Abstract
Phthalate esters, such as di(n-butyl) phthalate, (DBP), are synthetic chemical pollutants commonly used as plasticizers in the manufacture of plastics. In the present study, we investigated the effects of DBP in the testes of adult male quails (Coturnix cortunix japonica) exposed by oral gavage to variable doses of DBP (0 [control], 1, 10, 50, 200, and 400 mg/kgbw−d), for 30 days during the prepubertal period, using histo-morphometric and ultrastructural techniques. Generally, significant decreases in seminiferous tubular diameter (STD) and epithelial height (SEH) were observed predominantly at the highest DBP doses (200 and 400 mg/kg), as compared to medium (50 mg/kg), and lowest doses (1 and 10 mg/kg) as well as the control group. Ultrastructurally, apparent dose-specific degenerative changes were observed in the Leydig cells. The lowest DBP doses (1 and 10 mg/kg) did not produce significant effects on Leydig cell ultrastructure, whereas, at the highest doses (200 and 400 mg/kg), the Leydig cells were remarkably conspicuous in the interstitium and appeared foamy. There was a preponderance of electron-lucent lipid droplets which crowded out the normal organelles of the cell, as well as increases in the number of dense bodies in the cytoplasm. The smooth endoplasmic reticulum (sER) was less obvious, compacted, and wedged between the abundant lipid droplets and mitochondria. Taken together, these findings indicate that pre-pubertal exposure of precocious quail birds to DBP, produced parameter-specific histometric tubular changes, as well as dose-dependent cyto-structural derangement of the Leydig cells; which consequently may lead to overt reproductive impairments in the adult bird in the environment.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Components used in plastics, such as phthalate esters (PEs) are present in most consumer products including children’s toys and body care products (Hubinger and Havery 2006; Schettler 2006; Andrady and Neal 2009; Perico et al. 2022). PEs are also known as endocrine-disrupting compounds (EDCs) owing to their ability to modulate the endocrine system and thereby cause adverse effects on reproductive processes in humans and wildlife species in the environment (Diamanti-Kandarakis et al. 2009). Numerous wildlife species and humans are currently exposed to a wide variety of potential endocrine disrupters (ED), and at concentrations that produce varying effects due to species differences in the endocrine regulation of reproduction (Ito et al. 2005; De Lange et al. 2009). In most mammalian models, PEs, including di (n-butyl) phthalate (DBP), are known to induce testicular injury and adversely affect testicular differentiation and spermatogenesis by provoking germ cell loss, altered Leydig cell function and testicular atrophy (Ge et al. 2007; Spade et al. 2015; Walker et al. 2021).
Spermatogenesis represents a complex and dynamic process, and its development is hinged on precisely timed events (Hess and Franca 2009). It is well established that spermatogenesis is sensitive to environmental toxicants, particularly EDCs (Alam et al. 2010a; Yeung et al. 2011; Moody et al. 2013; Jenardhanan et al. 2016). Several EDCs affect reproductive function by inducing apoptosis in germ cells, thereby causing defective spermatogenesis (Cheng et al. 2011; Wan et al. 2013). Testicular toxicity resulting from exposure to PEs is age-dependent, with studies conducted on rats having shown that testicular damage due to PE exposure is more severe in sexually immature (pre-pubertal) than in mature adult male rats (David et al. 2000; Dalgaard et al. 2001; Hannas et al. 2011). Exposure of adult male rats to high doses of certain phthalates resulted in rapid and severe changes in the testis (Dalgaard et al. 2001). In testicular tissues, the primary cellular target of phthalate-induced exposure are Sertoli and/or Leydig cells, which exhibit biochemical and morphological changes (Moffit et al. 2007; Shirai et al. 2013; Wakui et al. 2013; Bello et al. 2014; 2019).
There are few in vivo or in vitro reproductive studies which characterize the adverse effects of phthalate-induced exposure, especially at the ultrastructural level (Andriana et al. 2004a, b; Tay et al. 2007; Shirai et al. 2013; Qin et al. 2018). Therefore, the characterization of chemically-induced cellular alterations, using various morphologic tools, has been shown to be a valid approach for assessment of the deleterious effects of environmental toxicants on organs and tissues (Creasy 2003), with a view to gaining a better insight into the mechanisms through which phthalate exposure causes tissue damage.
Despite extensive research into environmental influences on male reproductive health, the scope of the problem is largely unclear, and specifically, the reproductive health implications of phthalate-induced exposure are not well documented. In general, the mechanisms of toxicity of phthalates at the biological level, are either poorly understood or unknown. Furthermore, studies on the adverse effects of phthalate esters on avian male reproduction, with regard to precise histo-morphometric and ultrastructural evaluation of the seminiferous tubule, are scanty. It is possible that xenobiotic compounds, such as DBP, may induce different effects in birds than in mammals (Ottinger et al. 2005).
Therefore, the aim of the present study was to investigate the seminiferous tubular (histometric) and Leydig cell ultrastructural (subtle) changes in the testis of adult Japanese quail (Coturnix coturnix japonica) following a 30-day (repeated) exposure to di (n-butyl) phthalate DBP at pre-pubertal period, using histometric and ultrastructural techniques. It is hoped that the data obtained in this study would provide a clear morphological evidence of the detrimental effects of endocrine-disrupting properties of the environmental toxicant (DBP), in this specie.
Materials and methods
Chemicals
Di (n-butyl) phthalate (DBP) [CAS Number 84–74-2, technical grade, 99% purity] was purchased from Sigma-Aldrich (Pty) Ltd. (Johannesburg, South Africa). All other reagents were of the highest commercially available grade.
Animals, experimental design, and dosing considerations
Ninety (90) pre-sexed, 6-week-old male Japanese quails, Coturnix coturnix japonica weighing (180-200 g), procured from the Poultry section of the Irene Animal Improvement Research Station, Gauteng Province, Pretoria, were used for this study. The animals were housed, until 10 weeks of age, in battery cages with a dimension of 49 × 95 × 51 cm, in a well-ventilated room maintained at standard temperature (25 ± 2 °C), relative humidity of (25 ± 5%), and controlled photoperiod of 16L:8D light/dark cycle. (SANS Guidelines 2008). Throughout the experimental period, the animals were fed on a special (i.e., phthalate/bisphenol-A (BPA)-free), high-protein diet (ObaroFeeds™, Pretoria, South Africa), with drinking water provided ad libitum.
The experiment was designed in accordance with the avian toxicity testing studies (OECD Guidelines 2010). The animals were randomly divided into six groups (n = 15) with individuals exposed by oral gavage to different doses of DBP dissolved in corn oil (at 0 [control], 1, 10, 50, 200, and 400 mg/kg body weight), once daily for 30 consecutive days. The control group received only the corn oil base. These DBP doses were chosen based on previously published study (Bello et al. 2014); and to test the effects of environmentally-relevant (low) doses (i.e., 1–50 mg/kg) and doses that are regarded at the level of extreme and acute exposure conditions (i.e., 200 and 400 mg/kg). Further justification for the choice of doses was also based on the fact that the testicular cyto-morphological effects of DBP in avian species are limited and generally lacking in the literature, thereby warranting the use of a broad range of doses. In the rat model, for instance, the no-observed-adverse-effect-level (NOAEL) of DBP by intra-gastric lavage was 50 mg/kg/day (Mylchreest et al. 2000; Zhang et al. 2004); while a dose of 0, 15, and 35 µg DBP/L (Aoki et al 2011) and 0.1, 0.5.1.0, 5.0, or 10 ppm DBP (Lee and Veeramachaneni 2005) have been reported in fish and amphibians, respectively. Therefore, the choice of tested doses used in the present avian study was aimed to span possible environmentally-relevant concentrations, as well as to achieve a dose–response relationship of DBP exposure approach, useful for developing a mechanistic model that incorporates cellular responses in adult quail testis following pre-pubertal exposure to various DBP dose levels. After the last administration of DBP, the experimental and control animals were sacrificed, using an overdose of carbon dioxide (CO2) inhalation anesthesia. The testes were quickly excised and blocks of tissues were fixed for light (histological) and transmission electron microscopy techniques (TEM), as described below.
Transmission electron microscopy (TEM) procedure
Small blocks (i.e., 1 mm3) of testicular tissue were taken from each bird (control and DBP-treated groups); (n = 5 per group), and were immediately fixed by immersion in small Eppendorf™ tubes containing 4% glutaraldehyde in 0.13 M Millonig’s phosphate buffer at pH 7.4, for at least 24 h. The samples were then post-fixed in similarly buffered 1% Osmium tetroxide for 2 h, dehydrated in a series of graded ethanol concentrations, and embedded in epoxy-resin at a ratio of 1:2 for 1 h, 1:1 for 2 h, and 100% resin overnight. For each bird, three separate tissue blocks from each testis were prepared for microtomy. Semi-thin sections, of 1 µm thickness, were cut with a diamond knife and stained with toluidine blue. Stained sections were photographed with a DP 72 camera mounted on Olympus BX 63 microscope (Olympus Corporation, Tokyo, Japan). Ultra-thin (50–90 nm) sections of selected areas were cut on a Reichert-Jung Ultracut (C. Reichart AG, Vienna, Austria) using a diamond knife, collected onto copper grids, and stained with Reynold’s lead acetate and counterstained by using an aqueous saturated solution of uranyl citrate (Ayache et al. 2010). The sections were examined in a Phillip CM 10 transmission electron-microscope-TEM (Phillips Electron Optical Division, Eindhoven, Netherlands), operated at 80 kV. A mega view III side-mounted digital camera (Olympus Soft Imaging Solutions GmbH, Munster, Germany) was used to capture the images, and iTEM software (Olympus Soft Imaging Solutions GmbH, Münster, Germany) to adjust the brightness and contrast.
Histological and micro-stereological procedures
For light microscopic observations, testicular samples from DBP-treated and control groups were immediately fixed in 10% Neutral Buffered Formalin (NBF) for 24 h, dehydrated in ascending grades of ethanol, and embedded in paraffin; sectioned at 5 µm, and stained with hematoxylin and eosin (H&E), and subsequently examined under an Olympus BX 63® (Olympus Corporation, Tokyo, Japan) microscope.
Micro-stereological evaluation of the seminiferous tubular epithelium
Quantitative measurements of the seminiferous tubule were done on forty-two (42) H and E-stained histological sections per testis, using a stereological module of computer-assisted digital image analyzer (CellSens®Dimension ver 1.6 software program) running on a digital computer. The digitized images were acquired, using an Olympus BX63® (Olympus Corporation, Tokyo, Japan) microscope, fitted with an Olympus DP72 camera. The CellSens® Dimension stereological module works in tandem with the CellSens® Dimension Multiple Image Alignment (MIA) tools and was used to facilitate the creation of high-resolution, panoramic digital images, capable of covering the complete microscope stage. In this way, sequential imaging across different geometric parameters and/or areas of a specimen were possible. Briefly, sections of testicular tissue were examined at low magnification (10 ×), and then at high magnification (100 ×) for more detailed analyses of seminiferous tubular structure, as described by (Romano et al. 2010). These included analyzing the linear morphometry of the following parameters: seminiferous tubular diameter (STD), seminiferous epithelial height (SEH), seminiferous luminal diameter (SLD), and the area of the seminiferous tubule (AST).
In estimating STD, morphometric measurements of the seminiferous tubular epithelium were taken from one end of the basal lamina to another, the SEH measurements were taken as the linear length of the seminiferous tubule from the boundary layer (basal lamina) to the luminal edge, while SLD, were calculated as the longest measurement from one luminal edge to the other, while the cross-sectional areas of seminiferous tubules of circular transverse sections of seminiferous tubules were taken for each group (n = 7), as the AST measurements. For each seminiferous tubule parameter, measurements were performed on, at least, 20 round or nearly round tubular profiles, chosen randomly in each microslide (n = 7)/group. With the aid of a digitized mouse, at least three measurements were made, for each parameter, in each animal group. Captured data (digitized images) were automatically calculated by interactively sketching each of the transversely sectioned seminiferous tubule measurements and then averaged. These parameters were sequentially determined using a systematic, random sampling scheme (Gundersen and Jensen 1987; Cruz-Orive 1993), which allowed for an unbiased numerical estimation of the parameters.
Statistical analysis
All the micro-stereological data generated were expressed as mean ± standard error of the mean (SEM). After normal students’ t-testing for the homogeneity of variance in the dataset, a one-way analysis of variance (ANOVA) was applied to evaluate the differences between treatments for each parameter. Subsequently, the data were subjected to Duncan’s multiple comparison tests, used to make a comparison between DBP treatment groups, when and where appropriate. All statistical analyses were carried out, using Statistical Product and Service Solutions (SPSS) for Windows, 19th edition (IBM, IL, USA). The value of p < 0.05 was considered significant.
Results
Effects of DBP on Leydig cell morphology
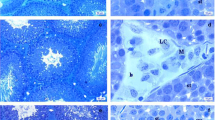
Figure 1A–C depicts the toluidine-blue (semi-thin) transverse sections of adult quail testes at various dosage regimes, as viewed under the bright-field microscopy. In quail testis fed by intragastric gavage with DBP (0 mg/kg) or low/median doses (1, 10, 50 mg/kg), the interstitium (Fig. 1A, B), showed isolated masses of normal Leydig cells with few lipids droplets. However, at high DBP doses (200 mg- and 400 mg/kg), the Leydig cell cytoplasm increasingly displayed aggregations of lipid droplets (Fig. 1C). Ultrastructurally, there were no evident morphological abnormalities in Leydig cells of adult quails fed with corn oil (control) or lowest doses of DBP (1 and 10 mg/kg) during the prepubertal period (Fig. 2A and B). However, in the medium (50 mg/kg) DBP dose group, apparently normal Leydig cells were seen together with abnormal Leydig cells in the interstitium (Fig. 3A). The latter (Fig. 3B) displayed numerous lipid droplets which crowded out the normal organelles of the cell, such as mitochondria and smooth endoplasmic reticulum (sER). On the other hand, significant changes in Leydig cells were observed in the highest (200 or 400 mg/kg) DBP dose groups. The Leydig cells were conspicuous in the interstitium because they appeared foamy (Fig. 4A). Some Leydig cell nuclei were irregular in shape because of lipid droplets that indented them (Fig. 4B), compared to the control group (Fig. 2A). In the neighborhood Leydig cells (Fig. 4C), there was an increase in the number of mitochondria (white asterisk) and dense bodies; as well as a preponderance of lipid droplets in the cytoplasm (Fig. 4D). In addition, the smooth endoplasmic reticulum (sER) was less obvious, and wedged between the abundant lipid droplets and mitochondria (Fig. 4E).
Photomicrograph of toluidine blue-stained (semi-thin) sections of the interstitium of adult quail testis taken from A DBP (0 mg/kg) control or low dose (DBP (1, 10 mg/kg) group and B medium-dose (DBP 50 mg/kg) groups with isolated masses of Leydig cells (white arrows) found in close association with blood vessels (Bv), and displaying a few lipids. Spermatogenesis appears normal. C is a high-powered view of the DBP-treated (200 mg or 400 mg/kg) group. Note the abundance of lipid droplets in the basal part of Sertoli cell cytoplasm (white squat arrows), but rare in the adluminal compartment (white arrowhead ). The intertubular space (Int) shows Leydig cell cytoplasm filled with numerous lipid droplets. Seminiferous epithelium (SE), Spermatogonia (Sp-g), Spermatocytes (Spt-cyt), Sertoli Cell (SC) and elongated spermatids (E-Spd) at various stages of differentiation. Toluidine blue stain (× 40)
A and B Transmission electron micrograph of the testis of DBP-(0 mg/kg) control or low doses DBP (1, 10 mg/kg) showing parts of seminiferous tubules and normal Leydig cells (LC) seen in the Interstitium. Seminiferous epithelium (SE), blood vessel (Bv), myofibroblast (Mc), boundary tissue (arrow heads). B High power view of part of a normal Leydig cell showing numerous mitochondria (Mt), abundant smooth endoplasmic reticulum (sER), a few lipid droplets (black arrows) and a mildly heterochromatic nucleus (Nc) Bar in Fig. 2A (5 µm), Bar in Fig. 2B (10 µm)
Electron micrograph section of the interstitium (Int) of the quail testis taken from DBP medium-dose (50 mg/kg) group. With the aid of a free-form (red, dash-line) demarcation, Leydig cell A appears normal, while the other, B, shows an abnormal accumulation of intracellular lipid droplets (white arrowheads), which crowded out normal organelles. The mitochondria (Mt) in both cells are, generally, of different sizes. Seminiferous epithelium (SE), Nucleus (Nc) Bar = 5 µm
A Survey electron micrograph of the testicular interstitium taken from a DBP high-dose (200 and 400 mg/kg) groups, displaying numerous, “foamy” Leydig cells (LC). B is high-powered view of (A) showing two neighboring Leydig cells (LC) with indented nuclei, due to lipid droplets (Lpd) in the interstitium. C is a high-powered view of a Leydig cell displaying numerous mitochondria (white asterisk), very few lipid droplets (black arrow) and dense bodies (arrowhead) in the cytoplasm. D and E (high-power view) are parts of Leydig cells, exhibiting numerous lipid droplets (Lpd) in the interstitium. In E, aside from the preponderance of lipid droplets, the dilated smooth endoplasmic reticulum (sER) is squashed between other organelles and abundant inclusion bodies (white arrow). Mt, mitochondria; Lpd, Lipid droplets; Spg, spermatogonia; SC, Sertoli cell; LC, Leydig cells; LC-cyt, Leydig cell cytoplasm; SE, seminiferous epithelium. Bars: A and D = 10 µm; B, C, and E = 5 µm
Effects of DBP on seminiferous tubulo-morphometry
In the current study, Fig. 5A-D, shows the graphical summaries of morphometric data of various testicular (i.e., STD, SEH, SLD, and AST) parameters, evaluated in both control and DBP-treated groups. Relatively, there was no significant change (p > 0.05) in STD values between low and medium (1, 10, 50 mg/kg) DBP-treated and control groups, but there was a slight decrease (p < 0.05) in STD at the highest (200 and 400 mg/kg) DBP dose groups, when compared to the control vehicle group (Fig. 5A). In contrast, the seminiferous tubules of all DBP-exposed quails showed apparent dose-specific reductions in germinal epithelial height. Furthermore, the maximum reduction in SEH occurred at the highest (200 and 400 mg/kg) DBP dose groups, compared with the controls, while the epithelial height in the medium (50 mg/kg) and lowest (1 and 10 mg/kg) DBP treatment groups were slightly reduced, relative to the control group (Fig. 5B). On the other hand, there was a slight but progressive increase (albeit not significant, p > 0.05) in SLD values, from low to the highest DBP dose levels (Fig. 5C), as compared to DBP control groups. AST values decreased as the dose level of DBP increased, such that the AST was highest at (1 mg- and 10 mg/kgbwt) DBP groups and lowest at (200 mg- and 400 mg/kgbwt) DBP dose groups (Fig. 5D).
Discussion
It was previously demonstrated that PEs, including DBP, are anti-androgenic environmental contaminants that cause adverse biological effects on male reproductive health, growth, and development in both human and wildlife (Foster et al. 2001; Oehlmann et al. 2009; Alam et al. 2010a; Bello et al. 2014, 2019). Since reproductive development is a continuous process throughout ontogeny in vertebrate species, it is susceptible to changes in physiology due to exposure to environmental contaminants at different stages of development, including structural differentiation and hormonal synthesis (Diamanti-Kandarakis et al. 2009). In addition, there has been considerable difficulty in creating universally accepted and reliable end-point(s) for exposure of avian species to EDCs due to a vast array of reproductive strategies. However, mammalian, reptilian, and piscean data have provided valuable insights on the likely mechanisms of action of EDCs. The impairment of testicular development as a result of PE exposure has been shown to be age-dependent, with mature animals being less sensitive than immature animals (Dalgaard et al. 2001). Although previous studies have shown compromised male copulatory behavior in the Japanese quail after exposure to EDCs, and, thus, providing a reliable and sensitive indicator of embryonic gonadal hormone exposure (Adkins 1979), few studies have investigated ultrastructural and morphometric changes in the testis following exposure to environmental contaminants. Therefore, the present study is unique, because it provides valuable information on PEs-mediated alterations of testicular variables and that may have consequences on avian reproductive health.
Effect of pre-pubertal exposure to DBP on the ultrastructure of Leydig cell
The Leydig cells, exclusively, are the primary sites of testicular androgen production in males (Griswold and Behringer 2009; Shima et al. 2013) Androgens are essential hormones necessary for the regulation of spermatogenesis. In this study, we observed that Leydig cells in the highest DBP (200 and 400 mg/kg) dose groups showed marked cyto-morphological changes, resulting in reduced cell size, compared with other treatment groups or controls. Our findings were in accordance with previous observations on the ultrastructural changes that included diminished amount of sER and a significant reduction in Leydig cell size, due to nuclear shrinkage and hyper-chromatization as reported by Blanco et al. (2010) in rodents. In our present study, Leydig cells in the DBP-exposed groups were characterized by a preponderance of electron-lucent lipid droplets which crowded out the normal organelles of the cell. Interestingly, the observed DBP-induced aggregation of lipid droplets in the cytoplasm may be indicative of either an increased lipid synthesis (lipidosis) or reduced utilization of lipids, and more specifically, cholesterol, by the cells. Increased synthesis and accumulation of lipid droplets in testes have been observed in pre-pubertal rats exposed to DBP (Alam et al. 2010b). Previously, Bell (1982) demonstrated that in certain tissues, di-(2-ethylhexyl) phthalate [DEHP] inhibited fatty acid synthesis. Lipid droplets in Leydig cells are generally rich in cholesteryl esters and serve as the reservoir of cholesterol for testosterone synthesis (Fujimoto and Parton 2011; Kraemer et al. 2013; Shen et al. 2016) and, hence, they may increase in number and/or size when the synthesis of testosterone is inhibited.
The exact mechanism by which DBP exposure produced lipidosis, as observed from the present study, is not well understood. However, considering that Leydig cell steroidogenesis begins with intracellular molecular trafficking (transport) of de-esterified substrate cholesterol from the lipid droplets into mitochondria (Hales et al. 2005; Manna et al. 2013; Tarique et al. 2019), it is probable that PEs, including DBP, inhibit steroid production by acting at the level of cholesterol transport across mitochondrial membranes, resulting in the accumulation of lipid droplets in the cytosol of the Leydig cells (Hu et al. 2010; Savchuk et al. 2015). In this context, the present study has demonstrated that a 30-day exposure of precocious Japanese quail testis to DBP, during the prepubertal period, could alter spermatogenesis in adult birds, especially at high dose levels. The main changes/effect being are cyto-morphological alterations in the Leydig cell, which changes could lead to deranged spermatogenesis and, consequently, infertility in this species. It is suggested that future studies are designed to involve more specific and in-depth indicators of degenerative changes such as apoptosis, along with other cyto-morphological approaches in the elucidation of biochemical events responsible for DBP anti-androgenic effects in the quail testis.
Effect of DBP on seminiferous tubular-histomorphometry
Several qualitative (Alam et al. 2010a; Ahbad and Barlas 2013; Moody et al. 2013; Shirai et al. 2013; Shono and Taguchi 2014) and quantitative (Auharek et al. 2010; Wakui et al. 2013) studies have been performed in order to detect testicular toxicity due to PE exposure in animals. Relatively, quantitative studies on the seminiferous tubule epithelium have received very little attention. Recent reports have shown altered testicular differentiation following prenatal and/or post-natal exposure to DBP (Alam et al. 2010a; Jobling et al. 2011; Ahbad and Barlas 2013; Giribabu et al. 2014). The STD is used as an important, functional evaluation of spermatogenetic activity in experimental and toxicological assays. In the present study, a significant reduction was observed in the STD of quails treated with high doses (200 and 400 mg/kg) of DBP, as compared to low doses or control groups. The decrease in tubular diameter with the higher dose of DBP was possibly due to the reduction of secretion of seminiferous tubular fluid. Alternatively, it could be due to cell death (apoptosis) or extensive sloughing of germ cells, as has been shown in rats and mice (Alam et al. 2010a; Zhu et al. 2010). According to Nakai et al. (1992), sloughed germ cells can block the efferent ductules and, consequently, decrease the tubular diameter due to fluid back-up. It has been established that there is a strong positive relationship between the seminiferous tubular diameter and spermatogenesis (Franca and Russell 1998; Franca and Godinho 2003). Nair et al. (2008), in Wistar rats, also reported that DBP exposure produced dose-dependent reproductive toxicity due to significant reductions in STD.
The current study has further revealed that the SEH was significantly reduced in all DBP-exposed groups, throughout the 30-day DBP exposure period. This was expected since it has been established that spermatogenesis is particularly sensitive to several environmental toxicants in rats (Wong and Cheng 2011; Manfo et al. 2014). The quail testis has also been shown to be susceptible to the effects of some environmental toxicants (Aire 2005; Bello et al. 2014, 2019). Furthermore, our present findings corroborate similar observations in rodents, showing that most phthalates adversely affect testicular differentiation; as well as adult spermatogenesis in the adult rodent by provoking germ cell loss, testicular atrophy, and altered Leydig cell function (Ge et al. 2007; Spade et al. 2015). It should be noted that SEH was reduced regardless of the DBP doses, indicating a possible suppression of active spermatogenesis because it is known that most phthalates, including DBP, disrupt active spermatogenesis at several stages (Boekelheide 2005; Boekelheide et al. 2009). The thinning of the seminiferous epithelium and subsequent decrease in seminiferous tubular diameter was probably due to cell death (apoptosis) and the sloughing of germ cells (Alam et al. 2010a; Zhu et al. 2010). These studies corroborate the current findings that the sloughing of germ cells in the seminiferous epithelium resulted in reduced SEH, as morphometric alterations may have been a consequence of germ cell apoptosis (Ünal et al. 2013). In contrast, a recent in utero study showed that gestational exposure to high DBP dose altered seminiferous tubule morphometry and inhibited the proliferation of fetal testicular somatic cells, but did not affect apoptosis (Boekelheide et al. 2009). This observation may indicate that decreased proliferation, rather than increased apoptosis, is the underlying mechanism of altered fetal development of seminiferous tubules in DBP-exposed animals or birds. The present study has further demonstrated the potential of computer-assisted micro-stereological analysis in assessing the seminiferous tubular function that ordinarily is not possible to determine or quantify due to subtle morphological alterations that are not amenable to routine light microscopy. It has also been shown that the progressive morphometric alterations seen in the seminiferous epithelium, were, in fact, due to the direct toxic effects of DBP on testicular tubular histo-dynamics. These observed effects have been dose-dependent (as seen from the observations from cyto-architectural changes) and parameter-specific, resulting in the thinning of SEH, increase in diameter of tubular lumen, decreases in Leydig cell size.
Numerous in vivo mammalian studies have shown DBP to disrupt the androgen-regulated development of the male reproductive tract (Mylchreest and Foster 2000; Kavlock et al. 2002; Saillenfait et al. 2008; de Falco et al. 2015). Specifically, these results have indicated that phthalates may induce diverse adverse effects depending on the timing of exposure, the sex of the animal, and the critical developmental window. In certain phthalates such as DEHP, low doses administered at pre- and post-natal stage or to prepubertal and the adult rat was able to induce functional perturbations of Leydig, Sertoli, and germ cells (Akingbemi et al. 2007; Sharpe et al. 2003; Saillenfait et al. 2008; Alam et al. 2010a; Johnson et al. 2012; Walker et al. 2021). Likewise, higher doses of DBP (200, 400, and 600 mg/kg) exposure have been reported to induce testicular malformations in the male, resulting into seminiferous tubular necrosis and the absence of spermatogenesis in rats (Aly et al. 2016).
On the other hand, phthalates have been shown to interfere with the regulation of the hypothalamic-pituitary–gonadal (HPG) axis by alterations of gonadotropin-releasing hormone (GnRH), luteinizing hormone (LH), and follicle-stimulating hormone (FSH) (Jin and Yang 2014; Meltzer et al. 2015; Hlisníková et al. 2020), leading to downstream consequences on some steroidogenic enzymes and altered sex hormones. These findings suggest that DBP-induced changes are anti-androgenic, potentially suppressing Leydig cell steroidogenesis and active spermatogenesis. And when the present study is viewed in parallel with our previous report (Bello et al. 2014), showing DBP effects on enzyme genes that regulate Leydig cell steroidogenesis without changing cellular testosterone levels, the DBP effect on cyto-architectural ultrastructural alterations may compromise the structural integrity of the affected Leydig cells and thereby modifying the form (i.e., structural cell organelles) and ultimately the cell functions; and with attendant downstream reproductive consequences on the steroidogenic machinery (Alam and Kurohmaru 2021). For instance, the increased size and preponderance of electron-lucent lipid droplets or more precisely, “the cholesteryl esters” by Leydig cells implies that cholesterol utilization for androgen, such as testosterone biosynthesis was decreased. Thus, the absence of effects on serum testosterone levels, as reported previously by Bello et al. (2014), may have significantly been altered in the experimental animals due to one or both of two factors, namely, increased Leydig cell numbers arising from phthalate-induced proliferation (Akingbemi et al. 2001, 2004) or a decrease in testosterone degradation and excretion from the body (Eveillard et al. 2009).
In conclusion, the present findings have provided information on the deleterious effects of DBP in the testes of adult male Japanese quail exposed to DBP prepubertally, as well as validating techniques that are able to determine chemically-induced cellular alterations on Leydig cell steroidogenic function, from both micro-stereological and ultrastructural assessment perspectives.
Data availability
The data that support the findings of this study are available from the corresponding author (UMB), upon reasonable request.
References
Adkins EK (1979) Effect of embryonic treatment with estradiol or testosterone on sexual differentiation of the quail brain: critical period and dose-response relationships. Neuroendocrinology 29:178–185. https://doi.org/10.1159/000122920
Ahbad MA, Barlas N (2013) Developmental effects of prenatal di-n-hexyl phthalate and dicyclohexyl phthalate exposure on reproductive tract of male rats: postnatal outcomes. Food Chem Toxicol 51:123–136. https://doi.org/10.1016/j.fct.2012.09.010
Aire TA (2005) Short-term effects of carbendazim on the gross and microscopic features of the testes of Japanese quails (Coturnix coturnix japonica). Anat Embryol 210:43–49. https://doi.org/10.1007/s00429-005-0001-0
Akingbemi BT, Youker RT, Sottas CM, Ge R-S, Katz E, Klinefelter GR, Zirkin BR, Hardy MP (2001) Modulation of rat Leydig cell steroidogenic function by di (2-ethylhexyl) phthalate. Biol Reprod 65:1252–1259. https://doi.org/10.1095/biolreprod65.4.1252
Akingbemi BT, Ge R, Klinefelter GR, Zirkin BR, Hardy MP (2004) Phthalate-induced Leydig cell hyperplasia is associated with multiple endocrine disturbances. PNAS 101:775–780. https://doi.org/10.1073/pnas.0305977101
Alam MS, Andrina BB, Tay TW, Tsunekawa N, Kanai Y, Kurohmaru M (2010a) Single administration of di(n-butyl) phthalate delays spermatogenesis in prepubertal rats. Tissue Cell 42:129–135. https://doi.org/10.1016/j.tice.2010.02.004
Alam MS, Ohsako S, Matsuwaki T, Zhu XB, Tsunekawa N, Kanai Y, Sone H, Tohyama C, Kurohmaru M (2010b) Induction of spermatogenic cell apoptosis in prepubertal rat testes irrespective of testicular steroidogenesis: a possible estrogenic effect of di(n-butyl) phthalate. Reproduction 139:427–437. https://doi.org/10.1530/rep-09-0226
Alam MS, Kurohmaru M (2021) Di-n-butyl phthalate diminishes testicular steroidogenesis by blocking the hypothalamic-pituitary-testicular axis: relationship with germ cell apoptosis in Japanese quail. Reprod Fertil Dev 33:319–332. https://doi.org/10.1071/rd20150
Aly HA, Hassan MH, El-Beshbishy HA, Alahdal AM, Osman AMM (2016) Dibutyl phthalate induces oxidative stress and impairs spermatogenesis in adult rats. Toxicol Ind Health 32:1467–1477. https://doi.org/10.1177/0748233714566877
Andrady AL, Neal MA (2009) Applications and societal benefits of plastics. Philos Trans R Soc Lond B Biol Sci 364:1977–1984. https://doi.org/10.1098/rstb.2008.0304
Andriana BB, Tay TW, Maki I, Awal MA, Kanai Y, Kurohmaru M, Hayashi Y (2004a) An ultrastructural study on cytotoxic effects of mono (2-ethylhexyl) phthalate (MEHP) on testes in Shiba goat in vitro. J Vet Sci 5:235–240. https://doi.org/10.4142/jvs.2004.5.3.235
Andriana BB, Tay TW, Tachiwana T, Sato T, Ishii M, Awal MA, Kanai Y, Kurohmaru M, Hayashi Y (2004b) Effects of mono (2-ethylhexyl) phthalate (MEHP) on testes in rats in vitro. Okajima Folia Anat Jap 80:127–136. https://doi.org/10.2535/ofaj.80.127
Auharek SA, deFranca LR, Mc Kinnell C, Jobling MS, Scott HM, Sharpe RM (2010) Prenatal plus postnatal exposure to di (n-butyl) phthalate and/or flutamide markedly reduces final Sertoli cell number in the rat. Endocrinology 151. https://doi.org/10.1210/en.2010-0108
Aoki KA, Harris CA, Katsiadaki I, Sumpter JP (2011) Evidence suggesting that di-n-butyl phthalate has antiandrogenic effects in fish. Environ Toxicol Chem 30:1338–1345. https://doi.org/10.1002/etc.502
Ayache J, Beaunier L, Boumendil J, Ehret G, Laub D (2010) Sample preparation handbook for transmission electron microscopy. Springer, New York, NY
Bell FP (1982) Effects of phthalate esters on lipid metabolism in various tissues, cells andorganelles in mammals. Environ Health Perspect 45:41–50. https://doi.org/10.1289/ehp.824541
Bello UM, Madekurozwa M-C, Groenewald HB, Aire TA, Arukwe A (2014) The effects on steroidogenesis and histopathology of adult male Japanese quails (Coturnix coturnix japonica) testis following pre-pubertal exposure to di(n-butyl) phthalate (DBP). Comp Biochem Physiol C Toxicol Pharmacol 166:24–33. https://doi.org/10.1016/j.cbpc.2014.06.005
Bello UM, Aire TA, Imam J, Abdulazeez J, Igbokwe CO (2019) Dose-specific morphological changes in the Sertoli cell of the adult male Japanese quails testes exposed to di(n-butyl) phthalate DBP prepubertally. FASEB J 33(S1):802–878. https://doi.org/10.1096/fasebj.2019.33.1_supplement.802.78
Blanco A, Moyano R, López AM, Blanco C, Flores-Acuña R, García-Flores JR, Espada M, Monterde JG (2010) Preneoplastic and neoplastic changes in the Leydig cells population in mice exposed to low doses of cadmium. Toxicol Ind Health 26:451–457. https://doi.org/10.1177/0748233710371111
Boekelheide K (2005) Mechanisms of toxic damage to spermatogenesis. J Natl Cancer Inst Monogr 34:6–8. https://doi.org/10.1093/jncimonographs/lgi006
Boekelheide K, Kleymenova E, Liu K, Swanson C, Gaido KW (2009) Dose-dependent effects on cell proliferation, seminiferous tubules, and male germ cells in the fetal rat testis following exposure to di(n-butyl) phthalate. Microsc Res Tech 72:629–638. https://doi.org/10.1002/jemt.20684
Cheng CY, Wong EW, Lie PP, Li MW, Su L, Siu ER, Yan HHN, Mannu J, Mathur PP, Bonanomi M, Silverstrini B, Mruk DD (2011) Environmental toxicants and male reproductive function. Spermatogenesis 1:2–13. https://doi.org/10.4161/spmg.1.1.13971
Creasy DM (2003) Evaluation of testicular toxicology: a synopsis and discussion of the recommendations proposed by the Society of Toxicologic Pathology. Birth Defects Res B Dev Reprod Toxicol 68:408–415. https://doi.org/10.1002/bdrb.10041
Cruz-Orive LM (1993) Systematic sampling in stereology. Bull Int Stat Inst 55:451–468
Dalgaard M, Nellemann MC, Lam HR, Sørensen IK, Ladefoged O (2001) The acute effects of mono (2-ethylhexyl) phthalate (MEHP) on testes of prepubertal Wistar rats. Toxicol Lett 122:69–79. https://doi.org/10.1016/S0378-4274(01)00348-4
David RM, Moore MR, Finney DC, Guest D (2000) Chronic toxicity of di (2-ethylhexyl) phthalatein rats. Toxicol Sci 55:433–443. https://doi.org/10.1093/toxsci/b55.2.433
De Falco M, Forte M, Laforgia V (2015) Estrogenic and anti-androgenic endocrine disrupting chemicals and their impact on the male reproductive system. Front Environ Sci 3:3. https://doi.org/10.3389/fenvs.2015.00003
De Lange HJ, Lahr J, Van der Pol JJ, Wessels Y, Faber JH (2009) Ecological vulnerability in wildlife: an expert judgement and multi criteria analysis tool using ecological traits to assess relative impact of pollulants. Environ Toxicol Chem 28:2233–2240. https://doi.org/10.1897/08-626.1
Diamanti-Kandarakis E, Bourguignon J-P, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore A (2009) Endocrine-disrupting chemicals: an endocrine society scientific statement. Endocr Rev 30:293–342. https://doi.org/10.1210/er.2009-0002
Eveillard A, Lassere F, deTayrac M, Polizzi A, Claus S, Canlet C, Mselli-Lakhal L, Gotardi G, Paris A, Guillou H, Martin PGP, Pineau T (2009) Identification of potential mechanisms of toxicity after di-(2-ethylhexyl)-phthalate (DEHP) adult exposure in the liver using a systems biology approach. Toxicol Appl Pharmacol 236:282–292. https://doi.org/10.1016/j.taap.2009.02.008
Foster PMD, Mylchreest E, Gaido KW, Sar M (2001) Effects of phthalate esters on the developing reproductive tract of male rats. Hum Reprod Update 7:231–235. https://doi.org/10.1093/humupd/7.3.231
Franca LR, Godiho CL (2003) Testis morphometry, seminiferous epithelium cycle length, and daily sperm production in domestic cats (Felis catus). Biol Reprod 68:1554–1561. https://doi.org/10.1095/biolreprod.102.010652
Franca LR, Russell LD (1998) The testis of domestic animals. In: Martinez F, Regadera J (eds) Male reproduction, a multidisciplinary overview. Churchill Livingstone, Madrid, Spain, pp 197–219
Fujimoto T, Parton RG (2011) Not just fat: the structure and function of the lipid droplet. Cold Spring Harb Perspect Biol 3:3. https://doi.org/10.1101/cshperspect.a004838
Ge R-S, Chen G-R, Tanrikut C, Hardy MP (2007) Phthalate ester toxicity in Leydig cells: developmental timing and dosage considerations. Reprod Toxicol 23:366–373. https://doi.org/10.1016/j.reprotox.2006.12.006
Giribabu N, Sainath SB, Reddy PS (2014) Prenatal di-n-butyl phthalate exposure alters reproductive functions at adulthood in male rats. Environ Toxicol 29:534–544. https://doi.org/10.1002/tox.21779
Griswold SL, Behringer RR (2009) Fetal Leydig cell origin and development. Sex Dev 3:1–15. https://doi.org/10.1159/000200077
Gundersen HJ, Jensen EB (1987) The efficiency of systematic sampling in stereology and its prediction. J Microsc 147:229–263. https://doi.org/10.1111/j.1365-2818.1987.tb02837.x
Hannas BR, Furr J, Lambright CS, Wilson VS, Foster PMD, Gray LE Jr (2011) Di-pentyl phthalate dosing during sexual differentiation disrupts fetal testis function and postnatal development of the male sprague-dawley rat with greater relative potency than other phthalates. Toxicol Sci 120:184–193. https://doi.org/10.1093/toxsci/kfq386
Hales DB, Allen JA, Shankara T, Janus P, Buck S, Diemer T, Hales KH (2005) Mitochondrial function in Leydig cell steroidogenesis. Ann NY Acad Sci 1061:120–134. https://doi.org/10.1196/annals.1336.014
Hess RA, Franca LRD (2009) Spermatogenesis and cycle of the seminiferous epithelium. In: Cheng CY(eds) Molecular mechanisns in Spermatogenesis Adv Exp Med Biol 636:1–15 Springer, New York, NY. https://doi.org/10.1007/978-0-387-09597-4_1
Hlisníková H, Petrovičová I, Kolena B, Šidlovská M, Sirotkin A (2020) Effects and mechanisms of phthalates’ action on reproductive processes and reproductive health: a literature review. Int J Environ Res Public Health 17:6811. https://doi.org/10.3390/ijerph17186811
Hu J, Zhang Z, Shen W-J, Azhar S (2010) Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr Metab (lond) 7:47. https://doi.org/10.1186/1743-7075-7-47
Hubinger JC, Havery DC (2006) Analysis of consumer cosmetic products for phthalate esters. J Cosmet Sci 57:127–137
Ito Y, Yokota H, Wang R, Yamanoshita O, Ichihara G, Wang H, Kurata Y, Takagi K, Nakajima T (2005) Species differences in the metabolism of di(2-ethylhexyl) phthalate (DEHP) in several organs of mice, rats, and marmosets. Archiv Toxicol 79:147–154. https://doi.org/10.1007/s00204-004-0615-7
Jenardhanan P, Panneerselvam M, Mathur PP (2016) Effect of environmental contaminants on spermatogenesis. Semin Cell Dev Biol 59:126–140. https://doi.org/10.1016/j.semcdb.2016.03.024
Jin JM, Yang WX (2014) Molecular regulation of hypothalamus–pituitary–gonads axis in males. Gene 551:15–25. https://doi.org/10.1016/j.gene.2014.08.048
Jobling MS, Hutchison GR, van den Driesche S, Sharpe RM (2011) Effects of di(n-butyl) phthalate exposure on foetal rat germ-cell number and differentiation: identification of age-specific windows of vulnerability. Int J Androl 34:23–42. https://doi.org/10.1111/j.1365-2605.2010.01140.x
Johnson KJ, Heger NE, Boekelheide K (2012) Of mice and men (and rats): phthalate-induced fetal testis endocrine disruption is species-dependent. Toxicol Sci 129:235–248. https://doi.org/10.1093/toxsci/kfs206
Kavlock R, Boekelheide K, Chapin R, Cunningham M, Faustman E, Foster P, Golub M, Henderson R, Hinberg I, Little R, Seed J, Shea K, Tabacova S, Tyl R,Williams P, Zacharewski T (2002) NTP center for the evaluation of risks to human reproduction: phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reprod Toxicol 16:529–653
Kraemer FB, Khor VK, Shen W-J, Azhar S (2013) Cholesterol ester droplets and steroidogenesis. Mol Cell Endocrinol 371:15–29. https://doi.org/10.1016/j.mce.2012.10.012
Lee SK, Veeramachaneni DR (2005) Subchronic exposure to low concentrations of di-n-butyl phthalate disrupts spermatogenesis in Xenopus laevis frogs. Toxicol Sci 84:394–407. https://doi.org/10.1093/toxsci/kfi087
Manfo FPT, Nantia EA, Mathur PP (2014) Effect of environmental contaminants on mammalian testis. Curr Mol Pharmacol 7:119–135. https://doi.org/10.2174/1874467208666150126155420
Manna PR, Cohen-Tannoudji J, Counis R, Garner CW, Huhtaniemi I, Kraemer FB, Stocco DM (2013) Mechanisms of action of hormone-sensitive lipase in mouse Leydig cells: its role in the regulation of the steroidogenic acute regulatory protein. J Biol Chem 2889:8505–8518. https://doi.org/10.1074/jbc.m112.417873
Meltzer D, Martinez-Arguelles DB, Campioli E, Lee S, Papadopoulos V (2015) In utero exposure to the endocrine disruptor di(2-ethylhexyl) phthalate targets ovarian theca cells and steroidogenesis in the adult female rat. Reprod Toxicol 51:47–56. https://doi.org/10.1016/j.reprotox.2014.12.005
Moody S, Goh H, Bielanowicz A, Rippon P, Loveland KL, Itman C (2013) Prepubertal mouse testis growth and maturation and androgen production are acutely sensitive to di-n-butyl phthalate. Endocrinol 154:3460–3475. https://doi.org/10.1210/en.2012-2227
Moffit JS, Bryant BH, Hall SJ, Boekelheide K (2007) Dose-dependent effects of sertoli cell toxicants 2, 5-hexanedione, carbendazim, and mono-(2-ethylhexyl) phthalate in adult rat testis. Toxicol Pathol 35:719–727. https://doi.org/10.1080/01926230701481931
Mylchreest E, Foster PM (2000) DBP exerts its antiandrogenic activity by indirectly interfering with androgen signaling pathways. Toxicol Appl Pharmacol 168:174–175. https://doi.org/10.1006/taap.2000.9031
Mylchreest E, Wallace DG, Cattley RC, Foster PM (2000) Dose-dependent alterations in androgen-regulated male reproductive development in rats exposed to di (n-butyl) phthalate during late gestation. Toxicol Sci 55:143–151. https://doi.org/10.1093/toxsci/55.1.143
Nair N, Bedwal S, Kumari D, Bedwal S, Bedwal RS (2008) Effect on histological and sperm kinetics in DBP exposed Wistar rats. J Environ Biol 29:769–772
Nakai M, Hess RA, Moore BJ, Guttroff RF, Strader LF, Linder RE (1992) Acute and long-term effects of a single dose of the fungicide carbendazim (methyl 2-benzimidazole carbamate) on the male reproductive system in the rat. J Androl 13:507–518
Organization for Economic Co-operation and Development (OECD) (2010) Revised draft proposal for a new OECD test guidelines ‘Avian Reproduction Toxicity in the Japanese Quail or Northern Bobwhite’. http://www.oecd.org/chemicalsafety/testing/avian-toxicity-testing.htm. Accessed 05 Dec 2019
Oehlmann J, Schulte-Oehlmann U, Kloas W, Jagnytsch O, Lutz I, Kusk KO, Wollenberger L, Santos EM, Paull GC, Van Look KJW, Tyler CR (2009) A critical analysis of the biological impacts of plasticizers on wildlife. Philos Trans R Soc Lond B Biol Sci 64:2047–2062. https://doi.org/10.1098/rstb.2008.0242
Ottinger MA, Quinn MJ Jr, Lavoie E, Abdelnabi MA, Thompson N, Hazelton JL, Wu JM, Beavers J, Jaber M (2005) Consequences of endocrine disrupting chemicals on reproductive endocrine function in birds: establishing reliable end points of exposure. Domest Anim Endocrinol 29:411–419. https://doi.org/10.1016/j.domaniend.2005.02.038
Perico G, Pereira J, Selbourne MDC, Limbo S, Pocas F (2022) Screening of diesters of ortho-phthalic acid in printed baby bibs in the European market. Packag Technol Sci 35:241–249. https://doi.org/10.1002/pts.2622
Qin X, Ma Q, Yuan J, Hu X, Tan Q, Zhang Z, Wang L, Xu X (2018) The effects of di-2-ethylhexyl phthalate on testicular ultrastructure and hormone-regulated gene expression in male rats. Toxicol Res 7:408–414
Romano RM, Romano MA, Bernardi MM, Furtado PV, Oliveira CA (2010) Prepubertal exposure to commercial formulation of the herbicide glyphosate alters testosterone levels and testicular morphology. Archiv Toxicol 84:309–317. https://doi.org/10.1007/s00204-009-0494-z
Saillenfait AM, Sabaté JP, Gallissot F (2008) Diisobutyl phthalate impairs the androgen-dependentreproductive development of the male rat. Reprod Toxicol 26:107–115. https://doi.org/10.1016/j.reprotox.2008.07.006
SANS Guidelines (2008) South African National Standard: the care and use of animals for scientific purposes. SABS Publisher, Pretoria, South Africa
Savchuk I, Söder O, Svechnikov K (2015) Mono-2-ethylhexyl phthalate stimulates androgen production but suppresses mitochondrial function in mouse Leydig cells with different steroidogenic potential. Toxicol Sci 145:149–156. https://doi.org/10.1093/toxsci/kfv042
Schettler T (2006) Human exposure to phthalates via consumer products. Int J Androl 29:134–139. https://doi.org/10.1111/j.1365-2605.2005.00567.x
Sharpe RM, McKinnell C, Kivlin C, Fisher JS (2003) Proliferation and functional maturation of Sertoli cells and their relevance to disorders of testis function in adulthood. Reproduction 125:769–784. https://doi.org/10.1530/rep.0.1250769
Shima Y, Miyabayashi K, Haraguchi S, Arakawa T, Otake H, Baba T, Matsuzaki S, Shishido Y, Akiyama H, Tachibana T, Tsutsui K, Morohashi K (2013) Contribution of Leydig and Sertoli cells to testosterone production in mouse fetal testes. Mol Endocrinol 27:63–73. https://doi.org/10.1210/me.2012-1256
Shirai M, Wakui S, Wempe MF, Mutou T, Oyama N, Motohashi M, Takahashi H, Kansaku N, Asari M, Hano H, Endou H (2013) Male Sprague-Dawley rats exposure to in utero di(n-butyl) phthalate: dose-dependent and age-related morphological change in Leydig cells smooth endoplasmic reticulum. Toxicol Pathol 41:984–991. https://doi.org/10.1177/0192623312474725
Shono T, Taguchi T (2014) Short-time exposure to mono-n-butyl phthalate (MBP)-induced oxidative stress associated with DNA damage and the atrophy of the testis in pubertal rats. Environ Sci Pollut Res Int 21:3187–3190. https://doi.org/10.1007/s11356-013-2332-3
Shen W-J, Azhar S, Kraemer FB (2016) Lipid droplets and steroidogenic cells. Exp Cell Res 340:209–214. https://doi.org/10.1016/j.yexcr.2015.11.024
Spade DJ, Hall SJ, Wilson S, Boekelheide K (2015) Di-n-butyl phthalate induces multinucleated germ cells in the rat fetal testis through a nonproliferative mechanism. Biol Reprod 93:1–10. https://doi.org/10.1095/biolreprod.115.131615
Tarique I, Vistro WA, Bai X, Yang P, Hong C, Huang Y, Haseeb A, Liu E, Gandahi NS, Xu M, Liu Y, Chen Q (2019) LIPOPHAGY: a novel form of steroidogenic activity within the LEYDIG cell during the reproductive cycle of turtle. Reprod Biol Endocrinol 17:(1)1–12. https://doi.org/10.1186/s12958-019-0462-2
Tay TW, Andriana BB, Ishii M, Choi EK, Zhu XB, Alam MS, Tsunekawa N, Kanai Y, Kurohmaru M (2007) An ultrastructural study on the effects of mono (2-ethylhexyl) phthalate on mice testes: cell death and sloughing of spermatogenic cells. Okajimas Folia Anat Jpn 83:123–130. https://doi.org/10.2535/ofaj.83.123
Ünal SG, Take G, Erdoğan D, Göktas G, Sahin E (2013) The effect of di-n-butyl phthalate on testis and the potential protective effects of resveratrol. Toxicol Ind Health 1:14. https://doi.org/10.1177/0748233713512364
Wakui S, Motohashi M, Satoh T, Shirai M, Mutou T, Takahashi H, Wempe MF, Endou H, Inomata T, Asari M (2013) Nuclear morphometric analysis of Leydig cells of male pubertal rats exposed in utero to di(n-butyl) phthalate. J Toxicol Pathol 26:439–446. https://doi.org/10.1293/tox.2013-0031
Walker C, Garza S, Papadopoulos V, Culty M (2021) Impact of endocrine-disrupting chemicals on steroidogenesis and consequences on testicular function. Mol Cell Endocrinol 527:111215
Wan HT, Mruk DD, Wong CK, Cheng CY (2013) Targeting testis-specific proteins to inhibit spermatogenesis: lesson from endocrine disrupting chemicals. Expert Opin Ther Targets 17:839–855. https://doi.org/10.1517/14728222.2013.791679
Wong EW, Cheng CY (2011) Impacts of environmental toxicants on male reproductive dysfunction. Trends Pharmacol Sci 32:290–299. https://doi.org/10.1016/j.tips.2011.01.001
Yeung BH, Wan HT, Law AYS, Wong CKC (2011) Endocrine disrupting chemicals: Multiple effects on testicular signaling and spermatogenesis. Spermatogenesis 1:231–239. https://doi.org/10.4161/spmg.1.3.18019
Zhang Y, Jiang X, Chen B (2004) Reproductive and developmental toxicity in F1 Sprague-Dawley male rats exposed to di-n-butyl phthalate in utero and during lactation and determination of its NOAEL. Reprod Toxicol 18:669–676
Zhu XB, Tay TW, Andriana BB, Alam MS, Choi EK, Tsunekawa N, Kanai Y, Kurohmaru M (2010) Effects of di-iso-butyl phthalate on testes of prepubertal rats and mice. Okajimas Folia Anat Jpn 86:129–136. https://doi.org/10.2535/ofaj.86.129
Acknowledgements
The authors wish to acknowledge technical assistance received from Dr. Lizette du Plessis (Electron Microscopy Unit, Ondersteeport campus) and Ms. Erna van Wilpe (Laboratory for Microscopy and Microanalysis, Hatfield campus) of the University of Pretoria. In addition, sincere thanks to Dr. Sifi Ibrahim of Université Amar Telidji, Laghouat, Algeria for help at the initial stage of data interpretation and analysis.
Funding
Open access funding provided by University of Pretoria. This study was supported by research grants to UMB, from the University of Pretoria and South African Veterinary Foundation (SAVF)/Norvatis Wildlife Foundation fund.
Author information
Authors and Affiliations
Contributions
Umar M. Bello: conceptualization, investigation, methodology, data curation, writing—original draft preparation, software, formal analysis, Mary-Cathrine Madekurozwa, Hermanus B. Groenewald, Tom A. Aire, and Augustine Arukwe: supervision, project administration, writing—review and editing, formal analysis, resources. All the authors read, reviewed, and approved the final version of the manuscript to be published.
Corresponding author
Ethics declarations
Ethical approval
The experimental procedures were performed according to the criteria outlined in the Guidelines for the Care and Use of Animals for Scientific purposes (SANS Guidelines 2008), and the animal study protocol was approved by the Institutional Animal Ethics Committee (AEC) of the Unversity of Pretoria vide Ethical Clearance (Certificate No. V058/12-Original document) issued. Precautions were taken during sampling and throughout the entire experiment to minimize animal suffering.
Consent to participate
All the authors of this work agree with the content and give their explicit consent to submit it. In addition, all the authors obtained the consent of the responsible authorities of the institute where the work was carried out before submitting the work.
Consent for publication
All the authors agree for consent for the publication and the current article does not contain data from any individual person.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bello, U.M., Madekurozwa, MC., Groenewald, H.B. et al. Changes in testicular histomorphometry and ultrastructure of Leydig cells in adult male Japanese quail exposed to di (n-butyl) phthalate (DBP) during the prepubertal period. Environ Sci Pollut Res 30, 55402–55413 (2023). https://doi.org/10.1007/s11356-023-25767-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-25767-2