mohamed MG, S-W Kuo, H-H Chou, Yahia IS, Wang G, Wen Z (2022) One-step construction of Y, C, and O tridoped g-C3N4 as a bifunctional photocatalyst for H2 evolution and organic pollutant degradation under visible light irradiation. Sustain Energy Fuels 6(16):3858–3871
Hilal A, Khan S, Khan W, Nongjai R, Khan I (2012) Study of structural, electrical and magnetic properties of Zn doped La0.67Sr0.33MnO3. J Alloys Compd 527:48–52
Hu X, Xu Y, Wang J, Ma J, Wang L, Jiang W (2022) Ligand-modified synthesis of shape-controllable and highly luminescent CsPbBr3 perovskite nanocrystals under ambient conditions. Inorg Chem Front 9(23):6080–6090
Hussien MSA, Yahia IS (2021) Hybrid multifunctional core/shell g-C3N4@TiO2heterojunctionnano-catalytic for photodegradation of organic dye and pharmaceutical compounds. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-12680-9
Hussien MSA, Mohammed MI, Yahia IS (2020a) Flexible photocatalytic membrane based on CdS/PMMA polymeric nanocomposite films: multifunctional materials. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-020-10305-1
Hussien MSA, Mohammed MI, Yahia IS (2020b) Multifunctional applications of graphene‑doped PMMA nanocomposite membranes for environmental photocatalytic. J Inorg Organomet Polym Mater. https://doi.org/10.1007/s10904-019-01433-4
Ismail D, Bahnemann W, Bannat I, Wark M (2009) J Phys Chem C 113:7429–7435
Ismail D, Bahnemann W, Robben L, Yarovyi V, Wark M (2010) Chem Mater 22:108–116
Jadli T, Mleiki A, Rahmouni H, Khirouni K, Hlil EK, Cheikhrouhou A (2021) Investigation of physical properties of manganite on example of Sm0.35Pr0.2Sr0.45MnO3"Physica B 600:412548. https://doi.org/10.1016/j.physb.2020.412548
Jayakumar G, Poomagal DS, Irudayaraj AA, Raj AD, Thresa SK, Akshadha P (2020) Study on structural, magnetic and electrical properties of perovskite lanthanum strontium manganite nanoparticles. https://doi.org/10.1007/s10854-020-04608-9
Kaewpanha M, Suriwong T, Wamae W, Nunocha P (2019) Synthesis and characterization of Sr-doped LaFeO3 perovskite by sol-gel auto-combustion method. J Physics: Conf Series 1259:012017. https://doi.org/10.1088/1742-6596/1259/1/012017
Kanakaraju D, Wong SP (2018) Photocatalytic efficiency of TiO2-biomass loaded mixture for wastewater treatment. J Chem 4314969. https://doi.org/10.1155/2018/4314969
Li X, Ma W, Liang D, Cai W, Zhao S, Zan Z (2022) High-performance CsPbBr 3@Cs4PbBr6/SiO2 nanocrystals via double coating layers for white light emission and visible light communication. eScience 2:646–654
Li L, Zhang H, Liu X, Sun P, Wang C, Wang X, Li B, Liang G, Chen Q (2019a) Structure and electromagnetic properties of La0.7Ca0.3-xKxMnO3 polycrystalline ceramics. Ceramics Int 45:10558–10564. https://doi.org/10.1016/j.ceramint.2019.02.120
Li Y, Zhang H, Liu X, Chen Q, Chen Q (2019b) Electrical and magnetic properties of La1-xSrxMnO3 (0.1≤ x≤ 0.25) ceramics prepared by sol–gel technique. Ceramics Int. https://doi.org/10.1016/j.ceramint.2019.05.159
Lijak J, Senoy T, Anantharaman MR (2016) A study on the magnetic properties of Gd–Sr based low band width manganites in their bulk and thin film forms and evidence for magnetization reversal in bulk Gd0.7Sr0.3MnO3. J Magn Magn Mater 398:174–182
Londoño-Calderón V, Rave-Osorio LC, Restrepo J, Játiva J, Jurado JF, Arnache O, Restrepo-Parra E (2018) Structural and magnetic properties of La1−x(Ca,Sr)xMnO3 powders produced by the hydrothermal method. J Supercond Nov Magn. https://doi.org/10.1007/s10948-018-4625-8
Medarde M, Mesot J, Lacorre P, Rosenkranz S, Fischer P, Gobrecht K (1995) High-pressure neutron-diffraction study of the metallization process in PrNiO3. Phys Rev B 52:9248–9258
Nguyen TT, LeMinh D (2012) Size effect on the structural andmagnetic properties of nanosized perovskite LaFeO3 prepared by different methods. Adv in Mater Sci Eng 2012:380306. https://doi.org/10.1155/2012/380306 6 pages
Nguyen MD, Tran H-V, Xu S, Lee TR (2021) Fe3O4 Nanoparticles: structures, synthesis, magnetic properties, surface functionalization, and emerging applications. Appl Sci 11:11301. https://doi.org/10.3390/app112311301
Pena MA, Fierro JLG (2001a) Chemical structures and performance of perovskite oxides. Chem Rev 101:1981
Pena MA, Fierro JLG (2001b) Chem Rev 101:1981–2018
Qin S, Liu D, Zuo Z, Sang Y, Zhang X, Zheng F, Liu H, Xu X-G (2010) UV-irradiation-enhanced ferromagnetism in BaTiO3. J Phys Chem Lett 1:238–241. https://doi.org/10.1021/jz900131x|
Qu X, Xie D, Cao L, Du F (2014) Synthesis and characterization of TiO2/ZrO2 coaxial core–shell composite nanotubes for photocatalytic applications. Ceram Int 40:12647–12653. https://doi.org/10.1016/j.ceramint.2014.04.111
Radaelli PG, Iannone G, Marezio M, Hwang HY, Cheong S-W, Jorgensen JD, Argyriou DN (1997) Structural effects on the magnetic and transport properties of perovskite A1−xAxMnO3 (x=0.25, 0.30). Phys Rev B 56:8265–8276
Rahmayeni R, Oktavia Y, Stiadi Y, Arief S, Zulhadjri Z (2020) Spinel ferrite of MnFe2O4 synthesized in Piper betle Linn extract media and its application as photocatalysts and antibacterial. J Dispersion Sci Technol. https://doi.org/10.1080/01932691.2020.1721011
Rostamnejadi A, Salamati H, Kameli P, Ahmadv H (2009) Superparamagnetic behavior of La0.67Sr0.33MnO3 nanoparticles prepared via sol–gel method. J Magn Magn Mater 321:3126–3131. https://doi.org/10.1016/j.jmmm.2009.05.035
Sakamoto M, Ahmed T, Begum S, Huq H (2019) Water pollution and the textile industry in Bangladesh: flawed corporate practices or restrictive opportunities? Sustainability 11(1951). https://doi.org/10.3390/su11071951.
Sfirloaga P, Malaescu I, Marin CN, Vlazan P (2019) The effect of partial substitution of Pd in LaMnO3 polycrystalline materials synthesized by sol–gel technique on the electrical performance. J Sol-Gel Sci Technol. https://doi.org/10.1007/s10971-019-05102-3
Shannon RD (1976) Revised effective ionic radii and studies of interatomic distances in halides and chalcogenides. Acta Cryst A 32:751
Shenoy MR, Ayyasamy S, Reddy MVV, Kadarkarai G, Suryakanth J, Tamilarasan S, Thangavelu S, Jeyaramane AC (2020) The effect of morphology-dependent surface charges of iron oxide on the visible light photocatalytic degradation of methylene blue dye. J Mater Sci Mater Electron 31:17703–17717
Shinde KP, Pawar SS, Shiragec PM, Pawar SH (2012) Studies on morphological and magnetic properties of La1-xSrxMnO3. Appl Surf Sci 258:7417–7420
Shinde KP, Pawar SS, Pawar SH (2011) Influence of annealing temperature on morphological and magnetic properties of La0.9Sr0.1MnO3. Appl Surf Sci 257:9996–9999. https://doi.org/10.1016/j.apsusc.2011.06.126
Shlapa Y, Solopan S, Belous A, Tovstolytkin A (2018) Effect of synthesis method of La1-xSrxMnO3 manganite nanoparticles on their properties. Nanoscale Res Lett 13:13. https://doi.org/10.1186/s11671-017-2431-z
Shui M, Yue LH, Xu ZD (2000) Acta Physico- Chimica Sinica 16:459–464
Smitha MG, Murugendrappa MV (2019) Effect of barium lanthanum manganite nano particle on the electric transport properties of polypyrrole at room temperature. J Mater Sci: Mater Electron. https://doi.org/10.1007/s10854-019-01421-x
Tran TH, Bach TC, Nguyen HP, Nguyen QH, Cong DS, Nguyen HN, Nguyen VT, NguyenTT Ho KH, Doan QK (2019) Phase transition of LaMnO3 nanoparticles prepared by microwave assisted combustion method. Mater Sci Semicond Process 89:121–125
Tütüncü HM, Srivastava GP (2008) Electronic structure and lattice dynamical properties of different tetragonal phases of BiFeO3. Phys Rev B 78:235209
Ulyanov AN, Yang D-S, Shin H-J, Kim K-j, Vasiliev AV, Savilov SV (2019) Valence, hybridization of electronic states and magnetic properties of La0.7Ca0.3Mn1-xScxO3 (x=0.0; 0.03, and 0.05) manganites, Journal of Alloys and Compounds. https://doi.org/10.1016/j.jallcom.2019.04.290
Utami RW, Rafsanjani RA, Triyono D (2019) Optical properties of La0.9Sr0.1Fe1-xMoxO3 (x = 0.1, 0.2, and0.3) perovskite material prepared by sol-gel method. J Phys: Conf Ser 1153:012072. https://doi.org/10.1088/1742-6596/1153/1/012072
Verma MK, Sharma ND, Choudhary N, Sharma S, Singh D (2019) Comparative study of La0.6R0.1Ca0.3Mn0.9Cr0.1O3 (R = La, Eu and Ho) nanoparticles: effect of A‑cation size and sintering temperature. J Mater Sci: Mater Electron 30:12328–12338. https://doi.org/10.1007/s10854-019-01591-8
Wiglusz RJ, Kordek K, Małecka M, Ciupa A, Ptak M, Pazik R, Pohl P, Kaczorowski D (2015) New approach in the synthesis of La1-xGdxFeO3perovskite nanoparticles structural and magnetic characterization. Dalton Trans 44:20067–20074. https://doi.org/10.1039/C5DT03378K
Xu H, Hu X, Zhang L (2008) Generalized low-temperature synthesis of nanocrystalline rare-earth orthoferrites LnFeO3 (Ln=La, Pr, Nd, Sm, Eu, Gd). Crystal Growth Design 8:2061–2065. https://doi.org/10.1021/cg800014b
Yang Q, Zuo X, Yao J, Zhang K, Zhang H, Khan MW, Wang W, Tang H, Wu M, Li G, Jin S (2019) La0.7Ca0.3MnO3 nanoparticles anchored on N-doped graphene: highly efficient bifunctional catalyst as counter electrode for dye-sensitized solar cells. J Electroanal Chem 844: 34–42
Yerkinova G, Balbayeva VJ, Inglezakis SG (2018) Poulopoulos, Photocatalytic treatment of a synthetic wastewater. IOP Conf Series: Mater Sci Eng 301:12143–12148. https://doi.org/10.1088/1757-899X/301/1/012143
Yousefi M, Ranjbar M (2017) Ultrasound and microwave-assisted co-precipitation synthesis of La0.75Sr0.25MnO3 perovskite nanoparticles from a new lanthanum (III) coordination polymer precursor. J Inorg Organomet Polym 27:633–640. https://doi.org/10.1007/s10904-017-0504-1
Zhang Z, Wu P, Chen L, Wang J (2010) Systematic variations in structural and electronic properties of BiFeO3 by A-site substitution. Appl Phys Lett 96:012905
Zhang G, Jianhua L (2010) Synthesis, electronic and magnetic properties of the double B mixed perovskite series La0.5Sr0.5Mn1-xFexO3. J Alloys Compd 507:47–52. https://doi.org/10.1016/j.jallcom.2010.07.162
Zhang R, Cao Y, Li H, Zhao Z, Zhao K, Jiang L (2019) The role of CuO modified La0·7Sr0·3FeO3perovskite on intermediate-temperature partial oxidation of methane via chemical looping scheme. Int J Hydrogen Energy. https://doi.org/10.1016/j.ijhydene.2019.12.082
Zhou Y, Zhu X, Li S (2018) Effect of heat treatment condition on magnetic, electrical transport and magnetoresistance properties of La0.67Sr0.33MnO3 manganite coatings. Ceramic Int 44(13, September 2018):15010–15018. https://doi.org/10.1016/j.ceramint.2018.05.130
Acknowledgements
It is a pleasure to acknowledge the financial support provided by Science, Technology & Innovation Funding Authority (STDF) Call/ Grant/ Program: Call 7, STDF Basic & Applied Research Grants (STDF-BARG), Basic & Applied Research Grants, Non-Industrial sector, National, with ID no. 37226.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This work was supported by Science, Technology & Innovation Funding Authority (STDF) Call/Grant/Program: Call 7, STDF Basic & Applied Research Grants (STDF-BARG), Basic & Applied Research Grants, Non-Industrial sector, National, with ID no. 37226. Author Mai S. A. Hussien has received research support from STDF.
Author information
Authors and Affiliations
Contributions
Conceptualization, software, and formal analysis: M.H. Ghozza, I.S. Yahia, Mai S.A. Hussien.
Visualization and resources: M.H. Ghozza, I.S. Yahia, Mai S.A. Hussien.
Data curation, writing—review and editing: M.H. Ghozza, I.S. Yahia, Mai S.A. Hussien.
Methodology, writing—review and editing, and project administration: M.H. Ghozza, I.S. Yahia, Mai S.A. Hussien.
Project administration and funding acquisition: Mai S. A. Hussien.
Supervision: M.H. Ghozza, I.S. Yahia, Mai S.A. Hussien.
Corresponding author
Ethics declarations
Ethics approval
This study follows all ethical practices during writing.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: George Z. Kyzas
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ghozza, M.H., Yahia, I.S. & Hussien, M.A. Structure, magnetic, and photocatalysis of La0.7Sr0.3MO3 (M = Mn, Co, and Fe) perovskite nanoparticles: Novel photocatalytic materials. Environ Sci Pollut Res 30, 61106–61122 (2023). https://doi.org/10.1007/s11356-023-26411-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26411-9