Abstract
The human monkeypox virus (MPXV) was first identified in 1959. Since then, the incidence of the disease has been sporadic. The endemic regions were identified in Africa’s central and western areas. However, the infection started to spread in 2017 to non-endemic regions such as North and South America, Europe, and Asia. Since May 2022, the non-endemic areas reported 62,635 till 20th September 2022. Although the monkeypox virus has a mortality of ≥ 10%, it showed only 82 mortalities worldwide in 2022. The common symptoms include chills, fever, fatigue, and skin lesions, and the complications include secondary respiratory tract infections, encephalitis, blindness, and severe diarrhea. The factors responsible for spreading the virus include improper handling and consumption of infected bushmeat, unprotected sexual intercourse, contact with an infected person, no smallpox vaccination, improper hygiene, lower diagnostic capacity, and strong travel history from the endemic regions. The therapeutic strategy is symptom-based treatment and supportive care. Antivirals and vaccines such as Tecovirimat, Brincidofovir, Cidofovir, Imvamune, and ACAM2000 have shown promising results. The primary purpose of the review is to perform an epidemiological study and investigate the pathobiology, diagnosis, prevention, treatment, and some associated complications of the monkeypox virus in 2022.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Monkeypox virus (MPXV) was first found in cynomolgus monkeys taken from Singapore to Copenhagen, Denmark, in 1959. It was known to cause pox in those monkeys (Afshar et al. 2022). MPXV is a highly virulent orthopoxvirus with high infectivity and mortality rates (≥ 10%). In 1970, the first confirmed patient of MPXV had discovered the Democratic Republic of Congo. The symptoms resembled a smallpox infection. There was a massive upsurge in the number of confirmed cases from 1981. This zoonotic disease became endemic in the rural central and western African regions. The infection is transmitted from humans and other animals such as mice, rats, prairie dogs, squirrels, and monkeys (Alakunle et al. 2020). Fifty-three confirmed cases were found in the USA in 2003 due to some household prairie dogs imported from Ghana (Ahmed et al. 2022). Israel reported 1 case in 2018 from a man who travelled from Nigeria. In 2019, a single case was reported in Singapore by a traveller from Nigeria. Three members of the same family became infected with MPXV in the UK after travelling from Nigeria in May 2021. Two more confirmed cases were found in Texas and Maryland, the USA, in July and November 2021, respectively. Both of them had a travel history from Nigeria. However, 2022 reported 36,513 cases from around the world (Afshar et al. 2022) and a trend of a sharp increase in the incidence of infection has been observed (Fig. 1). Practically, a detailed epidemiological study is difficult to conduct in this case due to a lack of reporting, confirmation, and similarity of the symptoms with smallpox.
Rapid increase in the cumulative cases of human monkeypox virus infection worldwide from 6th May to 20th September 2022 (Mathieu et al. 2015)
Mostly, cases are reported from subjects with a strong travel history from central and west African countries (Au et al. 2022). However, mathematical models suggest higher animal-to-human and lower human-to-human transmission. This includes contact with animal skin lesions, bodily fluids, and respiratory droplets directly or indirectly through clothes, utensils, etc. (Au et al. 2022). Human-to-human transmission is due to direct or close physical contact during sexual intercourse or other activity (Heskin et al. 2022). The risk factors include improper handling of bushmeat without proper precautions, contact with infected animals, heavily forested, rural areas, discontinuation of routine smallpox vaccination, taking care of infected individuals with MPXV without appropriate safeguards, and few cultural norms. Studies suggest that men and children have a higher risk of infection. Infected pregnant females have reported congenital anomalies and complications during childbirth (Cho and Wenner 1973).
The routes of viral entry include intradermal, oropharynx, and nasopharynx. The virus rapidly replicates and spreads to the local lymph nodes. The incubation period is generally 7–14 days (21 days maximum). The virus spreads and seeds to other organs post-incubation. Headache, fever, fatigue, profound weakness, erythema, skin hyperpigmentation, and lymph node swelling are some initial symptoms that develop post-incubation (Bragazzi et al. 2022). The progression of the disease leads to secondary infections, respiratory infections (such as pneumonia), loss of vision, encephalitis, and dehydration (through vomiting and diarrhea). Oral mucosal lesions followed by lesions concentrated in the face, soles, palms, and rashes in the rest of the body develop within 1–2 days of initial incubation. The lesions are 2–10 mm and go through macular, papular, vesicular, and pustular phases in 2–4 weeks. Five to seven days after the pustular stage, crusts develop over lesions which fall off after 7–14 days, and the patient is declared non-infective (Cho and Wenner 1973; Bragazzi et al. 2022; Rodriguez-Morales et al. 2022). The protocol suggesting complete isolation of the patient in a standard negative pressure chamber was given by the Centers for Disease Control and Prevention (CDC). It also recommended non-contact with the patient’s droplet and standard airborne precautions (Vivancos et al. 2022).
Structural characteristics of MPXV
The virus has been categorized under the Poxviridae family (Guagliardo et al. 2020). This family’s viruses include vaccinia, variola, and cowpox. The MPXV is a dumbbell-shaped, enveloped, and pleomorphic virus. The MPXV is nearly 200–250nm with a double-stranded DNA genome size of about 197kb consisting of non-overlapping open-reading frames (ORFs) >180 nucleotides in length (Alakunle et al. 2020). It consists of homologous genes from the vaccinia viruses at the terminal position of its genome. Nevertheless, MPXV consists of four ORFs at the inverted terminal repeats, which is different from vaccinia viruses, which have no ORFs at the inverted terminal repeats 9 (Antinori et al. 2022). The genome of MPXV was analyzed by Shchelkunov et al. (2002), which showed a similarity of the central conserved region with the other viruses of the orthopoxviruses (Shchelkunov et al. 2002). However, inverted terminal repetition (ITR) was found in the extreme right and left regions with tandem repeats. Simultaneous analysis and pathogenicity towards humans confirmed that MPVX showed a different genomic constitution than other orthopoxviruses (Kugelman et al. 2014).
Molecular mechanism of infection and pathogenesis
The MPXV is transmitted from infected animals or from infected humans to healthy humans (Fig. 2). The virus accumulates within the nasopharyngeal, oropharyngeal, or intradermal region. The virus replicates rapidly at the primary inoculation sites and then spreads to all the lymph nodes. The virus enters healthy human cells by cellular fusion or endocytosis. Upon entering the cytosol, the viral envelope uncoats releasing the DNA. The DNA replicates and undergoes transcription and translation in successive steps to form the proteins required for viral assembly (Kaler et al. 2022). Finally, the genetic material gets packaged within the viral envelope, and the mature virions are released. This process is called Primary Viremia. The mature virions further spread to the skin (to form skin lesions) and other tertiary organs (Fig. 3). This process is called Secondary Viremia, and the stage is called the Prodromal Stage (Kumar et al. 2022). The Central-African and Western-African strain have 173 and 171 unique functional genes and 56 and 53 virulent genes, respectively. The Central-African strain can activate T-cells by the T-cell receptor (TCR) pathway, thus inhibiting the host’s immune system to some extent. The MPXV is perceived to have a lower mutation rate than other viruses like human immunodeficiency virus (HIV) and severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). However, the viral samples from the current outbreak showed 40 mutations that might have occurred in 50 years. However, most of the mutations are for the survival of the virus and are non-lethal for the host (Kaler et al. 2022).
Schematic representation of monkeypox virus infection: a mechanism of transmission from the animal reservoir or other infected individuals, b pathobiology of the virus after entry into the host’s cell, c typical manifestations or symptoms of the infection, d progression of skin lesions (adapted from Alkhalil et al. 2010; Kaler et al. 2022; Kumar et al. 2022)
Manifestations of monkeypox virus infection in the form of skin lesions as per a case study reported by Adler et al. (2022)
Epidemiological study of MPXV infection of 2022
MPXV is generally self-limiting in nature. The symptoms resolve within 14–21 days after the appearance of the initial symptoms. Currently, two types of MPXV are found: the Western and Central-African strains. The mortality rate of the West-African type is around 3.6%, but sometimes, it may cause severe illness. In contrast, the Central-African type has a high mortality of approximately 10.6%. The epidemiological study of MPXV can be divided into two parts, endemic regions, and non-endemic regions. The endemic area includes the Democratic Republic of the Congo, Nigeria, Cameroon, the Central African Republic, and the Republic of the Congo (Table 1 and Fig. 4) (Ladnyj et al. 1972; Cho and Wenner 1973). The non-endemic areas include Europe, America, and Asia, which reported recent cases (Table 2 and Fig. 5). The first outbreak of MPXV in a non-endemic region was reported in 2003 in the USA due to some infected rodents and prairie dogs imported from Ghana (Cho and Wenner 1973).
Confirmed cases of MPXV in the endemic regions till 20th September 2022 (World Health Organization 2022c)
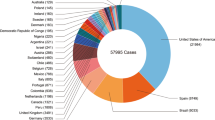
Total confirmed cases of MPXV in the non-endemic regions till 20th September 2022 (Mathieu et al. 2015)
The incidence of human monkeypox was sporadically reported from 1970 till 2017. However, the number of cases increased rapidly, with 2828 suspected cases and 69 mortalities in 5 endemic countries. In 2020, 6368 suspected cases were reported, with 231 mortalities in 7 countries. However, in 2021, more cases were reduced by 49% than in 2020 (Bunge et al. 2022). The leading cause of the upsurge is still unknown, but deforestation, contact of humans with wild animals, and bushmeat consumption could be one of the major causes. A total of 23,428 cumulative suspected cases with 589 mortalities have been reported from the endemic region since 2017 (Chakraborty et al. 2022). The fatality rate was around 2.5%. The Democratic Republic of the Congo includes approximately 96% of the cases in the endemic belt. Fatalities have been higher in young patients and HIV-infected individuals (Chakraborty et al. 2022; Oprea et al. 2022).
The first MPXV infection in 2022 outside the endemic region was reported in the UK from a patient with a travel history from Nigeria. The case was confirmed on 7th May 2022 and immediately isolated. Six more confirmed cases were reported between 13th and 15th May 2022 (World Health Organization 2022a). Two belonged to the same family as the case reported on 7th May. Most samples tested positive for the Western-African strain (Kugelman et al. 2014). At the end of May, 190 confirmed cases were recorded in the UK. The government of the UK gave particular emphasis on sexual contact, especially for patients who showed blisters within three weeks of sexual contact. On 7th June 2022, they declared MPXV as a “Notifiable Disease” and emphasized the diagnosis and contact tracing. The number of cases crossed a thousand by the end of June. Until 15th August 2022, 3201 confirmed cases were recorded (World Health Organization 2022a).
Apart from the UK, confirmed cases were reported from other European nations and non-European nations such as Argentina, Canada, French Guiana, the USA, the United Arab Emirates, Sudan, Belgium, Austria, Czechia, Denmark, France, Finland, Germany, Israel, Italy, Netherlands, Slovenia, Portugal, Spain, Switzerland, Sweden, Northern Ireland, United Kingdom of Great Britain, Australia, Benin, Poland, Latvia, Serbia, Luxemburg, and India (Nature 2022a; Oprea et al. 2022; World Health Organization 2022a). Cases began to increase from May 2022 among individuals with a travel history from the endemic African countries and the UK. The Centers for Disease Control and Prevention (CDC) emphasizes contact tracing and diagnosis of infected individuals in both USA and other countries. The CDC has guidelines for vaccination strategies, including post-exposure and pre-exposure prophylaxis. These vaccines include ACAM2000 and JYNNEOS, which are smallpox vaccines discussed in the later sections of this article (World Health Organization 2022b). India also reported a total number of 10 cases by 29th August 2022. The Ministry of Health and Family Welfare, Government of India, published the protocol for healthcare professionals and patients on 31st May 2022. However, the most alarming aspect is that the 4th confirmed case was reported from West Delhi, who had no travel history to any country (The Times of India 2022).
Diagnosis
The diagnosis of MPXV is generally made by polymerase chain reaction (PCR) for its sensitivity and accuracy. The sample includes skin lesions from different sites, like fluid from pustules, vesicles, exudates, and crusts (Saxena et al. 2022). The samples are collected from the suspected cases and stored in clean, sterilized tubes without the viral media for transport in cold storage conditions. Furthermore, diagnosis can be made through electron microscopy and molecular analysis of the sequenced results of PCR (Table 3) (Ibrahim et al. 1997; Espy et al. 2002; Nörz et al. 2022). The serological study can be conducted with specific immunoglobulin M within 5 days and specific immunoglobulin G within 8 days (Petersen et al. 2019). But currently, several companies have launched rapid diagnostic testing kits based on IgM/IgG antibodies. These tests are based on fluorescence immunochromatography, which uses whole human blood as a sample. Rapid tests can produce accurate results within 10–15 min (Aden et al. 2022).
Immunohistological analysis of the skin biopsies obtained from papular and vesiculopustular lesions revealed necrosis of keratinocytes, acanthosis, dermal lymphohistiocytic infiltrate, vacuolization of basal membrane, giant multinucleated epithelial cells, necrosis of epidermal layer, vasculitis, and increased number of neutrophils and eosinophils (Hofer 2022). Contact tracing requires contact tracing to record patient details, including age, initial symptoms, collection of specimens, date of the first appearance of rashes, lesion stage, location, travel history, vaccination status for smallpox, and confirmation of laboratory tests (Mileto et al. 2022).
Prevention
The exact mechanism of transmission of MPXV has yet to be identified. Transmission due to contact with bushmeat has been identified as essential in spreading the infection in endemic areas. Direct exposure to blood, inadequate precautions, and improper cooking are considered direct routes of exposure (Cho and Wenner 1973; Dhawan and Priyanka Choudhary 2022; Dhawan et al. 2022). Healthcare workers are at comparatively more risk of getting infected due to close contact with patients and the lack of inappropriate protective clothing, such as surgical masks and gloves (Dhawan et al. 2022). Prevention of bushmeat trade, such as import of non-human primates and rodents; proper precautions during handling of blood, meat, other body parts, and dead or decaying animals; immediate quarantine of infected animals showing initial symptoms of monkeypox; and proper cooking of meat-based products are considered essential precautions for preventing animal-to-human transmission (Minhaj et al. 2022). It is not easy to disinfect the MPXV as the marketed phenol-based disinfectants are not active against it. Solvents such as chloroform (at 60°C) and 20 nM caprylate (at 22°C) with high lipophilicity and lower pH are only effective and can take up to 2–3 h for complete disinfection (Di Guilo et al. 2004).
Orthopoxviruses are highly drying resistant, and this property is upregulated when the virus is released along with biological materials like blood, serum, dermal crust, saliva, or bodily secretions (Rheinbaben et al. 2007). Viruses like smallpox can remain contagious for several months in the infected individuals’ bedding blankets and personal clothes. The orthopoxviruses become more environmentally resistant at ambient temperatures, which increases to many folds at lower temperatures (Mahnel 1987). Cell-bound viruses present in the clinical isolates from the patient show more resistance than purified ones. For example, Vaccinia viruses in dried form can be stored for 35 weeks at 4 °C without any decrease in virulence (Essbauer et al. 2007). These characteristics can be highly correlated with MPXV due to the presence of an envelope, as an outer covering, like the other members of this genus. The environmental persistence of MPXV has been studied by Morgan et al. 2022, which identified the presence of viable viruses from porous surfaces such as fabrics (blankets, mattresses, towels, and underwear) and cellulosic materials (tissue papers), and also non-porous surfaces (wooden tabletop). However, this study was based on emergency response, and a further validated environmental sampling methodology is needed to design effective disinfection protocols for MPXV.
Diagnosis, contact tracing, quarantine, and isolation of human patients in controlled negative air-pressure isolation rooms with a travel history from central and western African regions are essential for breaking the chain of human-to-human transmission. Standard healthcare measurements, as recommended by CDC and WHO, are necessary (Centers for Disease and Prevention 2022). Standard nursing clothes and equipment should be provided to healthcare workers. The healthcare workers must be previously vaccinated with smallpox vaccines (Dhawan et al. 2022). Biological samples and specimens should be handled and transported according to the guidelines for transporting infectious materials, as provided by WHO. The government of lower- and middle-income countries, such as the countries of the endemic regions, should focus on building their healthcare infrastructure capacity. They should increase awareness among the population through surveillance programs, education, and training. Travel to the endemic regions should be highly controlled or strictly prohibited until a decrease in cases (Memariani et al. 2022).
Treatment
The current treatment protocol for human monkeypox infection includes only symptomatic management, secondary infection treatment, and supportive treatment because there is no specific treatment protocol available for MPXV. The Global Commission for the Certification of Smallpox Eradication (GCCSE) identified MPXV as a public health threat and recommended its vaccination, surveillance, and awareness program in 1980; WHO supported it from 1970 to 1986 (Cho and Wenner 1973). However, at the end of the smallpox eradication program, no scientific evidence was found supporting the smallpox vaccine’s effectiveness for monkeypox. However, a systematic surveillance program between 2005 and 2007, which divided the Democratic Republic of the Congo into nine healthcare zones, suggested a decrease in the risk of monkeypox infection by about 5.2 times among individuals vaccinated with the smallpox vaccine. It is because there are similar antigenic protein targets of smallpox and MPXV (Townsend et al. 2013).
Currently, WHO and CDC recommend the smallpox vaccine for healthcare workers, veterinary physicians, researchers, and the population. Studies suggest that viral-inhibitory and cross-neutralizing activity from immune subjects produces a heterogeneous and broad serum antibody response against MPXV, variola major virus (VARV), and vaccinia virus (Fig. 6) (Memariani et al. 2022). The only vaccine which gained marketing approval for both smallpox and monkeypox in the USA is JYNNEOS™, also called Imvanex or Imvamune (U.S. Food and Drug Administration 2021; Nature 2022b; Memariani et al. 2022). Surveillance data from the endemic countries suggest that this vaccine provides 85% protection and prevents the progression of the infection to its severe stages through the mechanism of action provided in Fig. 6. Adequate pre-clinical and clinical trial data can offer this vaccine’s effectiveness (Vivancos et al. 2022). Another vaccine is ACAM2000, but it is approved under the expanded-access Investigational New Drug Application (INDA) of the Food and Drug Administration (FDA). It is constituted of live vaccinia virus and is used in only adult populations with a more significant risk of getting the smallpox virus (Centers for Disease Control and Prevention 2022).
Schematic representation of the pathway for immune system activation by smallpox vaccines. Viral entry in the cell induces innate immunity which causes phagocytosis of the virus. Vaccination induces signalling pathways like Toll-like receptor (TLR) and retinoic acid-inducible gene I-like helicase (RLH), which along with the antigen-presenting cells produced from the innate response, activates the cytokines. The cytokines and helper T cells activate B cells which produce virus-specific antibodies (Moyron-Quiroz et al. 2009; Kennedy et al. 2009)
The drugs available for treating human monkeypox infection include Tecovirimat, Brincidofovir, Cidofovir, and Vaccinia Immune Globulin (VIG) (Petersen et al., 2019). The safety and effectiveness of Tecovirimat can only be confirmed by the pre-clinical data, as no clinical study results are currently available. It has shown efficacy against orthopoxviruses in animal studies. It can only be used under expanded-access INDA. The in vitro and in vivo study results suggest that Brincidofovir and Cidofovir effectively treat poxviruses. However, its use in severe cases of MPXV infection cannot be confirmed due to a lack of clinical trial data (Petersen et al. 2019; Saxena et al. 2022). Nevertheless, Cidofovir shows severe renal toxicity during cytomegalovirus (CMV) infection treatment. On the other hand, VIG can only be used in prophylactic therapy in case of severe deficiency of T-cells due to smallpox and monkeypox. However, still, VIG has not shown benefits as a first-line treatment regimen for human monkeypox (Memariani et al. 2022).
The burden of MPXV infection with co-associated complications
The differentiation of the disease from other diseases caused by different viruses of the orthopoxvirus family is quite difficult. The MPXV infection is self-limiting; however, it requires proper diagnosis and symptomatic and supportive therapy. The delay in treatment can cause the progression of the infection into severe complications. The presence of secondary conditions and disorders can delay the patient’s recovery.
HIV co-infection
Most patients with HIV co-infection showed genital, anogenital, and perianal rashes. A case study conducted by de Sousa et al. (2022) showed a 24-year-old HIV-positive man from Portugal showed initial symptoms of fever, fatigue, pain, and skin lesions (de Sousa et al. 2022). Upon testing the skin lesions from the perianal, genital, oral cavity, and abdomen region, the man, was confirmed with MPXV infection (de Sousa et al. 2022). He had 208 cells/mm3 CD4+ cells and more than 10,000,000 copies/mL of viral load, confirming acute HIV infection (de Sousa et al. 2022). Skin lesions in the papular stage were found in the genital and anogenital areas (de Sousa et al. 2022). The patient showed cervical and inguinal lymphadenopathy (de Sousa et al. 2022). Another 34-year-old male patient with HIV, MPXV, and syphilis infection from Prague, Czech Republic, developed a high fever, enlarged inguinal lymph node, and painless skin lesions near the abdominal and perianal region (Bížová et al. 2022). Both patients were associated with recent sexual intercourse. An interesting study found that smallpox vaccines can induce interferon-γ and -α, IL-1 and IL-2, tumor necrosis factors (TNFs), and other cytokines, providing a para-immunity against sexually transmitted diseases (Mayr et al. 1999). Mathematical models suggest that HIV co-infection with MPXV can increase the basic reproduction number and transmissibility of HIV among animals and human beings (Bhunu et al. 2012).
Lymphadenopathy
The enlargement of lymph nodes is known as lymphadenopathy. This complication is also associated with HIV and MPXV co-infection. It is due to the Primary Viremia stage of the viral replication, which takes place in the lymph node as discussed in the molecular mechanism of infection and pathogenesis. The complication can be understood with the help of an example of a 48-year-old HIV-positive man from France who had painful swelling in the peritonsillar region (Davido et al. 2022). Diagnosis revealed that the patient had voluminous unilateral cervical lymphadenopathy (Davido et al. 2022). However, there was no skin lesion; he had 900 cells/mm3 CD4+ count and less than 20 copies/mL of viral load (Davido et al. 2022).
Complications of the nervous system
The reported symptoms include anorexia, vesiculopapular rash, adenopathy, headache, and fatigue. Magnetic resonance imaging and electroencephalogram reveal meningeal amplification, edema, partial cortex, thalamus abnormalities, and polymorphonuclear pleocytosis of cerebrospinal fluid (Shafaati et al. 2022).
Complications of eye
Permanent loss of vision due to corneal scarring is one of the severe complications of conjunctivitis associated with MPXV infection (Hughes et al. 2014; Shafaati et al. 2022). Other manifestations in the eye include keratitis, photophobia, corneal ulcerations, blepharitis, peri- and orbital skin rashes, and per-auricular node enlargement (Shafaati and Zandi 2022). A study conducted by Hughes et al. (2014), between 2010 and 2013, revealed that 23.1% of the cases suffered from conjunctivitis, of which 61.8% were children under 10 years of age (Hughes et al. 2014).
Complications in children and pregnant women
Children, pregnant women, and immunocompromised patients form the vulnerable group for MPXV infection. The risk factors in children include encephalitis, keratitis, sepsis, and pneumonia. While in pregnant women, the infection becomes more severe in the third trimester, which includes infection of the fetus or even death of the fetus (Vouga et al. 2022).
Few case studies focusing on MPXV infection and treatment
-
An observational study was conducted retrospectively in the UK between 2018 and 2021, revealing the virus’s transmission. One patient acquired the virus within the healthcare facility. The other patient had a travel history from endemic areas and transmitted the virus to a child and an adult within the same household. Few patients were subjected to 200 mg/week of oral Brincidofovir, which showed elevation of metabolic enzyme; however, those receiving 600 mg/twice daily of oral Tecovirimat for 2 weeks had a shorter treatment duration with no adverse effects (Adler et al. 2022).
-
A study was conducted with 12 patients and 147 samples from Barcelona, Spain, between May and June 2022, which showed 67% of the cases showed viral load in feces, 75% in urine, 58% in semen, 83% in the nasopharyngeal region, and 92% in the rectum (Peiró-Mestres et al. 2022).
-
The risk of fatality due to MPXV increases due to HIV. However, if the viral load is kept low, then the risk decreases. A 30-year-old HIV-positive male from Melbourne, Australia, under treatment was infected with MPXV due to his recent travel from Europe. His treatment regimen consisted of Dolutegravir, Lamivudine, and Abacavir, which kept his viral load below 100 copies/mL. The patient showed genital rash after 3 days of the initial onset of symptoms which subsided within 5 days (Hammerschlag et al. 2022).
-
An observational study with 54 subjects (24% had HIV) in a sexual health center in London, UK showed common symptoms such as skin lesions at 1–4 sites consisting of oropharyngeal and anogenital lesions. Other common symptoms included fever and fatigue (Girometti et al. 2022).
-
The primary clinical manifestation of MPXV is skin lesions. However, asymptomatic cases have been found among patients of a male sexual health center in Belgium. A total of 224 male subjects were used in the study, where oropharyngeal and anorectal samples were collected and tested by MPXV-specific polymerase chain reaction (PCR). The subjects tested negative after 21–37 days by PCR. However, none showed any clinical manifestations or contact with infected individuals (De Baetselier et al. 2022).
-
There is a considerable shift from travel-associated MPXV cases to autochthonous cases reported between May and June 2022 in Berlin, Germany. The study considered 262 cases, out of which 21% of cases had a travel history, while 79% of cases had no travel history. However, 58% of cases visited public gatherings during the infectious period (Selb et al. 2022).
Future projections
Bisanzio and Reithinger created a simulation-based model with 50 million subjects based on location and socioeconomic status (Table 4) (Bisanzio and Reithinger 2022). The model emphasizes the non-endemic regions of Europe with higher income than the endemic regions of Africa. Factors such as travel background from the endemic areas, recent contact with patients, and genetic variability are considered. For the study, the researchers introduced 3, 30, and 300 infected individuals within the healthy population, and the spread of the infection will be within 18, 118, and 402 individuals. However, the spread of the disease can be limited due to vaccination and isolation of the individuals showing initial symptoms. The outbreak can last for 23–37 weeks after the initial introduction of infected individuals. Preventive measures such as vaccination, contact tracing, and isolation of infected individuals showed a reduction in the average duration of the outbreak between 60.9 and 75.5%. Till June 2020, no clear evidence of sexual transmission was found. However, recent cases strongly suggest sexual transmission, especially among men. Other constraints like reduction of gatherings, avoiding contact with bodily fluids, and lesions reduced the number of cases to a greater extent (Zumla et al. 2022; Bisanzio and Reithinger 2022).
MPXV infection burden during the COVID-19 pandemic
The initial spread of coronavirus disease (COVID-19) from Wuhan, China, in December 2019 was travel-related, just like the spread of MPXV from the endemic areas. Nevertheless, later, in the mid-2020s, a community spread of COVID-19 became a pandemic. However, there were certain differences between the spread of COVID-19 and MPXV. The first difference is that severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is an RNA virus, while MPXV is a DNA virus (Palmore and Henderson 2022). This characteristic makes SARS-CoV-2 unable to repair the mutations as quickly as other DNA viruses, and thus, more new strains keep evolving within a short period (Bisanzio and Reithinger 2022). Therefore, SARS-CoV-2 has a much higher infectivity rate than MPXV. The SARS-CoV-2 was not limited to only humans but also spread to other animals (Palmore and Henderson 2022). But MPXV is only found within the bushmeat of the central and western-African regions, and a few dogs are imported from the endemic areas. COVID-19 not only spreads from the droplets produced during cough but also from the aerosol which was present in the air of a contaminated area; it is found that MPXV only spreads due to close contact with patients and animals, their skin lesions, cough droplets, and consumption of infected bushmeat. Unlike COVID-19, the MPXV rarely spreads asymptomatically (Palmore and Henderson 2022).
The primary focus of the healthcare facility of a country is to build the capacity to increase the number of diagnostic tests, contact tracing, vaccination with smallpox vaccines, and create awareness among the citizen to avoid the risks of another pandemic. The primary concern with COVID-19 and MPXV is co-infection development within the population. COVID-19 is already found to develop co-infection with Mycoplasma pneumoniae, Acinetobacter baumannii, Klebsiella pneumoniae, Streptococcus pneumoniae, Streptococcus pneumoniae, influenza B virus, metapneumovirus, human immunodeficiency virus, and rhinovirus/enterovirus (Lai et al. 2022). Co-infection with MPXV can lead to a new and more infectious variant of SARS-CoV-2 (Farahat et al., 2022; Chatterjee et al. 2022). This can be prevented using smallpox vaccination programs in endemic-prone areas. The risk of co-infection further increases in sexual transmission, especially in the case of men (Zhu et al. 2022). There is a higher risk of HIV, COVID-19, and MPXV infection, which can cause more single-nucleotide polymorphisms (SNPs) to develop within MPXV, making it more virulent (Zhu et al. 2022). A man from California, USA, recently contracted monkeypox after contracting COVID-19 in late June (Hammerschlag et al. 2022). The man had red lesions along his arms, legs, back, and neck (Daily Mail Online 2022).
The endemic regions of Africa are facing significant healthcare challenges during the COVID-19 pandemic. That is because their healthcare system is currently occupied with diagnosing, treating, and preventing COVID-19, paying less attention to the emerging threat of MPXV. Lack of testing capacity, high cost of treatment, lack of animal surveillance, and lack of proper differentiation between MPXV and smallpox are causing a delay in treatment and prevention of the cases (Uwishema et al. 2022). Furthermore, the patients are stigmatized from going to the nearby healthcare facility for proper diagnosis and treatment. International organizations such as World Health Organization (WHO), World Organization for Animal Health (OIE), Food and Agriculture Organization (FAO), and One Health are collaborating to build capacity in the central and western African regions (Uwishema et al. 2022; Okyay et al. 2022).
Conclusion
The recent COVID-19 pandemic has impacted the immune system of a huge population of the world today. Exposure to SARS-CoV-2 has made individuals prone to secondary co-infections. Moreover, frequent mutations in the virus also have affected the development of herd immunity as was speculated initially. In the background of this pandemic, several infectious diseases like MPXV were being neglected, which is a matter of concern. The increased transmissibility of MPXV is primarily due to natural selection and mutation for a long period. The exact molecular mechanism of MPXV infection is yet to be elucidated and requires extensive research. Sametime, lifestyle-related co-morbidities have added some risk for the contraction of MPXV. The current pandemic also have exposed the weaknesses of the public health sectors across the world and imposed a burden on the states towards diagnosis, treatment, vaccine development, and containment of infectious diseases.
However, after the initial jolt, the scientific community is gradually regrouping to address these shortcomings. WHO declared monkeypox virus infection as an international public health emergency on 23rd July 2022 (World Health Organization 2022d). WHO is providing constant support for its member states in surveillance, contact tracing, clinical investigation, education, training, precaution, prevention, and treatment of the infected individuals (World Health Organization 2022c). WHO and CDC recommended and published interim guidelines for its member states to follow. Integrated Disease Surveillance and Response (IDSR) programs have been established in the endemic regions. The MPXV has been the topmost priority for this program. An enhanced surveillance system has been set up in the states of Nigeria, River states, Bayelsa, and Delta. National Multisectoral Emergency Operations Centre has been upgraded to level II in Nigeria (Velavan and Meyer 2022). There is an increment in the number of laboratory tests in Nigeria, the Democratic Republic of Congo, and the Central African Republic. Hotspot regions have been identified and sealed off in the Central African Republic (Espy et al. 2002). The full-length genome sequence of the MPXV has recently been published and is helping diagnose the infection in European countries. The non-endemic countries have established strict surveillance on travellers from the endemic regions (Ibrahim et al. 1997). There is an increase in diagnostic tests, such as PCR capacity, in countries such as Germany, Netherlands, Portugal, France, and Belgium. Recent diagnostic results revealed the presence of mainly the Western-African virus type in Europe. European Union, USA, and Canada have recently approved the vaccinia virus strain with the virus’s genetically modified Ankara Bavarian Nordic (MVA-BN) strain to prevent human monkeypox (Centers for Disease Control and Prevention 2022; Memariani and Memariani et al. 2022; Rodriguez-Morales 2022).
The current treatment methodology for MPXV is based on the other members of the poxvirus and requires a more validated and targeted treatment approach. Hence, smallpox vaccination should be mandatorily provided until the development of MPXV-specific vaccines to prevent the spread of the infection to some extent. The high-risk population should be educated regarding preventative measures and encouraged to maintain personal hygiene. Identifying animal reserves is one of the most significant steps to contain the infection and assess the risk of transmission to the human population. Standard disease monitoring protocols should be applied based on serological surveys and genomic studies. In conclusion, proactive scientific measures by an individual and the states in meeting the abovementioned demands may help to avert another pandemic in this world.
Data availability
Not applicable.
References
Aden TA, Blevins P, York SW, Rager S, Balachandran D, Hutson CL et al (2022) Rapid diagnostic testing for response to the Monkeypox outbreak - laboratory response Network, United States, May 17-June 30, 2022. MMWR Morb Mortal Wkly Rep 71:904–907. https://doi.org/10.15585/mmwr.mm7128e1
Adler H, Gould S, Hine P, Snell LB, Wong W, Houlihan CF, Osborne JC, Rampling T, Beadsworth MB, Duncan CJ, Dunning J, Fletcher TE, Hunter ER, Jacobs M, Khoo SH, Newsholme W, Porter D, Porter RJ, Ratcliffe L et al (2022) Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis 22:1153–1162. https://doi.org/10.1016/S1473-3099(22)00228-6
Afshar ZM, Rostami HN, Hosseinzadeh R, Janbakhsh A, Pirzaman AT, Babazadeh A, Aryanian Z, Sio TT, Barary M, Ebrahimpour S (2022) The reemergence of monkeypox as a new potential health challenge: a critical review. Authorea Preprints. https://doi.org/10.22541/au.165446104.43472483/v1
Ahmed M, Naseer H, Arshad M, Ahmad A (2022) Monkeypox in 2022: a new threat in developing. Ann Med Surg 78:103975. https://doi.org/10.1016/j.amsu.2022.103975
Alakunle E, Moens U, Nchinda G, Okeke MI (2020) Monkeypox virus in Nigeria: infection biology, epidemiology, and evolution. Viruses 12:1257. https://doi.org/10.3390/v12111257
Alkhalil A, Hammamieh R, Hardick J, Ichou MA, Jett M, Ibrahim S (2010) Gene expression profiling of monkeypox virus-infected cells reveals novel interfaces for host-virus interactions. Virol J. 7:173. https://doi.org/10.1186/1743-422X-7-173
Antinori A, Mazzotta V, Vita S, Carletti F, Tacconi D, Lapini LE, D'Abramo A, Cicalini S, Lapa D, Pittalis S, Puro V (2022) Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Eurosurveillance 27:2200421. https://doi.org/10.2807/1560-7917.ES.2022.27.22.2200421
Bhunu CP, Mushayabasa S, Hyman JM (2012) Modelling HIV/AIDS and monkeypox co-infection. Appl Math Comput 218:9504–9518. https://doi.org/10.1016/j.amc.2012.03.042
Bížová B, Veselý D, Trojánek M, Rob F (2022) Coinfection of syphilis and monkeypox in HIV positive man in Prague. Czech Republic. Travel Med Infect Dis 49:102368. https://doi.org/10.1016/j.tmaid.2022.102368
Bragazzi NL, Kong JD, Mahroum N, Tsigalou C, Khamisy-Farah R, Converti M, Wu J (2022) Epidemiological trends and clinical features of the ongoing monkeypox epidemic: a preliminary pooled data analysis and literature review. Available at SSRN. https://doi.org/10.1002/jmv.27931
Bunge EM, Hoet B, Chen L, Lienert F, Weidenthaler H, Baer LR et al (2022) The changing epidemiology of human monkeypox-a potential threat? A systematic review. PLoS Negl Trop Dis. 16:e0010141. https://doi.org/10.1371/journal.pntd.0010141
Centers for Disease Control and Prevention (2022) Treatment information for healthcare professionals. https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html#:~:text=Tecovirimat%20(also%20known%20as%20TPOXX%2C%20ST%2D246)&text=CDC%20holds%20an%20expanded%20access,a%20pill%20or%20an%20injection. Accessed 8 July 2022
Chatterjee A, Saha R, Mishra A, Shilkar D, Jayaprakash V, Sharma P et al (2022) Molecular determinants, clinical manifestations and effects of immunization on cardiovascular health during COVID-19 pandemic era - a review. Curr Probl Cardiol 101250:101250. https://doi.org/10.1016/j.cpcardiol.2022.101250
Cho CT, Wenner HA (1973) Monkeypox virus. Bacteriol Rev 1973:1–8. https://doi.org/10.1080/22221751.2022.2095309
Daily Mail Online (2022) ‘Incredibly bad luck’: California man says he caught COVID and monkeypox at the same time - leaving him in excruciating pain. https://www.dailymail.co.uk/health/article-11039491/Incredibly-bad-luck-Californian-man-says-caught-Covid-monkeypox-time.html. Accessed 23 July 2022
de Sousa D, Patrocínio J, Frade J, Correia C, Borges-Costa J, Filipe P (2022) Human monkeypox coinfection with acute HIV: an exuberant presentation. Int J STD AIDS 33(10):936–938. https://doi.org/10.1177/09564624221114998
Dhawan M, Priyanka Choudhary OP (2022) Emergence of monkeypox: risk assessment and containment measures. Travel Med Infect Dis 49:102392. https://doi.org/10.1016/j.tmaid.2022.102392
Dhawan M, Emran TB, Islam F (2022) The resurgence of monkeypox cases: reasons, threat assessment, and possible preventive measures. Travel Med Infect Dis 49:102367. https://doi.org/10.1016/j.tmaid.2022.102367
Di Guilo DB, Eckburg PB (2004) Human monkeypox: an emerging zoonosis. Lancet Infect. Dis 4:15–25. https://doi.org/10.1016/S1473-3099(03)00856-9
Espy MJ, Cockerill FR III, Meyer RF, Bowen MD, Poland GA, Hadfield TL, Smith TF (2002) Detection of smallpox virus DNA by light cycler PCR. J Clin Microbiol 40:1985–1988. https://doi.org/10.1128/JCM.40.6.1985-1988.2002
Essbauer S, Meyer H, Porsch-Özcürümez M, Pfeffer M (2007) Long-lasting stability of vaccinia virus (orthopoxvirus) in food and environmental samples. Zoonoses Public Health 54:118–124. https://doi.org/10.1111/j.1863-2378.2007.01035.x
Farahat RA, Abdelaal A, Shah J, Ghozy S, Sah R, Bonilla-Aldana DK et al (2022) Monkeypox outbreaks during COVID-19 pandemic: are we looking at an independent phenomenon or an overlapping pandemic? Ann Clin Microbiol Antimicrob 21:26. https://doi.org/10.1186/s12941-022-00518-2
Ghate SD, Suravajhala P, Patil P, Vangala RK, Shetty P, Rao RSP (2022) Molecular detection of monkeypox and related viruses – challenges and opportunities. https://doi.org/10.31219/osf.io/jtdnz
Girometti N, Byrne R, Bracchi M, Heskin J, McOwan A, Tittle V, Gedela K, Patel S, Gohil J, Nugent D, Suchak T, Dickinson M, Feeney M, Mora-Peris B, Stegmann K, Plaha K, Davies G, Moore LSP, Mughal N et al (2022) Demographic and clinical characteristics of confirmed human monkeypox virus cases in individuals attending a sexual health centre in London, UK: an observational analysis. Lancet Infect Dis 22(9):1321–1328. https://doi.org/10.1016/S1473-3099(22)00411-X
Guagliardo SAJ, Monroe B, Moundjoa C, Athanase A, Okpu G, Burgado J et al (2020) Asymptomatic orthopoxvirus circulation in humans in the wake of a monkeypox outbreak among chimpanzees in Cameroon. Am J Trop Med Hyg 102:206–212. https://doi.org/10.4269/ajtmh.19-0467
Heskin J, Belfield A, Milne C, Brown N, Walters Y, Scott C, Bracchi M, Moore LS, Mughal N, Rampling T, Winston A (2022) Transmission of monkeypox virus through sexual contact–a novel route of infection. J Infect 85(3):334–363. https://doi.org/10.1016/j.jinf.2022.05.028
Hofer U (2022) Case series of monkeypox infections. Nat Rev Microbiol 7:1. https://doi.org/10.1038/s41579-022-00757-2
Hughes C, McCollum A, Pukuta E, Karhemere S, Nguete B, Shongo Lushima R et al (2014) Ocular complications associated with acute monkeypox virus infection. DRC. Int J Infect Dis. 21:276–277. https://doi.org/10.1016/j.ijid.2014.03.994
Ibrahim MS, Esposito JJ, Jahrling PB, Lofts RS (1997) The potential of 5' nuclease PCR for detecting single-base polymorphism in Orthopoxvirus. Mol Cell Probes 11:143–147. https://doi.org/10.1006/mcpr.1996.0093
Kaler J, Hussain A, Flores G, Kheiri S, Desrosiers D (2022) Monkeypox: a comprehensive review of transmission, pathogenesis, and manifestation. Cureus 14:e26531. https://doi.org/10.7759/cureus.26531
Kennedy RB, Ovsyannikova IG, Jacobson RM, Poland GA (2009) The immunology of smallpox vaccines. Curr Opin Immunol 21:314–320. https://doi.org/10.1016/j.coi.2009.04.004
Kugelman JR, Johnston SC, Mulembakani PM, Kisalu N, Lee MS, Koroleva G et al (2014) Genomic variability of monkeypox virus among humans, Democratic Republic of the Congo. Emerg Infect Dis 20:232–239. https://doi.org/10.3201/eid2002.130118
Kumar N, Acharya A, Gendelman HE, Byrareddy SN (2022) The 2022 outbreak and the pathobiology of the monkeypox virus. J Autoimmun 131:102855. https://doi.org/10.1016/j.jaut.2022.102855
Ladnyj ID, Ziegler P, Kima E (1972) A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ 46:593–597
Lai C-C, Wang C-Y, Hsueh P-R (2022) Co-infections among patients with COVID-19: the need for combination therapy with non-anti-SARS-CoV-2 agents? J Microbiol Immunol Infect 53:505–512. https://doi.org/10.1016/j.jmii.2020.05.013
Mahnel H (1987) Experimental results of the stability of poxviruses under laboratory and environmental conditions. Zentralbl Veterinarmed B. J Vet Med 34:449–464
Mathieu E, Dattani S, Ritchie H, Spooner F, Roser M (2015) Monkeypox. OurWorldInData
Mayr A, Mayr B (1999) A new concept in prophylaxis and therapy: paramunization by poxvirus inducers. Pesq. Vet. Bras 19:3–4. https://doi.org/10.1590/S0100-736X1999000300001
Memariani M, Memariani H (2022) Multinational monkeypox outbreak: what do we know and what should we do? Irish J Med Sci 1971:1–2. https://doi.org/10.1007/s11845-022-03052-4
Mileto D, Riva A, Cutrera M, Moschese D, Alessandro M, Meroni L, Giacomelli A, Bestetti G, Rizzardini G, Gismondo MR, Antinori S (2022) New challenges in human monkeypox outside Africa: a review and case report from Italy. Travel Med Infect Dis 49:102386. https://doi.org/10.1016/j.tmaid.2022.102386
Minhaj FS, Ogale YP, Whitehill F, Schultz J, Foote M, Davidson W, Hughes CM, Wilkins K, Bachmann L, Chatelain R, Donnelly M, Mendoza R, Downes BL, Roskosky M, Barnes M, Gallagher GR, Basgoz N, Ruiz V, Kyaw N et al (2022) Monkeypox Response Team 2022. Monkeypox outbreak - nine states, May 2022. MMWR. Morb Mortal Wkly Rep 71:764–769. https://doi.org/10.15585/mmwr.mm7123e1
Morgan CN, Whitehill F, Doty JB, Schulte J, Matheny A, Stringer J, Delaney LJ, Esparza R, Rao AK, McCollum AM (2022) Environmental persistence of monkeypox virus on surfaces in household of person with travel-associated infection, Dallas, Texas, USA, 2021. Emerg Infect Dis 28:1982–1989. https://doi.org/10.3201/eid2810.221047
Moyron-Quiroz JE, McCausland MM, Kageyama R, Sette A, Crotty S (2009) The smallpox vaccine induces an early neutralizing IgM response. Vaccine 28:140–147. https://doi.org/10.1016/j.vaccine.2009.09.086
Nature (2022a) Monkeypox goes global: why scientists are on alert. https://doi.org/10.1038/d41586-022-01421-8. Accessed 25 May 2022
Nature (2022b) Monkeypox vaccination begins — can the global outbreaks be contained?. https://doi.org/10.1038/d41586-022-01587-1. Accessed 9 July 2022
Okyay RA, Bayrak E, Kaya E, Şahin AR, Koçyiğit BF, Taşdoğan AM, Avcı A, Sümbül HE (2022) Another epidemic in the shadow of COVID 19 pandemic: a review of monkeypox. Proteins 7:10. https://doi.org/10.14744/ejmo.2022.2022
Oprea C, Ianache I, Piscu S, Tardei G, Nica M, Ceausu E et al (2022) First report of monkeypox in a patient living with HIV from Romania. Travel Med Infect Dis 49:102395. https://doi.org/10.1016/j.tmaid.2022.102395
Petersen E, Kantele A, Koopmans M, Asogun D, Yinka-Ogunleye A, Ihekweazu C et al (2019) Human monkeypox: epidemiologic and clinical characteristics, diagnosis, and prevention. Infect Dis Clin North Am 33:1027–1043. https://doi.org/10.1016/j.idc.2019.03.001
Rheinbaben FV, Gebel J, Exner M, Schmidt A (2007) Environmental resistance, disinfection, and sterilization of poxviruses. In: Mercer AA, Schmidt A, Weber O (eds) Poxviruses. Basel, Birkhäuser Basel, pp 397–405
Rodriguez-Morales AJ (2022) Monkeypox and the importance of cutaneous manifestations for disease suspicion. Microbes, Infect Chemother 2:e1450. https://doi.org/10.54034/mic.e1450
Shafaati M, Zandi M (2022) Monkeypox virus neurological manifestations in comparison to other orthopoxviruses. Travel Med Infect Dis 49:102414. https://doi.org/10.1016/j.tmaid.2022.102414
Shchelkunov SN, Totmenin AV, Safronov PF, Mikheev MV, Gutorov VV, Ryazankina OI et al (2002) Analysis of the monkeypox virus genome. Virology 2002:172–194. https://doi.org/10.1006/viro.2002.1446
The Times of India (2022) India’s 4th monkeypox case in Delhi, patient has no foreign travel history. https://timesofindia.indiatimes.com/india/indias-4th-monkeypox-case-in-delhi-patient-has-no-foreign-travel history/articleshow/93096145.cms. Accessed 1 August 2022
Townsend MB, Keckler MS, Patel N, Davies DH, Felgner P, Damon IK, Karem KL (2013) Humoral immunity to smallpox vaccines and monkeypox virus challenge: proteomic assessment and clinical correlations. J Virol 87:900–911. https://doi.org/10.1128/JVI.02089-12
U.S. Food & Drug Administration 2021 JYNNEOS. https://www.fda.gov/vaccines-blood-biologics/jynneos (accessed 15 August 2022)
Uwishema O, Adekunbi O, Peñamante CA, Bekele BK, Khoury C, Mhanna M et al (2022) The burden of monkeypox virus amidst the COVID-19 pandemic in Africa: a double battle for Africa. Ann Med Surg (Lond) 80:104197. https://doi.org/10.1016/j.amsu.2022.104197
Velavan TP, Meyer CG (2022) Monkeypox 2022 outbreak: an update. Tropical Med Int Health 27:604–605. https://doi.org/10.1111/tmi.13785
Vivancos R, Anderson C, Blomquist P, Balasegaram S, Bell A, Bishop L, Brown CS, Chow Y, Edeghere O, Florence I, Logan S (2022) Community transmission of monkeypox in the United Kingdom, April to May 2022. Eurosurveillance 27:2200422. https://doi.org/10.2807/1560-7917.ES.2022.27.22.2200422
World Health Organization (2022a) Monkeypox - United Kingdom of Great Britain and Northern Ireland. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON383. Accessed 8 July 2022
World Health Organization (2022b) Multi-country monkeypox outbreak: situation update. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON396. Accessed 12 July 2022
World Health Organization (2022c) Outbreaks and emergencies bulletin. https://www.afro.who.int/health-topics/disease-outbreaks/outbreaks-and-other-emergencies-updates . Accessed 2 July 2022
World Health Organization (2022d) WHO director-general declares the ongoing monkeypox outbreak a public health emergency of international concern. https://www.who.int/europe/news/item/23-07-2022-who-director-general-declares-the-ongoing-monkeypox-outbreak-a-public-health-event-of-international-concern . Accessed 23 July 2022
Zhu F, Li L, Che D (2022) Monkeypox virus under COVID-19: caution for sexual transmission - correspondence. Int J Surg 104:106768. https://doi.org/10.1016/j.ijsu.2022.106768
Zumla A, Valdoleiros SR, Haider N, Asogun D, Ntoumi F, Petersen E, Kock R (2022) Monkeypox outbreaks outside endemic regions: scientific and social priorities. Lancet. Infect Dis 400:23. https://doi.org/10.1016/S1473-3099(22)00354-1
Au NH, Portillo MT, Marwah A, Thomas-Bachli A, Demarsh PA, Khan K, Bogoch II (2022) Potential for monkeypox exportation from west and Central Africa through global travel networks. J Travel Med. https://doi.org/10.1093/jtm/taac072
Bisanzio D, Reithinger R (2022) Projected burden and duration of the 2022 Monkeypox outbreaks in non-endemic countries. Lancet Microbe. https://doi.org/10.1016/S2666-5247(22)00183-5
Chakraborty C, Bhattacharya M, Nandi SS, Mohapatra RK, Dhama K, Agoramoorthy G (2022) Appearance and re-appearance of zoonotic disease during the pandemic period: long-term monitoring and analysis of zoonosis is crucial to confirm the animal origin of SARS-CoV-2 and monkeypox virus. Vet Q 1-1. https://doi.org/10.1080/01652176.2022.2086718
Davido B, D'Anglejan E, Baudoin R, Dahmane L, Chaud A, Cortier M et al (2022) Monkeypox outbreak 2022: an unusual case of peritonsillar abscess in a person previously vaccinated against smallpox. J Travel Med 29(6). https://doi.org/10.1093/jtm/taac082
De Baetselier I, Van Dijck C, Kenyon C, Coppens J, Van den Bossche D, Smet H, Liesenborghs L, Fien V, Block T, Eric F, Koen V, Marjan VE (2022) Asymptomatic monkeypox virus infections among male sexual health clinic attendees in Belgium. SSRN Electron J. https://doi.org/10.2139/ssrn.4142074
Hammerschlag Y, MacLeod G, Papadakis G, Adan Sanchez A, Druce J, Taiaroa G, Savic I, Mumford J, Roberts J, Caly L, Friedman D, Williamson DA, Cheng AC, McMahon JH (2022) Monkeypox infection presenting as genital rash, Australia, May 2022. Euro Surveill. 27. https://doi.org/10.2807/1560-7917.ES.2022.27.22.2200411
Nörz D, Brehm TT, Tang HT, Grewe I, Hermanussen L, Matthews H et al (2022) Clinical characteristics and comparison of longitudinal qPCR results from different specimen types in a cohort of ambulatory and hospitalized patients infected with monkeypox virus. J Clin Virol 105254. https://doi.org/10.1016/j.jcv.2022.105254
Palmore TN, Henderson DK (2022) Adding new fuel to the fire: monkeypox in the time of COVID-19-implications for health care personnel. Ann Intern Med M22-1763: Advance online publication. https://doi.org/10.7326/M22-1763
Peiró-Mestres A, Fuertes I, Camprubí-Ferrer D, Marcos MÁ, Vilella A, Navarro M, Rodriguez-Elena L, Riera J, Català A, Martinez MJ, Blanco JL (2022) Frequent detection of monkeypox virus DNA in saliva, semen, and other clinical samples from 12 patients, Barcelona, Spain, May to June 2022. Euro Surveill 27. https://doi.org/10.2807/1560-7917.ES.2022.27.28.2200503
Saxena SK, Ansari S, Maurya VK, Kumar S, Jain A, Paweska JT et al (2022) Re-emerging human monkeypox: a major public-health debacle. J Med Virol. https://doi.org/10.1002/jmv.27902
Selb R, Werber D, Falkenhorst G, Steffen G, Lachmann R, Ruscher C, McFarland S, Bartel A, Hemmers L, Koppe U, Stark K, Bremer V, Jansen K (2022) A shift from travel-associated cases to autochthonous transmission with Berlin as epicentre of the monkeypox outbreak in Germany, May to June 2022. Euro Surveill 27. https://doi.org/10.2807/1560
Vouga M, Nielsen-Saines K, Dashraath P, Baud D (2022) The monkeypox outbreak: risks to children and pregnant women. Lancet Child Adolesc Health. https://doi.org/10.1016/S2352-4642(22)00223-1
Acknowledgements
Ankit Majie is thankful to AICTE, New Delhi, for awarding the fellowship under PG-Scholarship for qualifying Graduate Pharmacy Aptitude Test (GPAT-2021); Rajdeep Saha is thankful to Birla Institute of Technology, Mesra, for providing the Institute Research Fellowship.
Author information
Authors and Affiliations
Contributions
Data collection and original draft preparation were performed by Ankit Majie. Conceptualization and investigation of the concerned topic were performed by Rajdeep Saha. Provision of necessary resources, supervision, reviewing, and editing was done by Biswatrish Sarkar. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Since the identification of the monkeypox virus in 1959, the spread of the infection has been sporadic.
• However, there has been a multiple-country infection outbreak since May 2022.
• Currently, no established treatment options are available; only precautionary, supportive, and symptomatic care is necessary.
• The World Health Organization (WHO), on 23rd July 2022, declared monkeypox virus infection an international public health emergency.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Majie, A., Saha, R. & Sarkar, B. The outbreak of the monkeypox virus in the shadow of the pandemic. Environ Sci Pollut Res 30, 48686–48702 (2023). https://doi.org/10.1007/s11356-023-26098-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26098-y